ABSTRACT
Mooren’s ulcer (MU) is a chronic and painful ulcerative keratitis that is difficult to diagnose, especially when concealed beneath the pterygium, which is a common, benign, wedge-shaped, fleshy tissue growth of the conjunctiva extending onto the cornea. The coexistence of MU and pterygium is extremely rare. A 41-year-old man presented with a 2-month history of unprovoked redness, pain, and blurred vision in the right eye. Corneal epithelial defects around the pterygium head were noted upon slit-lamp examination and fluorescein staining. The patient was initially misdiagnosed with a corneal epithelial defect and pterygium. The initial treatments with anti-inflammatory and corneal epithelial growth promotion tear agents failed. Anterior segment optical coherence tomography (AS-OCT) showed corneal stromal lysis thinning, and in vivo confocal microscopy (IVCM) revealed marked inflammatory cell infiltration and stromal degeneration. We suspected the pathology was an immune-related or tumor-related corneal ulcer. The MU concealed beneath the pterygium was diagnosed by histopathological examination of a biopsy specimen that presented typical localized loss of the corneal epithelium and Bowman’s layer, stromal degeneration, and inflammatory cell infiltration. Finally, we performed lamellar keratoplasty (LKP) combined with pterygium excision surgery. The patient recovered with no complications or recurrence during the 1-year follow-up period. Few cases of MU concealed beneath the pterygium have been reported. It is beneficial to rule out the pathological changes concealed beneath the pterygium, combined with multiple means of examination such as slit-lamp examination, AS-OCT, and IVCM. A histopathological examination should be performed to establish a diagnosis.
Introduction
Mooren’s ulcer (MU) is a chronic, spontaneous, and painful ulcerative keratitis that initially affects the peripheral cornea and progressively spreads centrally [Citation1,Citation2]. MU is rare worldwide, with an incidence of approximately 0.03% in China [Citation3] and its etiopathogenesis remains unclear [Citation2,Citation4]. Several studies have reported that MU is an autoimmune disease targeting the corneal stroma [Citation5,Citation6]. Chi et al have identified cornea-associated antigens in the corneal stroma [Citation7]. Risk factors include intestinal parasitic infection [Citation8], chronic hepatitis C infection [Citation9,Citation10], ocular trauma [Citation11,Citation12], corneal surgery [Citation13], and infection [Citation3,Citation5].
The diagnosis of MU is based on medical history, ophthalmological examination (slit-lamp examination, optical coherence tomography [OCT], or in vivo confocal microscopy [ICVM]), and laboratory tests: rheumatoid factor concentration, titers for antinuclear antibody, antistreptolysin O, human leukocyte antigen B27, anti-SSA and -SSB antibodies, and an erythrocyte suspension rate. The diagnosis also rules out other causes of ulceration at the edge of the cornea, such as Terrien’s degeneration, blepharokeratoconjunctivitis (BKC), granulomatosis with polyangiitis (GPA), rheumatoid arthritis, and systemic lupus erythematosus [Citation14].
Pterygium is a common, raised, benign, fleshy, wedge-shaped tissue on the marginal cornea, extending from the conjunctiva to the cornea [Citation15]. The coexistence of MU and pterygium is an extremely rare clinical condition that is easily misdiagnosed [Citation16–18]. Herein, we report a case of MU concealed beneath the pterygium that was first misdiagnosed as a corneal epithelial defect.
Materials and methods
Patient and examinations of the ocular surface
A 41-year-old male patient visited the Xiamen Eye Center, and an informed consent for publication was obtained. This case report was approved by the Ethics Committee of Xiamen University affiliated Xiamen Eye Center, and written informed consent was obtained from the patient to use the images of eye and other medical examination results in this manuscript.
The best spectacle-corrected visual acuity (BSCVA) was measured using a standard logarithmic visual acuity chart designed by a Chinese researcher and presented in the Snellen chart system [Citation19]. Images were taken with a slit lamp digital video camera (SL 990 N DIGITAL CSO, Costruzione Strumenti Oftalmici, SL 990 N, Italy). The AS-OCT images were taken with the Ophthalmic Optical Coherence Tomography System (RTVue XR, Optovue Inc., USA). In vivo confocal microscopy (IVCM) images of the cornea were taken with the Heidelberg Retina Tomograph (Heidelberg Engineering GmbH, Germany).
Hematoxylin and eosin staining
Fresh tissue samples were fixed in a tissue fixation solution consisting of ethanol and polyol (XW-RS-018, Guangzhou Xiuwei, China) for 24 hours then processed and embedded in paraffin. Sample sections 5 μm thick were cut and stained with hematoxylin (Harris, BA4097, BaSO Biotechnology, China) and eosin (Eosin, BA4099, BaSO Biotechnology) using a standard Harris’s H&E protocol. Images were captured using a BX53F2 microscope (Olympus Corp., Japan) with a camera from OLY-0.5X (Logene, China).
Results
The patient presented with unprovoked redness, severe pain, photophobia, lacrimation, and vision loss in the right eye, which had lasted for approximately 1 month. During the past 2 years, the patient noticed a membranous growth in his right eye with no ocular discomfort. There was no history of trauma, surgery to the right eye; and there was no systemic immune system diseases or parasitic infections. Because of corneal epithelium defects around the head of the pterygium, the patient was first diagnosed with pterygium with a corneal epithelial defect in the right eye by a local practitioner, followed by treatment with tobramycin dexamethasone eye drops for anti-inflammatory and recombinant bovine basic fibroblast growth factor eye drops to promote the wound-healing of corneal epithelium at the local hospital over a period of 1 month. However, ocular symptoms and signs did not improve substantially. The pain progressively worsened and became more pronounced at night.
The patient was then admitted to our hospital. The best corrected visual acuity (BSCVA) was 0.8 (0.1 in the LogMAR chart was abnormal as compared to the normal value of 0) in the right eye and 1.0 in the left eye. Intraocular pressure (IOP) was normal in both eyes. The examination by slit-lamp microscopy with a fluorescein dye revealed conjunctival congestion and nasal invasion of fibrous membranous material approximately 3 mm into the limbus, ring-shaped thinning of the peripheral cornea with infiltration, and epithelial defects in the lesions with no obvious secretions ( a1-a2) and the left was normal (Supplemetary s-). Moreover, anterior segment optical coherence tomography (AS-OCT) revealed thinning of cornea with stromal lysis ( b1-b2). IVCM revealed marked infiltration with a larger number of Langerhans cells and epithelial cell detachment in the lesion area, stromal exposure, loose structure, a small amount of inflammatory cell infiltration, deep stromal density, and invasion of new blood vessels ( and Supplementary s-).
Figure 1. The clinical characteristics from examinations of the patient’s right eye. (a) Slit-lamp microscopy examination. A pterygium in the nasal interpalpebral zone and a ring-shaped peripheral corneal ulcer with an infiltrated, overhanging edge along the head of the pterygium. (b) Fluorescein staining shows ring-shaped thinning of the peripheral cornea with infiltration and epithelial defects in the lesions with no obvious secretions. (c-d) AS-OCT showing corneal stromal lysis thinning. (b) thinnest point of the lesion measures 212 μm. (blue arrow) (e-g) IVCM examination. (e-f) revealed a marked inflammatory cell infiltration with epithelial cell detachment in the lesion area (yellow arrows), (g) a larger number of Langerhans cells (orange arrows), (h) has stromal exposure and loose structure, (i) a small number of infiltrating inflammatory cells and (j) shows deep stromal (keratocytes) densification and scar formation (light orange arrow), and invasion of new blood vessels (red arrows).
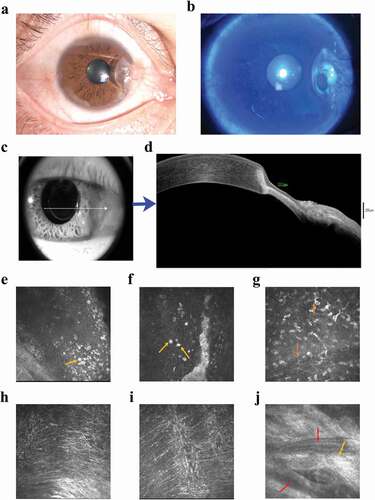
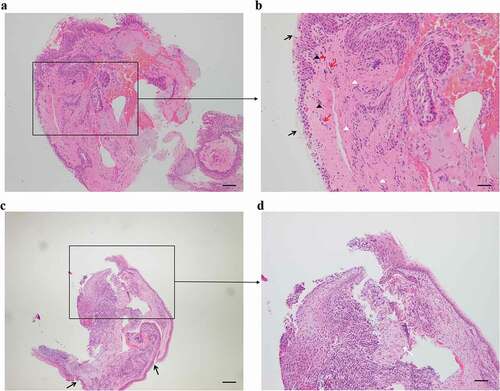
Based on the morphological findings, clinical signs and symptoms, we speculated that this condition was either an immune-related or tumor-related corneal ulcer coexisting with a pterygium. Except for an elevated erythrocyte sedimentation rate (ESR) of 32.1 mm/h (normal range: 0–15 mm/h), laboratory tests for systemic autoimmune diseases were all negative for rheumatoid factor, antistreptolysin O, human leukocyte antigen B27, anti-SSA and -SSB antibody, antinuclear antibody, and anti-neutrophil cytoplasmic antibody titers (). A histopathological examination of small tissue samples was performed to clarify the diagnosis and the next step of treatment. The typical histopathology of the pterygium was presented; corneal biopsy showed that the corneal epithelium was partially absent, and the anterior elastic membrane was invisible. Additionally, the stroma was infiltrated with lymphocytes, and some stromal degeneration showed the characteristics of MU (). Peripheral corneal ulcers caused by systemic diseases, such as granulomatosis with polyangiitis (GPA), rheumatoid arthritis, and systemic lupus erythematosus, were excluded based on comprehensive laboratory test results for systemic autoimmune diseases as stated above. Based on ulcer morphology, physical examination, and histopathological examination, the patient was diagnosed with MU in his right eye.
Table 1. Patient’s primary laboratory tests.
Figure 2. Hematoxylin-eosin staining of the biopsies (a and b zoom in insert). The typical histopathology of the pterygium. (b insert) shows an increase in goblet cells and epithelial hyperplasia of the conjunctiva (black arrows), invasion of new blood vessels (red arrows), hyperplasia of fibroblast cells (white triangles), stroma degeneration (white arrow), lymphocyte infiltration (red triangles) and neutrophils (black triangles). (c and d zoom in insert) Corneal tissue (c) with localized epithelial detachment and loss of the anterior elastic membrane (black arrows). (d insert) Fibrous degeneration of the superficial stroma with infiltration of inflammatory cells (white arrow) and a large infiltration of inflammatory cells in the deep stroma. Scale bar = 200 μm.
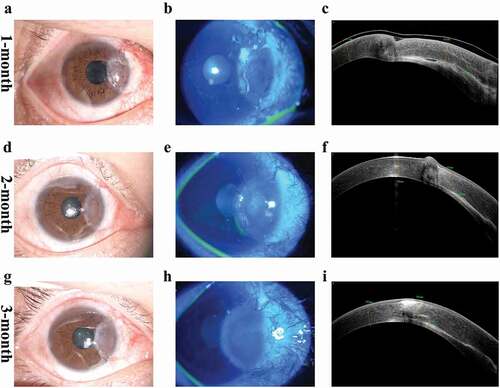
We then performed lamellar keratoplasty (LKP) and pterygium excision in the right eye to maintain corneal structure and prevent disease progression. The main surgical procedure is shown in Supplementary s- a-f). This graft gradually integrated with the patient’s corneal stroma and was completely re-epithelialized with invasive neovascularization during the 3- month follow-up period. (). A year later, due to the preventative strategies for coronavirus disease 2019 (COVID-19), the patient could not visit the outpatient clinic for follow-up which was conducted by telephone. At that time, the patient reported no eye pain or vision loss.
Figure 3. Postoperative slit-lamp ocular images, blue fluorescein staining, and AS-OCT black and white images at 1 month, 2 months, and 3 months. (a–c) At one month, the graft showed edema, fluid leakage at the graft-host interface and beginning of neovascularization. (d-f) at 2 months, the edema of the graft gradually vanished, fluid was absorbed and graft was closely attached to the recipient bed. (g-i) At 3 months, the graft had complete re-epithelialization and cornea exhibited a smooth anterior curve.
Discussion
Clinical manifestations of MU include severe pain, intense reddening, photophobia, and epiphora. The diagnosis of MU requires the absence of any ocular infection or systemic rheumatological diseases known to cause peripheral corneal ulceration, which is easily confused with Terrien’s degeneration, BKC, GPA, and other marginal corneal ulcers induced by rheumatoid arthritis or systemic lupus erythematosus [Citation14]. Terrien’s degeneration commonly occurs at the superior or superonasal aspect of the cornea [Citation20] and has fewer symptoms, such as pain, photophobia, lacrimation, and slow progression [Citation21]. BKC induces marginal corneal ulcers mostly located at the superior or superonasal aspect of the cornea and has a chronic inflammatory disorder of the eyelid [Citation22]. Corneal ulcers induced by GPA [Citation23,Citation24], rheumatoid arthritis [Citation25], and systemic lupus erythematosus [Citation26] are commonly secondary to systemic diseases. With the progression of redness, severe pain, photophobia, lacrimation, and vision loss, the patient’s condition was highly related to MU.
The corneal epithelial defect and corneal ulcer concealed beneath the pterygium makes it more challenging to clarify the diagnosis, especially for immune-related or tumor-related corneal ulcers. In such cases, a histopathological examination is helpful for the diagnosis. The histopathological characteristics of MU include lymphocyte infiltration and corneal stroma lysis or degeneration [Citation7]. However, Ding et al reported Terrien’s marginal degeneration is characterized by innumerable intracellular and extracellular vacuoles in the affected stroma and the absence of inflammatory cell infiltration [Citation21]. The tumor, such as squamous cell carcinoma, dermoid and conjunctival intraepithelial neoplasia, usually presents as atypical anaplasia, hyperplasia, or dysplasia [Citation27]. Based on the symptoms and multiple methods of examination (slit-lamp examination, AS-OCT, and IVCM) combined with histopathological examination, this patient was diagnosed with MU coexisting with a pterygium. Hence, histopathology played a crucial role in clarifying our diagnosis.
The treatments for MU vary from medication to surgery [Citation1]. Most medical treatments are based on immunomodulation, and surgical treatments that mainly involve removing diseased tissue and restoring corneal integrity. The common complications after surgery are graft-host interface leakage, graft rejection, and initial disease recurrence [Citation28]. MU recurrence remains a clinical challenge, with a recurrence rate of 25.5% [Citation3]. Kakizaki et al [Citation18]. Suggested that the head of a pterygium might induce a chronic inflammatory response in the cornea and the development of MU. The available literature does not provide much information on treating MU with pterygium.
Conservative medications were ineffective for our patient. We presume the corneal ulcer failed to recover completely because the coexisting pterygium persistently stimulated the immune response and irregular apophysis-induced symptoms, including ocular irritation and foreign body sensation. Therefore, we performed an LKP together with pterygium excision as seen in Supplementary s-. Because of preventive strategies for COVID-19, this patient could not undergo frequent outpatient follow-ups. The graft was integrated with the recipient corneal stroma and completely re-epithelialized with invasive neovascularization during the 3-month outpatient follow-up period. The patient did not complain of eye pain or vision loss during the 1-year telephone follow-up period. Previous studies have shown that initial postoperative recurrence occurs mostly within 6 months [Citation14]. Therefore, we concluded that the patient’s ulcer did not recur during the follow-up period.
Conclusion
In conclusion, MU can be easily misdiagnosed, resulting in inappropriate treatment. MU coexisting with a pterygium is extremely rare. The combination of histopathology, symptoms, signs, and multiple examinations, such as slit-lamp, AS-OCT, and IVCM, can effectively diagnose marginal corneal ulcers concealed beneath the pterygium. Combined surgery with LKP and pterygium excision may be an effective therapeutic approach for maintaining the corneal structure and preventing the progression of MU concealed beneath the pterygium.
Supplemental Material
Download TIFF Image (12.2 MB)Supplemental Material
Download TIFF Image (23 MB)Supplemental Material
Download TIFF Image (2 MB)Acknowledgments
All authors have read and approved the manuscript. Specifically, YJ and SO designed this study, performed the literature search, and wrote the manuscript. XF, ZX, and HW also helped design this study and held to collect the clinical data.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplemental data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/01478885.2022.2137666
Additional information
Funding
References
- Alhassan MB, Rabiu M, Agbabiaka IO. Interventions for mooren’s ulcer. Cochrane Database Syst Rev. 2014;(1):CD006131.
- Yang L, Xiao J, Wang J, et al. Clinical characteristics and risk factors of recurrent mooren’s ulcer. J Ophthalmol. 2017;2017:8978527.
- Chen J, Xie H, Wang Z, et al. Mooren’s ulcer in China: a study of clinical characteristics and treatment. Br J Ophthalmol. 2000;84(11):1244–1249.
- Alhassan MB, Rabiu M, Agbabiaka IO. Interventions for Mooren’s ulcer. Cochrane Database Syst Rev. 2011;15(6):CD006131.
- Dong Hyun Kim M, Mee Kum Kim M, PhD Joo Youn Oh M, et al. Mooren’s ulcer in a cornea referral practice in Korea. Ocul Immunol Inflamm. 2016;2016:55–59.
- Shinomiya K, Ueta M, Sotozono C, et al. Immunohistochemical analysis of inflammatory limbal conjunctiva adjacent to Mooren’s ulcer. Br J Ophthalmol. 2013;97(3):362–366.
- Chi H, Hao W, Qi X, et al. A proteomic approach towards understanding the pathogenesis of Mooren’s ulcer. Exp Eye Res. 2021;205:108509.
- Agarwal P, Singh D, Sinha G, et al. Bilateral Mooren’s ulcer in a child secondary to helminthic infestation of the gastrointestinal tract. Int Ophthalmol. 2012;32(5):463–466.
- Aaltonen V, Alavesa M, Pirilä L, et al. Case report: bilateral Mooren ulcer in association with hepatitis C. BMC Ophthalmol. 2017;17(1):239.
- Yeo JH, Kim KW, Kim JC. Mooren’s ulcerative keratitis after systemic pegylated interferon alpha2a in chronic hepatitis C. Can J Ophthalmol. 2017;52(5):e163–e167.
- Joondeph HC, McCarthy WL Jr., Rabb M, et al. Mooren’s ulcer: two cases occurring after cataract extraction and treated with hydrophilic lens. Ann Ophthalmol. 1976;8(2):187–194.
- Kim J, Kim MK, Wee WR, et al. Mooren ulcer in a child wearing orthokeratology Contact Lenses. Eye Contact Lens. 2018;44(4):e13–e15.
- Sangwan VS, Gupta S, Das S. Cataract surgery in ocular surface diseases: clinical challenges and outcomes. Curr Opin Ophthalmol. 2018;29(1):81–87.
- Chen J, Xie H, Wang Z, et al. Mooren’s ulcer in China: a study of clinical characteristics and treatment. Br J Ophthalmol. 2000;84(11):1244–1249.
- Murube J. Pterygium: its treatment with beta therapy. Ocul Surf. 2009;7(1):3–9.
- He N, Song W, Gao Y. Treatment of Mooren’s ulcer coexisting with a pterygium using an intrastromal lenticule obtained from small-incision lenticule extraction: case report and literature review. J Int Med Res. 2021;49(6):3000605211020246.
- Kim EC, Jun A, Kim MS, et al.Mooren ulcer occurring at donor site after contralateral conjunctivolimbal autograft for recurrent pterygium. Cornea. 2012;31(11):1357–1358.
- Kakizaki H, Zako M, Nakano T, et al. A case of mooren’s ulcer associated with a pterygium. Jpn J Ophthalmol. 2005;49(6):542–543.
- Wang S, Hao X, Ma X, et al. Associations between poor vision, vision-related behaviors and mathematics achievement in Chinese students from the CNAEQ-PEH 2015. Int J Environ Res Public Health. 2020;17(22):8561.
- Galvis V, Tello A, Niño CA, et al. Terrien Marginal Degeneration: clinical Characteristics and Outcomes. Am J Ophthalmol. 2016;164:151–152.
- Ding Y, Murri MS, Birdsong OC, et al. Terrien marginal degeneration. Surv Ophthalmol. 2019;64(2):162–174.
- Hammersmith KM. Blepharokeratoconjunctivitis in children. Curr Opin Ophthalmol. 2015;26(4):301–305.
- Moosig F, Lamprecht P, Gross WL. Wegener’s granulomatosis: the current view. Clin Rev Allergy Immunol. 2008;35(1–2):19–21.
- Ebrahimiadib N, Modjtahedi BS, Roohipoor R, et al. Successful treatment strategies in granulomatosis with polyangiitis-associated peripheral ulcerative keratitis. Cornea. 2016;35(11):1459–1465.
- Stylianides A, Jones MN, Stewart RM, et al. Rheumatoid arthritis-associated corneal ulceration: mortality and graft survival. Ophthalmology. 2013;120(4):682–686.
- Cao Y, Zhang W, Wu J, et al. Peripheral Ulcerative Keratitis Associated with autoimmune disease: pathogenesis and treatment. J Ophthalmol. 2017;2017:7298026.
- Shields CL, Demirci H, Karatza E, et al. Clinical survey of 1643 melanocytic and nonmelanocytic conjunctival tumors. Ophthalmology. 2004;111(9):1747–1754.
- Huang T, Wang Y, Ji J, et al. Evaluation of different types of lamellar keratoplasty for treatment of peripheral corneal perforation. Graefes Arch Clin Exp Ophthalmol. 2008;246(8):1123–1131.
