Abstract
The opioid agonist hydromorphone is indicated for the management of severe acute and chronic pain given that alternate treatments are insufficient. While the genotoxicity profile of hydromorphone is well investigated, little is known about the genotoxic potential of its impurities. In this study, 2,2-bishydromorphone was tested in silico and in vitro for both its mutagenic potential in an Ames test performed with Salmonella typhimurium and Escherichia coli tester strains up to a maximum concentration of 5 mg per plate in the absence and presence of metabolic activation. Furthermore, it was tested for its ability to induce micronuclei in TK6 cells in a micronucleus test up to a maximum concentration of 500 µg/mL with or without an exogenous metabolic activation system. 2,2-Bishydromorphone did not reveal any potential for inducing mutagenicity or clastogenicity under the conditions of the respective tests and is therefore considered non-mutagenic and non-clastogenic/aneugenic in vitro. These results are in line with negative in silico quantitative structure-activity relationship (QSAR) prediction for 2,2-bishydromorphone mutagenicity and clastogenicity and provide evidence of good correlation of in silico and in vitro data. Conclusively, these studies add important new clinically relevant information on the safety of hydromorphone as the impurity of 2,2-bishydromorphone is proven to be non-mutagenic and non-clastogenic.
Introduction
Hydromorphone hydrochloride (hydromorphone) is a hydrogenated ketone of morphine and an opioid analgesic indicated for the management of acute and chronic pain in settings, where non-opioid analgesics are considered insufficient (Fresenius Kabi Canada Ltd Citation2019).
Since hydromorphone cannot be manufactured as a 100% pure medicinal product, it contains some impurities and/or degradation products. As the patient safety is of utmost importance for medicinal products and the respective therapy, all impurities must fulfill well-defined pharmacologic criteria. In this context, it needs to be ensured that such impurities are not more toxic than the parental drug. One of the most critical toxicological endpoints of concern is mutagenicity and clastogenicity because of the enhanced risk of cancer.
The mutagenic and clastogenic potential of a medicinal product is not only related to the active pharmaceutical ingredient (API) but also to the API degradants (impurities) present or formed in the product. Hydromorphone was not mutagenic in the in vitro bacterial reverse mutation assay (Ames test) and not clastogenic in neither the in vitro human lymphocyte chromosome aberration assay nor the in vivo micronucleus test (MNT) but positive in the in vitro mouse lymphoma assay in the presence of metabolic activation (Center for Drug Evaluation and Research Citation2010). However, the weight of evidence suggests that hydromorphone is not considered as mutagenic or clastogenic.
Although the parent drug product is not of mutagenic concern, it does not mean that its impurities or degradation products can be considered as non-mutagenic as well. A prominent example currently being under scientific discussion are ranitidine-containing products (Wagner and Colombo Citation2020). Ranitidine is examined to be not mutagenic itself; however, scientific literature indicates that N-Nitrosodimethylamine (NDMA), a potential mutagen/carcinogen to humans, might be formed under certain physiological conditions (European Medicines Agency Citation2020).
Currently, there is a lack of information concerning the mutagenic and clastogenic effects of the hydromorphone impurity 2,2-bishydromorphone. As this impurity was identified in Fresenius Kabi’s hydromorphone hydrochloride, its potential mutagenic and clastogenic potential was examined.
The aim of the present study was to determine the mutagenic and clastogenic potential of 2,2-bishydromorphone via predictive in silico QSAR analysis in an initial step. Furthermore, experimental in vitro Ames and MNT were performed to support the in silico predictions of the mutagenic and clastogenic potential. Lastly, an in silico/in vitro data correlation should be drawn as in particular limited data and correlation of in silico/in vitro data regarding clastogenicity is available for pharmaceutical impurities. Based on the comparable molecular structures of the API hydromorphone and the impurity 2,2-bishydromorphone, we hypothesize that 2,2-bishydromorphone does not exert mutagenic and clastogenic properties as well.
Materials and methods
Chemicals and reagents
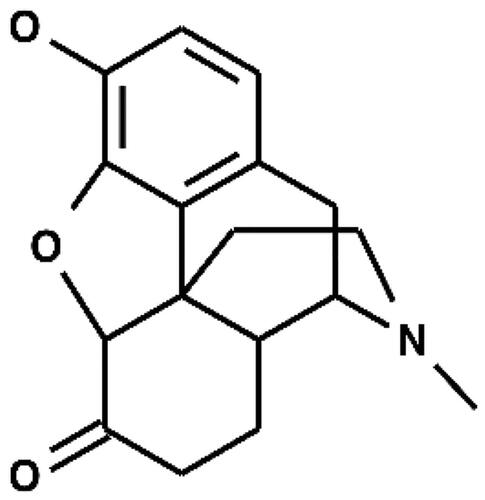
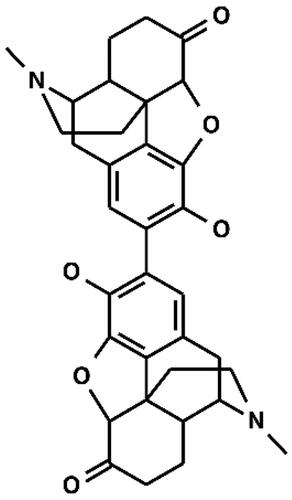
The structures of hydromorphone (CAS No. 466–99-9) and 2,2-bishydromorphone are given in and , respectively. 2,2-Bishydromorphone (CAS No. 109648–80-8, purity 97%, Lot No. B102286P194) was supplied by Mallinckrodt Pharmaceuticals (St. Louis, MO, USA). The vehicle for preparation of impurity formulation composed of dimethylsulfoxide (DMSO) in both Ames and MNT and was supplied by Sigma-Aldrich, Inc (CAS No. 67–68-5, Lot No. SHBH9867). Positive controls 2-aminoanthracene (CAS No. 613–13-8, Lot No. STBD3302V), ICR-191 acridine (CAS No. 17070–45-0, Lot No. SLBR0485V), 2-nitrofluorene (CAS No. 607–57-8, Lot No. S43858V), sodium azide (CAS No. 26628–22-8, Lot No. MKBX7529V), mitomycin C (CAS No. 50–07-7, Lot No. SLBN5647V), vinblastine sulfate (CAS No. 143–67-9, Lot No. 107M4057V), and cyclophosphamide (CAS No. 6055–19-2, Lot No. MKBX1822V) were obtained from Sigma-Aldrich, Inc (St. Louis, MO, USA), whereas 4-nitroquinoline-N-oxide (CAS No. 56–57-5, Lot No. LC15235V) was received from Supelco, Inc.
Cells and culture conditions
Four histidine-auxotrophic Salmonella typhimurium strains TA98, TA100, TA1535, TA1537, and one tryptophan-auxotrophic Escherichia coli strain WP2 uvrA were used for the Ames test and obtained from Molecular Toxicology, Inc. (Boone, NC, USA). The human lymphoblast cell line TK6 was originally obtained from Pfizer Global Research and Development (Groton, CT, USA) and used for the MNT. The 9000 × g liver supernatant fraction (S9) Aroclor™ 1254-treated rats is obtained from Molecular Toxicology, Inc. (Boone, NC, USA) and used in both tests. S. typhimurium and E. coli strains were incubated at 36 °C to 38 °C for approximately two days, whereas TK6 cells were incubated at 36 °C to 38 °C, 4% to 6% CO2 for 4 to 44 h.
Experimental design
QSAR analysis
A toxicological QSAR analysis using the Leadscope Model Applier’s Genetox statistical models (version 2.4.5) was conducted to examine the mutagenic and clastogenic potential of impurity 2,2′-bishydromorphone.
Ames test
The Ames test was performed according to OECD Guideline for testing of chemicals – Test guideline No. 471 (Citation2020), ICH Guideline S2(R1) (Citation2011), Maron and Ames (Citation1983) and in compliance with Good Laboratory Practice. Four histidine-auxotrophic S. typhimurium strains (TA98, TA100, TA1535, and TA1537) and one tryptophan-auxotrophic E. coli strain (WP2 uvrA) were cultured following the respective provider instruction. The mutagenic potential of 2,2-bishydromorphone was assessed in triplicates in two independent experiments in the presence and absence of an external metabolic activation system from Aroclor™ 1254-treated rats (S9-fraction, final concentration of 1.4%) in an initial assay (plate incorporation method) performed with eight concentrations (25–5000 µg/plate) followed by a confirmatory assay (pre-incubation method) performed with six concentrations (100–5000 µg/plate). Appropriate vehicle controls (DMSO: 100 µL/plate) and positive controls (without S9-fraction: 0.5 µg/plate ICR 191 acridine for TA1537; 2.5 µg/plate 2-nitrofluorene for TA98; 1.0 µg/plate sodium azide for TA100 and TA1535; 2.0 µg/plate 4-nitroquinoline-N-oxide for WP2 uvrA; with S9-fraction: 10 µg/plate 2-aminoanthracene for WP2 uvrA; 2.5 µg/plate 2-aminoanthracene for TA98, TA100, TA1535, and TA1537) were included in both assays. All plates were checked for test article-induced cytotoxicity (reduction in the background lawn or a > 50% reduction in the mean number of revertant colonies compared to the vehicle control) and evaluated macroscopically for precipitation of test article and microscopically for thinning of the background lawn. Results are expressed as mean number of revertant colonies compared to the vehicle control.
Micronucleus test
The MNT was carried out following OECD Guideline for testing of chemicals – Test guideline No. 487 (Citation2016) and ICH Guideline S2(R1) (Citation2011) and in compliance with Good Laboratory Practice. Briefly, TK6 cells were seeded a cell density of 1.3 to 1.8 × 105 cells/mL and cultured following the respective provider instruction. The potential of 2,2-bishydromorphone to induce micronuclei in TK6 cells was assessed in duplicate cultures exposed to the test article at six concentrations (15.6–500 µg/mL) in the presence and absence of an external metabolic activation system from Aroclor™ 1254-treated rats (S9-fraction, final concentration of 0.34%).
These concentrations were selected based on a preliminary cytotoxicity range-finding assay. TK6 cells were exposed to 2,2-bishydromorphone for 4 h with and without metabolic activation system (harvest time 44 h) and 27 h without metabolic activation system (harvest time 27 h). Cells without S9-fraction were cultured in complete culture medium (CCM), whereas cells during incubation with S9-fraction were cultured in incomplete culture medium (ICM) followed by incubation in CCM until harvest.
Appropriate vehicle controls (DMSO: 1%) and positive controls (4 h without S9-fraction: 0.0625 µg/mL mitomycin C; 27 h without S9-fraction: 11.9 µg/mL vinblastine sulfate, 4 h with S9-fraction: 0.003 µg/mL cyclophosphamide) were included in all experiments. After harvest, cells were treated with hypotonic KCl solution, fixed in methanol:glacial acetic acid mix and slides were prepared for micronuclei evaluation followed by staining with acridine orange solution.
All cell cultures were checked for test article-induced cytotoxicity (relative population doubling in test article-treated cells compared to the vehicle control), pH change, and evaluated visually for precipitation of the test article. Micronucleus frequencies were analyzed in 2000 cells per culture (4000 cells per concentration). Three test article concentrations (125, 250, and 500 µg/mL), the vehicle control and one concentration of the positive control were scored for micronucleated cells in each treatment condition. Results are expressed as mean percentage of micronucleated cells compared to the vehicle control.
Statistical analysis
Mean colony counts and standard deviation were calculated for evaluation of Ames test results.
Accordingly, data are presented as mean ± SD in the tables. For the MNT, a One-Tailed Fisher’s Exact test (San et al. Citation2002) was performed on the total number of cells with micronuclei, comparing the treated groups to the results obtained from the concurrent vehicle control group, as needed. The detection of dose–response trends, if needed, was carried out using the Cochran-Armitage test (Margolin et al. Citation1986). Differences were considered statistically significant at p ≤ 0.05. Statistical analysis was performed by MSTAT system.
Results
QSAR analysis
The weight of evidence from the leadscope indicates that impurity 2,2′-bishydromorphone is unlikely to be a bacterial mutagen and should not be considered clastogenic in vitro or in vivo (Fresenius Kabi Deutschland GmbH 2020).
Ames test
In the initial plate incorporation assay (), 2,2-bishydromorphone was tested at 25, 50, 100, 250, 500, 1000, 2500, and 5000 μg/plate and did not show any sign of precipitation or cytotoxicity (i.e., reduction in the background lawn or a > 50% reduction in the mean number of revertant colonies compared to the vehicle control) in any strain either with or without metabolic activation (Aroclor™ 1254-induced rat liver S9 microsomal fraction). Neither precipitation nor cytotoxicity were observed with 2,2-bishydromorphone in the confirmatory assay () using the pre-incubation method when tested at concentrations of 100, 250, 500, 1000, 2500, and 5000 μg/plate.
Table 1. Summary results of plate incorporation and pre-incubation experiment for 2,2-bishydromorphone in the Salmonella typhimurium/Escherichia coli reverse mutation assay.
In both assays, criteria for a negative response were met for all tester strains with and without metabolic activation. The mean number of revertant colonies was comparable to historical control ranges at all concentrations for all tester strains with and without metabolic activation. Additionally, data from the vehicle and positive controls demonstrated the validity and sensitivity of this test system for detecting chemical mutagens with and without metabolic activation.
Taken together, these data support the conclusion that 2,2-bishydromorphone is negative for mutagenic activity in the S. typhimurium strains TA1535, TA1537, TA98, TA100, and in the E. coli strain WP2 uvrA, with and without metabolic activation, under the conditions of this assay (Fresenius Kabi Deutschland GmbH 2018b).
Micronucleus test
2,2-Bishydromorphone was evaluated for the potential to induce micronuclei in TK6 cells during short (4 h) and long (27 h) incubations with or without an exogenous metabolic activation system (Aroclor™ 1254-induced rat liver S9 microsomal fraction) (). In the range-finding cytotoxicity assay, target concentrations of 2,2-bishydromorphone from 0.977 to 500 μg/mL were examined and neither precipitation nor cytotoxicity were detected.
Table 2. Summary results of cytotoxicity and micronucleated cells for 2,2-bishydromorphone in the micronucleus test.
Based on results of the range-finding assay, concentrations of 2,2-bishydromorphone used during the MNT ranged from 15.6 to 500 μg/mL for the 4-h treatments with and without metabolic activation and the 27-h treatment without metabolic activation. Cytotoxicity or precipitates were not observed in the 4-h treatments with and without metabolic activation, or the 27-h treatment without metabolic activation.
Evaluation of the three highest concentrations tested, 125, 250, and 500 μg/mL, did not reveal statistically significant increases in the percent of micronucleated cells in test article-treated cultures and the concurrent vehicle control under any assay condition. The percent of micronucleated cells in vehicle and positive control cultures were comparable to the historical control data and therefore indicate the validity and sensitivity of the test system.
Conclusively, 2,2-bishydromorphone was considered negative for inducing micronuclei in TK6 cells in the 27-h treatment without metabolic activation and in the 4-h treatments with and without metabolic activation under the conditions of this test system (Fresenius Kabi Deutschland GmbH Citation2018a).
Discussion
Hydromorphone impurity 2,2-bishydromorphone was tested for its mutagenic and clastogenic potential via predictive in silico QSAR analysis and in vitro AMES and MNT. The most important finding of our study is that 2,2-bishydromorphone exerted neither mutagenic (), nor clastogenic () properties (Fresenius Kabi Deutschland GmbH Citation2018a, 2018b, 2020). These findings provide evidence of valid in silico and in vitro data correlation of the mutagenic and clastogenic potential of 2,2-bishydromorphone. Conclusively, limits for this non-mutagenic impurity can therefore be set according to the ICH Q3B guideline ‘Impurities in new drug products.’
The genotoxicity safety profile of the parent medicinal product hydromorphone has been well characterized (Center for Drug Evaluation and Research Citation2010). Briefly, hydromorphone was not predicted to reveal mutagenic and clastogenic potential via in silico analysis and the absence of a mutagenic potential was confirmed in different in vitro experiments, while the in vitro results for clastogenicity were contradictory. Hydromorphone was not clastogenic in the in vitro human lymphocyte chromosome aberration assay but showed clastogenic potential in the in vitro mouse lymphoma assay in the presence of metabolic activation. Although hydromorphone was judged to be non-mutagenic and non-clastogenic, it cannot be excluded that the positive clastogenicity result might be due to additional impurities.
The examined 2,2-bishydromorphone was shown to be clearly negative for mutagenicity and clastogenicity; however, additional impurities have not been investigated within Fresenius Kabi’s studies due to time and cost reasons but may be worth to be examined in the future. A large number of impurities for hydromorphone exist, and a complete genotoxic data package does not exist for each of these impurities.
Further hydromorphone impurities, such as hydromorphone N-oxide, have also previously been shown to be negative for both mutagenicity and clastogenicity in genotoxicity studies (Center for Drug Evaluation and Research Citation2010), whereas no experimental mutagenicity/clastogenicity data is publicly available for another hydromorphone impurity, namely, dihydroxyhydromorphone. Although in silico analysis of dihydroxyhydromorphone showed negative prediction for mutagenicity and clastogenicity, in silico and in vitro data correlation remains still unclear.
Based on the genotoxicity tests performed for 2,2-bishydromorphone, we provide a valuable contribution for another impurity with valid in silico/in vitro data correlation. The genotoxicity profile of a chemical is one of the most important characteristics as it crucially drives the human risk assessment. The initial step to assess the mutagenic (DNA reactive) potential of a compound is the performance of a predictive in silico QSAR analysis followed by in vitro tests such as bacterial reverse mutation assay (Ames test) to either confirm or overrule identified structural alerts from in silico analysis. Thus, a correlation of predicted in silico and experimental in vitro/in vivo data is crucial for valid interpretation of results.
For mutagenicity, valid correlation between predicted in silico data and experimental in vitro data is crucial and has been well established within the last decade as thousands of chemicals and pharmaceuticals have been tested and published under the European REACH program by the European Chemical Agency (ECHA). In contrast, limited data and correlation of in silico/in vitro data regarding clastogenicity are available for pharmaceutical impurities. Since the introduction of the ICH M7 guideline, clastogenicity testing for impurities does no longer belong to the standard testing battery. Thus, presented 2,2-bishydromorphone impurity clastogenicity data provide essential clastogenicity information and fill a data gap that has been there up to now.
Reliable in silico and in vitro correlation can support the application of the 3 R (replacement, reduction, refinement) principle to minimize in vivo experiments performed with laboratory animals. However, further improvement of the statistical QSAR tools and methods is mandatory to increase both sensitivity and specificity and to reduce the number of false positive or negative predictions. This can for instance be achieved by combining different sensitive structural alerts for genotoxicity endpoints as previously reported by Benigni (Citation2021). Briefly, a large database of more than 6000 genotoxicants was analyzed with structural alerts implemented in the OECD QSAR Toolbox resulting in a maximum of 3% false negative predictions only. However, cautious follow-up examination of predicted positives should be applied by searching for data from structural similar compounds or experimentation if necessary. In absence of any data supporting the outcome of a QSAR prediction, further in vitro experiments are mandatory as a QSAR prediction as stand-alone method will never be sufficient to meet regulatory requirements. Taken together, these studies add important new clinically relevant information on the safety of hydromorphone as the impurity of 2,2-bishydromorphone is proven to be non-mutagenic and non-clastogenic.
Author contributions
DF and MKB designed the study and interpreted the data. SBH conducted the in vitro experiments. DF drafted the article and MKB, MW, and SBH revised it critically and finally approved the version to be submitted.
Acknowledgements
We thank the following employee of Charles River Skokie, USA for her participation in the study: Nikita D. Navalkar for formulation analysis of the test item.
Disclosure statement
The authors report no conflict of interest. DF, MKB, and MW are employees of Fresenius Kabi Deutschland GmbH.
References
- Benigni, R., 2021. In silico assessment of genotoxicity. Combinations of sensitive structural alerts minimize false negative predictions for all genotoxicity endpoints and can single out chemicals for which experimentation can be avoided. Regulatory Toxicology and Pharmacology: RTP, 126 (2021), 105042.
- Center for Drug Evaluation and Research, 2010. Pharmacology review(s). Application number: 21-217s000. EXALGO (hydromorphone hydrochloride) extended release tablets. Neuromed Pharmaceuticals, LTD. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/021217s000PharmR.pdf
- European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP), 2020. Assessment report ranitidine. EMEA/H/A + 31/1491. https://www.ema.europa.eu/en/documents/referral/ranitidine-article-31-referral-assessment-report_en.pdf
- Fresenius Kabi Canada Ltd, 2019. Hydromorphone hydrochloride injection, USP (prescribing information). Health Canada Control # 253887. https://www.fresenius-kabi.com/en-ca/documents/HYDROmorphone-2mg-EN-PM.pdf
- Fresenius Kabi Deutschland GmbH, 2018a. 2,2’Bis hydromorphone 2 HCl in vitro micronucleus assay in TK6 cells. Charles River Study Report no. 01111007.
- Fresenius Kabi Deutschland GmbH, 2018b. 2,2’Bis hydromorphone 2 HCl Salmonella typhimurium-Escherichia coli/reverse mutation assay. Charles River Study Report no. 01111006.
- Fresenius Kabi Deutschland GmbH, 2020. Toxicological analysis of 2,2’-bishydromorphone using the leadscope model applier’s genetox statistical models. ForthTox Report no. TCS0121s.
- ICH Guideline S2(R1), 2011. Guidance on genotoxicity testing and data interpretation for pharmaceuticals intended for human use. https://www.ich.org/page/safety-guidelines
- Margolin, B.H., et al., 1986. Statistical analyses for in vitro cytogenetic assays using Chinese hamster ovary cells. Environmental Mutagenesis, 8 (2), 183–204.
- Maron, D.M., and Ames, B.N., 1983. Revised methods for the Salmonella mutagenicity test. Mutation Research, 113 (3-4), 173–215.
- OECD Guideline for testing of chemicals – Test guideline No. 471, 2020. Bacterial reverse mutation test. https://www.oecd-ilibrary.org/environment/test-no-471-bacterial-reverse-mutation-test_9789264071247-en
- OECD Guideline for testing of chemicals – Test guideline No. 487, 2016. In vitro mammalian cell micronucleus test. https://www.oecd.org/chemicalsafety/test-no-487-in-vitro-mammalian-cell-micronucleus-test-9789264264861-en.htm
- San, R.H.C., et al., 2002. Genetic toxicology. In: M. J. Derelanko, M. A. Hollinger, eds. Handbook of toxicology. 2nd ed. New York, NY: CRC Press, 597–620. https://muhammadsubchi.files.wordpress.com/2010/04/buku-handbook-of-toxicology-2nd-edition.pdf
- Wagner, J.A., and Colombo, J.M., 2020. Medicine and media: the ranitidine debate. Clinical and Translational Science, 13 (4), 649–651.