Abstract
The upper Tennessee River Basin contains the highest density of our nation's caves; yet, little is known regarding speleogenesis or Fe and Mn biomineralization in these predominantly epigenic systems. Mn:Fe ratios of Mn and Fe oxide-rich biofilms, coatings, and mineral crusts that were abundant in several different caves ranged from ca. 0.1 to 1.0 as measured using ICP-OES. At sites where the Mn:Fe ratio approached 1.0 this represented an order of magnitude increase above the bulk bedrock ratio, suggesting that biomineralization processes play an important role in the formation of these cave ferromanganese deposits. Estimates of total bacterial SSU rRNA genes in ferromanganese biofilms, coatings, and crusts measured approximately 7×107–9×109 cells/g wet weight sample. A SSU-rRNA based molecular survey of biofilm material revealed that 21% of the 34 recovered dominant (non-singleton) OTUs were closely related to known metal-oxidizing bacteria or clones isolated from oxidized metal deposits. Several different isolates that promote the oxidation of Mn(II) compounds were obtained in this study, some from high dilutions (10–8–10–10) of deposit material. In contrast to studies of caves in other regions, SSU rRNA sequences of Mn-oxidizing bacterial isolates in this study most closely matched those of Pseudomonas, Leptothrix, Flavobacterium, and Janthinobacterium. Combined data from geochemical analyses, molecular surveys, and culture-based experiments suggest that a unique consortia of Mn(II)-oxidizing bacteria are abundant and promoting biomineralization processes within the caves of the upper Tennessee River Basin.
Introduction
Within the continental United States, carbonate bedrock underlies 20% of the land area east of the Mississippi River, forming an intricate karst hydrologic network with numerous caves (Christman and Culver Citation2001; White et al. Citation1995). As such, the southern Appalachians contain numerous caves: of the 50,000 cave systems known to exist in the United States, ca. 14% occur within the state of Tennessee (Barton and Jurado Citation2007). However, to date only a few studies describe the microbial communities of Appalachian cave systems (Angert et al. Citation1998; Campbell et al. Citation2011; Engel et al. Citation2001; Shapiro and Pringle Citation2010; Simon et al. Citation2003), and none of these address in a significant way the geomicrobiology of an epigenic cave system, which is the primary method of speleogenesis in cave and karst systems worldwide (White et al. Citation1995).
Ferromanganese deposits are quite common in caves in the southern Appalachians. However, our knowledge of the microbial consortia associated with cave ferromanganese deposits is limited to research conducted in cave systems located in the southwest United States (Cunningham et al. Citation1995; Northup et al. Citation2003; Spilde et al. Citation2005, Citation2006), which formed via hypogene speleogensis (characteristically deeper cave formation via sulfuric acid in ascending groundwater) rather than epigene speleogensis (typically shallow cave formation via carbonic acid in descending meteoric water). Therefore questions remain unanswered regarding 1) the nature of these shallow, epigenic cave systems in the southern Appalachians, 2) how similar these shallow cave systems are to their deeper, hypogene counterparts and 3) to what extent surface waters and biota influence microbial consortia and biomineralization processes within shallow cave systems. Answers to these questions require basic exploratory efforts in order to generate hypotheses that provide a framework and direction for future research endeavors. This point is particularly salient regarding research within understudied environments, such as the cave systems of the southern Appalachians.
The formation of cave mineral deposits and speleothems was long thought to be controlled primarily by abiotic processes as a result of microsite environmental conditions (e.g., temperature, pH, solution chemistry), and changes in redox conditions (Barton and Northup Citation2007; Engel et al. Citation2004; Northup and Lavoie Citation2001). However, more recent geomicrobiology research lends support to the hypothesis that microbes play a role in the formation and dissolution of cave mineral deposits via direct and indirect metabolic activities and biomineralization processes (Barton and Luiszer Citation2005; Cañaveras et al. Citation2006; Jones Citation2001; Melim et al. Citation2001; Northup et al. Citation1997; de los Ríos et al. Citation2011; Spilde et al. Citation2005; Taboroši Citation2006). In particular, microbial reactions have been shown to promote the formation of cave manganese oxide and ferromanganese (mixed Fe and Mn oxides) deposits, such as crusts (Northup et al. Citation2000, 2003; Spilde et al. Citation2005), manganese flowstones (Gradziński et al. Citation1995), rock coatings (Allouc and Harmelin Citation2001; Peck Citation1986), and manganese stromatolites (Rossi et al. Citation2010).
Cave ferromanganese deposits may contain Mn oxide, Mn hydroxide, and Mn oxyhydroxide minerals (collectively referred to hereafter as Mn oxides), and the mineralogy of these deposits can be quite complex (Onac and Forti Citation2011; Post Citation1999; White et al. Citation2009). The oxidation of Mn(II) to Mn(III) or Mn(IV) is kinetically inhibited in the absence of a catalyst at near-neutral pH of most environments. Microorganisms are known to catalyze the oxidation of Mn(II) compounds, increasing reaction rates up to five orders of magnitude relative to abiotic oxidation rates (Nealson et al. Citation1988; Dixon and Skinner Citation1992; Francis and Tebo Citation2002). Therefore, rapid Mn(III/IV) oxide depositional rates, especially those which exceed predicted abiotic reaction rates in a given environment, are a strong indication of microbial involvement in deposit formation (Nealson et al. Citation1988).
Mn oxide minerals have highly charged surfaces and are biogeochemically active, demonstrating the ability to degrade humic substances (Sunda and Kieber Citation1994), scavenge reactive oxygen species (Archibald and Fridovich Citation1981; Daly et al. Citation2004; Ghosal et al. Citation2005; Learman et al. Citation2011), concentrate rare earth elements (Onac et al. Citation1997), and influence trace metal bioavailability (Nelson et al. Citation1999; Post Citation1999; Kay et al. Citation2001; Manceau et al. Citation2002; Villalobos et al. Citation2005; Toner et al. Citation2006) and speciation (Fendorf et al. Citation1992; White et al. Citation2009). Biogenic oxides, which tend to have higher percentages of vacancies and smaller particle sizes (Learman et al. Citation2011; Webb et al. Citation2005), demonstrate an increased sorptive capacity relative to abiotically produced oxides (Nelson et al. Citation1999). Therefore, biogenic Mn oxides may exert a greater impact on local geochemistry than abiotically generated deposits.
In this study, we examined the geomicrobiology of ferromanganese deposits in an epigene cave system located in the cave-rich but poorly studied southern Appalachian karst region. Using a combination of molecular-based SSU rRNA analysis and culture-based methodologies, we demonstrate that several different species of Mn(II)-oxidizing bacteria are abundant and promoting biomineralization processes within the caves. Molecular evidence from the primary study site, Carter Saltpeter Cave, suggests that the Mn-oxidizing microbial consortia in ferromanganese deposits within shallow cave systems harbor a unique signature when compared to similar deposits in deep cave systems located within the southwest United States.
Methods
Field Description
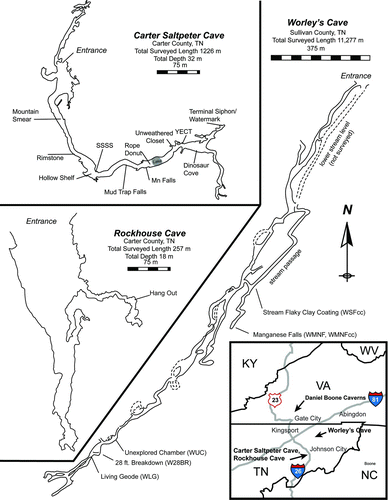
The study area (, inset) is comprised of several epigenic caves, all located in the Ordovician Knox Group (Oder Citation1934) within the upper Tennessee River Basin. The primary study site, Carter Saltpeter Cave (Carter County, TN, ), herein referred to as CSPC, is a shallow cave system at a depth of approximately 30 m. It is an epigenic cave system typical of those found within the Appalachian region, and evidence of anthropogenic impact is widespread throughout the system. Rockhouse Cave (R) is located less than 2 km east of CSPC, and the two systems are hydrologically connected (Gao et al. 2006a, 2006b). Worley's Cave, located 24 km northeast of CSPC in Sullivan County, TN, is frequently visited by humans and contains a subterranean creek system that exits the cave and flows to the south fork of the Holstein River. Recent work (Y. Gao, unpublished data) has demonstrated that Worley's cave is hydrologically connected to sinkholes in nearby farm fields and therefore may be susceptible to agricultural runoff. In contrast to these three anthropogenically impacted caves, Daniel Boone Caverns (herein referred to as DBC) in Scott County, VA is gated and access is controlled by the landowner. Therefore, this cave is rarely visited. DBC is located in an isolated forest location on the top of a ridge and is not subject to agricultural or municipal runoff. It contains several pools and drip networks, but does not have an extensive subsurface hydrologic system.
Fig. 1 Maps of the primary study sites: Carter Saltpeter Cave, Rockhouse Cave, and Worley's Cave. A map of Daniel Boone Caverns was not available at the time of this writing. Sampling locations are labeled with hash marks. Regional map inset shows the relative location of all four cave systems that are included in this study. Carter Saltpeter Cave survey conducted on February 8, 1981 by L. Adams, R. Knight, R. Page, and T. Wilson. Rockhouse Cave survey conducted on May 6, 1980 by L. Adams, T. Gingrich, and D. Nelms. Worley's Cave survey conducted from December 22, 1971 - August 8, 1973 by M. Adams, T. Anderson, C. Booth, R. Bowery, J. Cox, T. Harrison, D. Mire, A. Powers, D. Powers, and J. Powers. All cave maps adapted by S.K. Carmichael (color figure available online).
Sample Collection
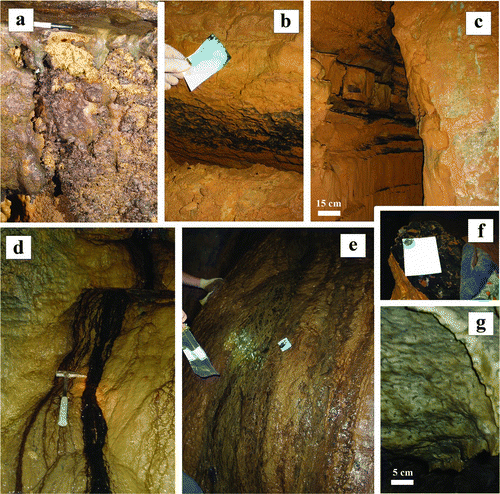
Samples () were collected periodically, in roughly three month intervals, from July 2009 to September 2011 in four cave systems. Ferromanganese deposits were identified as black/chocolate brown biofilms, coatings, or mineral-rich crusts that coat cave walls and speleothems. Deposits were screened for the presence of Mn oxides using 0.04% Leucoberbein Blue (LBB), a redox indicator that is oxidized by Mn(III) or Mn(IV) to produce a bright blue color change (Krumbein and Altmann Citation1973). LBB tests were conducted by scraping the deposit surface using Whatman chromatography paper, flushing the sample with LBB, and looking for the production of the color change described above. Deposit morphology was highly variable within systems (), with LBB-positive samples collected from biofilms, ferromanganese coatings and crusts, and ferromanganous micronodules. Samples were collected aseptically by scraping the deposit surface, placed in a cooler on ice or dry ice and transported back to the lab for immediate plating/inoculation (for culturing), DNA extraction (for clone libraries and qPCR), or fixation (for microscopy). Field samples for electron microscopy were fixed in the field using gluteraldehyde with sodium phosphate buffer.
Table 1 A descriptive summary of samples obtained from cave ferromanganese deposits in this study
Fig. 2 Examples of ferromanganese deposit types found in the upper Tennessee River Valley karst network: a) coatings on cave coral at Dino Cove (CSPC); b) coatings on massive nontronite clay deposits (DBC); c) coatings on 5 cm thick calcite layers within cave walls (DBC); d) biofilm associated with a groundwater seep at Mn Falls (CSPC); e) coating associated with a groundwater seep at Mud Trap Falls (CSPC); f) coatings on calcite layers within cave walls show LBB+ signature (DBC); and g) biovermiculations (R). Cave systems are abbreviated as follows: Carter Saltpeter Cave (CSPC), Daniel Boone Caverns (DBC), and Rockhouse Cave (R) (color figure available online).
Geochemistry and Biogeochemistry
A subset of cave ferromanganese samples were analyzed at Appalachian State University to determine substrate mineralogy using a Shimadzu 6000 powder X-ray diffractometer with a Cu X-ray source and measured from 5–80° 2θ. Species were identified using the PDF/4+ Minerals Database. A subset of samples were collected for metal analyses in December 2009 (Worley's Cave) and January 2010 (CSPC), lyophilized over a 48 hour period, then digested for metal analyses following US EPA SW846 Method 3051A: Microwave Assisted Acid Digestion of Sediments, Sludges, Soils, and Oils (Link et al. Citation1998; SW-846 EPA Method 3051A Citation2007). Elemental analysis was performed in duplicate on several rock, coating, and biofilm samples to determine total Mn and Fe content using a Varian 710-ES Inductively Coupled Plasma-Optical Emission Spectrometer (ICP-OES).
Real-time Quantitative PCR
Ten samples from three cave systems were analyzed using real-time quantitative PCR to quantify the relative abundance of Archaea and Bacteria in the CSPC, Rockhouse, and Worley's cave systems. Primers were selected for each domain that demonstrated broad coverage over the 16S rRNA region. The forward primer 338F 5′-TCCTACGGGAGGCAGCAGT-3′ (Nadkarni et al. Citation2002) was paired with the reverse primer 518R 5′-ATTACCGCGGCTGCTGG-3′ (Einen et al. Citation2008) to target the bacterial 16S rRNA gene sequence. The primer pair 967F 5′-AATTGGCGGGGGAGCAC-3′ / 1060R 5′-GGCCATGCACCWCCTCTC-3′ (Cadillo-Quiroz et al. Citation2006) was selected to target the archaeal 16S rRNA gene sequence. Amplifications were performed in triplicate on an Applied BiosystemsTM 7300 Real-Time PCR System (Carlsbad, CA) using MaximaTM SYBR Green/ROX qPCR Master Mix (Fermentas, Glen Burnie, MA) with 2 ng sample DNA/well. Calibration curves for quantification were generated using one of the following standards: 1) plasmid DNA containing the SSU rRNA gene from Rhodobacter sp. CR07–74 (Bacteria, range of 102–109 target copies/μL) or 2) genomic DNA extracted using the Qiagen DNeasy Blood and Tissue Kit (Valencia, CA) from Methanoregula boonei 6A8 (Archaea, range of 102–107 target copies/μL). Circular plasmid DNA standards have been reported to cause overestimation of sample cell number using quantitative PCR (Hou et al. Citation2010). To circumvent this potential issue, plasmid DNA standards were lineraized by restriction digest using BssHII (New England BioLabs, Ipswich, MA). rRNA operon copy numbers in bacterial cells are variable (Fogel et al. Citation1999) and change based on environmental conditions (Klappenbach et al. Citation2000). Therefore, SSU rRNA gene copy number was normalized in experimental results using the average copy number for Archaea (1.07 copies/cell) and Bacteria (4.08 copies/cell) as reported by the Ribosomal RNA Operon Copy Number Database (Klappenbach et al. Citation2001) in March, 2010.
Fluorescence Direct Counts
Fluorescence direct counts were performed on samples from Mn Falls and Mud Trap Falls (CSPC) to validate real-time quantitative PCR results. Upon receipt in the lab, 0.1 g (wet weight) of biofilm material was mixed 1:10 w/v with 0.1% (final concentration) sodium pyrophosphate (Na4P2O7.10 H2O) and vortexed for ten minutes to disrupt cell clumps and homogenize the material. Samples were fixed in a 4% paraformaldehyde solution and stored at 4°C overnight. Samples were then re-suspended by vortexing, and a 5 μL sample was then applied to a slide and evenly spread over 484 mm2 surface area. Samples were stained with 1 μg/mL (final concentration) DAPI (4,6-diamino-2-phenylindole), and Citiflour Antifadent Mounting Medium AF1 (Electron Microscopy Sciences, Hatfield, PA) was applied to prevent bleaching of the DAPI fluorescent signal. Fluorescence direct counts were conducted at 100X magnification on an Olympus Bx51 fluorescence microscope. Fields of view were randomly selected and counted until a minimum of 300 cells/sample were visualized and recorded.
Bacterial and Archaeal Clone Library Construction
DNA was extracted from two LBB-positive cave deposits, Mn Falls and Mud Trap Falls, located within the primary study site (CSPC) using a bead beating protocol with the Fast DNA Spin Kit for Soil (MP Biomedicals, Solon, OH). The concentration of extracted DNA was determined using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE). Extracted DNA was used to create a total of four clone libraries. For Bacteria, one library each was created from DNA extracted from light and dark material at the Mn Falls site in CSPC. This site appeared to be highly active as it was strongly LBB positive and had streamers of Mn-oxide-rich biofilm material flowing down the cave wall at the time of sampling. Both dark and light material was sampled in attempt to detect a significant enrichment in one or more groups that may be implicated in Mn(II)-oxidation.
Small clone libraries were constructed since the main goal was to evaluate the most abundant OTUs, rather than rare OTUs, in each sample. Approximately 3 ng of environmental DNA was used as a template for the PCR amplification of bacterial DNA using the primer 27F 5′-AGAGTTTGATCMTGGCTCAG-3′ (Lane Citation1991) combined with a modified version of 1492R primer 5′-RGYTACCTTGTTACGACTT-3′ (for Bacteria) (Emerson and Moyer Citation2002). Each 25 μL PCR reaction contained, in final concentrations, 1.25 U AmpliTaq Gold (Applied Biosystems, Carlsbad, CA), 200 μM each primer, 1X PCR Gold Buffer (Applied Biosystems, Carlsbad, CA), 2 mM MgCl2 Solution (Applied Biosystems, Carlsbad, CA), 200 μM each dNTP, and 2X BSA (New England Biolabs, Ipswich, MA). A MJ Mini Personal Thermal Cycler (Bio-Rad, Hercules, CA) was used for all PCR-amplification reactions. The amplification protocol for bacterial clone libraries was as follows: an initial denaturation of 95°C for 10 min, followed by 30 cycles of 94°C for 5 min, 64°C for 90 s, 72°C for 3 min, and a final extension of 72°C for 7 min. PCR amplifications were conducted in triplicate, and amplified PCR products were pooled before purification using a Montage® PCR Purification Kit (Millipore, Billerica, MA).
For Archaea, one library was created from DNA extracted from the Mn Falls and Mud Trap Falls sites. Approximately 3 ng of environmental DNA was used as a template for the PCR amplification of archaeal DNA using the using the 109F 5′-ACKGCTCAGTAACACGT-3′ and 912R 5′-CTCCCCCGCCAATTCCTTTA-3′ primer pair for Archaea (Lueders and Friedrich Citation2000). Each 25 μL PCR reaction contained, in final concentrations, 1.25 U AmpliTaq Gold (Applied Biosystems, Carlsbad, CA), 200 μM each primer, 1X PCR Gold Buffer (Applied Biosystems, Carlsbad, CA), 2 mM MgCl2 Solution (Applied Biosystems, Carlsbad, CA), 200 μM each dNTP, and 2X BSA (New England Biolabs, Ipswich, MA). The amplification protocol for archaeal clone libraries is as follows: an initial denaturation of 94°C for 5 min, followed by 35 cycles of 94°C for 1 min, 54°C for 1 min, 72°C for 90 s, and a final extension of 72°C for 6 min. PCR amplifications were conducted in triplicate, and amplified PCR products were pooled before purification using a Montage® PCR Purification Kit (Millipore, Billerica, MA).
PCR products were cloned into TOPO TA pcr®2.1 vectors (Invitrogen, Carlsbad, CA), and plasmid DNA extracted from transformants using the QIAprep Spin Miniprep Kit (Qiagen, Valencia, CA) was screened by sequencing using the M13F (-20) primer. 96 well plates of glycerol stocks were prepared for each sample site using each primer set and sequenced using M13F(-20) and M13R(-27) primers. Sequencing was conducted using a Sanger platform at Beckman-Coulter Genomics (Danvers, MA). Chimeric sequences were eliminated from analysis prior to consensus sequence construction. Sequences from both bacterial and archaeal libraries were pooled (creating a ca. 180 sequence bacterial library and 65 sequence archaeal library) for DOTUR analysis in order to make OTU determinations (Schloss and Handelsman Citation2005). Representative sequences for each OTU for Archaea or each dominant OTU (as defined by two or more sequence representatives) for Bacteria were chosen based on sequence length and quality. For the bacterial dominant OTUs, additional sequencing of transformant plasmid DNA was conducted using primers 357F 5′-CCTACGGGAGGCAGCAG-3′, 926R 5′-CCGYCWATTCMTTTRGT TT-3′, and 1098R 5′-GGGTYKCGCTCGTTGC-3′ to obtain full-length (ca. 1500 bp) SSU rRNA gene sequences. Contigs were assembled using Sequencher sequence analysis software (Version 5.0, Build 7082, Gene Codes Corporation, Ann Arbor, MI). Good's nonparametric coverage was estimated using the equation [1-(n/N)] x 100, where “N” is the total number of clones evaluated and “n” is the number of singleton OTUs (Good Citation1953).
For phylogenetic analysis, additional sequences of interest were selected using ARB (Ludwig et al. Citation2004) and the NCBI taxonomic database (Johnson et al. Citation2008). OTU and additional sequences of interest were aligned using the on-line SILVA aligner (Pruesse et al. Citation2007). Phylogenetic trees were constructed using the PHYLIP software package (Felsenstein Citation2004) by conducting both neighbor-joining and maximum likelihood analysis. Clone sequences of archaeal OTUs and dominant bacterial OTUs were deposited in GenBank under the accession numbers JN820160-JN820219.
Isolation of Mn(II)-oxidizing Microorganisms on Agar Media
Mn-oxide-rich samples from ferromanganese biofilms, micronodules, and coatings on rock walls and speleothems were collected in an attempt to cultivate Mn(II)-oxidizing cave microorganisms. Samples were transported to the lab on ice and immediately plated on a variety of media designed to target the phylogenetically diverse array of Mn-oxidizers. A modified version of AY media (Santelli et al. Citation2011) was created by supplementing the media post-autoclaving with 100 μM MnCl2. A modified version of Burk's nitrogen-free medium (Mohandas Citation1988) was created to target putative nitrogen-fixing, Mn(II)-oxidizing microorganisms by substituting an equimolar concentration of succinic acid, disodium salt for sucrose as a carbon source and amending the media (post-autoclaving) with 100 μM MnCl2 and 3.7 mM FeCl3. A novel media, Nitrate Mineral Salts (NMS), was designed for this study to target methylotrophic Mn(II)-oxidizers. NMS contains (in g L−1) 1 MgSO4·7H20, 0.14 CaCl2·2H20, 1 KNO3, 0.27 KH2PO4, 0.3 NaH2PO4, 1 mL trace element solution (containing in mg L−1 1000 EDTA, 400 FeSO4·7H20, 250 CuSO4·5H20, 20 ZnSO4·7H20, 6 MnCl2·4H20, 60 H3BO3, 40 CoCl2·6H20, 2CaCl2·2H20, 4 NiCl2·6H20, 6 Na2Mo4·2H20). pH of the media was adjusted ca. 7.1–7.2 before autoclaving, and 15 g agar was added for plates. NMS media was supplemented post-autoclaving with sterile 0.02 M Hepes buffer pH 7.2, 5 μM ferrous ammonium citrate, 0.2% v/v vitamin solution for J medium (Tebo et al. 2007), and 100 μM MnCl2. For agar plates, a 0.05% v/v methanol was added as a carbon source; a 50:50 CH4(g):air mix was used as the sole carbon source for liquid media. A new medium, FMO2, was designed for this study by S.L. Bräuer and contains (in g L−1) 10 mL Major Metals 1 solution (containing in g L−1 12 NaCl, 1.2 KCl, 5 MgCl2·6H20, 1 KH2PO4, 2 NH4Cl, 1 CaCl2·2H20), 1 mL 1000X Trace Metal 1 Solution with NTA (containing in g L−1 0.15 CoCl2·6H20, 0.15 ZnCl2, 0.05 H3BO3, 0.02 NiCl2·6H20, 0.01 Na2Mo4·2H20, 0.4 FeCl2·4H20, 0.1 MnSO4·4H20, 3 MgSO4·7H20, 0.1 CaCl2·2H20, 0.01 CuSO4·5H20, 0.18 AlK(SO4)2·12H20, 1.5 NTA), and 0.05 yeast extract. pH of the media was adjusted to ca. 7.0–7.2 before autoclaving, and either 15 g agar or Gelrite gellan gum [an alternative solidifying agent (Hara et al. Citation2009)] was added for plates. The media was supplemented post-autoclaving with sterile 0.02 M Hepes buffer pH 7.2, 5 μM ferrous ammonium citrate, 0.2% v/v vitamin solution for J medium (Tebo et al. Citation2007), 100 μM MnCl2, and either 10 mM arabinose, 10 mM succinate, or 0.05% casamino acids as a carbon source (final concentrations). Plates were inoculated by spreading 80 μL of a 1% v/v Mn oxide rich sample in 0.02M Hepes buffer pH 7.2 on agar-solidified media. All cultures were incubated in the dark at 10°C to mimic environmental conditions within caves. Some plates were incubated under full oxygen conditions, while others were incubated microaerophilically (roughly 10% atmospheric air in a sealed environment), with the recognition that oxygen limited environments constitute important niches in cave systems (Portillo and Gonzalez Citation2009). Mn(II)-oxidation was confirmed in isolates by LBB testing; LBB-positive isolates were re-streaked for isolation a minimum of three times on the equivalent growth medium.
Serial Dilutions
Serial dilutions to extinction were conducted in Cellstar 96-well culture plates (greiner bio-one, Monroe, NC) using three different media types: FMO2 media with either 10% 2M arabinose, 10% 2M succinate, or 10% casamino acids as a carbon source. Inocula from the most dilute sample that grew and produced dark brown/black precipitates was transferred to the equivalent agar-solidified growth medium and were re-streaked for isolation a minimum of three times. Mn-oxidation was confirmed in isolates by LBB testing. All cultures were incubated in the dark at 10°C, mimicking environmental conditions within caves.
Identification of Isolates
Once a colony was isolated, a colony PCR reaction was used to screen the microorganism for phylogenetic placement using the universal bacterial primer 357F 5′-CCTACGGGAGGCAGCAG-3′. For screening, each 25 μL PCR reaction contained final concentrations of 1X PCR Master Mix (Fermentas, Glen Burnie, MD) and 0.2 μM each primer (27F and a modified version of 1492R, detailed above). DNA was added to each reaction by touching an isolated colony with a sterile pipette tip and washing the tip in the reaction mixture. The amplification protocol for screening isolates is as follows: an initial denaturation of 95°C for 10 min, followed by 30 cycles of 94°C for 1 min, 55°C for 90 sec, 72°C for 3 min, and a final extension of 72°C for 7 min. Isolates of interest were cloned as previously described using TOPO TA pcr®2.1 vectors (Invitrogen, Carlsbad, CA), and plasmid DNA containing the SSU rRNA gene sequence was sequenced using M13F(-20), M13R(-27), 357F 5′-CTACGGGAGGCAGCAG-3′, 926R 5′-CCGYCWATTCMTTTRAGTTT-3′, and 1098R 5′-GGGTYKCGCTCGTTGC-3′ primers to obtain a full-length (ca. 1500 bp) SSU rRNA gene sequence for phylogenetic placement as described above. Contigs were assembled using Sequencher sequence analysis software (Version 5.0, Build 7082, Gene Codes Corporation, Ann Arbor, MI). Isolate sequences were deposited in GenBank under the accession numbers JN820147-JN820159.
Detection of in-situ Microbial Mn-oxidation with Scanning Electron Microscopy
Environmental samples were examined using scanning electron microscopy (SEM) to visualize microbial morphologies associated with ferromanganese deposits. In addition to direct sampling of rock or sediment substrate, polycarbonate 0.45 μm filters were affixed to glass slides with dots of plumber's glue and set in various locations in CSPC for a period of six months or more. Upon collection, slides and substrate samples were placed in a sterile falcon tube and preserved in a 2.5% gluteraldehyde mixture, then dehydrated by soaking the filters in a series of 50%, 75%, and 85% ethanol in water solutions, followed by soaking two times in 100% ethanol. Samples were treated for a minimum of two hours in each ethanol solution. Samples were then critically point dried using liquid CO2 in a Polaron critical point dryer and imaged using a FEI Quanta 200 Environmental Scanning Electron Microscope with an EDAX Genesis XM energy dispersive X-ray spectrometer for elemental analysis.
Table 2 Biogeochemical analyses of ferromanganese deposits located in cave systems within the upper Tennessee River Basin
Transmission Electron Microscopy and Elemental Analysis
Mn-oxidizing bacterial cultures were examined using a JEOL JEM-1400 transmission electron microscope (TEM) equipped with an Oxford INCA energy dispersive X-ray detector (EDS) to confirm the presence of Mn oxides associated with bacterial cells. Samples were mounted on Formvar Carbon Type-B, 200 mesh Cu TEM grids (Ted Pella, Redding, CA) by diluting liquid cultures 1:5 using sterile deionized water and applying 5 μL dilution to each grid. TEM grids were allowed to air dry in a laminar flow cabinet. This process was repeated a total of three times, with a total volume of 15 μL of diluted culture applied to each grid, and then carbon coated. Samples were initially imaged using transmission electron microscopy and spot analyzed with EDS to confirm the presence of Mn deposits. Several samples were selected for elemental mapping via scanning transmission electron microscopy (STEM) to confirm the locations of Mn within the sample. In all cases, EDS spot analysis (data not shown) demonstrated the presence of concentrated Mn deposits associated with bacterial cells.
Results
Geochemical and Biogeochemical Analyses
All four cave systems (CSPC, Worley's, Rockhouse, and DBC, ) contain a variety of speleothem formations (flowstone, dripstone, soda straws, corrosion residue, ) and are particularly enriched in ferromanganese oxide deposits. The likely source of Fe(II) and Mn(II) necessary for the formation of these deposits is the bedrock dolomite of the Knox Dolomite Supergroup. The Knox Dolomite is a limestone, dolomite, and shale sequence with dolomite containing ca. 88–445 ppm Mn and ca. 1340–7050 ppm Fe, and typical Mn:Fe ratios of 0.001–0.256 (Montañez Citation1994).
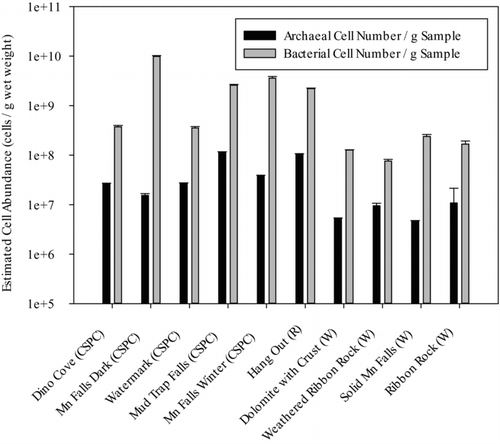
Fig. 3 Real-time qPCR quantification of SSU rRNA gene copy number of total Archaea and Bacteria in samples from Carter Saltpeter (CSPC), Rockhouse (R), and Worley's (W) Caves. SSU rRNA gene copy number was normalized by 1.77 copies (for Archaea) and 4.08 copies (for Bacteria) to obtain estimates of cell abundance. Analyses were conducted in triplicate and error bars represent standard error.
Weathering and corrosion of the Knox Dolomite bedrock by infiltration of meteoric water containing carbonic acid produces a nontronite (smectite-type) clay residuum in addition to calcite speleothems in all four study sites, including flowstones, rimstones, and popcorn-like micronodules (cave coral). We observe ferromanganese crusts, coatings, and/or biofilms occurring on all substrate types. Geochemical analyses show that the concentration of total Mn in these crusts, coatings, and biofilms ranges from 10.9 ppm–327.5 ppm (). The Mn:Fe ratios in cave ferromanganese deposits vary between cave systems and within cave deposits, ranging from 0.06–1.07 (). However, no obvious trends have emerged that indicate a possible effect of substrate mineralogy or water content on Mn:Fe ratios in these deposits. For example, samples taken from different locations with similar substrate compositions in Carter Saltpeter Cave show both the second highest (0.91) and lowest (0.06) Mn:Fe ratios. Notably, the Mn:Fe ratio at all sample sites, with the exception of CSPC Watermark (), was enriched relative to the average Mn:Fe ratio for Knox Group rocks in that region (ca. 0.07) as reported by Montañez (Citation1994). At sites where the Mn:Fe ratio approaches 1 (e.g. Ribbon Rock and Mn Falls), this would represent nearly an order of magnitude increase above the bulk bedrock ratio.
Quantification of Bacterial and Archaeal Abundance
Our qPCR results () demonstrated that total bacterial SSU rRNA genes in Mn oxide-rich samples from Mn Falls and Mud Trap Falls (CSPC- ) represented approximately 9 × 109 cells/g wet weight, a number that was confirmed via fluorescence direct cell counts. Total bacterial SSU rRNA genes from other ferromanganese coatings and crusts () were up to two orders of magnitude lower, containing an estimated 7 × 107–2 × 109 cells/g wet weight. Archaeal SSU rRNA gene sequences from cave samples were estimated to represent from 5 × 106–1 × 108 cells/g wet weight, with an average of 2.7 × 107 cells/g wet weight in biofilm samples vs. 3.8 × 107 cells/g wet weight in ferromanganese coatings and crusts. Archaeal percentages were highest at several sample sites within the CSPC and Worley's cave systems: Dino Cove (ca. 7%), Ribbon Rock (ca. 6%), Watermark (ca. 7%), and Weathered Ribbon Rock (ca. 11%). Overall, bacterial SSU rRNA gene copies represented on average 95% (89–99%) of the total estimated microbial (bacterial and archaeal) cell numbers at each site, while those of Archaea represented on average 5% (0.2%–11%).
Bacterial and Archaeal Community Structure
In this study, a SSU rRNA clone library survey was conducted on two ferromanganese-rich deposits located in close physical proximity within the CSPC system, Mn Falls (, , OTUs from this sample are prefaced by a F in and ) and Mud Trap Falls (, OTUs from this sample are prefaced by a T in and ). Using 98% sequence similarity to define archaeal operational taxonomic units (OTUs), analysis of archaeal libraries revealed 26 unique OTUs out of 65 total sequences from the Mn and Mud Trap Falls sites. Rarefaction analysis of sequencing data indicated the development of an asymptotic trend (data not shown); therefore, sampling efforts were sufficient in capturing the archaeal diversity within the microbial community. Good's nonparametic coverage estimator indicated approximately 66–75% coverage in the archaeal libraires.
Fig. 4 Neighbor-joining tree based on Thaumarchaeal SSU rRNA gene sequences obtained from biofilms found in Carter Saltpeter Cave, Carter County, TN in this study. The number of sequences from each library [Mn Falls (MNF) and Mud Trap Falls (MTF)] representing a particular OTU is given in parentheses following the NCBI accession number. Alignments were created using the on-line SILVA aligner. Dendogram was created using PHYLIP. Bootstrapping values are shown for nodes that were supported >50% of the time and with maximum-likelihood analysis (data not shown). Aquifex pyrophilus and Thermotoga maritima were used as outgroups. Branch lengths indicate the expected number of changes per sequence position.
![Fig. 4 Neighbor-joining tree based on Thaumarchaeal SSU rRNA gene sequences obtained from biofilms found in Carter Saltpeter Cave, Carter County, TN in this study. The number of sequences from each library [Mn Falls (MNF) and Mud Trap Falls (MTF)] representing a particular OTU is given in parentheses following the NCBI accession number. Alignments were created using the on-line SILVA aligner. Dendogram was created using PHYLIP. Bootstrapping values are shown for nodes that were supported >50% of the time and with maximum-likelihood analysis (data not shown). Aquifex pyrophilus and Thermotoga maritima were used as outgroups. Branch lengths indicate the expected number of changes per sequence position.](/cms/asset/9e637ef8-5e55-4a99-a752-cfdbf5f54d82/ugmb_a_769651_o_f0004g.gif)
Thaumarchaeal sequences were binned into 3 operational taxonomic units (OTUs/species) (), representing ca. 40% of the total archaeal sequences in cave biofilm clone libraries. According to top BLAST hits, these OTUs were related to clone sequences from a variety of environments, from sediment, to the deep subsurface, freshwater systems, and other ferromanganese deposits. Thaumarchaeal OTUs represented members of the Marine Group 1 Thaumarchaea and SAGMA Groups 1 and 2. The dominant Thaumarchaeal sequence in the present study was OTU TDO2, sharing 98% identity over a 765 bp alignment to a clone sequence isolated from freshwater ferromanganous micronodules and sediments by Stein et al. (Citation2001). Euryarchaeal sequences from clone libraries were binned into 23 OTUs (), representing ca. 60% of the total archaeal sequences in the clone library data. OTUs were closely related to other clones isolated from soil, freshwater and marine systems, and low-temperature environments. Members of the Deep Sea Hydrothermal Vent Group 6 (DSHV6) represented 29% of the total archaeal sequence types in cave biofilm clone libraries, and 45% of the Euryarchaeal sequences.
Bacterial community composition in the CSPC system was much more diverse than archaeal community composition, a pattern that is consistent with most environmental surveys, including caves (Chelius and Moore Citation2004; Macalady et al. Citation2006; Macalady et al. Citation2007; Northup et al. Citation2003). Using a 97% identity cutoff, there were 114 unique OTUs out of ca. 180 total sequences. Rarefaction analysis revealed no evidence of the development of asymptotic trend (data not shown), indicating that sampling efforts were not sufficient to measure the full extent of diversity within the biofilm communities. Good's nonparametric coverage estimator indicated approximately 53–57% coverage for the bacterial libraries. For phylogenetic analysis, data were further reduced into 34 dominant OTUs representing ca. 100 sequences (, , and ). A dominant OTU was defined as representing two or more sequences in the clone library, and it is important to note that this approach would result in an underestimation of diversity in the clone library as ca. 80 singleton OTUs were eliminated from downstream phylogenetic analysis.
Fig. 5 Neighbor-joining tree based on Euryarchaeal SSU rRNA gene sequences obtained from biofilms found in Carter Saltpeter Cave, Carter County, TN in this study. The number of sequences from each library [Mn Falls (MNF) and Mud Trap Falls (MTF)] representing a particular OTU is given in parentheses following the NCBI accession number. Alignments were created using the on-line SILVA aligner. Dendogram was created using PHYLIP. Bootstrapping values are shown for nodes that were supported >50% of the time and with maximum-likelihood analysis (data not shown). Aquifex pyrophilus and Thermotoga maritima were used as outgroups. Branch lengths indicate the expected number of changes per sequence position.
![Fig. 5 Neighbor-joining tree based on Euryarchaeal SSU rRNA gene sequences obtained from biofilms found in Carter Saltpeter Cave, Carter County, TN in this study. The number of sequences from each library [Mn Falls (MNF) and Mud Trap Falls (MTF)] representing a particular OTU is given in parentheses following the NCBI accession number. Alignments were created using the on-line SILVA aligner. Dendogram was created using PHYLIP. Bootstrapping values are shown for nodes that were supported >50% of the time and with maximum-likelihood analysis (data not shown). Aquifex pyrophilus and Thermotoga maritima were used as outgroups. Branch lengths indicate the expected number of changes per sequence position.](/cms/asset/97d31cba-7445-4821-974e-16651403fdf1/ugmb_a_769651_o_f0005g.gif)
Fig. 6 Neighbor-joining tree inferring the phylogenetic relationship between cultured strains* (asterisks) and those found in clone libraries in Carter Saltpeter Cave, Carter County, TN in this study for sequences clustering in the Beta- and Gammaproteobacteria. Isolates that oxidize Mn continually are indicated by a +; isolates that oxidize Mn intermittently are indicated by a ±. Putative Nitrogen-fixing, Mn(II)-oxidizers are indicated by an N; putative methylotrophic Mn(II)-oxidizers are indicated by an M. Source of isolation is noted immediately before accession number. The number of sequences from each library [Mn Falls Light (L) and Mn Falls Dark (D)] representing a particular OTU is given in parentheses following the NCBI accession number. Alignments were created using the on-line SILVA aligner. Dendogram was created using PHYLIP. Bootstrapping values are shown for nodes that were supported >50% of the time and with maximum-likelihood analysis (data not shown). Aquifex pyrophilus and Thermotoga maritima were used as outgroups. Branch lengths indicated the expected number of changes per sequence position (see scale bar).
![Fig. 6 Neighbor-joining tree inferring the phylogenetic relationship between cultured strains* (asterisks) and those found in clone libraries in Carter Saltpeter Cave, Carter County, TN in this study for sequences clustering in the Beta- and Gammaproteobacteria. Isolates that oxidize Mn continually are indicated by a +; isolates that oxidize Mn intermittently are indicated by a ±. Putative Nitrogen-fixing, Mn(II)-oxidizers are indicated by an N; putative methylotrophic Mn(II)-oxidizers are indicated by an M. Source of isolation is noted immediately before accession number. The number of sequences from each library [Mn Falls Light (L) and Mn Falls Dark (D)] representing a particular OTU is given in parentheses following the NCBI accession number. Alignments were created using the on-line SILVA aligner. Dendogram was created using PHYLIP. Bootstrapping values are shown for nodes that were supported >50% of the time and with maximum-likelihood analysis (data not shown). Aquifex pyrophilus and Thermotoga maritima were used as outgroups. Branch lengths indicated the expected number of changes per sequence position (see scale bar).](/cms/asset/7137e07c-f805-4b72-aa7f-887f539a6e60/ugmb_a_769651_o_f0006g.gif)
Fig. 7 Neighbor-joining tree inferring the phylogenetic relationship between cultured strains* (asterisks) and those found in clone libraries in Carter Saltpeter Cave, Carter County, TN in this study for sequences clustering in the Bacteroidetes, Chlorobi, and Actinobacteria. Isolates that oxidize Mn(II) continually are indicated by a +; isolates that oxidize Mn(II) intermittently are indicated by a ±. Source of isolation is noted immediately before accession number. The number of sequences from each library [Mn Falls Light (L) and Mn Falls Dark (D)] representing a particular OTU is given in parentheses following the NCBI accession number. Alignments were created using the on-line SILVA aligner. Dendogram was created using PHYLIP. Bootstrapping values are shown for nodes that were supported >50% of the time and with maximum-likelihood analysis (data not shown). Aquifex pyrophilus and Thermotoga maritima were used as outgroups. Branch lengths indicate the expected number of changes per sequence position (see scale bar).
![Fig. 7 Neighbor-joining tree inferring the phylogenetic relationship between cultured strains* (asterisks) and those found in clone libraries in Carter Saltpeter Cave, Carter County, TN in this study for sequences clustering in the Bacteroidetes, Chlorobi, and Actinobacteria. Isolates that oxidize Mn(II) continually are indicated by a +; isolates that oxidize Mn(II) intermittently are indicated by a ±. Source of isolation is noted immediately before accession number. The number of sequences from each library [Mn Falls Light (L) and Mn Falls Dark (D)] representing a particular OTU is given in parentheses following the NCBI accession number. Alignments were created using the on-line SILVA aligner. Dendogram was created using PHYLIP. Bootstrapping values are shown for nodes that were supported >50% of the time and with maximum-likelihood analysis (data not shown). Aquifex pyrophilus and Thermotoga maritima were used as outgroups. Branch lengths indicate the expected number of changes per sequence position (see scale bar).](/cms/asset/b3eeea97-7acb-4c92-8742-07b91796f585/ugmb_a_769651_o_f0007g.gif)
Fig. 8 Neighbor-joining tree inferring the phylogenetic placement of SSU rRNA gene sequences obtained from biofilms found in Carter Saltpeter Cave, Carter County, TN in this study for sequences clustering in the Alpha- and Deltaproteobacteria as well as Planctomycetes, Verrucomicrobia, and Acidobacteria. The number of sequences from each library [Mn Falls Light (L) and Mn Falls Dark (D)] representing a particular OTU is given in parentheses following the NCBI accession number. Alignments were created using the on-line SILVA aligner. Dendogram was created using PHYLIP. Bootstrapping values are shown for nodes that were supported >50% of the time and with maximum-likelihood analysis (data not shown). Aquifex pyrophilus and Thermotoga maritima were used as outgroups. Branch lengths indicate the expected number of changes per sequence position (see scale bar).
![Fig. 8 Neighbor-joining tree inferring the phylogenetic placement of SSU rRNA gene sequences obtained from biofilms found in Carter Saltpeter Cave, Carter County, TN in this study for sequences clustering in the Alpha- and Deltaproteobacteria as well as Planctomycetes, Verrucomicrobia, and Acidobacteria. The number of sequences from each library [Mn Falls Light (L) and Mn Falls Dark (D)] representing a particular OTU is given in parentheses following the NCBI accession number. Alignments were created using the on-line SILVA aligner. Dendogram was created using PHYLIP. Bootstrapping values are shown for nodes that were supported >50% of the time and with maximum-likelihood analysis (data not shown). Aquifex pyrophilus and Thermotoga maritima were used as outgroups. Branch lengths indicate the expected number of changes per sequence position (see scale bar).](/cms/asset/872f5f02-f99c-4f96-b475-7a21f31a2151/ugmb_a_769651_o_f0008g.gif)
Dominant bacterial OTUs from relatively small (ca. 96 sequences) clone libraries of a cave biofilm represented a diverse taxonomic array, with sequences from recovered clones in the libraries representing members of the Bacteroidetes (26%), Betaproteobacteria (20%), Alphaproteobacteria (15%), Acidobacteria (12%), Gammaproteobacteria (10%), Verrucomicrobia (7%), Planctomycetes (5%), Chlorobi (2%), and Deltaproteobacteria (2%). Several dominant bacterial OTUs identified in this study were related to clones and environmental isolates from freshwater and marine systems, sediment, contaminated ecosystems, other cave systems, and ferromanganese deposits. Some (ca. 10%) of the 34 dominant OTUs were related to known Mn-oxidizers such as Leptothrix [OTU BF2AO7, , 100% identical over a 1,485 bp alignment to Leptothrix cholodnii SP-6 (Emerson and Ghiorse Citation1992)] and Pseudomonas (OTUs BF2B07 and BF2E03, ). In addition, some (ca. 11%) of the 34 dominant OTUs were related to known Fe-oxidizers (Leptothrix spp.) or clones isolated from oxidized iron deposits (OTUs BF2C07 and BF2C10, ). Combined, 21% of the recovered clone sequences represented by dominant OTUs were related to known metal-oxidizers or clones isolated from oxidized metal environments.
Isolation of Mn(II)-oxidizing Microorganisms
Six Mn(II)-oxidizing isolates obtained from CSPC ferromanganese deposits clustered within the Gammaproteobacterial subphylum and are near members of the genus Pseudomonas, a common group of known Mn(II)-oxidizing microorganisms (, ). Cultures N4, T4, T2, and N3 were isolated on a modified version of Burk's N-free media (Mohandas Citation1988), although we have not yet established if these isolates are capable of N2 fixation. Isolate N3 is 97% identical over a 1,502 bp alignment to OTU BF2E03, and isolates N4 and T4 are 97% identical (over a 1,181 and 1,473 bp alignment, respectively) to OTU BF2B07. Isolate Mn Falls 11 is a close relative (99% identity over a 1,128 bp sequence alignment) of Pseudomonas putida, a model organism used in the study of the molecular mechanisms involved in Mn(II)-oxidation (Geszvain and Tebo Citation2010).
Table 3 Media used to isolate Mn(II)-oxidizing bacteria in this study
Two Mn(II)-oxidizing isolates obtained from ferromanganese deposits within CSPC clustered within the Betaproteobacteria (, ): Janthinobacterium sp. A6 and Leptothrix sp. G6. Janthinobacterium sp. A6 oxidizes Mn(II) in liquid culture, and appears to oxidize Mn extracellularly, since clumps of Mn oxides were loosely associated with cells (). Isolate A6 was obtained from a serial dilution culture containing 2.5×10–8g wet weight biofilm material. Isolate A6 oxidized Mn(II) intermittently during maintenance of the culture, with newly inoculated cultures periodically losing the capability to oxidize Mn(II). However, the sequenced culture was oxidizing Mn(II), as confirmed by LBB testing.
Fig. 9 TEM microscopy of the sheath-forming isolate Leptothrix sp. G6 and Janthinobacterium sp. A6. TEM micrographs (scale bars represent1 μm) demonstrated the presence of electron-dense Mn deposits associated with bacterial cells. STEM P and Mn elemental maps (scale bars represent 800 nm) revealed the location of phosphorous and manganese within bacterial cells.
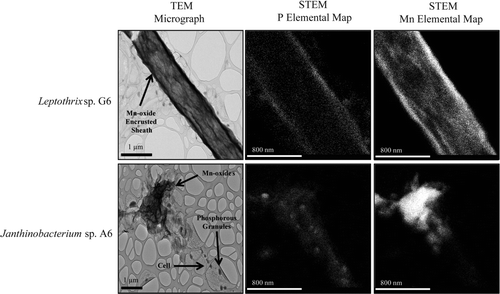
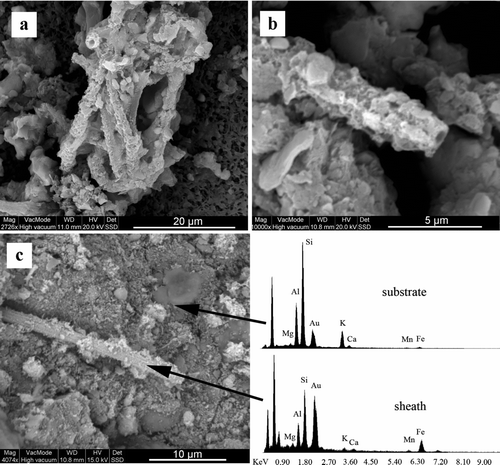
Cave isolate Leptothrix sp. G6 oxidizes Mn along the sheath (). This organism is 99% identical over a 1,485 bp alignment to the Mn-oxidizing, sheath-forming Leptothrix cholodnii (formerly L. discophora) SP-6 (Emerson and Ghiorse Citation1992), and it was obtained from a serial dilution culture containing 2.5 × 10–8 g wet weight deposit material. Leptothrix-like morphologies, as evidenced by straight, hollow sheaths coated in Mn and Fe, were detected in in-situ based glass slide incubations () at the Mud Trap Falls seep and in SEM images of sample material from DBC's Top of V site ().
Fig. 10 SEM images (a,b) of a polycarbonate filter mounted on a glass slide and incubated for 6 months in situ at the top of the Mud Trap Falls seep (CSPC) revealed Leptothrix-like microbial morphologies. SEM micrograph of Daniel Boone Caverns Top of V site (c) revealed a putative Leptothrix; however, the hollow structure of the putative sheath was not verified. EDS spectra of the filament is enriched in Fe and Mn as compared to EDS spectra of the background material.
Two Mn(II)-oxidizing isolates obtained from ferromanganese deposits within CSPC clustered within the Bacteroidetes (): Flavobacterium sp. E8 and Flavobacterium sp. MTFA, which oxidize Mn(II) in liquid culture (). Members of this genus have previously been reported to oxidize Mn(II) (Ford and Mitchell Citation1990; Nealson Citation1978; Santelli et al. Citation2010). Flavobacterium sp. E8 is 98% identical over a 1,387 bp alignment to the Mn(II)-oxidizing Flavobacterium sp. DS2psk4b, which was isolated from coal mine drainage (Santelli et al. Citation2010). Isolate E8 was obtained from a serial dilution culture containing 2.5×10–10g wet weight biofilm material.
A single isolate clustering within the Actinobacteria, Arthrobacter sp. L (, ), was obtained from a ferromanganese deposit located within Daniel Boone Caverns. Arthrobacter isolate L oxidizes Mn(II) in liquid culture; members of this genus have been reported to demonstrate this capability (Schweisfurth et al. Citation1978). Arthrobacter sp. L is 98% identical over a 1,457 bp alignment to its closest cultivated relative, Arthrobacter methylotrophus, a facultative methylotroph isolated from an enrichment culture containing dimethylsulfone as the sole source of carbon and energy (Borodina et al. Citation2000, Citation2002). Interestingly, in addition to Mn(II)-oxidizing microorganisms, two putative methylotrophic Mn(II)-oxidizers were isolated in this study: a member of the Gammaproteobacteria, Acinetobacter sp. V1 (, ), and a member of the Bacteroidetes, Flavobacterium sp. V2 (, ). Both isolates were capable of growth in liquid NMS media, which was designed to target methanotrophic Mn oxidizers by using methane as a sole carbon source. However, methanotrophy has not yet been confirmed in these strains.
Discussion
The Cave Geochemical Environment
Iron and manganese are the fourth and fifth most abundant elements in the Earth's crust, respectively (Edwards et al. Citation2004; Tebo et al. Citation2007), where Fe outweighs Mn by a ratio of ca. 58:1 in the upper continental crust (Turekian and Wedepohl Citation1961; Wedepohl Citation1995). The predominance of Fe over Mn in a variety of natural systems is well documented in studies of marine (Edwards et al. Citation2004; Nitahara et al. Citation2011) and freshwater systems (Johnson et al. Citation2012; Stein et al. Citation2001). However, in cases where the concentration of Mn is equal to or outweighs the concentration of Fe (Gradziński et al. Citation1995; Krumbein and Jens Citation1981) or where secondary mineral deposits are enriched in metal concentration relative to substrate geochemistry (Cunningham et al. Citation1995; Northup et al. Citation2003; Spilde et al. Citation2005, 2006), biomineralization processes are invoked as a causal factor in the formation of Mn-enriched geochemical environments.
These microbial biomineralization processes contribute to ferromanganese oxide accretion in this study, where Mn concentrations were enriched relative to bedrock concentrations and where the Mn:Fe ratio approached 1:1, such as Worley's Ribbon Rock and CSPC Mn Falls (). A Mn:Fe ratio of ca. 1:1 in cave ferromanganese deposits is a common ratio found in deep, oligotrophic systems in the southwest United States such as Lechuguilla and Spider Caves (Northup et al. Citation2003; Spilde et al. Citation2005). Previous research demonstrates the role of microsite geochemistry in establishing environmental niches (Engel et al. Citation2010; Macalady et al. Citation2008; Rossmassler et al. Citation2012), structuring microbial communities (Barton et al. Citation2007; Goldscheider et al. Citation2006; Shabarova and Pernthaler Citation2010), and influencing mineral precipitation (Frierdich et al. Citation2011) and composition (Post Citation1999; White et al. Citation2009) in subsurface karst systems. However, the role of microsite geochemistry on biomineralization in shallow, epigenic cave systems may be masked by agricultural, anthropogenic, and soil geochemical inputs.
Ferromanganese Biofilm Community Composition
In the present study, qPCR data indicated that bacterial cells represented on average 95% of the total microbial cells at sample sites. Most notably, the range of detection of bacterial cell numbers in our study (7.5 × 107 to 9.8 × 109 cells/mL) was two to four orders of magnitude higher than numbers typically reported within similar, pH neutral cave systems (Barton et al. Citation2006; Northup et al. Citation2000). In addition, Mn oxide-rich biofilm samples were estimated to contain 9.85 × 109 total microbial cells/g wet weight, a quantity that is two orders of magnitude greater than studies of ferromanganese deposits in Spider and Lechuguilla Caves (Spilde et al. Citation2005). Deep cave systems with limited energy supply and minimal human impact are considered to be oligotrophic environments, and recent research indicates that they are low-biomass environments with high levels of microbial diversity (Northup and Lavoie Citation2001; Barton et al. Citation2004; Boston et al. Citation2006; Hunter et al. Citation2004). The total cell count reported in the present study of surface influenced shallow caves is higher than that found in deeper, oligotrophic caves; however, it is not outside the range of numbers reported in studies of other environmental systems. Microbial cell abundance has been estimated to range from 1.29 × 109 to 7.6 × 1010 cells/mL in marine sediments (Nitahara et al. Citation2011), cold seep microbial mats (Grünke et al. Citation2011), arable soil (Torsvik et al. Citation2002), and filamentous microbial mats in sulfidic springs (Engel et al. Citation2004).
At the time of sampling, the CSPC Mn Falls site was thought to be heavily impacted by organic input from sewage effluent (Carmichael et al. 2013). Bacterial biomass in cave pools has been shown to increase proportionally with organic carbon input (Simon and Buikema Jr. Citation1997), and cell concentrations in the range of 1 × 109 cells/mL have been reported in sewage (Fierer and Lennon Citation2011). Therefore, high cell counts at sites within the CSPC system could be indicative of high levels of anthropogenic impact (e.g., nutrient loading), due in part to the shallow, surface-influenced depths of these caves. In fact, clone library data corroborate this idea as many of the clones obtained here were related to sequences obtained from fecal contaminated environments (see , OTUs BF2B07, BF2E04, BF2F03, BLD10, and BLB01). However, it is important to note that if the environment has selected for microbes adapted to high nutrient loading, it is likely that many of these organisms may have above average SSU rRNA operon copy numbers (Klappenbach et al. Citation2000). Therefore, it is possible that the total cell number has been overestimated.
In general, a high degree of diversity within systems is supported by microbial metabolic versatility (Whitman et al. Citation1998) and the development of mutualistic associations in biofilm communities leading to the interdependency of organisms within the community (Fierer and Lennon Citation2011). The SSU rRNA data from the present study are suggestive of the presence of a variety of microbial metabolic strategies within the CSPC Mn Falls and Mud Trap Falls communities, as clone sequences obtained in this study are closely related to methanogens (OTU G01, ), hydrocarbon degraders (OTU BF2E03, ), ammonia-oxidizers (OTU BF2F03, ), denitrifiers (OTU BLB01, ), and iron or manganese-oxidizers. Several lines of evidence from the SSU rRNA molecular-based survey of CSPC suggest that metal-oxidation plays an important role in the formation of ferromanganous biofilms within the cave system.
Members of the Proteobacteria (47% of the sequences represented by dominant OTUs in the present study) are common constituents of cave clone libraries and have been detected in RNA-based surveys as metabolically active members of cave microbial consortia (Portillo et al. Citation2008). Molecular work from the present study indicates that Leptothrix are present in detectable numbers within the CSPC Mn Falls biofilm community. In fact, 6% of the total library sequences (from both libraries) were represented by OTU BF2A07 (), suggesting that both samples included live cells from the growing edge of the Leptothrix sheaths (Fleming et al. Citation2011). Additionally, the organisms representing these OTUs were phylogenetically related (99% identity over a 1,489 bp sequence alignment) to the Leptothrix strain isolated from a high dilution suggesting that they play an important role in the biomineralization of Mn in southern Appalachian cave systems. Leptothrix spp. are known to be capable of both Fe and Mn oxidation (Spring Citation2006; van Veen et al. Citation1978), and in situ slides of Leptothrix from CSPC Mud Trap Falls demonstrate both Mn and Fe oxidation, thus confirming the role of microbes in the formation of cave ferromanganese biofilms and crusts.
Although based on small sample size, molecular analyses indicate that the most abundant and detectable populations in CSPC ferromanganese deposits (e.g., Leptothrix, Pseudomonas, and Flavobacterium-related organisms) are distinct from the populations identified by Northup et al. (Citation2003) in a survey of the microbial communities inhabiting ferromanganese deposits in Lechuguilla and Spider Caves, which included taxa related to Hyphomicrobium, Pedomicrobium, Leptospirillum, Stenotrophomonas, and Pantoea-related organisms. These two distinct microbial communities may exhibit a level of functional redundancy in Fe- and Mn-biomineralization capacity within the two cave systems. Evidence from this study indicates that shallow cave systems may harbor Mn-oxidizing consortia with a unique signature in comparison to other cave systems, although deeper sequencing efforts may reveal more overlap.
Questions exist regarding the nature of these community differences, and whether these differences relate to differences in nutrient input in anthropogenically impacted vs. pristine cave systems, or simply the fact that these two cave systems differ in both regional geology and hydrology. Lechuguilla Cave has been described as deep and oligotrophic (Northup et al. Citation2003), whereas a parallel study of CSPC indicates that it has been severely impacted by nutrient loading from the surface (Carmichael et al. Citation2013). Future research should attempt to elucidate the cause of these observed differences, with an initial focus on the increased role of surface impact in shallow vs. deep cave systems.
Archaea, like Bacteria, are ubiquitous within the environment (DeLong Citation1992) and play a key role in the maintenance of biogeochemical cycles (Goldscheider et al. Citation2006). Yet, Archaea remain understudied members of microbial communities, particularly in caves. The role of Archaea in the formation and transformation of cave ferromanganese deposits is debatable, although molecular evidence of Archaea in clone libraries generated from cave ferromanganese deposits (Northup et al. 2003) has led some investigators (Tebo et al. Citation2005) to speculate regarding the possible existence of a new class of Archaea capable of Mn-biomineralization.
In the present study, Archaea represented ca. 5.0% of the total microbial population in a cave biofilm and ferromanganese coating. Archaeal community diversity was relatively low in comparison to bacterial community diversity; however, this trend is consistent with some cave systems (Macalady et al. Citation2007; Legatzki et al. Citation2011), marine ferromanganese crusts (Nitahara et al. Citation2011), and some other environments (DeLong Citation1992). The archaeal community in this study was dominated by members of Thaumarchaea, primarily Marine Group 1 OTU TDO2. This group accounted for 37% of the total archaeal diversity in the library, and 92% of the Thaumarchaeal diversity. At least one clone from the SAGMA groups 1 and 2 of the Thaumarchaea was also recovered and this clone (TG02) shared 92% identity to one recovered from Lechuguilla Cave, clone CV1B4 (Northup et al. Citation2003, and ). Members of the Rice Cluster V and Deep Sea Hydrothermal Vent Group 6 (DSHV6) Euryarchaea were the second and third most dominant archaeal groups, representing 32% and 29% respectively of the total archaeal diversity captured in this study.
Of the six DSHV6 OTUs identified in this study, four (FD02, FF04, TD04, and TD06) were less than 95% similar to their closest relatives as determined by BLAST analysis. This high degree of divergence (Amann et al. Citation1995) indicates that these sequences represent novel lineages unique to CSPC or other similar systems. Several findings emphasize the need to ascertain the functional role of Archaea in cave biogeochemical cycling: 1) the dominance of sequences related to Thaumarchaeal OTU TD02 in the CSPC Mn(II)-oxidizing biofilm community, 2) recent evidence from other studies of metabolically active Thaumarchaea in similar cave systems (Gonzalez et al. Citation2006), and 3) the recovery of several novel Euryarchaeal lineages in CSPC.
Mn-biomineralization in Cave Ferromanganese Deposits
The present study is significant due to the broad taxonomic array of bacteria isolated from cave ferromanganese deposits that demonstrate the ability to oxidize Mn(II) in culture. The isolation of a Janthinobacterium sp. capable of Mn(II)-oxidation represents the first report of a Mn(II)-oxidizing member from a cave, although members of this genus have been both detected in clone libraries, as well as isolated from desert varnish (Northup et al. Citation2010). This finding expands the phylogenetic diversity of known cave Mn-oxidizers to include new genera and provides further support for the importance of this process to the microbial cell. In addition, this study represents the first report of cave isolates among the genera Flavobacterium and Arthrobacter that also demonstrated Mn-biomineralization capacity in vitro. Flavobacterium (Ikner et al. Citation2007) and Arthrobacter (Ikner et al. Citation2007; Laiz et al. Citation2000) species have been isolated from cave systems in prior studies, although the Mn-biomineralization capacity of these isolates was not established.
Iron and manganese oxidizing Leptothrix spp. are commonly isolated from freshwater systems and are particularly abundant at redox interfaces (Spring Citation2006; van Veen et al. 1978). However, Leptothrix spp. typically lose their sheath-forming capacity in culture, so isolation of a close relative of the sheath-forming strain SP-6 from a high dilution is unusual and noteworthy. The isolation of a Mn(II)-oxidizing Leptothrix from CSPC represents the first reported isolation of this organism from a cave in over twenty years (Moore Citation1981; Peck Citation1986), although recent work has demonstrated the presence of Leptothrix-like morphologies in caves using scanning electron microscopy (de los Ríos et al. Citation2011; Florea et al. Citation2011; Frierdich et al. Citation2011). In-situ based cultivation efforts from this study ( and 10b) demonstrate that Leptothrix spp. are actively oxidizing Mn in the field, corroborating in-vitro cultivation results. Combined data from this and other studies suggests that Leptothrix spp. may be widespread in epigenic cave systems. Further, results demonstrate that Mn-oxidizing Leptothrix spp. are particularly abundant and active in our study sites.
Over 30 different metal oxide and hydroxide minerals can be found in caves (Hill and Forti Citation1997) though it is important to note that not all black or dark brown deposits in caves are Mn oxides (Gázquez et al. Citation2012; Hill Citation1982) and it is unlikely that all are formed by microbes. However, Mn-oxidizing microbes have been shown to produce several of the Mn oxide minerals (such as birnessite and todorokite) commonly found in caves (Frierdich et al. Citation2011; Gadd Citation2007; Northup and Lavoie Citation2001; Santelli et al. Citation2010; Spilde et al. Citation2005). The isolation of three Mn(II)- biomineralizing organisms from high dilutions (2.5×10–8 g wet weight and 2.5×10–10 g wet weight) is suggestive of the environmental relevance of these genera (Janthinobacterium, Leptothrix, and Flavobacterium) in the formation of cave ferromanganese deposits in the upper Tennessee River Basin. The diversity of isolates with inferred biomineralization capacity discovered in this study, combined with evidence of in situ Mn-oxidation by Leptothrix spp. demonstrates the importance of these organisms in cave biogeochemical cycles.
Conclusions
The present study represents the first geomicrobiological analysis of ferromanganese deposits within the cave-rich, yet understudied southern Appalachian karst system. SSU rRNA based molecular surveys at Mn-enriched sites reveal the genetic potential for Mn-biomineralization within these deposits. Biomineralization capacity was inferred via culture-dependent surveys, which resulted in the isolation of a broad taxonomic array of Mn(II)-oxidizing bacteria from cave ferromanganese deposits. Combined molecular, cultivation-based, and in situ-based evidence demonstrate that Mn(II)-oxidizing bacteria are abundant and environmentally relevant species in cave ferromanganese deposits. Further, at the present sequencing depth, results suggest that microbial communities contributing to the accretion of ferromanganese deposits in these shallow cave systems share little taxonomic similarity with those in deeper cave systems. The Mn-oxidizing bacteria identified here likely play a role in mediating cave biogeochemical cycles, forming and transforming the cave mineral environment, and are vital contributors (via functional diversity) to the maintenance of cave microbial consortia (Warren and Kauffman Citation2003) within these fragile and unique cave systems.
Acknowledgments
The authors would like to thank Dr. Clara Chan for assistance with electron microscopy and sample preparation, and Dr. Guichuan Hou and Oliver Burns for assistance with light, confocal, and electron microscopy. We thank Dr. Yongli Gao, Taylor Burnham, Seth Hewitt, John Rossi, Milton Starnes, Robbie Winters, and John Matthews for providing field expertise and scouting locations of cave ferromanganese deposits, and to the landowners of the caves for site access. We are appreciative of assistance from Dr. Carol Babyak, Dr. Shea Tuberty, Daniel Jackson, and Yosuke Sakamachi in preparing samples and in conducting ICP-OES analyses. We are deeply grateful for the assistance of Dr. Trevor Craig, Zach Anderson, Jared Butler, Kornelia Galior, Ashley Hawkins, Daniel Parker, Leigh Anne Roble, Marlie Shelton, and Bryan Zorn in culture maintenance, field assistance, and procedural development. Partial support was provided through a National Science Foundation grant 0935270 to S. Bräuer, two North Carolina Space Grant New Investigators Program Awards to S. Carmichael and to S. Bräuer, and through a North Carolina Space Grant Graduate Research Fellowship awarded to M.J. Carmichael. Support was also provided by Appalachian State University.
© Mary J. Carmichael, Sarah K. Carmichael, Cara M. Santelli, Amanda Strom, and Suzanna L. Bräuer
References
- Allouc , J and Harmelin , J G . 2001 . Mn-Fe deposits in shallow cryptic marine environment: examples in northwestern Mediterranean submarine caves . B Soc Geol Fr , 172 : 765 – 778 .
- Amann , R I , Ludwig , W and Schleifer , K-H . 1995 . Phylogenetic identification and in situ detection of individual microbial cells without cultivation . Microbiol Rev , 59 : 143 – 169 .
- Angert , E R , Northup , D E , Reysenbach , A-L , Peek , A S , Goebel , B M and Pace , N R . 1998 . Molecular phylogenetic analysis of a bacterial community in Sulphur River, Parker Cave, Kentucky . Am Mineral , 83 : 1583 – 1592 .
- Archibald , F S and Fridovich , I . 1981 . Manganese and defenses against oxygen toxicity in Lactobacillus plantarum . J Bacteriol , 145 : 442 – 451 .
- Barton , H A and Jurado , V . 2007 . What's up down there? Microbial diversity in caves . Microbe , 2 : 132 – 138 .
- Barton , H A and Luiszer , F . 2005 . Microbial metabolic structure in a sulfidic cave hot spring: potential mechanisms of biospeleogenesis . J Cave Karst Stud , 67 : 28 – 38 .
- Barton , H A and Northup , D E . 2007 . Geomicrobiology in cave environments: past, current and future perspectives . J Cave Karst Stud , 69 : 163 – 178 .
- Barton , H A , Taylor , M R and Pace , N R . 2004 . Molecular phylogenetic analysis of a bacterial community in an oligotrophic cave environment . Geomicrobiol J , 21 : 11 – 20 .
- Barton , H A , Taylor , N M , Kreate , M P , Springer , A C , Oehrle , S A and Bertog , J L . 2007 . The impact of host rock geochemistry on bacterial community structure in oligotrophic cave environments . Int J Speleol , 36 : 93 – 104 .
- Barton , H A , Taylor , N M , Lubbers , B R and Pemberton , A C . 2006 . DNA extraction from low-biomass carbonate rock: An imporved method with reduced contamination and the low-biomass contaminant database . J Microbiol Meth , 66 : 21 – 31 .
- Borodina , E , Kelly , D P , Rainey , F A , Ward-Rainey , N L and Wood , A P . 2000 . Dimethylsulfone as a growth substrate for novel methylotrophic species of Hyphomicrobium and Arthrobacter . Arch Microbiol , 173 : 425 – 437 .
- Borodina , E , Kelly , D P , Schumann , P , Rainey , F A , Ward-Rainey , N L and Wood , A P . 2002 . Enzymes of dimethylsulfone metabolism and the phylogenetic characterization of the facultative methylotrophs Arthrobacter sulfonivorans sp. nov., Arthrobacter methylotrophus sp. nov., and Hyphomicrobium sulfonivorans sp. nov . Arch Microbiol , 177 : 173 – 183 .
- Boston , P J , Hose , L D , Northup , D E and Spilde , M N . 2006 . “ The microbial communities of sulfur caves: a newly appreciated geologically driven system on Earth and potential model for Mars ” . In Perspectives on Karst Geomorphology, Hydrology, and Geochemistry , Edited by: Harmon , R S and Wicks , C M . 331 – 344 . Boulder , Colorado : Geological Society of America .
- Cadillo-Quiroz , H , Braüer , S , Yashiro , E , Sun , C , Yavitt , J and Zinder , S . 2006 . Vertical profiles of methanogenesis and mathanogens in two contrasting acidic peatlands in central New York State, USA . Environ Microbiol , 8 : 1428 – 1440 .
- Campbell , J W , Watson , A , Watson , C , Ball , H and Pirkle , R . 2011 . Escherichia coli, other coliform, and environmental chemoheterotrophic bacteria in isolated water pools from six caves in northern Alabama and northwestern Georgia . J Cave Karst Stud , 73 : 75 – 82 .
- Cañaveras , J C , Cuezva , S , Sanchez-Moral , S , Lario , J , Laiz , L , Gonzalez , J M and Saiz-Jimenez , C . 2006 . On the origin of fiber calcite crystals in moonmilk deposits . Naturwissenschaften , 93 : 27 – 32 .
- Carmichael , S , Carmichael , M , Strom , A , Johnson , K , Roble , L , Gao , Y and Bräuer , S . 2013. Sustained anthropogenic impact in Carter Saltpeter Cave, Carter County, Tennessee and the potential effects on Mn cycling . J Cave Karst Stud [accepted]. ,
- Chelius , M K and Moore , J C . 2004 . Molecular phylogenetic analysis of Archaea and Bacteria in Wind Cave, South Dakota . Geomicrobiol J , 21 : 123 – 134 .
- Christman , M C and Culver , D C . 2001 . The relationship between cave biodiversity and available habitat . J Biogeogr , 28 : 367 – 380 .
- Cunningham , K I , Northup , D E , Pollastro , R M , Wright , W G and Larock , E J . 1995 . Bacteria, fungi and biokarst in Lechuguilla Cave, Carlsbad Caverns National Park, New Mexico . Environ Geol , 25 : 2 – 8 .
- Daly , M J , Gaidamakova , E , Matrosova , V , Vasilenko , A , Zhai , M , Venkateswaran , A , Hess , M , Omeichenko , M V , Kostandarithes , H , Makarova , K , Wackett , L , Fredrickson , J K and Ghosal , D . 2004 . Accumulation of Mn(II) in Deinococcus radiodurans facilitates gamma-radiation resistance . Science , 306 : 1025 – 1028 .
- de los Ríos , A , Bustillo , M , Ascaso , C and Carvalho , M R . 2011 . Bioconstructions in ocherous speleothems from lave tubes on Terceira Island (Azores) . Sediment Geol , 236 : 117 – 128 .
- DeLong , EF. 1992 . Archaea in coastal marine environments . Proc Natl Acad Sci USA , 89 : 5685 – 5689 .
- Dixon , J B and Skinner , H CW . 1992 . “ Manganese minerals in surface environments ” . In Biomineralization Processes of Iron and Manganese , Edited by: Skinner , H CW and Fitzpatrick , R W . 31 – 59 . Cremlingen , , Germany : Catena Verlag .
- Edwards , K J , Bach , W and McCollum , T M . 2004 . Neutrophilic iron-oxidizing bacteria in the ocean: their habitats, diversity, and roles in mineral deposition, rock alteration, and biomass production in the deep sea . Geomicrobiol J , 21 : 393 – 404 .
- Einen , J , Thorseth , I H and Øvreås , L . 2008 . Enumeration of Archaea and Bacteria in seafloor basalt using real-time quantitative PCR and fluorsecence microscopy . FEMS Microbiol Lett , 282 : 182 – 187 .
- Emerson , D and Ghiorse , W C . 1992 . Isolation, cultural maintenance, and taxonomy of a sheath-forming strain of Leptothrix discophora and characterization of manganese-oxidizing activity associated with the sheath . Appl Environ Microbiol , 58 : 4001 – 4010 .
- Emerson , D and Moyer , C L . 2002 . Neutrophilic Fe-oxidizing bacteria are abundant at the Loihi Seamount hydrothermal vents and play a major role in Fe oxide deposition . Appl Environ Microbiol , 68 : 3085 – 3093 .
- Engel , A S , Meisinger , D B , Porter , M L , Payn , R A , Schmid , M , Stern , L A , Schleifer , K H and Lee , N M . 2010 . Linking phylogenetic and functional diversity to nutrient spiraling in microbial mats from Lower Kane Cave (USA) . ISME J , 4 : 98 – 110 .
- Engel , A S , Porter , M L , Kinkle , B K and Kane , T C . 2001 . Ecological assessment and geological significance of microbial communities from Cesspool Cave, Virginia . Geomicrobiol J , 18 : 259 – 274 .
- Engel , A S , Porter , M L , Stern , L A , Quinlan , S and Bennett , P C . 2004 . Bacterial diversity and ecosystem function of filamentous microbial mats from aphotic (cave) sulfidic springs dominated by chemolithoautotrophic “Epsilonproteobacteria . FEMS Microbiol Ecol , 51 : 31 – 53 .
- Engel , A S , Stern , L A and Bennett , P C . 2004 . Microbial contributions to cave formation: New insights into sulfuric acid speleogenesis . Geology , 32 : 369 – 372 .
- Felsenstein , J. 2004 . “ PHYLIP (Phylogeny Inference Package) version 3.68 ” . In Distributed by the author Department of Genome Sciences , University of Washington . Seattle
- Fendorf , S E , Fendorf , M , Sparks , D L and Gronsky , R . 1992 . Inhibitory mechanisms of Cr(III) oxidation by δ-MnO2 . J Colloid Interface Sci , 153 : 37 – 54 .
- Fierer , N and Lennon , J T . 2011 . The generation and maintenance of diversity in microbial communities . Am J Bot , 98 : 439 – 448 .
- Fleming , E J , Langdon , A E , Martinez-Garcia , M , Stepanauskas , R , Poulton , N J , Masland , E DP and Emerson , D . 2011 . What's new is old: resolving the identity of Leptothrix ochracea using single cell genomics, pyrosequencing and FISH . PLoS ONE , 6 : e17769
- Florea , L J , Noe-Stinson , C L , Brewer , J , Fowler , R , Kearns , J B and Greco , A M . 2011 . Iron oxide and calcite associated with Leptothrix sp. biofilms within an estavelle in the upper Floridan aquifer . Int J Speleol , 40 : 205 – 219 .
- Fogel , G B , Collins , C R , Li , J and Brunk , C F . 1999 . Prokaryotic genome size and SSU rDNA copy number: estimation of microbial relative abundance form a mixed population . Microb Ecol , 38 : 93 – 113 .
- Ford , T and Mitchell , R . 1990 . The ecology of microbial corrosion . Advances in Microbial Ecology , 11 : 231 – 262 .
- Francis , C A and Tebo , B M . 2002 . Enzymatic manganese(II) oxidation by metabolically-dormant spores of diverse Bacillus species . Appl Environ Microbiol , 68 : 874 – 880 .
- Frierdich , A J , Hasenmueller , E A and Catalano , J G . 2011 . Composition and structure of nanocrystalline Fe and Mn oxide cave deposits: Implications for trace element mobility in karst systems . Chem Geol , 284 : 82 – 96 .
- Gadd , GM. 2007 . Geomycology: biogeochemical transformations of rocks, minerals, metals and radionuclides by fungi, bioweathering and bioremediation . Mycol Res , 111 : 3 – 49 .
- Gao , Y , Benfield , R , Jones , S , Burnham , T and Gibson , N . 2006 . Groundwater tracing in the Rockhouse Cave System, Carter County, Tennessee. In: Proceedings of the Sixteenth Tennessee Water Resources Symposium. Burns, TN , 3B – 20 . Montgomery Bell State Park .
- Gao , Y , Gibson , N , Burnham , T and Evanshen , B . 2006 . Water chemistry and quality changes in the Rockhouse Cave System, Carter County, Tennessee. In: Proceedings of the Sixteenth Tennessee Water Resources Symposium. Burns, TN , 1B – 3 . Montgomery Bell State Park .
- Gázquez , F , Calaforra , J-M , Rull , F , Forti , P and García-Casco , A . 2012 . Organic matter of fossil origin in the amberine speleothems from El Soplao Cave (Cantabria, Northern Spain) . Int J Speleol , 41 : 113 – 123 .
- Geszvain , K and Tebo , B M . 2010 . Identification of a two-component regulatory pathway essential for Mn(II) oxidation in Pseudomonas putida GB-1 . Appl Environ Microbiol , 76 : 1224 – 1231 .
- Ghosal , D , Omelchenko , M V , Gaidamakova , E K , Matrosova , V Y , Vasilenko , A , Venkateswaran , A , Zhai , M , Kostandarithes , H M , Brim , H , Makarova , K S , Wackett , L P , Fredrickson , J K and Daly , M J . 2005 . How radiation kills cells: survival of Deinococcus radiodurans and Shewanella oneidensis under oxidative stress . FEMS Microbiol Rev , 29 : 361 – 375 .
- Goldscheider , N , Hunkeler , D and Rossi , P . 2006 . Review: microbial biocenoses in pristine aquifers and an assessment of investigative methods . Hydrogeol J , 14 : 926 – 941 .
- Gonzalez , J M , Portillo , M C and Saiz-Jimenez , C . 2006 . Metabolically active Crenarchaeota in Altamira Cave . Naturwissenschaften , 93 : 42 – 45 .
- Good , IJ. 1953 . The population frequencies of species and the estimation of population parameters . Biometrika , 40 : 237 – 264 .
- Gradziński , M , Banaś , M and Uchman , A . 1995 . Biogenic origin of manganese flowstones from Jaskinia Czarna Cave, Tatra Mts., Western Carpathians . Annales Societatis Geologorum Poloniae , 65 : 19 – 27 .
- Grünke , S , Felden , J , Lichtschlag , A , Girnth , A-C , De Beer , D , Wenzhöfer , F and Boetius , A . 2011 . Niche differentiation among mat-forming, sulfide-oxidizing bacteria at cold seeps of the Nile Deep Sea Fan (Eastern Mediterranean Sea) . Geobiology , 9 : 330 – 348 .
- Hara , S , Hashidoko , Y H , Desyatkin , R V , Hatano , R and Tahara , S . 2009 . High rate of N2 fixation by East Siberian Cryophilic soil bacteria as determined by measuring acetylene reduction in Nitrogen-poor medium solidified with Gellan Gum . Appl Environ Microbiol , 75 : 2811 – 2819 .
- Hill , C and Forti , P . 1997 . “ Oxides and hydroxides ” . In Cave Minerals of the World , Edited by: Hill , C and Forti , P . 123 – 136 . Huntsville , , Alabama : National Speleological Society .
- Hill , CA. 1982 . Origin of black deposits in caves . Natl Speleol Soc Bull , 44 : 15 – 19 .
- Horner-Devine , M C , Silver , J M , Leibold , M A , Bohannan , B JM , Colwell , R K , Fuhrman , J A , Green , J L , Kuske , C R , Martiny , J BH , Muyzer , G , Øvreås , L , Reysenbach , A-L and Smith , V H . 2007 . A comparison of taxon co-occurence patterns for macro- and microorganisms . Ecology , 88 : 1345 – 1353 .
- Hou , Y , Zhang , H , Miranda , L and Lin , S . 2010 . Serious overestimation in quantitative PCR by circular (supercoiled) plasmid standard: microalgal pcna as the model gene . PLoS ONE , 5 : e9545
- Hunter , A J , Northup , D E , Dahm , C N and Boston , P J . 2004 . Persistent coliform contamination in Lechuguilla cave pools . J Cave Karst Stud , 66 : 102 – 110 .
- Ikner , L A , Toomey , R S , Nolan , G , Neilson , J W , Pryor , B M and Maier , R M . 2007 . Culturable microbial diversity and the impact of tourism in Kartchner Caverns, Arizona . Microb Ecol , 53 : 30 – 42 .
- Johnson , K , Carmichael , M J , McDonald , W , Rose , N , Windelspecht , M , Pitchford , J , Karatan , E and Brauer , S . 2012 . Increased abundance of Gallionella spp., Leptothrix spp. and total bacteria in response to enhanced Mn and Fe concentrations in a disturbed southern Appalachian high elevation wetland . Geomicrobiol J , 29 : 124 – 138 .
- Johnson , M , Zaretskaya , I , Raytselis , Y , Merezhuk , Y , McGinnis , S and Madden , T L . 2008 . NCBI BLAST: a better web interface . Nucleic Acids Res , 36 : W5 – W9 .
- Jones , B. 2001 . Microbial activity in caves—A geological perspective . Geomicrobiol J , 18 : 345 – 357 .
- Kay , J , Conklin , M , Fuller , C and O’Day , P . 2001 . Processes of nickel and cobalt uptake by a manganese oxide forming sediment in Pinal Creek, Globe Mining District, Arizona . Environ Sci Technol , 35 : 4719 – 4625 .
- Klappenbach , J A , Dunbar , J M and Schmidt , T M . 2000 . rRNA operon copy number reflects ecological strategies of bacteria . Appl Environ Microbiol , 66 : 1328 – 1333 .
- Klappenbach , J A , Saxman , P R , Cole , J R and Schmidt , T M . 2001 . rrndb: the Ribosomal RNA Operon Copy Number Database . Nucleic Acids Res , 29 : 181 – 184 .
- Krumbein , W E and Altmann , H J . 1973 . A new method for the detection and enumeration of manganese oxidizing and reducing microorganisms . Helgölander Wissenschaftlichen Untersuchungen , 25 : 347 – 356 .
- Krumbein , W E and Jens , K . 1981 . Biogenic rock varnishes of the Negev Desert (Israel) an ecological study of iron and manganese transformation by cyanobacteria and fungi . Oecologia , 50 : 25 – 38 .
- Laiz , L , Groth , I , Schumann , P , Zezza , F , Felske , A , Hermosin , B and Saiz-Jimenez , C . 2000 . Microbiology of the stalactites from Grotta dei Cervi, Porto Badisco, Italy . Inter J Microbiol , 3 : 25 – 30 .
- Lane , DJ. 1991 . “ 16S and 23S rRNA sequencing ” . In Nucleic Acid Techniques in Bacterial Systematics , Edited by: Stackebrandt , E and Goodfellow , M . 115 – 148 . New York : John Wiley .
- Learman , D R , Voelker , B M , Vazquez-Rodriguez , A I and Hansel , C M . 2011 . Formation of manganese oxides by bacterially generated superoxide . Nat Geosci , 4 : 95 – 98 .
- Learman , D R , Wankel , S D , Webb , S M , Martinez , N , Madden , A S and Hansel , C M . 2011 . Coupled biotic-abiotic Mn(II) oxidation pathway mediates the formation and structural evolution of biogenic Mn oxides . Geochim Cosmochim Acta , 75 : 6048 – 6063 .
- Legatzki , A , Ortiz , M , Neilson , J W , Dominguez , S , Andersen , G L , Toomey , R S , Pryor , B M , Pierson , L S III and Maier , R M . 2011 . Bacterial and archaeal community structure of two adjacent calcite speleothems in Kartchner Caverns, Arizona, USA . Geomicrobiol J , 28 : 99 – 117 .
- Link , D D , Walter , P J and Kingston , H M . 1998 . Development and validation of the new EPA microwave-assiste leach method 3051A . Environ Sci Technol , 32 : 3628 – 3632 .
- Ludwig , W , Strunk , O , Westram , R , Richter , L , Yadhukumar , H M , Buchner , A , Lai , T , Steppi , S , Jobb , G , Förster , W , Brettske , I , Gerber , S , Ginhart , A W , Gross , O , Grumann , S , Hermann , S , Jost , R , König , A , Liss , T , Lümann , R , May , M , Nonhoff , B , Reichel , B , Strehlow , R , Stamatakis , A , Stuckmann , N , Vilbig , A , Lenke , M , Ludwig , T , Bode , A and Schleifer , K H . 2004 . ARB: a software environment for sequence data . Nucl Acids Res , 32 : 1363 – 1371 .
- Lueders , T and Friedrich , M . 2000 . Archaeal population dynamics during sequential reduction processes in rice field soil . Appl Environ Microbiol , 66 : 2732 – 2742 .
- Macalady , J L , Dattagupta , S , Schaperdoth , I , Jones , D S , Druschel , G K and Eastman , D . 2008 . Niche differentiation among sulfur-oxidizing bacterial populations in cave waters . ISME J , 2 : 590 – 601 .
- Macalady , J L , Jones , D S and Lyon , E H . 2007 . Extremely acidic, pendulous cave wall biofilms from the Frasassi cave system, Italy . Environ Microbiol , 9 : 1402 – 1414 .
- Macalady , J L , Lyon , E H , Koffman , B , Albertson , L K , Meyer , K , Galdenzi , S and Mariani , S . 2006 . Dominant microbial Populations in limestone-corroding stream biofilms, Frasassi cave system, Italy . Appl Environ Microbiol , 72 : 5596 – 5609 .
- Manceau , A N , Tamura , M A , MacDowell , M A , Celestre , R S , Soblett , R E , Sposito , G and Padmore , H A . 2002 . Deciphering Ni sequestration in soil ferromanganese modules by combining X-ray fluorescence, absorption, and diffraction at micrometer scales of resolution . Am Mineral , 87 : 1494 – 1499 .
- Melim , L A , Shinglman , K M , Boston , P J , Northup , D E , Spilde , M N and Queen , J M . 2001 . Evidence for microbial involvement in pool finger precipitation, Hidden Cave, New Mexico . Geomicrobiol J , 18 : 311 – 329 .
- Mohandas , S. 1988 . Nitrogen fixation in tomato (Lycopersicon esculentum Mill ‘Pusa Ruby’) . Plant Soil , 107 : 219 – 225 .
- Montañez , IP. 1994 . Late diagenetic dolomitization of the Lower Ordovician, Upper Knox Carbonates: a record of the hydrodynamic evolution of the southern Appalachian Basin . AAPG Bull , 78 : 1210 – 1239 .
- Moore , GW. 1981 . Manganese deposition in limestone caves. In: Beck BF, ed. Proceedings of the International Congress of Speleology 2 . : 642 – 645 .
- Nadkarni , M A , Martin , F E , Jacques , N A and Hunter , N . 2002 . Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set . Microbology , 148 : 257 – 266 .
- Nealson , KH. 1978 . “ The isolation and characterization of marine bacteria which catalyze manganese oxidation ” . In Environmental Biogeochemistry and Geomicrobiology , Edited by: Krumbein , W E . Vol. 3 , 847 – 858 . Ann Arbor , MI : Ann Arbor Science Publishers, Inc . Methods, Metals and Assessment
- Nealson , K H , Tebo , B M and Rosson , R A . 1988 . Occurrence and mechanisms of microbial oxidation of manganese . Adv Appl Microbiol , 33 : 279 – 318 .
- Nelson , Y M , Lion , L W , Shuler , M L and Ghiorse , W C . 1999 . Lead binding to metal oxide and organic phases of natural aquatic biofilms . Limnol Oceanogr , 4 : 1715 – 1729 .
- Nitahara , S , Kato , S , Urabe , T , Usui , A and Yamagishi , A . 2011 . Molecular characterization of the microbial community in hydrogenetic ferromanganese crusts of the Takuyo-Daigo Seamount, northwest Pacific . FEMS Microbiol Lett , 321 : 121 – 129 .
- Northup , D E , Barns , S M , Yu , L E , Spilde , M N , Schelble , R T , Dano , K E , Crossey , L J , Connolly , C A , Boston , P J , Natvig , D O and Dahm , C N . 2003 . Diverse microbial communities inhabiting ferromanganese deposits in Lechuguilla and Spider Caves . Environ Microbiol , 5 : 1071 – 1086 .
- Northup , D E , Dahm , C N , Melim , L A , Spilde , M N , Crossey , L J , Lavoie , K H , Mallory , L M , Boston , P J , Cunningham , K I and Barns , S M . 2000 . Evidence for geomicrobiological interactions in Guadalupe Caves . J Cave Karst Stud , 62 : 80 – 90 .
- Northup , D E and Lavoie , K H . 2001 . Geomicrobiology of caves: a review . Geomicrobiol J , 18 : 199 – 222 .
- Northup , D E , Reysenbach , A-L and Pace , N R . 1997 . “ Microorganisms and speleothems ” . In Cave Minerals of the World. Huntsville , Edited by: Hill , C and Forti , P . 261 – 266 . Alabama : National Speleological Society .
- Northup , D E , Snider , J R , Spilde , M N , Porter , M L , van de Kamp , J L , Boston , P J , Nyberg , A M and Bargar , J R . 2010 . Diversity of rock varnish bacterial communities from Black Canyon, New Mexico . J Geophys Res , 115 ( G2 )
- Oder , CRL. 1934 . Preliminary subdivision of the knox dolomite in East Tennessee . J Geol , 42 : 469 – 497 .
- Onac , B P and Forti , P . 2011 . State of the art and challenges in cave mineral studies . Studia UBB Geologia , 56 : 29 – 38 .
- Onac , B P , Pedersen , R B and Tysseland , M . 1997 . Presence of rare-earth elements in black ferromanganese coatings from Vântului Cave (Romania) . J Cave Karst Stud , 59 : 128 – 131 .
- Peck , SB. 1986 . Bacterial deposition of iron and manganese oxides in North American caves . Natl Speleol Soc Bull , 48 : 26 – 30 .
- Portillo , M C and Gonzalez , J M . 2009 . Sulfate-reducing bacteria are common members of bacterial communities in Altamira Cave (Spain) . Sci Total Environ , 407 : 1114 – 1122 .
- Portillo , M C , Gonzalez , J M and Saiz-Jimenez , C . 2008 . Metabolically active microbial communities of yellow and grey colonizations on the walls of Altamira Cave, Spain . J Appl Microbiol , 104 : 681 – 691 .
- Post , JE. 1999 . Manganese oxide minerals: crystal structures and economic and environmental significance . Proc Natl Acad Sci USA , 96 : 3447 – 3454 .
- Pruesse , E , Quast , C , Knittel , K , Fuchs , B M , Ludwig , W , Peplies , J and Glöckner , F . 2007 . SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB . Nucl Acids Res , 35 : 7188 – 7196 .
- Rossi , C , Lozano , R P , Isanta , N and Hellstrom , J . 2010 . Manganese stromatolites in caves: El Soplao (Cantabria, Spain) . Geology , 38 : 1119 – 1122 .
- Rossmassler , K , Engel , A S , Twing , K I , Hanson , T E and Campbell , B J . 2012 . Drivers of Episilonproteobacterial community composition in sulfidic caves and springs . FEMS Microbiol Ecol , 79 : 421 – 432 .
- Santelli , C , Pfister , D , Lazarus , D , Sun , L , Burgos , W and Hansel , C . 2010 . Promotion of Mn(II) Oxidation and Remediation of Coal Mine Drainage in Passive Treatment Systems by Diverse Fungal and Bacterial Communities . Appl Environ Microbiol , 76 : 4871 – 4875 .
- Santelli , C , Webb , S M , Dohnalkova , A and Hansel , C . 2011 . Diversity of Mn oxides produced by Mn(II)-oxidizing fungi . Geochim Cosmochim Acta , 75 : 2762 – 2776 .
- Schloss , P D and Handelsman , J . 2005 . Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness . Appl Environ Microbiol , 71 : 1501 – 1506 .
- Schweisfurth , R , Eleftheriadis , D , Gundlach , H , Jacobs , M and Jung , W . 1978 . “ Microbiology of the precipitation of manganese ” . In Environmental Biogeochemistry and Geomicrobiology Volume 3: Methods, Metals, and Assessment , Edited by: Krumbein , W . 923 – 928 . Ann Arbor , MI : Ann Arbor Science Publishers Inc .
- Shabarova , T and Pernthaler , J . 2010 . Karst pools in subsurface environments: collectors of microbial diversity or temporary residence between habitat types . Environ Microbiol , 12 : 1061 – 1074 .
- Shapiro , J and Pringle , A . 2010 . Anthropogenic influences on the diversity of fungi isolated from caves in Kentucky and Tennessee . Am Midl Nat , 163 : 76 – 86 .
- Simon , K S , Benfield , E F and Macko , S A . 2003 . Food web structure and the role of epilithic biofilms in cave streams . Ecology , 84 : 2395 – 2406 .
- Simon , K S and Buikema , A L Jr. 1997 . Effects of organic pollution on an Appalachian cave: Changes in macroinvertebrate populations and food supplies . Am Midl Nat , 138 : 387 – 401 .
- Spilde , M N , Northup , D E and Boston , P J . 2006 . Ferromanganese deposits in the caves of the Guadalupe Mountains . : 161 – 166 . In: Land L, Lueth VW, Raatz W, Boston PJ, Love DW, eds. The New Mexico Geological Society Guidebook, 57th Field Conference, Caves and Karst of Southeast New Mexico
- Spilde , M N , Northup , D E , Boston , P J , Schelble , R T , Dano , K E , Crossey , L J and Dahm , C N . 2005 . Geomicrobiology of cave ferromanganese deposits: a field and laboratory investigation . Geomicrobiol J , 22 : 99 – 116 .
- Spring , S. 2006 . “ The genera Leptothrix and Sphaerotilus ” . In The Prokaryotes Volume 5: Proteobacteria: The Alpha and Beta Subclasses , Edited by: Dworkin , M , Falkow , S , Rosenberg , E , Schleifer , K-H and Stackebrandt , E . p758 – 777 . New York : Springer .
- Stein , L Y , La Duc , M T , Grundi , T J and Nealson , K H . 2001 . Bacterial and archaeal populations associated with freshwater ferromanganous micronodules and sediments . Environ Microbiol , 3 : 10 – 18 .
- Sunda , W G and Kieber , D J . 1994 . Oxidation of humic substances by manganese oxides yields low-molecular-weight organic substrates . Nature , 367 : 62 – 64 .
- SW-846 EPA Method 3051A . 2007 . “ Microwave assisted acid digestion of sediments, sludges, soils, and oils ” . In Test Methods for Evaluating Solid Waste, 3rd Edition, Update IIIB , Washington , DC : US Environmental Protection Agency .
- Taboroši , D. 2006 . “ Biologically influenced carbonate speleothems ” . In Perspectives on karst geomorphology, hydrology, and geochemistry , Edited by: Harmon , R S and Wicks , C . 307 – 317 . Geological Society of America .
- Tebo , B M , Clement , B G and Dick , G J . 2007 . “ Biotransformations of Manganese ” . In Manual of Environmental Microbiology , Edited by: Hurst , C J , Crawford , R L , Garland , J L , Lipson , D A , Mills , A L and Stetzenbach , L D . 1223 – 1238 . Washington , DC : ASM Press .
- Tebo , B M , Johnson , H A , McCarthy , J K and Templeton , A S . 2005 . Geomicrobiology of manganese(II) oxidation . Trends Microbiol , 13 : 421 – 428 .
- Toner , B , Manceau , A , Webb , S M and Sposito , G . 2006 . Zinc sorption to biogenic hexagonal-birnessite particles within a hydrated bacterial biofilm . Geochim Cosmochim Acta , 70 : 27 – 43 .
- Torsvik , V , Øvreås , L and Thingstad , T F . 2002 . Prokaryotic diversity- magnitude, dynamics, and controlling factors . Science , 296 : 1064 – 1066 .
- Turekian , K K and Wedepohl , K H . 1961 . Distribution of the elements in some major units of the Earth's crust . Geol Soc Am Bull , 72 : 175 – 192 .
- van Veen , W L , Mulder , E G and Deinema , M H . 1978 . The Sphaerotilus-Leptothrix Group of Bacteria . Microbiol Rev , 42 : 329 – 356 .
- Villalobos , M , Bargar , J and Sposito , G . 2005 . Mechanisms of Pb(II) sorption on a biogenic manganese oxide . Environ Sci Technol , 39 : 569 – 576 .
- Warren , L A and Kauffman , M E . 2003 . Microbial geoengineers . Science , 299 : 1027 – 1029 .
- Webb , S M , Tebo , B M and Bargat , J R . 2005 . Structural characterization of biogenic Mn oxides produced in seawater by the marine Bacillus sp. strain SG-1 . Am Mineral , 90 : 1342 – 1357 .
- Wedepohl , KH. 1995 . The composition of the continental crust . Geochim Cosmochim Acta , 59 : 1217 – 1232 .
- White , W B , Culver , D C , Herman , J S , Kane , T C and Mylroie , J E . 1995 . Karst lands . Am Sci , 83 : 450 – 459 .
- White , W B , Vito , C and Scheetz , B E . 2009 . The mineralogy and trace element chemistry of black Manganese oxide deposits from caves . J Cave Karst Stud , 71 : 136 – 143 .
- Whitman , W B , Coleman , D C and Wiebe , W J . 1998 . Prokaryotes: the unseen majority . Proc Natl Acad Sci USA , 95 : 6578 – 6583 .