Abstract
The synthesis and testing of thousands of chemical compounds is an essential process in the development of small molecule drug therapies. Over time, even a small research group can generate tens of thousands of compounds and a vast amount of data that is associated with the compounds. One of the challenges faced by research groups is how to manage these compound collections. This review considers chemical compound libraries from a records management perspective and was written based on personal experience working in drug discovery and with knowledge gleaned during postgraduate study in archival systems. A review of the four dimensions – create, capture, organise and pluralise – of the Records Continuum Model has been employed to address some of the issues associated with the management of large chemical compound libraries. This review is not a technical description of how to create a compound library management system; rather, it serves as a starting point for drug development scientists to consider applying archival science theories to improve chemical compound management.
Introduction
Records continuum theory has provided a means by which to treat records, not just as fixed documents, but as information objects that serve as evidence of the functions and transactions which cause their creation.Footnote 1 Archives are not simply document repositories; rather, they are active entities that are related to the records, activities and actors involved in the creation and use of the records.Footnote 2 The Records Continuum Model (RCM) developed by Frank Upward and colleagues was first published in 1996 and is a culmination of the evolution of continuum thinking in Australia.Footnote 3
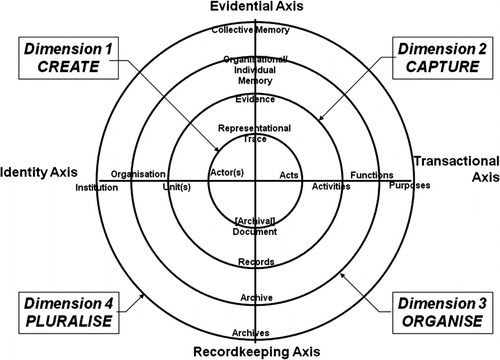
The model presented by Upward (see Figure ) uses concentric circles to display the four dimensions: create, capture, organise and pluralise. The axes of evidence, identity, transactionality and recordkeeping intersect across the dimensions, with the words at the points of the axes being representative of an array of issues that are important to recordkeeping.Footnote 4 A more thorough explanation of all of the labels has been provided elsewhere.Footnote 5 However, it is important to note that the axes and dimensions are interrelated and that the continuum is not linear, allowing for the consideration of recordkeeping from multiple starting points of the continuum or through simultaneous review of the dimensions.Footnote 6
In recent years, there has been a paradigm shift away from viewing records as fixed objects, leading the field of archival science to challenge the notion of what constitutes a record and what constitutes archives. Records are not just considered to be embedded within fixed hierarchical organisations, but are situated within networks of workflow functionality.Footnote 7 This report will provide an overview of the workflows associated with creating and screening compounds during drug discovery and then consider management of the associated records through a reading of the RCM.Footnote 8 Events relating to the creation and storage of compounds will be described through the dimensions of the continuum, followed by a discussion of the ways in which an archival approach to compound library management can ensure the integrity of the library as archives.Footnote 9
Background
Research into the design of new small molecule drug therapies involves the synthesis of hundreds or thousands of novel molecules by synthetic chemists. Once the physical sample is prepared, analytical data is generated to provide evidence of each compound’s chemical structure and purity (described further in the next section ‘Compound library management and the records continuum’).Footnote 10 Compounds are then tested by biologists in various assays to determine their efficacy against a specific biological target. The data generated during biological screening adds value to the evidentiary nature of a compound as a potential drug candidate. All of the data generated throughout the workflow of compound synthesis to screening establishes the compound as a record and a compound collection as an archive.
Large pharmaceutical companies will maintain libraries containing hundreds of thousands of compounds and have automated robotic systems and dedicated staff for managing and manipulating these libraries. Smaller research groups might initially have a small compound library, but, over time, the library size can grow to tens of thousands of compounds. As the compound library and associated data collection grows, an organisation is forced to consider their library management practices to ensure that the integrity of the library is maintained and also that compounds and relevant data are stored such that re-access and re-use are possible.
The field of compound management became prominent with the advent of high-throughput screening, as the logistics of managing collections of hundreds of thousands of chemical compounds became crucial to drug development groups.Footnote 11 Much of the available literature about compound library management is written by members of large companies with fully automated systems for the maintenance of large libraries.Footnote 12 The issue of library integrity in these reviews focuses on the specific storage conditions, such as low humidity, the importance of an inert atmosphere and optimal temperatures. One of the most thorough reviews on compound library management by Chan and Hueso-Rodríguez includes schematic representation of compound management processes and describes the automated systems and informatics tools used to manage a collection.Footnote 13 The authors also highlight the importance of having a group dedicated to the management, control and retrieval of library compounds. They provide a fairly comprehensive analysis of the logistics involved in the manipulation of a very large compound library. However, while the report by Chan and Hueso-Rodríguez and similar reviews on compound management describes the use of well-established automated systems, they do not illustrate the decision-making processes involved in system development. Also, the incorporation of legacy compounds and data into a new library system is rarely mentioned (if at all) in compound management literature.
For a small group without the resources for specialist robotics and customised informatics systems, the management of an ever-growing compound library can seem overwhelming. Any new system must be able to manage new compounds as they are created, as well as being able to absorb the existing library and large quantities of legacy data. The scientists’ equivalent of a dark and dusty basement packed with boxes of old yellowing files may be fridges and freezers full of compounds in a variety of vessel types and the metadata available to help identify each compound may be incomplete. How does a small group with limited resources approach the complex task of upgrading the management of their compound library?
Some of the key issues that require consideration are:
| • | Should a dedicated person or group be employed to manage the compound library? | ||||
| • | How should the current storage space be utilised and will new fit-for-purpose spaces be required? | ||||
| • | What sorts of storage vessels should be used and how will they be labelled? | ||||
| • | How will information about the compounds be catalogued and stored? | ||||
An approach to compound management is considered in this review through reading the RCM and using the model to assist with the decision-making processes involved in creating and administering a compound library.
In order to review the records continuum, it is important to consider the nature of records in an archival context and also how scientists consider records. It was identified in the InterPARES 2 project report that there is a strong focus on data management in the sciences, but records receive far less consideration.Footnote 14 Although scientists do not necessarily consider records management as part of their everyday work, they do understand the need for the authenticity and reliability of research data, so that they can provide accurate evidence to support their hypotheses and research claims. Authenticity, reliability and accuracy are the features that differentiate records from other information sources.Footnote 15 Also, records have ‘transactionality’, as they serve as evidence of actions. This is the whole purpose of creating scientific data – to provide evidence of experiments.
It may be possible to take archiving and records management practices from the more ‘traditional’ library and archives settings and apply them in scientific research and development, because scientists already understand the importance of the core principles of records management. Applying archival theory, such as the records continuum, to compound library management could help ensure that the compounds are true records and accurate evidence of an organisation’s research efforts.
Compound library management and the records continuum
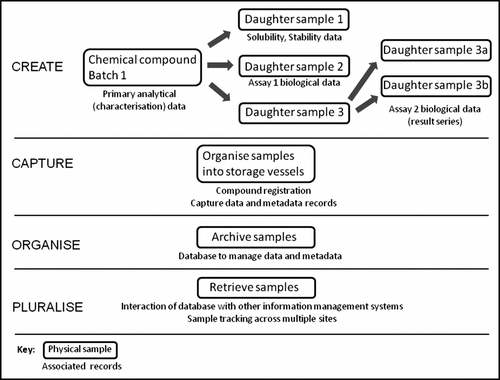
The RCM has been a valuable theory in archival science, as it represents the multi-dimensional nature of recordkeeping and archiving.Footnote 16 The dimensions – create, capture, organise and pluralise – will now be considered from a chemical compound management perspective. Figure describes some of the events associated with these dimensions of the RCM. Two different aspects of the library need to be considered: the physical samples themselves and the data associated with the samples.
Dimension 1: create
The ‘create’ dimension is quite complex when considering chemical compound management. The creation of the physical compound itself is not the result of a single transaction, such as mixing two chemicals to create a final product. Often a series of chemical reactions are performed over several days or weeks before the final compound, which will be tested in a biological assay, is generated.Footnote 17 Throughout this process, a series of intermediate compounds are created, and each of these has associated characterisation data.Footnote 18 Whilst each intermediate compound is itself a record, it is also a proto-record for the final compound. The characterisation data for each compound is evidence that the compound has the desired chemical structure, and all of the intermediates and associated data serve as evidence of the transactions performed by chemists (as actors) that occurred to generate the final compound. For each final compound, many different types of assays can be performed, generating many sub-samples and a large amount of data and metadata. The group of actors also extends to include the biologists that screen the compounds.
To provide the samples to biologists for screening, the parent compound is subdivided into daughter samples, and each of these daughters can be used for a different purpose.Footnote 19 The compounds are screened to determine their efficacy against a biological target and serve as evidence of the early stages of a drug development programme.
Dimension 2: capture
At the ‘capture’ stage, compounds are transferred to storage vessels and a series of characterisation data is collected. The metadata related to the compound (such as the chemical structure, purity, the name of the creating chemist and the synthesis date) is registered into a database and the compound is given a unique ID number. From the parent compound, a series of daughter samples will be generated for various purposes in addition to the primary biological screening, including stability and solubility testing and a variety of other assays. The data associated with all of the analyses and screening is also captured at this stage.
Deriving daughter samples from the parent can be reviewed through reading the records continuum in reverse, from capture back to creation. The continuum approach does not require creation and capture to be single events, but allows for consideration of these states through time. This non-linear approach is important in revealing a flaw that can occur with compound management at the create and capture stages. With the preparation of each daughter sample, a new record is created. However, the processes associated with the capture of the sample and its associated data are not always performed. Daughter samples are not always given unique IDs, and metadata that traces the daughter to its parent may not be created. If there is a failure somewhere in a process (for example, decomposition of a sample), it might not be possible to trace which other related samples have been affected and, thus, the integrity of the data is compromised. The records that are considered essential to accompany the creation and capture of the parent compound should therefore also accompany all other creation and capture events, so as to ensure that the compounds serve as accurate evidence of the core business of the group.
Dimension 3: organise
A compound archive should allow the compounds (as records) to serve as evidence of their functions through time and as an accurate memory of an individual’s activities and a whole organisation. Consideration of appropriate storage conditions for the compound library is crucial to ensure that integrity is maintained throughout the life of the library. Much of the compound management literature previously mentioned describes appropriate physical storage conditions for chemical compounds to prevent their decomposition, so the details of such storage will not be considered further here. Instead, a review of the continuum and how it can assist in the decision-making processes relating to compound storage will be discussed.
Initially, the creation of compounds for drug screening is dictated by the specific biological target that is the focus of a project team. It can be tempting to group compounds in archives by project, with the archives managed from within the project team, so that the compounds are readily accessible by actors involved in their creation and primary use. This approach privileges the ‘creation’ dimension and does not consider all of the actors and functions that may be associated with the compounds in the future. Over time, the library may be screened and re-screened on various projects, and subsets of one project may become part of another. Compound storage by project therefore becomes complicated over time. An alternative approach is to ensure that any relevant project information is captured amongst the compound data, but to archive the compound in the context of the entire library, not just within one project collection. Appropriate data and metadata should be archived in parallel with the compound to ensure ease of retrieval independent of a compound’s initial purpose or time of creation.
Dimension 4: pluralise
The ‘pluralise’ dimension of the records continuum is concerned with the way in which archives are integrated into a broader framework.Footnote 20 For chemical compound archives that are created for internal use within a research group, the relevance of pluralisation is not always immediately obvious. The integration aspect may not relate how the compound archives could be accessed by an external organisation, but rather how the archives integrate with pre-existing information management systems within the organisation.
Consideration of the arrangement of the compound archives and associated metadata at both the organisation and pluralisation dimensions can significantly impact how readily individual compounds and groups of compounds can be accessed in the future. As described above, consideration of possible future uses of compounds and not just their initial use can affect how the compounds are organised into the archive. For example, compounds could be stored in solution in 96-well plate format (see Figure 3) in a freezer.Footnote 21 This method of storage requires a significant amount of work at the ‘creation’ dimension, but will minimise storage space and cost when compared with compound storage in individual vials, which is especially important as the library grows over time. However, if single compounds are required for future use, an entire plate of 96 compounds must be thawed in order to access one compound. Repetition of freeze-thaw cycles can increase the likelihood of compound decomposition, yet storage of the compounds at higher temperatures to minimise freeze-thaw cycles can also lead to significant decrease in compound integrity.Footnote 22 For these reasons, it may be more beneficial to store compounds in individual vials as dry samples without solvent. The initial cost may be higher, but the library’s integrity is preserved in the long-term. Considering preservation at the time of creation highlights the importance of reading the whole continuum through each stage of library management.
The metadata associated with the compound archives is as valuable to a research group as the compounds themselves. The metadata provides information about the chemical structure, purity and biological activity of the compounds and ensures that the compound can be re-synthesised. It is this data that will be scrutinised internally or by external organisations to determine whether a drug development project is suitable for progression into clinical development. The data may also need to be accessed for patent publication, so the integrity of the library and data is essential for the integrity of the organisation’s intellectual property. The system that stores the compounds and metadata therefore requires flexibility, so that relevant information can be readily accessed and re-packaged for a variety of purposes. Therefore, all of these elements need to be considered, in order to ensure that the archives are readily accessible for future purposes.
An additional external mandate that needs to be considered with chemical compound archives is compliance with occupational health and safety (OH&S) and dangerous goods regulations. Chemical compounds prepared for research purposes are not subjected to a full assessment of their toxicity to determine their safety. Therefore, the compounds are all treated as toxic and need to be stored, handled and transported in accordance with the National Occupational Health and Safety Commission (NOHSC) code of practice for the storage and handling of dangerous goods.Footnote 23 A risk assessment should be performed, detailing the potential hazards associated with compound management and storage, thus ensuring that any storage space meets OH&S requirements and also that any additional metadata is created.
Evidence, identity, transactionality and recordkeeping
The previous sections described compound management events by reviewing the RCM from creation and working outwards. What follows considers alternate readings of the continuum, in order to inform decisions regarding compound archiving.
To begin with, an ‘outside-in’ review of the continuum along the recordkeeping axis considers the requirements for organisation of compounds into archives and how these requirements can dictate events that occur at the ‘create’ and ‘capture’ dimensions. The archives may initially need to be readily and frequently accessed by scientists as compounds are used in current research. The same compounds may then need to be stored for several years and re-accessed by different actors for different projects. For current, frequent access, the archives needs to be stored on the same site as the users, and compounds need to have appropriate accompanying metadata, so the physical coordinates of a particular compound in a fridge or storage room are available for easy access. If specific storage vessels are required for archiving purposes, then these vessels could be introduced at the capture stage. Also, the data and metadata necessary for management of the archives could be generated when the compounds are created and captured. Planning for future uses and future library growth will also ensure that an appropriate space is dedicated to the compound archive. By considering how the compounds will be archived before they are created prevents resources being required to reorganise the records at the stage of archiving and improves the efficiency of compound management.
Reviewing the evidential and identity axes of the RCM indicates that the compound library needs to serve as the memory of the organisation. To minimise the risk that the memory could be lost, a duplicate of the entire archive could be stored on a separate site under conditions that ensure the integrity of the compounds. Considerations such as this, as regards the organise and pluralise dimensions, impact upon the creation phase. If a duplicate of all compounds is required for off-site archives, then the quantity prepared at the beginning needs to be increased. Considering organisation before creation ensures that enough of a compound is generated for both current and future uses. Such risk mitigation is easily put into practice, by ensuring that scientists are aware of the immediate use, as well as the archiving requirements, for the compounds that they synthesise, so that the appropriate quantities are made. Also, specialist compound management facilities are available that enable off-site storage of a duplicated library.Footnote 24
In recordkeeping systems, the authenticity of a record is essential for that record to serve as evidence of the functions and transactions for which it was created.Footnote 25 This is certainly true when considering compounds as records in a drug development process. Biological screening of compounds that have decomposed or have been mislabelled can lead to drawing false conclusions based on the data generated from such screening. Time and money are wasted by creating data based on these invalid records. Ensuring the integrity of a chemical library by quality control analysis can be very time-consuming and costly and is sometimes even considered a luxury in a research group with limited resources. The prevailing approach to compound screening in time-constrained research groups is to screen for functional activity first and then to only review the quality of compounds of interest. The problem with this approach is that weeks can be wasted on invalid data; time that would have been saved by ensuring the quality of the library from the beginning. Approaching compound management from an archivist’s perspective highlights the importance of the integrity of records as evidence. A compound archive can be created with processes in place to monitor the quality of the compounds and ensure their continuing integrity.
A review of the RCM can support the decisions associated with creating a chemical compound archive. In addition, continuum thinking can be applied when considering how legacy data is incorporated into an archive. The requirements for archives to act as evidence of an organisation should also be met by old compounds being introduced into a new system. There is often reluctance within research groups to destroy old compounds, not only because it would seem to be a waste of the resources that went into preparing the compounds, but also because of the fear that such disposal will result in the loss of crucial future results. However, as previously described, much time can be wasted collecting invalid data. If old compounds have not been kept under storage conditions that ensure their composition and integrity, then they need to be re-evaluated before entering new archives.
Disposal schedules are very useful in records management and archiving, as they relate not only to the destruction of records, but also to the retention, transfer and archiving of records.Footnote 26 Generating a disposal schedule relating to compound management and storage would provide valuable information to all members of a research group. The schedule could detail the data that is required at the create and capture stages to ensure the validity of each record. In addition, the schedule could include guidelines for the archiving of analytical data and associated metadata and provide workflows for managing the physical organisation of samples into archives.
Conclusions
In reviewing compound management from a records continuum perspective, it becomes apparent that a system that ensures the integrity of archives is not self-sustaining. It requires active management through constant appraisal of the records and the processes and transactions in which they are involved. The ideal compound library manager requires a scientific background and experience working in a laboratory environment, but they would also benefit from training in information management and archival sciences. A compound library manager with these cross-disciplinary skills to manage the library and associated data and databases would ensure the integrity of the library as archives and could help to introduce an accountability framework to govern the creation, capture, organisation and pluralisation of the records.
Approaching chemical compound library management through a reading of the RCM has revealed a number of ways in which archival science can add value to the field of compound management. A non-linear evaluation of the records continuum highlights events that can be introduced at the creation and capture stages, which allow for an effective compound archive to be generated and maintained.
The compounds are not only evidence of the process involved in their synthesis, but also evidence of the work performed by actors and evidence of a drug development programme. When collected in archives, the compounds serve as the memory of the group and their core business. The integrity of the records as evidence is therefore crucial, and the design of the archives should incorporate features that ensure all functions and uses are preserved.
Considering chemical compounds as records highlights the complexity that can be associated with defining a record. Postmodern approaches to records management can help accommodate the notion of compounds and associated data embedded within workflow functionality. When a compound is initially created, without any confirmed information about its chemical structure or its physical and biological properties, is it a record yet or is it just white powder? As data relating to the chemical structure and physical and biological properties are created, the compound becomes a true record, continually in a state of becoming. The compound itself and each new piece of associated data are fixed at the time of creation, but this ‘record collective’ is continually changing for each compound, as its purpose changes through time. Approaching a compound as a record collective can have a significant impact on the way in which compound archives are created and managed.
This review does not provide specific details on how to create the ideal compound library management system. Rather this reading of the RCM provides a reference point for those involved in compound management to consider such management from an archivist’s perspective. It allows compound managers to exploit the wealth of information published by recordkeepers and archivists to assist in the creation and management of high quality compound archives.
Acknowledgements
The author would like to thank Sue McKemmish for her encouragement and valuable input during the preparation of this paper.
Notes
1F Upward, ‘Structuring the Records Continuum Part One: Post-Custodial Principles and Properties’, Archives and Manuscripts, vol. 24, no. 2, November 1996, pp. 268–85.
2F Upward, ‘The Records Continuum’, in S McKemmish, M Piggott, B Reed and F Upward (eds), Archives: Recordkeeping in Society, Centre for Information Studies, Wagga Wagga, 2005, pp. 197–222.
3The development of the RCM is described by Upward, ‘Structuring the Records Continuum Part One’ and S McKemmish, F Upward and B Reed, ‘Records Continuum Model’, in MJ Bates and M Maack (eds), Encyclopedia of Library and Information Sciences, VRV Press, Boca Raton, 2010, pp. 4447–59.
4S McKemmish, ‘Topic 6 Archival Frameworks: The Records Continuum Model’, Lecture Notes, FIT5087 Archival Systems, Monash University, Melbourne, 14 April 2010.
5S McKemmish, F Upward and B Reed in the article ‘Records Continuum Model’ describe the evolution of continuum thinking in Australia and details how each dimension and each axis point on the continuum addresses requirements for effective records management. F Upward in 'Structuring the Records Continuum Part One: Post-Custodial Principles and Properties' describes the structural properties of the continuum model, considering records as logical entities, and emphasises the need for integrating recordkeeping into societal processes and purposes.
6B Reed, ‘Reading the Records Continuum: Interpretations and Explorations’, Archives and Manuscripts, vol. 33, no. 1, May 2005, pp. 18–43.
7T Cook, ‘Archival Science and Postmodernism: New Formulations for Old Concepts’, Archival Science, vol. 1, no. 1, 2001, pp. 3–24.
8‘Screening’ or ‘assaying’ compounds the process of testing a compound to determine particular properties. Biological screening tests the efficacy of a compound against a specific biological target.
9Knowledge of the processes used for compound management comes from firsthand experience and observations made while working in small research groups in the drug development industry. This report was written while studying archival science theory, with a view to apply contemporary theories and practices in archiving and records management to the practices within the field of laboratory science.
10‘Analytical data’ describes a variety of data types used to confirm the chemical structure of a compound.
11WP Janzen and IG Popa-Burke, ‘Advances in Improving the Quality and Flexibility of Compound Management’, Journal of Biomolecular Screening, vol. 14, no. 5, June 2009, pp. 444–51. ‘High-throughput screening’ is the automated process of rapidly screening thousands of compounds against biological targets.
12PE Ilouga, D Winkler, C Kirchhoff, B Schierholz and J Wölcke, ‘Investigation of 3 Industry-Wide Applied Storage Conditions for Compound Libraries’, Journal of Biomolecular Screening, vol. 12, no. 1, February 2007, pp. 21–32; WW Keighley and TP Wood, ‘Compound Library Management: An Overview of an Automated System’, in WP Janzen (ed.), Methods in Molecular Biology, Vol. 190: High Throughput Screening: Methods and Protocols, Humana Press, New Jersey, 2002, pp. 129–52; SL Matson, M Chatterjee, DA Stock, JE Leet, EA Dumas, CD Ferrante, WE Monahan, LS Cook, J Watson, NJ Cloutier, MA Ferrante, JG Houston and MN Banks, ‘Best Practices in Compound Management for Preserving Compound Integrity and Accurately Providing Samples for Assays’, Journal of Biomolecular Screening, vol. 14, no. 5, June 2009, pp. 476–84.
13JA Chan and JA Hueso-Rodríguez, ‘Compound Library Management’, in WP Janzen (ed.), Methods in Molecular Biology, Vol. 190: High Throughput Screening: Methods and Protocols, Humana Press, New Jersey, 2002, pp. 117–27.
14Y Hackett, W Underwood and P Eppard, ‘Part One – Case and General Studies in the Artistic, Scientific and Governmental Sectors: Focus Task Force Report’, in L Duranti and R Preston (eds), International Research on Permanent Authentic Records in Electronic Systems (InterPARES) 2: Experiential, Interactive and Dynamic Records, Associazione Nazionale Archivistica Italiana, Padova, 2008, available at <http://www.interpares.org/display_file.cfm?doc=ip2_book_part_1_focus_task_force.pdf >, accessed 19 August 2011.
15B Reed, ‘Records’, in S McKemmish, M Piggott, B Reed and F Upward (eds), Archives: Recordkeeping in Society, Centre for Information Studies, Wagga Wagga, 2005, pp. 101–30.
16S McKemmish, ‘Topic 6 archival frameworks: the records continuum model’, Lecture notes, FIT5087 Archival Systems, Monash University, Melbourne, Lecture presented 14 April 2010, p. 4.
17A series of chemical transformations is required to generate a compound that is tested. Throughout the process, a number of compounds are generated, but the ‘final compound’ refers to the compound that is submitted to be assayed.
18‘Characterisation’ is the process by which the chemical structure of a compound is determined. Characterisation involves collecting analytical data.
19The ‘parent compound’ refers to the primary batch of a compound sample. When a portion of a compound is removed from a batch, this portion is referred to as a ‘daughter sample’.
20McKemmish, Upward and Reed, ‘Records continuum model’ in MJ Bates and M Maack (eds), Encyclopedia of Library and Information Sciences, VRV Press, Boca Raton, 2010, p. 4.
21A ‘96-well plate’ is a vessel with 8 x 12 wells used for storage and screening of small quantities and chemical compounds (see Figure ).
22Ilouga et al, ‘Investigation of 3 industry-wide applied storage conditions for compound libraries’, pp. 21, 31.
23National Occupational Health and Safety Commission, ‘Storage and Handling of Workplace Dangerous Goods’, National Code of Practice NOHSC: 2017 (2001), National Occupational Health and Safety Commission, Sydney, 2001.
24Griffith University, ‘Queensland Compound Library’, available at <http://www.griffith.edu.au/science-aviation/queensland-compound-library>, accessed 13 January 2013.
25R Hartland, S McKemmish and F Upward, ‘Documents’, in S McKemmish, M Piggott, B Reed and F Upward (eds), Archives: Recordkeeping in Society, Centre for Information Studies, Wagga Wagga, 2005, pp. 75–100.
26S McKemmish, ‘Topic 7 Appraisal Frameworks, Strategies and Tools: Part 1 Appraisal Processes and Disposal Tools’, Lecture Notes, FIT5087 Archival Systems, Monash University, Melbourne, 21 April 2010.