ABSTRACT
Australia’s temperate woodlands are among the most heavily modified biomes globally. I summarise some of the work on birds in south-eastern Australia that identifies drivers of bird biodiversity loss and the effectiveness of management interventions. I particularly focus on studies by the Sustainable Farms project at The Australian National University which show that: (1) Bird occurrence is associated with the amount of woody vegetation cover at site, farm and landscape scales. (2) Planting to increase woodland cover has greater relative positive effects on birds than grazing control. However, grazing of plantings has inherently negative impacts. (3) There are different broad structural types of woodland (old growth, regrowth and replantings) and each supports different bird assemblages. (4) The highest bird biodiversity occurs on farms which support all three woodland structural types, as well as other natural assets like paddock trees and fallen timber. (5) Long-term data show that while some woodland species are increasing, twice as many species are declining. Despite the body of information on woodland birds, substantial knowledge gaps remain. These include understanding of: (1) the role of fire in woodland bird dynamics and habitat suitability, and (2) demographic processes like bird breeding success and how it affects long-term site occupancy. Bird biodiversity in Australian agricultural landscapes formerly dominated by temperate woodlands will be best supported by: (1) ceasing land clearing, (2) greater woodland regeneration and woodland planting, (3) limiting livestock grazing, and (4) limiting the impacts of the Noisy Miner (Manorina melanocephala).
Introduction
Globally, agriculture and land clearing has had massive negative impacts on biodiversity, including birds (Intergovernmental Science-policy Platform on Biodiversity and Ecosystem Services (IPBES) Citation2019). Australia is no exception, especially as a result of widespread landscape transformation for agriculture, including livestock grazing, cropping and mixed broadacre farming (Walker et al. Citation1993; Williams and Price Citation2011; Norton and Reid Citation2013; Dickman and Lindenmayer Citation2022). As a result, ecosystems such as the temperate woodlands of south-eastern Australia are amongst the most heavily modified biomes globally (Fischer et al. Citation2009). For example, an estimated 12–15 billion trees have been cleared in the Murray-Darling Basin since European settlement (Walker et al. Citation1993), and much of this would have been temperate woodland (Prober and Thiele Citation1995; Hobbs and Yates Citation2000). Cleared pastures and scattered paddock trees now characterise vast areas that were once extensive temperate woodland (Gibbons and Boak Citation2002; Manning et al. Citation2006) with little remaining of other important natural ecosystems such as temperate grasslands (Department of the Environment and Energy Citation2016). For the purposes of this review article, the broad definition of woodland follows that of Hobbs and Yates (Citation2000) and relates to the height of the trees and inter-tree spacing. In addition, the body of work on which this paper is based relates primarily to temperate woodlands dominated by eucalypts, especially Box-Gum Grassy Woodlands.
The major transformations in land cover that have occurred in the temperate woodlands of south-eastern Australia have had significant impacts on biodiversity, including native birds. Indeed, many millions of birds are likely to have been killed either directly or indirectly as a result of vegetation clearing for agriculture (Cogger et al. Citation2017). In addition, patterns of connectivity have been altered for some bird species (Cooper and Walters Citation2002) with likely disruptions to key functional processes such as gene flow (Sunnucks Citation2016; Radford et al. Citation2021). Temperate woodland environments are also characterised by relatively large numbers of exotic carnivores (Legge et al. Citation2017; Lindenmayer et al. Citation2020b) that can prey on birds as well as exotic herbivores and native herbivores that can degrade bird habitats (Bennett et al. Citation2020; Lindenmayer et al. Citation2020b). As a result of major changes in landscapes, bird communities have been substantially altered (Ford et al. Citation2001; Ford Citation2011) and are now often dominated by large numbers of hyper-aggressive honeyeaters such as the Noisy Miner (Manorina melanocephala) (Montague-Drake et al. Citation2009; Mac Nally et al. Citation2012; Maron et al. Citation2013) which have negative impacts on smaller-bodied bird species (Grey et al. Citation1997; Westgate et al. Citation2021b; Hingee et al. Citation2022).
Extensive modification of temperate woodlands and associated environmental problems such as land degradation through soil loss, declining vegetation condition, secondary salinity and biodiversity loss, have led to major remediation programs to tackle these problems. These include efforts to fence and protect patches of remnant vegetation from high-intensity livestock grazing (Spooner and Briggs Citation2008; Fischer et al. Citation2009), restoration of vegetation through deliberate planting (Munro et al. Citation2007; Mac Nally et al. Citation2010; Lindenmayer et al. Citation2010b, Citation2018a; Haslem et al. Citation2021), and the provision of artificial habitat elements such as nest boxes (Goldingay et al. Citation2015; Lindenmayer et al. Citation2015). It is important to quantify the effectiveness of these and other kinds of management interventions for key groups of biota (Doerr et al. Citation2017) such as birds, and, more broadly, to identify the factors that influence the distribution and abundance of birds in Australian agricultural environments, including those environments that were formerly temperate woodland-dominated ecosystems. This understanding is critical given that: (1) agricultural and pastoral land comprises ~ 55% of Australia’s terrestrial land mass (Department of Agriculture Water and Environment Citation2021), and (2) a large proportion of Australia’s woodland bird fauna has been forecast to be lost by 2050 without concerted conservation action (Recher Citation1999).
Over the past few decades, many research groups and individuals have completed key studies of the effects on woodland birds of landscape change, including landscape conversion and livestock grazing (Fruedenberger and Brooker Citation2004; Radford et al. Citation2005, Citation2021; Major Citation2010; Ford Citation2011; Haslem et al. Citation2021) and, conversely, attempts to mitigate their impacts through targeted management interventions such as planting, livestock grazing management (Martin et al. Citation2006; Debus et al. Citation2017; Lindenmayer et al. Citation2018b) and control of Noisy Miner populations (Grey et al. Citation1997; Mac Nally et al. Citation2012; Maron et al. Citation2013; Melton et al. Citation2021). It is not possible to do justice to that complete body of work in the limited space available here. On this basis, the overarching aim of this paper is to summarise key findings of research and management significance, with a focus on long-term work on temperate woodland birds in livestock grazing and mixed cropping agricultural landscapes in south-eastern Australia. A particular focus is on the body of work conducted by the Sustainable Farms group at The Australian National University over the past two decades (see , ). Using these studies and those elsewhere in Australian temperate woodlands, I outline important results within general themes for woodland bird conservation and management. These are generally framed as statements of general outcomes that can be communicated to farmers and natural resource management practitioners. I also present a new conceptual model of the drivers of change in the presence and/or assemblage composition of temperate woodland birds in south-eastern Australia and approaches to mitigate (and potentially reverse) those declines (). I conclude with key knowledge gaps that require closing to enhance temperate woodland bird conservation in agricultural Australia.
Figure 1. Map of the location of long-term studies of birds in temperate woodlands of south-eastern Australia. Across these large-scale studies, a total of 872 sites (each comprising a 200 m transect; see ) have been surveyed repeatedly on an annual and biannual basis for between 12 and 23 years.
Figure 2. Conceptual model of the drivers of change in the presence and/or assemblage composition of temperate woodland birds in south-eastern Australia and approaches to mitigate (and potentially reverse) those declines. The top part of the diagram illustrates how the interacting processes of land clearing and grazing can lead to habitat loss and habitat fragmentation and can have direct effects on bird site occupancy or their impacts can be mediated through influences on populations of competitors and predators such as the Noisy Miner and the Grey and Pied Butcherbird (Westgate et al. Citation2021b). The bottom part of the conceptual diagram illustrates the direct and indirect effects of revegetation and grazing control on bird occurrence. Note that attempts to reduce fragmentation by directly connecting patches of native vegetation have not been illustrated as the effects on birds of boosting connectivity relative to simply increasing the amount of vegetation cover remain unclear (Lindenmayer et al. Citation2020a).
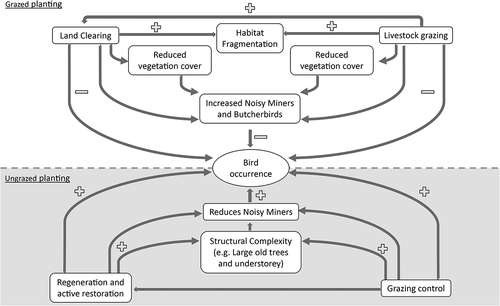
Table 1. Summary of six large-scale, long-term studies maintained by The Australian National University in which sites were surveyed repeatedly for temperate woodland birds with the aim of determining the impact of land use changes and management interventions on avian biodiversity.
Background
Large-scale studies by the Sustainable Farms project at The Australian National University span true experiments, natural experiments, and observational investigations (sensu Cunningham and Lindenmayer Citation2016) with some running for up to 23 years, and all based on repeated counts of birds at permanent long-term monitoring sites (Hingee et al. Citation2022) (). Several smaller studies have been connected to, or nested within, these larger scale studies such as those on bird breeding success in woodland patches (e.g. Okada et al. Citation2016; Belder et al. Citation2018, Citation2019), or the effectiveness of culling programs for the Noisy Miner; Beggs et al. Citation2019, Citation2020).
Repeated surveys of woodland birds have been accompanied by assessments of vegetation structure and plant species composition, as well as quantification of spatial and temporal changes in the amount of woody vegetation cover in the broader landscape. Data also have been gathered on bird life history attributes (e.g. body size, breeding system, diet), long-term average climate, and annual weather for use as covariates in statistical analyses of the factors influencing the occurrence of bird species at our long-term field sites.
Insights from the various large-scale, long-term bird monitoring projects and associated extensive, high quality datasets have helped create a new conceptual model of both the drivers of decline of temperate woodland birds and, conversely, where management interventions might be effective in arresting that decline and, in some cases, even promote recovery (). This conceptual model has helped structure the key insights which feature in the remainder of this article.
The body of work summarised here does not explore in detail the effects of some key areas of woodland bird ecology such as the impacts of pesticide use on breeding success (e.g. see Olsen et al. Citation1993) and the effects of abundant native herbivores such as macropods on bird occurrence (see Howland et al. Citation2016), or prey for birds such as invertebrate assemblages (Barton et al. Citation2011; Barton et al. Citation2009).
Key insights
Long-term monitoring and understanding the temporal trajectory of bird populations
Long-term data gathered in temperate woodlands have enabled quantification of temporal changes in bird biota over the past ~ two decades. For example, data on 108 bird species gathered between 2002 and 2015 on the South West Slopes region of southern New South Wales, indicated that 30 species had declined whereas 14 species had increased (Lindenmayer et al. Citation2018d). Nectarivores and large-bodied birds declined across all broad types of vegetation (old growth woodland, regrowth woodland and plantings), whereas small-bodied species increased, particularly in plantings. Resident but not migratory species declined, with this trend strongest in restoration plantings. Common birds tended to decline whereas rare birds tended to increase, with effects for both most pronounced in plantings. Thus, the work revealed that patterns of temporal change were linked with life history attributes and broad vegetation type (e.g. plantings versus old growth).
Long-term trends in patterns of bird occurrence spanned both the Millennium drought (van Dijk et al. Citation2013) and a subsequent wet period and would not have been apparent from short-term (cross-sectional) studies. This highlights the importance of long-term monitoring for quantifying temporal trends in patterns of occurrence of woodland birds, especially in environments characterised by marked inter-annual differences in weather conditions. A key challenge will be to ensure other long-term monitoring programs are established and existing programs maintained. These additional programs are needed to determine whether the patterns documented in one region are replicated in others and, in turn, create a broader, species-level assessment for temporal changes in occurrence.
Increased tree cover has marked positive effects on bird biodiversity
Much has been written about the loss of tree cover in temperate woodland landscapes in south-eastern Australia (Walker et al. Citation1993; Gibbons and Boak Citation2002; Fischer et al. Citation2009; Ward et al. Citation2019). Efforts to reverse this loss and boost tree cover can have marked positive effects on bird species richness and occurrence (Cunningham et al. Citation2014a, Citation2014b) (including bird species of conservation concern sensu Montague-Drake et al. Citation2009). Indeed, the amount of vegetation cover is a more important driver of bird occurrence (including for bird species of conservation concern) than the connectivity of that vegetation (Lindenmayer et al. Citation2020a). This is possibly because most species are relatively mobile and can colonise patches with empty territories (Driscoll and Lindenmayer Citation2009; although see Cooper and Walters Citation2002).
Doubling the amount of native woody vegetation cover at landscape, farm and individual site scales increases the number of species recorded at these scales by a factor of 3, 2 and 0.7, respectively (Cunningham et al. Citation2014b). Importantly, species such as the Noisy Miner and exotic pest species like the Common Starling (Sturnus vulgaris) are less likely to occur in areas with greater amounts of native vegetation cover (Cunningham et al. Citation2014a; Westgate et al. Citation2021a). The positive effects on bird biodiversity of increasing the amount of vegetation cover (largely through natural regeneration and planting programs) is particularly pronounced in highly disturbed landscapes with very limited amounts of remaining native vegetation (Cunningham et al. Citation2008 - but see Mac Nally et al. Citation2010).
In summary, boosting native vegetation cover has inherently positive effects on the majority of woodland bird species. This likely occurs through creating more habitat for particular taxa and making agricultural landscapes less attractive for hyper-aggressive species such as the Noisy Miner.
There are key attributes of temperate woodland remnants that are important
The temperate woodlands of south-eastern Australia are characterised by three broad kinds of native vegetation cover. These are old growth woodland, plantings, and regrowth woodland (that has regenerated after past cutting or fire, or from seeds shed by large old paddock trees) (Ikin et al. Citation2015). These broad vegetation types vary in structure and composition, such as the prevalence of large old trees, tree canopy width and depth, stocking rate, and a wide range of other stand attributes (Vesk et al. Citation2008; Ikin et al. Citation2015).
Remnant old growth woodlands are critically important habitat for a wide range of temperate woodland bird species (Montague-Drake et al. Citation2009; Watson et al. Citation2014). However, not all woodland remnants are equal, with some having particular attributes that make them more likely to be occupied by woodland birds than others, especially woodland birds of conservation concern. Habitat variables of particular importance include the amount of leaf litter, the volume of fallen timber, and the prevalence of mistletoe (Montague-Drake et al. Citation2009). Notably, the suite of attributes that makes a patch of remnant woodland suitable for occupancy varies markedly between species (Montague-Drake et al. Citation2009).
Larger patches of remnant woodland are important for many species of woodland birds, including birds of conservation concern (Zanette et al. Citation2000; Montague-Drake et al. Citation2009). Of such larger patches, those of particular importance in the Australian States of New South Wales and Queensland are Travelling Stock Reserves. These cover millions of hectares and are public land that was used to drive cattle from farm to market in the era before motorised transport. They are also used for livestock grazing when fodder is depleted on private land, such as during droughts (Lentini et al. Citation2011).
Travelling Stock Reserves have been subject to less land clearing and less intensive livestock grazing relative to other areas of temperate woodland. As a result, they support vegetation in better structural condition than elsewhere in agricultural landscapes (Lindenmayer et al. Citation2012b; O’Loughlin et al. Citation2017) and are characterised by greater biodiversity, including bird biota (Lindenmayer et al. Citation2010a, Citation2012b). Small-bodied, non-seed eating and open-nesting woodland-dependent bird species are significantly more likely to occur in Travelling Stock Reserves than in woodlands on private land that are subject to conventional livestock grazing regimes (Lindenmayer et al. Citation2012b).
Although large patches of woodland are those with the greatest conservation significance for woodland-dependent birds, this does not mean that small patches are without value (Wintle et al. Citation2019). For example, ‘patches’ as small as a single paddock tree can facilitate connectivity by acting as stepping stones to assist movement through agricultural landscapes (Fischer and Lindenmayer Citation2002). In addition, birds can successfully breed in patches as small as 1.3 ha (Belder et al. Citation2019). Moreover, ensembles of small patches can support many species of woodland birds, particularly when the vegetation they support is in relatively good condition (Fischer and Lindenmayer Citation2002).
Many patches of temperate woodland are in poor condition as a result of a range of interacting factors including overgrazing by domestic livestock, limited or no natural regeneration and tree recruitment, tree dieback, a paucity of large old trees, and the prevalence of invasive plants (Sato et al. Citation2019). Management actions such as fencing, grazing control, and the establishment of native understorey and midstorey vegetation can significantly improve the condition of woodland remnants (Lindenmayer et al. Citation2010b, Citation2018a, Citation2018b; Sato et al. Citation2019).
In summary, given the extent of past and current land clearing, all native vegetation in temperate woodlands is important for birds, even patches as small as a single paddock tree. Woodland remnants in good condition are particularly important to protect (Montague-Drake et al. Citation2009), but interventions to improve vegetation condition ae also essential (Lindenmayer et al. Citation2010b, Citation2018a, Citation2018b; Sato et al. Citation2019).
Regrowth woodland is important, but old growth woodland should never be cleared to promote regrowth woodland
For the purposes of this paper, regrowth temperate woodland can be broadly defined as vegetation that has regrown for at least 10+ years after previous clearing, firewood cutting or burning. Regrowth woodland is structurally different to both old growth woodland and replanted woodland, including in terms of stem density, large tree abundance, canopy cover, and other attributes (Ikin et al. Citation2015). These structural differences, in turn, influence habitat suitability for birds, with different species assemblages observed in areas of regrowth versus plantings, and old growth (Lindenmayer et al. Citation2012a). The different assemblages of birds in these different vegetation types also respond differently to temporal variation in weather variations such as between droughts and more mesic periods (Lindenmayer et al. Citation2018c). Regrowth woodland appears to be valuable for a greater range of bird species (as reflected by bird body size) and over a greater range of weather and long-term climatic conditions than plantings and old growth woodlands (Lindenmayer et al. Citation2018c). However, that does not mean that plantings or old growth woodland should be cleared and replaced by regrowth woodland. Indeed, the reverse is the case; all three broad structural kinds of temperate woodland are important to conserve. This is because the full suite of bird species characteristic of temperate woodland ecosystems is likely to occur only on those farms that support a range of key vegetation assets, including regrowth woodland, old growth woodland, and replantings (Cunningham et al. Citation2008; Lindenmayer et al. Citation2012a). Farms characterised by a range of native vegetation types and other key natural assets typically support more species (Cunningham et al. Citation2008). Other natural assets that boost bird biodiversity at a whole of farm level include scattered paddock trees, fallen timber, and native grasslands on a farm (Cunningham et al. Citation2008).
Regrowth vegetation is also important because the trees it contains can mature and eventually become old growth woodland that is in decline in many parts of the temperate woodland estate as a result of, for example, senescing and ageing trees (Manning et al. Citation2013), direct and indirect damage by livestock grazing (Fischer et al. Citation2009), and wildfire (Crane et al. Citation2016). In addition, regrowth woodland is cheaper to establish than plantings and therefore can lead to larger scale revegetation in many extensively cleared agricultural areas (Lindenmayer et al. Citation2018e).
Whilst regrowth woodland has considerable conservation value for birds, that does not mean that old growth woodland can be cleared and its loss offset by allowing natural regeneration to occur elsewhere on a farm. Old growth and regrowth woodland are structurally and functionally different kinds of habitats for woodland birds and both need protection and their condition maintained or improved. It is therefore important to avoid ‘bioperverse’ land management policies that trigger increased land clearing and, in turn, increased biodiversity loss. This is implicit in data which repeatedly show the importance for woodland birds of high levels of native vegetation cover (see above). Hence, vegetation management policies should ensure that existing old growth woodland is maintained, existing regrowth is not cleared, and new areas of regrowth are recruited. (Lindenmayer et al. Citation2018c).
Establishing plantings is good – but following design principles can make them better
Deliberate planting of temperate woodlands has long been a widely employed restoration strategy in many agricultural landscapes in south-eastern Australia (Munro et al. Citation2007; Mac Nally et al. Citation2010; Lindenmayer et al. Citation2018b; Haslem et al. Citation2021). Plantings are disproportionately valuable on farms where there is limited remnant native vegetation (Cunningham et al. Citation2008). Plantings are important habitats for several bird species (e.g. Flame Robin Petroica phoenicea, Red-capped Robin Petroica goodenovii, and Rufous Whistler Pachycephala rufiventris) relative to other broad kinds of structural vegetation types such as old growth woodland and regrowth woodland (Cunningham et al. Citation2008; Lindenmayer et al. Citation2012b). These findings have been documented in agricultural landscapes in northern Victoria and southern and central New South Wales, but different responses by these species may occur in temperate woodlands elsewhere in eastern and south-western Australia.
The establishment of plantings increases levels of woody vegetation cover and, in turn, boosts the functional richness of bird assemblages at larger scales (Ikin et al. Citation2019). Hence, revegetation programs add key ecosystem functions played by birds, particularly those insectivores, nectarivores, and canopy and understorey foragers, that would otherwise be rare or absent in areas without plantings (Ikin et al. Citation2019). Extra ecological roles played by enhanced levels of functional richness from revegetation programs in south-eastern Australia include greater pest insect suppression, more seed dispersal, and pollination services (Ikin et al. Citation2019).
There are some key design principles for plantings which can boost their value for bird biodiversity (Lindenmayer et al. Citation2010b). ‘Good’ plantings for temperate woodland birds are those that: (1) are fenced and not grazed (Lindenmayer et al. Citation2018b), (2) are large in size (Lindenmayer et al. Citation2010b), although many species nevertheless show signs of breeding activity in small plantings (Belder et al. Citation2019), (3) connected to other plantings (Lindenmayer et al., Citation2007), (4) support an understorey (Lindenmayer et al. Citation2010b, Citation2018b), (5) are located in gullies (Lindenmayer et al. Citation2010b), (6) contain natural assets such as paddock trees, fallen timber, rocky outcrops or farm dams (Lindenmayer et al. Citation2010b), and (7) are located near other plantings and woodland remnants (Lindenmayer et al. Citation2010b). For instance, large and wide plantings are characterised by less ‘edge’ habitat than smaller, narrower plantings and support higher levels of bird biodiversity (Lindenmayer et al. Citation2010b). As an example, species such as the Grey Fantail (Rhipidura albiscapa) tend to avoid plantings with a large amount of edge (Lindenmayer et al. Citation2016). Finally, it is important that plantings are established using native plants and not exotic species such as willows. This is because of the potential of willows to boost populations of introduced bird species like the Common Starling and Goldfinch (Carduelis carduelis) (Lindenmayer et al. Citation2018e).
The value of plantings for birds varies not only in terms of the attributes of particular plantings and the surrounding landscape, but also in response to long-term climate and short-term weather. Plantings are particularly important for small-bodied birds (Fischer et al. Citation2008), but especially so in wet years and in broader landscapes characterised by wetter long-term climate (Lindenmayer et al. Citation2018c). That is, the most pronounced positive effects of high rainfall are in plantings in areas with a wet long-term climate. An important consequence of this finding is that a predicted drying of the climate as a result of climate change may limit the ability of populations of small birds to increase during extended periods of wet weather.
In summary, there is an increasing body of empirical knowledge on the value of plantings for woodland bird biodiversity, particularly more densely stocked areas where the Noisy Miner is less common relative to other parts of agricultural landscapes (Lindenmayer et al. Citation2010b). These empirical studies highlight the value for birds of specific design principles associated with their size, shape, location, plant species composition, stand structure connectedness and landscape context. As indicated in the following section, how plantings are managed also matters for bird biodiversity.
Grazing of plantings is bad for birds
The value for birds of plantings in south-eastern Australia can be undermined by intensive, continuous grazing by domestic livestock (Lindenmayer et al. Citation2018b). Negative impacts of grazing arise because of its effects on leaf litter and midstorey vegetation cover – places where birds feed and nest (Lindenmayer et al. Citation2018b). Intensive grazing also can promote pest species in plantings such as the Common Starling (Tulloch et al. Citation2016).
While the problem of high-intensity grazing on birds in plantings is well established, the impacts on bird biodiversity of lower intensity livestock grazing regimes remain unclear, and may even be positive in some circumstances. This includes grazing regimes like short-term ‘crash’ grazing for biomass reduction and control of swards of exotic grasses before maturation and seed dispersal (Lunt et al. Citation2007). A key strategy for managing plantings is to avoid exposing them to high-intensity grazing them wherever possible. The issue of grazing pressure in plantings is increasingly important because fences are deteriorating 20–30 years after being established under past initiatives like the Australian Government’s Natural Heritage Trust (Lindenmayer et al. Citation2018b). It will therefore be important to restore fencing to ensure that grazing pressure in plantings can be controlled and bird biodiversity values maintained.
Management interventions often work – but not always
Management interventions like grazing control, fencing, the deliberate planting of trees and shrubs, and the establishment of nest boxes have been employed in an attempt to improve woodland condition and recover woodland biodiversity, including birds. The success of different management interventions has been variable. Grazing control is critically important as it can enable tree recruitment (Sato et al. Citation2019), maintain levels of leaf litter, and reduce the amount of bare ground – all of which can have positive impacts on woodland birds (Lindenmayer et al. Citation2012b, Citation2018b). The deliberate planting of woodland trees also can have positive impacts on a range of bird species (see above section on tree plantings) as can enhancement planting of understorey shrubs within old growth woodland (Lindenmayer et al. Citation2018a).
Some management interventions have met with varied success for woodland birds. These include the establishment of nest boxes such as in plantings where hollow-bearing trees are rare (Lindenmayer et al. Citation2015) and where they have been deployed as an offset for removing large old paddock trees (Lindenmayer et al. Citation2017a). In these cases, nest boxes were often occupied by: (1) pest species such as the Common Starling, (2) animals that may compete for natural hollows with birds like the European Honeybee (Apis melifera) (Cunningham et al. Citation2022), and/or (3) bird species that are already common in agricultural landscapes and for which targeted conservation efforts are not needed (like the Galah Eolophus roseicapilla and Eastern Rosella Platycercus eximius) (Lindenmayer et al. Citation2015, Citation2017a). In some cases, the limited effectiveness of the establishment of nest boxes as a form of management intervention may have been associated with poor quality installation, the use of boxes that were not fit for purpose for birds of conservation concern, or both (Lindenmayer et al. Citation2017a).
Temperate woodlands, including plantings, need management to maintain or promote their conservation value for birds. This includes management interventions to improve their condition and in turn enhance habitat suitability or to directly provide key resources like nesting cavities. A key part of woodland bird conservation is establishing robust monitoring programmes to quantify the effectiveness of management interventions. Work to date suggests that interventions like planting programs and grazing control have had greater success in contributing to bird conservation (including species of conservation concern) than programmes like providing nest boxes. More careful installation of better designed boxes may obviate these problems, although additional work is required to determine if this is the case. Given such current uncertainty, the removal of natural cavities in existing large old hollow-bearing trees should be avoided wherever possible.
Noisy Miners are bad – but can be limited by strategic planting
Many studies have established that large numbers of the Noisy Miner can have negative impacts on other temperate woodland birds, particularly small-bodied species (Montague-Drake et al. Citation2011; Maron et al. Citation2013; Mortelliti et al. Citation2016; Westgate et al. Citation2021b). The effects of the Noisy Miner on other bird taxa are particularly pronounced in the presence of the Grey Butcherbird (Cracticus torquatus) and Pied Butcherbird (Cracticus nigrogularis) (Westgate et al. Citation2021b). There also may be productivity relationships between the occurrence of the Noisy Miner and other woodland birds. For example, Montague-Drake et al. (Citation2011) found that when the abundance of the Noisy Miner was low, the abundance of small-bodied bird species was high, especially on high productivity woodland sites.
Given the extensive distribution, high abundance, and major negative impacts of the Noisy Miner, much effort has been expended on attempts to control them and help promote the recovery of other woodland birds. Culling has been widely used as a control method (reviewed by Melton et al. Citation2021). Some culls of this species have been successful (Grey et al. Citation1997; Crates et al. Citation2020). Others have not, with rapid recolonisation of sites where birds had initially been extensively culled (Davitt et al. Citation2018; Beggs et al. Citation2019). An interesting outcome of the review of culling studies was that although removals do not result in a reduction in Noisy Miners below a threshold at which they no longer impact on smaller-bodied birds, such actions often facilitated an increase in other woodland birds (Melton et al. Citation2021), including breeding by species targeted for conservation such as the Regent Honeyeater (Anthochaera phrygia) (Crates et al. Citation2020). However, it remains unclear how long the benefits of culling persist and how much sustained removal pressure is required for Noisy Miner control to be effective (Beggs et al. Citation2019; Melton et al. Citation2021). It is possible that in the future there will be animal welfare concerns raised about culling of the Noisy Miner and this may underpin additional efforts to implement strategies of control by modifying habitat such as the establishment of densely stocked areas of replanted woodland or the establishment of understorey underplantings in existing old growth woodland.
Other work on temperate woodland birds, including on the Noisy Miner, has produced insights valuable for control efforts: (1) The occurrence of the Noisy Miner is lower in landscapes with higher levels of native vegetation cover (Cunningham et al. Citation2014a). (2) The occurrence of the Noisy Miner is reduced in plantings with a wattle understorey (Lindenmayer et al. Citation2010b) and in old growth woodlands where there is enhancement planting in the understorey (Lindenmayer et al. Citation2018a). (3) The synergistic effects of the Noisy Miner, Grey Butcherbird and Pied Butcherbird are reduced where woodland vegetation cover includes a dense midstorey (Westgate et al. Citation2021b). And, (4) Bird recovery is strongest where Noisy Miner control is part of a suite of threat mitigation strategies, including increasing the amount of native vegetation cover (Tulloch et al. Citation2016).
In summary, much remains to be learnt about the most effective ways to control overabundant populations of the Noisy Miner. Culling appears to have some short-term benefits. However, its long-term efficacy remains unclear (Beggs et al. Citation2019; Melton et al. Citation2021). Moreover, culling over large areas and on a repeated basis may be financially unsustainable, approaching $1 billion across the entire temperate woodland biome (see Beggs et al. Citation2019). In some cases, the long-term control of populations of the Noisy Miner might be better achieved by ensuring that understorey and midstorey vegetation is established in plantings and old growth woodland. In others, Noisy Miner control might be most effective when it is part of an integrated approach to threat mitigation that entails other strategies such as facilitating natural regeneration and deliberate planting that increase the amount of vegetation cover (Tulloch et al. Citation2016) and promoting stand structural complexity such as through greater midstorey vegetation cover (Lindenmayer et al. Citation2010b) and understorey enhancement (Lindenmayer et al. Citation2018a).
Which management intervention is best?
As outlined above, several kinds of management interventions can be employed to enhance woodland bird biodiversity in south-eastern Australia. Tulloch et al. (Citation2016) showed that the greatest gains in patch colonisation for all bird species (and species otherwise in decline) accrued from increasing tree cover, relative to the removal of the Noisy Miner, and grazing control. However, instigating more than one management action (and thereby simultaneously tackling more than just one threat) such as increasing tree cover and removing the Noisy Miner, had the greatest effect, promoting levels of patch colonisation by 10% overall bird occurrence and by 19% for declining species (Tulloch et al. Citation2016). A combined effort is important because the majority of woodland bird species are negatively affected by more than one threat.
Landscape context matters
Landscape context refers to the amount, condition, and connectivity of the vegetation surrounding a given site or patch (Forman Citation1995). Landscape context influences the occurrence of woodland birds in old growth and regrowth woodland patches as well as plantings (Haslem et al. Citation2021). For example, plantings surrounded by other plantings or near patches of remnant vegetation generally support more species than isolated plantings (Lindenmayer et al. Citation2010b).
The impacts of landscape context on temperate woodland birds have been tested directly in southern New South Wales (Lindenmayer et al. Citation2019). Birds inhabiting woodland patches have been monitored as the surrounding landscape was converted from one dominated by pastures with scattered paddock trees to one dominated by 10000ha exotic Radiata Pine (Pinus radiata) plantation stands (Lindenmayer et al. Citation2019). At the same time, a matched set of temperate woodland patches was monitored in adjacent grazed landscapes (where there was no plantation establishment (Lindenmayer et al. Citation2019). In the 23+ years since plantation establishment, most woodland bird species in the woodland patches embedded within the plantation have been lost and replaced by forest taxa (Lindenmayer et al. Citation2019). In addition, bird assemblages in woodland patches located on farms up to 6 km away from the plantation have been affected (Lindenmayer et al. Citation2019). These results highlight not only the importance of landscape context effects on woodland birds, but also how far impacts such as those associated with large-scale plantation establishment can be propagated across landscapes.
In summary, an understanding of the effects of landscape context is critical in appraising the effectiveness for woodland birds of management interventions like restoration programs. Landscape context is important in other large-scale landscape transformations in vegetation cover such as the conversion of semi-cleared woodland vegetation to plantation-dominated environments, as is occurring in many parts of Australia and globally (Watson et al. Citation2014; Ghazoul et al. Citation2019). Assessments of the impacts on birds of such land cover conversions are essential, even when already heavily disturbed areas are being targeted for transformation.
Factors at multiple scales are important in influencing bird occurrence
Much of the work on bird responses in temperate woodlands in south-eastern Australia has been at the site level; for example, in individual plantings or individual patches of remnant vegetation. However, factors at other scales are important (Cunningham et al. Citation2014a, Citation2014b; Haslem et al. Citation2021). Hingee et al. (Citation2022) showed that factors at individual patch, landscape and regional scales influenced site occupancy by woodland birds. These included vegetation structure, the presence of the Noisy Miner, temperature and rainfall, and weather extremes. Notably, there was a surprising lack of redundancy in covariate effects at different scales. These findings suggest that fine-scale studies of woodland bird occurrence will be enhanced by ensuring they account for factors like climate, whereas large scale investigations need to accommodate finer-scaled factors like the structure of woodland vegetation (Hingee et al. Citation2022).
In other cases, the same variable can influence bird responses at multiple scales. For example, the amount of native woody vegetation cover has significant and generally positive, effects on bird species richness, the richness of bird species of conservation concern, and the occurrence of many individual bird species at a site, farm and landscape scales (a scale of ~10 000 ha) (Cunningham et al. Citation2014a, Citation2014b). Notably, these results suggest that restoration efforts in a given landscape will lead not only to increased bird species richness in that landscape, but also on sites and farms within that landscape. Moreover, restoration at a landscape scale can lead to an increase in bird species richness on farms and sites where no restoration has taken place. This underscores the bird biodiversity benefits at multiple scales that are associated with large (landscape) scale restoration programs. (Cunningham et al. Citation2014a, Citation2014b).
In summary, scale matters in understanding both: (1) the range of key factors influencing the occurrence of woodland bird biota, and (2) how individual variables like tree cover influence bird occurrence at site to landscape scales. This understanding is, in turn, critical for guiding restoration programs as well as determining what factors affect why birds occur where they do.
Birds may not be good indicators of other groups like frogs, reptiles and mammals
Woodland birds have sometimes been proposed as biodiversity indicators (reviewed by Lindenmayer and Likens Citation2010), including in temperate woodlands (Lambeck Citation1997; Brooker Citation2002; Fruedenberger and Brooker Citation2004). This proposal is often built on the premise that if suitable habitats for particular bird species or birds per se are conserved, maintained or restored, then other groups of biota also will benefit. Thus, for example, environments that support high levels of bird biodiversity are hypothesised to be similarly rich in species for other taxonomic group such as reptiles, amphibians, and mammals. This concept is sometimes termed cross-taxon congruence (Westgate et al., Citation2014). To date there is only limited evidence that, at least for Australian temperate woodland environments, birds are good indicators of the richness and/or abundance of other groups, (e.g. reptiles) (Yong et al. Citation2018). There is likely to be several reasons for this outcome. First, some kinds of habitats on farms such as rocky outcrops, which are important for groups like reptiles (Michael and Lindenmayer Citation2018) are not favoured environments for the vast majority of bird taxa. Similarly, farm dam environments which can be important environments for some species of amphibians in agricultural landscapes (Hazell et al. Citation2001) and also water birds (Hamilton et al. Citation2017) support relatively few temperate woodland birds. Conversely, whilst vegetation such as plantings provide habitat for some bird species (Lindenmayer et al. Citation2010b, Citation2016, Citation2018c), groups like cavity-dependent arboreal marsupials are largely absent from these environments (Lindenmayer et al. Citation2017b). Second, whilst there can still be relatively diverse assemblages of birds in some areas of temperate woodland, other groups such as native mammals in agricultural Australia have suffered many regional or even global extinctions with assemblages now highly depauperate relative to pre-European colonisation.
Even within bird assemblages, few individual species appear to be good indicators of the richness of the remainder of the bird community (Lindenmayer et al. Citation2014). This is possibly because of inter-specific differences in habitat requirements, both in remnant old growth woodland (see Montague-Drake et al. Citation2009) and in plantings (Lindenmayer et al. Citation2010b). Indeed, a species such as the Superb Parrot (Polytelis swainsonii) is what could be deemed to be an ‘anti-indicator species’ in that few other native bird species are strongly associated with it (Lindenmayer et al. Citation2014). This is probably because of their strong association with relatively open areas such as croplands (which are unsuitable for many other woodland birds).
The limited indicator value of birds for other groups, or the indicator value of particular bird species within bird assemblages, does not lessen the importance of conservation actions to conserve birds. Rather, it means that considered conservation actions will often need to be targeted for particular species or sets of species as opposed to biodiversity per se. In addition, birds are a group with which many in the farming community can relate (as opposed to reptiles, for example). On this basis, efforts to conserve and/or restore temperate woodlands for birds will often have support from farmers and other natural resource managers.
General discussion
There have been long-held concerns about the persistence of populations of many species of birds in Australian temperate woodland ecosystems (Recher Citation1999; Ford et al. Citation2001; Ford Citation2011). Effective conservation of bird biodiversity in these ecosystems requires a good understanding of temporal change in species occurrence, key drivers of change, and how species respond to management actions such a grazing control (e.g. fencing), competitor removal (e.g. the removal of the Noisy Miner), and habitat augmentation (e.g. the provision of nest boxes). Work to date indicates that interventions such as grazing control and the establishment of restored woodland vegetation have positive effects on temperate woodland birds (Lindenmayer et al. Citation2012b, Citation2018b). Evidence for the success of other interventions like the removal of the Noisy Miner and provision of nest boxes is less convincing (Lindenmayer et al. Citation2017a; Beggs et al. Citation2019; but see Crates et al. Citation2020). This suggests a need to ensure that the objectives of a given management action are well articulated before it is implemented. It also highlights the value of monitoring management interventions to determine whether they have been effective. A key additional finding has been the importance for birds of different natural assets on farms. These assets include patches of old growth remnant vegetation, regrowth woodland, plantings, paddock trees, fallen timber, native grasslands and farm dams. The highest bird biodiversity at the farm level typically occurs on those farms with all of these assets (Cunningham et al. Citation2008). Despite the large body of work generated to date, there are still major knowledge gaps and several of these are discussed in the following section.
Knowledge gaps
The role and importance of fire
Temperate woodland environments have experienced recurrent wildfires throughout their evolution, including by fires lit by First Nations people (Hobbs Citation2002). However, the use of fire in woodlands management, including in south-eastern Australia, has been limited in the past 220 years, likely because of perceived risks to farm infrastructure. Nevertheless, both prescribed fires and wildfires have had major impacts of some key natural assets on farms such as large old paddock trees (Crane et al. Citation2016). Moreover, the ways in which fires burn in agricultural landscapes appear to be somewhat different from patterns of fire behaviour in forest ecosystems (e.g. see Collins et al. Citation2014). There is considerable interest in employing cultural burning practices in temperate woodland environments and this should be done in ways that maintain the Intellectual Property of First Nations groups, and capitalises on opportunities for cross-cultural learning, including through integrating Indigenous knowledge and western science. Existing large-scale, long-term monitoring programmes would be a valuable foundation around which to the test biodiversity responses to the addition of fire management in temperate woodland ecosystems.
A greater focus on Travelling Stock Reserves in New South Wales and Queensland
There is a need for greater understanding of the connectivity, habitat and other ecological roles played by networks of woodland vegetation such as those in Travelling Stock Reserves in New South Wales and Queensland. Divestment of these areas in States like New South Wales has been proposed (Possingham and Nix Citation2008), meaning they could be subject to the same levels of clearing and livestock grazing as has occurred in large parts of the remaining agricultural landscape. This would likely have significant negative effects on bird biodiversity (Lindenmayer et al. Citation2010a) and environmental regulations are needed to prevent this from happening. Indeed, I argue that Travelling Stock Reserves need more, not less protection. They also need more, not less management to enhance their condition, especially given their exceptional value for bird and other biodiversity.
Beyond patterns of bird occurrence to key demographic processes
The vast majority of studies of birds in temperate woodland environments have been ‘pattern-based’ assessments of bird occurrence (Belder et al. Citation2019). However, there is a lack of knowledge about key demographic process like bird breeding success, recruitment, and mortality (Selwood et al. Citation2009, Citation2015; Okada et al. Citation2016; Belder et al. Citation2018, Citation2019) (but see Zanette et al. Citation2000). This knowledge is crucial in understanding the effectiveness of management actions like the removal of the Noisy Miner (Beggs et al. Citation2020; Crates et al. Citation2020). It is also important to ensure that areas of woodland like those dominated by replantings are not ecological traps, with individuals living in them but never breeding successfully and contributing to long-term population recovery (see Belder et al. Citation2019). Such kinds of information is also instrumental in determining what attributes of patches have an important impact of fecundity (e.g. Zanette et al. Citation2000) as well as on successful inter-patch dispersal (e.g. Walters et al., Citation1999).
Maintaining long-term monitoring
Many insights about temperate woodland birds have been discovered only through long-term monitoring. The value of such studies is undisputed (Hughes et al. Citation2017). However, perhaps more challenging than highlighting their value, is to determine how to maintain them, even though the importance of monitoring programmes typically increases the longer they run (Lindenmayer and Likens Citation2018).
The maintenance of long-term studies remains a fundamental issue in Australia, with past initiatives to address this problem having been axed (Lindenmayer et al. Citation2017). New funding and institutional arrangements are needed to initiate, support and then maintain long-term monitoring in Australia. One solution might be the establishment of a new Long-Term Ecological Network akin to that in the USA and many other countries globally. Another might be to create a new Australian Government agency responsible for maintaining long-term ecological monitoring programs across a range of ecosystems. Data from such monitoring programs in temperate woodlands will be critical for gauging the performance and environmental credentials of initiatives like the Beef Sustainability Framework (https://www.sustainableaustralianbeef.com.au/) and the Sheep Sustainability Framework (https://www.sheepsustainabilityframework.com.au/) that aim to integrate production and conservation objectives in agricultural landscapes.
One way to reduce the cost and logistical burden of large-scale, long-term monitoring would be to enlist the support of citizen scientists. Many of the surveys in the temperate woodlands reported in this article were completed with the assistance of expert volunteer ornithologists. However, and critically, a strong experimental framework was imposed around these survey efforts to ensure a robust empirical approach to field data collection, consistency in survey effort, and the gathering of datasets of sufficient quality to enable subsequent statistical analyses.
Better integrating bird conservation and agricultural production
Finally, a major challenge will be to develop approaches to better integrate wildlife conservation (including the conservation of birds) with agricultural production. Part of such integration will pivot around better farm planning to help farmers and identify which natural assets occur where on their farms and then devising management interventions to ensure those assets are better protected, and their condition enhanced. Investments such as those through carbon markets that result in long-term biodiverse carbon storage on farms could be critical in this respect, including stewardship schemes where farmers are payed for demonstrably improved conservation outcomes on their land (https://www.agsteward.com.au/program/index.html). These kinds of schemes will be essential if protection and restoration efforts are to occur at the scales required to truly address bird biodiversity loss in temperate agricultural Australia.
Conclusions
There is little doubt that the vast human-generated changes to Australia’s temperate woodlands have had enormous negative impacts on native biodiversity, including woodland birds. Many species are continuing to decline (Lindenmayer et al. Citation2018d) and some regional extinctions are likely in the coming decades. However, there is a growing body of empirical evidence of the factors which help contribute to the recovery of at least some of the declining bird species, particularly efforts to increase woody vegetation cover as well as grazing control (Tulloch et al. Citation2016). The hope in the future is that approaches to enhance bird biodiversity on farms – such as environmental stewardship programs – will be well-designed and guided by the insights that have been described here and which have resulted from decades of intensive research and monitoring.
Acknowledgements
I thank Dr Kate Buchanan for suggesting this overview article be written. Dr Richard Beggs and two anonymous reviewers provided useful comments which substantially improved earlier versions of the manuscript. I thank the outstanding statistical scientists with whom I have worked and have been responsible for helping develop robust experimental designs and subsequent cutting-edge analyses. These include Dr Wade Blanchard, Professor Ross Cunningham, and the late Professor Jeff Wood. I thank all those outstanding ecologists including post-graduate students and field staff with whom I have worked on farms over the past ~25 years. I gratefully thank volunteers from the Canberra Ornithologists Group for assistance with field surveys. Tabitha Boyer assisted with many aspects of manuscript preparation.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Barton, P. S., Manning, A. D., Gibb, H., Lindenmayer, D. B., and Cunningham, S. A. (2009). Conserving ground-dwelling beetles in an endangered woodland community: Multi-scale habitat effects on assemblage diversity. Biological Conservation 142, 1701–1709. doi:10.1016/j.biocon.2009.03.005
- Barton, P. S., Manning, A. D., Gibb, H., Wood, J. T., Lindenmayer, D. B., and Cunningham, S. A. (2011). Experimental reduction of native vertebrate grazing and addition of logs benefit beetle diversity at multiple scales. Journal of Applied Ecology 48, 943–951. doi:10.1111/j.1365-2664.2011.01994.x
- Beggs, R., Tulloch, A. I. T., Pierson, J., Blanchard, W., Crane, M., and Lindenmayer, D. B. (2019). Patch-scale culls of an overabundant bird defeated by immediate recolonization. Ecological Applications 29, e01846. doi:10.1002/eap.1846
- Beggs, R., Tulloch, A. I. T., Pierson, J., Blanchard, W., Crane, M., and Lindenmayer, D. B. (2020). An empirical test of the mechanistic underpinnings of interference competition. Oikos 129, 93–105. doi:10.1111/oik.06583
- Belder, D. J., Pierson, J. C., Ikin, K., and Lindenmayer, D. B. (2018). Beyond pattern to process: Current themes and future directions for the conservation of woodland birds through restoration plantings. Wildlife Research 45, 473–498. doi:10.1071/wr17156
- Belder, D. J., Pierson, J. C., Ikin, K., Blanchard, W., Westgate, M. J., Crane, M., and Lindenmayer, D. (2019). Is bigger always better? Influence of patch attributes on breeding activity of birds in box-gum grassy woodland restoration plantings. Biological Conservation 236, 134–152. doi:10.1016/j.biocon.2019.05.015
- Bennett, A., Duncan, D. H., Rumpff, L., and Vesk, P. A. (2020). Disentangling chronic regeneration failure in endangered woodland ecosystems. Ecosphere 11, e02998. doi:10.1002/ecs2.2998
- Brooker, L. (2002). The application of focal species knowledge to landscape design in agricultural lands using the ecological neighbourhood as a template. Landscape and Urban Planning 60, 185–210. doi:10.1016/S0169-2046(02)00055-5
- Cogger, H., Dickman, C., Ford, H., Johnson, C., and Taylor, M. (2017). ‘Australian Animals Lost to Bulldozers in Queensland 2013–15.’ (WWF-Australia: Sydney, Australia.)
- Collins, L., Penman, T. D., Price, O. F., and Bradstock, R. A. (2014). Adding fuel to the fire? Revegetation influences wildfire size and intensity. Journal of Environmental Management 150, 196–205. doi:10.1016/j.jenvman.2014.11.009
- Cooper, C. B., and Walters, J. R. (2002). Experimental evidence of disrupted dispersal causing decline of an Australian passerine in fragmented habitat. Conservation Biology 16, 471–478. doi:10.1046/j.1523-1739.2002.00346.x
- Crane, M., Lindenmayer, D. B., Cunningham, R. B., and Stein, J. (2016). The effect of wildfire on scattered trees, ‘keystone structures’, in agricultural landscapes. Austral Ecology 45, 145–153. doi:10.1111/aec.12414
- Crates, R., Rayner, L., Webb, M., Stojanovic, D., Wilkie, C., and Heinsohn, R. (2020). Sustained and delayed noisy miner suppression at an avian hotspot. Austral Ecology 45, 636–643. doi:10.1111/aec.12878
- Cunningham, R. B., Lindenmayer, D. B., McGregor, C., Crane, M., and Michael, D. (2008). The combined effects of remnant vegetation and replanted vegetation on farmland birds. Conservation Biology 22, 742–752. doi:10.1111/j.1523-1739.2008.00924.x
- Cunningham, R. B., Lindenmayer, D. B., Barton, P., Ikin, K., Crane, M., Michael, D., Okada, S., et al. (2014a). Cross-sectional and temporal relationships between bird occupancy and vegetation cover at multiple spatial scales. Ecological Applications 24, 1275–1288. doi:10.1890/13-0872.1
- Cunningham, R. B., Lindenmayer, D. B., Crane, M., Michael, D. R., Barton, P. S., Gibbons, P., Okada, S., et al. (2014b). The law of diminishing returns: Woodland birds respond to native vegetation cover at multiple spatial scales and over time. Diversity and Distributions 20, 59–71. doi:10.1111/ddi.12145
- Cunningham, R., and Lindenmayer, D. B. (2016). Approaches to landscape scale inference and design issues. Current Landscape Ecology Reports 2, 42–50. doi:10.1007/s40823-016-0019-4
- Cunningham, S. A., Crane, M. J., Evans, M. J., Hingee, K. L., and Lindenmayer, D. B. (2022). Density of feral western honey bee (Apis mellifera) colonies in fragmented woodland indicates potential for large ecosystem impacts. Scientific Reports 12, 3603. doi:10.1038/s41598-022-07635-0
- Davitt, G., Maute, K., Major, R. E., McDonald, P. G., and Maron, M. (2018). Short-term response of a declining woodland bird assemblage to the removal of a despotic competitor. Ecology and Evolution 8, 4771–4780. doi:10.1002/ece3.4016
- Debus, S., Martin, W. K., and Lemon, J. M. (2017). Changes in woodland bird communities as replanted woodland matures. Pacific Conservation Biology 23, 359–371. doi:10.1071/PC16028
- Department of Agriculture Water and Environment. (2021). ‘Snapshot of Australian Agriculture 2021.’ https://www.awe.gov.au/abares/products/insights/snapshot-of-australian-agriculture-2021 [Verified 19 March 2022].
- Department of the Environment and Energy. (2016). Natural Temperate Grassland of the South Eastern Highlands: A Nationally Protected Ecological Community. (Department of the Environment and Energy: Canberra, Australia.)
- Dickman, C. R., and Lindenmayer, D. (2022). Australia’s environment. In ‘Paradigm Shift.’ (Ed. S. Williams.) (Springer: Berlin.)
- Doerr, V. A. J., Davies, M. J., Doerr, E. D., Prober, S., Murphy, H., McGinness, H., and Hoffmann, B. (2017). ‘Knowledge Bank of Management Effectiveness: Technical Guide.’ (CSIRO: Canberra, Australia.)
- Driscoll, D. A., and Lindenmayer, D. B. (2009). Empirical test of metacommunity theory using an isolation gradient. Ecological Monographs 79, 485–501. doi:10.1890/08-1114.1
- Fischer, J., and Lindenmayer, D. B. (2002). Small patches can be valuable for biodiversity conservation: Two case studies on birds in southeastern Australia. Biological Conservation 106, 129–136. doi:10.1016/S0006-3207(01)00241-5
- Fischer, J., Lindenmayer, D. B., and Montague-Drake, R. (2008). The role of landscape texture in conservation biogeography: A case study on birds in south-eastern Australia. Diversity & Distributions 14, 38–46.
- Fischer, J., Stott, J., Zerger, A., Warren, G., Sherren, K., and Forrester, R. I. (2009). Reversing a tree regeneration crisis in an endangered ecoregion. Proceedings of the National Academy of Sciences 106, 10386–10391. doi:10.1073/pnas.0900110106
- Ford, H. A., Barrett, G. W., Saunders, D. A., and Recher, H. F. (2001). Why have birds in the woodlands of southern Australia declined? Biological Conservation 97, 71–88. doi:10.1016/S0006-3207(00)00101-4
- Ford, H. A. (2011). The causes of decline of birds of eucalypt woodlands: Advances in our knowledge over the last 10 years. Emu - Austral Ornithology 111, 1–9. doi:10.1071/MU09115
- Forman, R. T. T. (1995). ‘Land Mosaics: The Ecology of Landscapes and Regions.’ (Cambridge University Press: New York.)
- Fruedenberger, D., and Brooker, L. (2004). Development of the focal species approach for biodiversity conservation in the temperate agricultural zones of Australia. Biodiversity and Conservation 13, 253–274. doi:10.1023/B:BIOC.0000004320.43567.f7
- Ghazoul, J., Bugalho, M., and Keenan, R. (2019). Forests: Economic perks of plantations. Nature 570, 307. doi:10.1038/d41586-019-01878-0
- Gibbons, P., and Boak, M. (2002). The value of paddock trees for regional conservation in an agricultural landscape. Ecological Management & Restoration 3, 205–210. doi:10.1046/j.1442-8903.2002.00114.x
- Goldingay, R. L., Rueegger, N. N., Grimson, M. J., and Taylor, B. D. (2015). Specific nest box designs can improve habitat restoration for cavity-dependent arboreal mammals. Restoration Ecology 23, 482–490. doi:10.1111/rec.12208
- Grey, M. J., Clarke, M. F., and Loyn, R. H. (1997). Initial changes in the avian communities of remnant eucalypt woodlands following a reduction in the abundance of noisy miners, Manorina melanocephala. Wildlife Research 24, 631–648. doi:10.1071/WR96080
- Hamilton, A. J., Conort, C., Bueno, A., Murray, C. G., and Grove, J. R. (2017). Waterbird use of farm dams in south-eastern Australia: Abundance and influence of biophysical and landscape characteristics. Avian Research 8. Art. 2. doi:10.1186/s40657-016-0058-x
- Haslem, A., Clarke, R. H., Holland, G. J., Radford, J. Q., Stewart, A., and Bennett, A. F. (2021). Local management or wider context: What determines the value of farm revegetation plantings for birds? Journal of Applied Ecology 58, 2552–2565. doi:10.1111/1365-2664.13988
- Hazell, D., Cunnningham, R., Lindenmayer, D., Mackey, B., and Osborne, W. (2001). Use of farm dams as frog habitat in an Australian agricultural landscape: Factors affecting species richness and distribution. Biological Conservation 102, 155–169. doi:10.1016/S0006-3207(01)00096-9
- Hingee, K., Westgate, M., and Lindenmayer, D. B. (2022). Complementary drivers of bird biodiversity across spatial scales. Journal of Biogeography 49, 979–992. in press. doi:10.1111/jbi.14330
- Hobbs, R. J., and Yates, C. J. (Eds.) (2000). Temperate Eucalypt Woodlands in Australia: Biology, Conservation, Management and Restoration. (Surrey Beatty and Sons: Chipping Norton.)
- Hobbs, R. J. (2002). Fire regimes and their effects in Australian temperate woodlands. In ‘Flammable Australia. The Fire Regimes and Biodiversity of a Continent.’ (Eds R. A. Bradstock, J. E. Williams, and A. M. Gill.) pp. 305–326. (Cambridge University Press: Melbourne.)
- Howland, B. W. A., Stojanovic, D., Gordon, I. J., Radford, J., Manning, A. D., and Lindenmayer, D. B. (2016). Birds of a feather flock together: Using trait-groups to understand the effect of macropod grazing on birds in grassy habitats. Biological Conservation 194, 89–99. doi:10.1016/j.biocon.2015.11.033
- Hughes, B. B., Beas-Luna, R., Barner, A. K., Brewitt, K., Brumbaugh, D. R., Cerny-Chipman, E. B., Close, S. L., et al. (2017). Long-term studies contribute disproportionately to ecology and policy. BioScience 67, 271–281. doi:10.1093/biosci/biw185
- Ikin, K., Mortelliti, A., Stein, J., Michael, D., Crane, M., Okada, S., Wood, J., et al. (2015). Woodland habitat structures are affected by both agricultural land management and abiotic conditions. Landscape Ecology 30, 1387–1403. doi:10.1007/s10980-015-0193-5
- Ikin, K., Barton, P. S., Blanchard, W., Crane, M., Stein, J., and Lindenmayer, D. B. (2019). Avian functional responses to landscape recovery. Proceedings of the Royal Society B: Biological Sciences 286, 20190114. doi:10.1098/rspb.2019.0114
- Intergovernmental Science-policy Platform on Biodiversity and Ecosystem Services (IPBES). (2019). ‘IPBES Global Assessment Summary for Policymakers.’ (Bonn, Germany: United Nations.)
- Lambeck, R. J. (1997). Focal species: A multi-species umbrella for nature conservation. Especies focales: Una sombrilla multiespecifica para conservar la naturaleza. Conservation Biology 11, 849–856. doi:10.1046/j.1523-1739.1997.96319.x
- Legge, S., Murphy, B. P., McGregor, H., Woinarski, J. C. Z., Augusteyn, J., Ballard, G., Maxwell, M., et al. (2017). Enumerating a continental-scale threat: How many feral cats are in Australia? Biological Conservation 206, 293–303. doi:10.1016/j.biocon.2016.11.032
- Lentini, P. E., Fischer, J., Gibbons, P., Lindenmayer, D. B., and Martin, T. G. (2011). Australia’s stock route network. 1. A review of its values and implications for future management. Ecological Management & Restoration 12, 119–127. doi:10.1111/j.1442-8903.2011.00591.x
- Lindenmayer, D. B., Cunningham, R., Crane, M., Michael, D., and Montague-Drake, R. (2007). Farmland bird responses to intersecting replanted areas. Landscape Ecology 22, 1555–1562.
- Lindenmayer, D. B., and Likens, G. E. (2010). Direct Measurement Versus Surrogate Indicator Species for Evaluating Environmental Change and Biodiversity Loss. Ecosystems 14, 47–59. doi:10.1007/s10021-010-9394-6
- Lindenmayer, D. B., Cunningham, R. B., Crane, M., Montague-Drake, R., and Michael, D. (2010a). The importance of temperate woodland in travelling stock reserves for vertebrate biodiversity conservation. Ecological Management & Restoration 11, 27–30. doi:10.1111/j.1442-8903.2010.00509.x
- Lindenmayer, D. B., Knight, E. J., Crane, M. J., Montague-Drake, R., Michael, D. R., and MacGregor, C. I. (2010b). What makes an effective restoration planting for woodland birds? Biological Conservation 143, 289–301. doi:10.1016/j.biocon.2009.10.010
- Lindenmayer, D. B., Northrop-Mackie, A. R., Montague-Drake, R., Crane, M., Michael, D., Okada, S., and Gibbons, P. (2012a). Not all kinds of revegetation are created equal: Revegetation type influences bird assemblages in threatened Australian woodland ecosystems. PLOS One 7, e34527. doi:10.1371/journal.pone.0034527
- Lindenmayer, D. B., Wood, J., Montague-Drake, R., Michael, D., Crane, M., Okada, S., MacGregor, C., et al. (2012b). Is biodiversity management effective? Cross-sectional relationships between management, bird response and vegetation attributes in an Australian agri-environment scheme. Biological Conservation 152, 62–73. doi:10.1016/j.biocon.2012.02.026
- Lindenmayer, D. B., Zammit, C., Attwood, S. A., Burns, E., Shepherd, C. L., Kay, G., and Wood, J. (2012c). A novel and cost-effective monitoring approach for outcomes in an Australian biodiversity conservation incentive program. PLOS One 7, e50872.
- Lindenmayer, D. B., Lane, P. W., Westgate, M. J., Crane, M., Michael, D., Okada, S., and Barton, P. S. (2014). An empirical assessment of the focal species hypothesis. Conservation Biology 28, 1594–1603. doi:10.1111/cobi.12330
- Lindenmayer, D. B., Crane, M., Blanchard, W., Okada, S., and Montague-Drake, R. (2015). Do nest boxes in restored woodlands promote the conservation of hollow-dependent fauna? Restoration Ecology 24, 244–251. doi:10.1111/rec.12306
- Lindenmayer, D. B., Lane, P. W., Barton, P. S., Crane, M., Ikin, K., Michael, D. R., and Okada, S. (2016). Long-term bird colonization and turnover in restored woodlands. Biodiversity and Conservation 25, 1587–1603. doi:10.1007/s10531-016-1140-8
- Lindenmayer, D. B. (2017). Save Australia’s ecological research. Science 357, 557. doi:10.1126/science.aao4228
- Lindenmayer, D. B., Crane, M., Evans, M. C., Maron, M., Gibbons, P., Bekessy, S., and Blanchard, W. (2017a). The anatomy of a failed offset. Biological Conservation 210, 286–292. doi:10.1016/j.biocon.2017.04.022
- Lindenmayer, D. B., Mortelliti, A., Ikin, K., Pierson, J., Crane, M., Michael, D., and Okada, S. (2017b). The vacant planting: Limited influence of habitat restoration on patch colonization patterns by arboreal marsupials in south-eastern Australia. Animal Conservation 20, 294–304. doi:10.1111/acv.12316
- Lindenmayer, D. B., and Likens, G. E. (2018). ‘‘Effective Ecological Monitoring’.’ (CSIRO Publishing: Melbourne.)
- Lindenmayer, D. B., Blanchard, W., Crane, M., Michael, D., and Florance, D. (2018a). Size or quality. What matters in vegetation restoration for bird biodiversity in endangered temperate woodlands? Austral Ecology 43, 798–806. doi:10.1111/aec.12622
- Lindenmayer, D. B., Blanchard, W., Crane, M., Michael, D., and Sato, C. (2018b). Biodiversity benefits of vegetation restoration are undermined by livestock grazing. Restoration Ecology 26, 1157–1164. doi:10.1111/rec.12676
- Lindenmayer, D. B., Lane, P., Crane, M., Florance, D., Foster, C. N., Ikin, K., Michael, D., et al. (2018c). Weather effects on birds of different size are mediated by long-term climate and vegetation type in endangered temperate woodlands. Global Change Biology 25, 675–685. doi:10.1111/gcb.14524
- Lindenmayer, D. B., Lane, P., Westgate, M., Scheele, B., Foster, C., Sato, C., Ikin, K., et al. (2018d). Tests of predictions associated with temporal changes in Australian bird populations. Biological Conservation 222, 212–221. doi:10.1016/j.biocon.2018.04.007
- Lindenmayer, D. B., Michael, D. R., Crane, M., Florance, D., and Burns, E. (2018e). ‘Restoring Farm Woodlands for Wildlife.’ (CSIRO Publishing: Melbourne.)
- Lindenmayer, D. B., Blanchard, W., Westgate, M. J., Foster, C., Banks, S. C., Barton, P. S., Crane, M., et al. (2019). Novel bird responses to successive large-scale, landscape transformations. Ecological Monographs 89, e01362. doi:10.1002/ecm.1362
- Lindenmayer, D. B., Blanchard, W., Foster, C. N., Scheele, B. C., Westgate, M. J., Stein, J., Crane, M., et al. (2020a). Habitat amount versus connectivity: An empirical study of bird responses. Biological Conservation 241, 108377. doi:10.1016/j.biocon.2019.108377
- Lindenmayer, D. B., Lane, P., Westgate, M., Scheele, B., Florance, D., Crane, M., Crane, C., et al. (2020b). Long-term mammal and nocturnal bird trends are influenced by vegetation type, weather and climate in temperate woodlands. Austral Ecology 45, 813–824. doi:10.1111/aec.12928
- Lunt, I. D., Eldridge, D. J., Morgan, J. W., and Witt, G. B. (2007). A framework to predict the effects of livestock grazing and grazing exclusion on conservation values in natural ecosystems in Australia. Australian Journal of Botany 55, 401–415. doi:10.1071/BT06178
- Mac Nally, R., Dr Vries, L., and Thomson, J. R. (2010). Are replanted floodplain forests in southeastern Australia providing bird biodiversity benefits. Restoration Ecology 18, 85–94. doi:10.1111/j.1526-100X.2008.00430.x
- Mac Nally, R., Bowen, M., Howes, A., McAlpine, C. A., and Maron, M. (2012). Despotic, high-impact species and the subcontinental scale control of avian assemblage structure. Ecology 93, 668–678. doi:10.1890/10-2340.1
- Major, R. (2010). Fragmentation responses of birds, insects, spiders and genes – Diverse lessons for woodland conservation. In ‘Temperate Woodland Conservation and Management.’ (Eds D. B. Lindenmayer, A. F. Bennett, and R. J. Hobbs.) pp. 199-208. (CSIRO Publishing: Melbourne.)
- Manning, A. D., Fischer, J., and Lindenmayer, D. B. (2006). Scattered trees are keystone structures - implications for conservation. Biological Conservation 132, 311–321. doi:10.1016/j.biocon.2006.04.023
- Manning, A. D., Gibbons, P., Fischer, J., Oliver, D., and Lindenmayer, D. B. (2013). Hollow futures? Tree decline, lag effects and hollow-dependent species. Animal Conservation 16, 395–403. doi:10.1111/acv.12006
- Maron, M., Grey, M. J., Catterall, C. P., Major, R. E., Oliver, D. L., Clarke, M. F., Loyn, R. H., et al. (2013). Avifaunal disarray due to a single despotic species. Diversity and Distributions 19, 1468–1479. doi:10.1111/ddi.12128
- Martin, T. G., McIntyre, S., Catterall, C. P., and Possingham, H. P. (2006). Is landscape context important for riparian conservation? Birds in grassy woodland. Biological Conservation 127, 201–214. doi:10.1016/j.biocon.2005.08.014
- Melton, C. B., Reside, A. E., Simmonds, J. S., Mcdonald, P. G., Major, R. E., Crates, R., Catterall, C. P., et al. (2021). Evaluating the evidence of culling a native species for conservation benefits. Conservation Science and Practice 3, e549. doi:10.1111/csp2.549
- Michael, D. R., Wood, J. T., Crane, M., Montague-Drake, R., and Lindenmayer, D. B. (2014). How effective are agri-environment schemes for protecting and improving herpetofaunal diversity in Australian endangered woodland ecosystems?. Journal of Applied Ecology 51, 494–504.
- Michael, D., and Lindenmayer, D. B. (2018). ‘Rocky Outcrops in Australia.’ (CSIRO Publishing: Melbourne.)
- Montague-Drake, R. M., Lindenmayer, D. B., and Cunningham, R. B. (2009). Factors affecting site occupancy by woodland bird species of conservation concern. Biological Conservation 142, 2896–2903. doi:10.1016/j.biocon.2009.07.009
- Montague-Drake, R., Lindenmayer, D. B., Cunningham, R. B., and Stein, J. (2011). A reverse keystone species affects the landscape distribution of woodland avifauna: A case study using the Noisy Miner (Manorina melanocephala) and other Australian birds. Landscape Ecology 26, 1383–1394. doi:10.1007/s10980-011-9665-4
- Mortelliti, A., Ikin, K., Tulloch, A. I. T., Cunningham, R., Stein, J. A. R., Michael, D., and Lindenmayer, D. B. (2016). Surviving with a resident despot: Do revegetated patches act as refuges from the effects of the noisy miner (Manorina melanocephala) in a highly fragmented landscape? Diversity and Distributions 22, 770–782. doi:10.1111/ddi.12444
- Munro, N., Lindenmayer, D. B., and Fischer, J. (2007). Faunal response to revegetation in agricultural areas of Australia: A review. Ecological Management & Restoration 8, 199–207. doi:10.1111/j.1442-8903.2007.00368.x
- Norton, D., and Reid, N. (2013). ‘Nature and Farming.’ (CSIRO Publishing: Melbourne.)
- O’Loughlin, T., O’Loughlin, L. S., Michael, D. R., Wood, J. T., Waudby, H. P., Falcke, P., and Lindenmayer, D. B. (2017). The importance of travelling stock reserves for maintaining high-quality threatened temperate woodlands. Australian Journal of Botany 65, 507–516. doi:10.1071/BT17114
- Okada, S., Lindenmayer, D. B., Wood, J. T., Crane, M. J., and Pierson, J. C. (2016). How does a transforming landscape influence bird breeding success? Landscape Ecology 32, 1039–1048. doi:10.1007/s10980-017-0507-x
- Olsen, P., Fuller, P., and Marples, T. G. (1993). Pesticide-related eggshell thinning in Australian raptors. Emu - Austral Ornithology 93, 1–11. doi:10.1071/MU9930001
- Possingham, H. P., and Nix, H. A. (2008). ‘The Long Paddock Scientists’ Statement.’ 6. http://www.wilderness.org.au/files/long-paddock-scientists-statement.pdf
- Prober, S., and Thiele, K. R. (1995). Conservation of the Grassy White Box Woodlands: Relative contributions of size and disturbance to floristic composition and diversity of remnants. Australian Journal of Botany 43, 349–366. doi:10.1071/BT9950349
- Radford, J. Q., Bennett, A. F., and Cheers, G. J. (2005). Landscape-level thresholds of habitat cover for woodland-dependent birds. Biological Conservation 124, 317–337. doi:10.1016/j.biocon.2005.01.039
- Radford, J. Q., Amos, V., Harrisson, K., Sunnucks, P., and Pavlov, A. (2021). Functional connectivity and population persistence in woodland birds: Insights for management from a multi-species conservation genetics study. Emu - Austral Ornithology 121, 147–159. doi:10.1080/01584197.2021.1903331
- Recher, H. F. (1999). The state of Australia’s avifauna: A personal opinion and prediction for the new millenium. Australian Zoologist 31, 11–29. doi:10.7882/AZ.1999.003
- Sato, C. F., Florance, D., and Lindenmayer, D. B. (2019). Drivers of temperate woodland condition through time in an agricultural landscape. Land Degradation & Development 30, 1357–1367. doi:10.1002/ldr.3325
- Selwood, K., Mac Nally, R., and Thomson, J. R. (2009). Native bird breeding in a chronosequence of revegetated sites. Oecologia 159, 435–446. doi:10.1007/s00442-008-1221-9
- Selwood, K. E., Clarke, R. H., Cunningham, S. C., Lada, H., McGeoch, M. A., and Mac Nally, R. (2015). A bust but no boom: Responses of floodplain bird assemblages during and after prolonged drought. Journal of Animal Ecology 84, 1700–1710. doi:10.1111/1365-2656.12424
- Spooner, P. G., and Briggs, S. V. (2008). Woodlands on farms in southern New South Wales: A longer-term assessment of vegetation changes after fencing. Ecological Management & Restoration 9, 33–41. doi:10.1111/j.1442-8903.2008.00385.x
- Sunnucks, P. (2016). Towards modelling persistence of woodland birds: The role of genetics. Emu - Austral Ornithology 111, 19–39. doi:10.1071/mu10008
- Tulloch, A. I. T., Mortelliti, A., Kay, G., Florance, D., and Lindenmayer, D. B. (2016). Using empirical models of species colonization under multiple threatening processes to identify complementary threat-mitigation strategies. Conservation Biology 30, 867–882. doi:10.1111/cobi.12672
- van Dijk, A. I., Beck, H. E., Crossbie, R. S., de Jeu, R. A., Liu, Y. Y., Podger, G. M., Timbal, B., et al. (2013). The Millennium Drought in southeast Australia (2001-2009); natural and human causes and implications for water resources, ecosystems, economy, and society. Water Resources Research 49, 1040–1057. doi:10.1002/wrcr.20123
- Vesk, P., Nolan, R., Thomson, J. W., Dorrough, J. W., and Mac Nally, R. (2008). Time lags in the provision of habitat resources through revegetation. Biological Conservation 141, 174–186. doi:10.1016/j.biocon.2007.09.010
- Walker, J., Bullen, F., and Williams, B. G. (1993). Ecohydrological changes in the Murray-Darling Basin. I. The number of trees cleared over two centuries. Journal of Applied Ecology 30, 265–273. doi:10.2307/2404628
- Walters, J. R., Ford, H. A., and Cooper, C. B. (1999). The ecological basis of sensitivity of brown treecreepers to habitat fragmentation: A preliminary assessment. Biological Conservation 90(1), 13–20.
- Ward, M. S., Simmonds, J. S., Reside, A. E., Watson, J. E. M., Rhodes, J. R., Possingham, H. P., Trezise, J., et al. (2019). Lots of loss with little scrutiny: The attrition of habitat critical for threatened species in Australia. Conservation Science and Practice 1, doi:10.1111/csp2.117
- Watson, S. J., Luck, G. W., Spooner, P. G., and Watson, D. M. (2014). Land-use change: Incorporating the frequency, sequence, time span, and magnitude of changes into ecological research. Frontiers in Ecology and the Environment 12, 241–249. doi:10.1890/130097
- Westgate, M. J., Barton, P. S., Lane, P. W., and Lindenmayer, D. B. (2014). Global meta-analysis reveals low consistency of biodiversity congruence relationships. Nature Communications 5, 3899.
- Westgate, M. J., Crane, C., Smith, D., O’Malley, C., Siegrist, A., Florance, D., Lang, E., Hingee, K., Scheele, B. C., and Lindenmayer, D. B. (2021a). Improved management of farm dams increases vegetation cover, water quality and macroinvertebrate biodiversity. Ecology and Evolution 12, e8636. doi:10.1002/ece3.8636
- Westgate, M. J., Mason, M., Florance, D., and Lindenmayer, D. B. (2021b). Synergistic impacts of aggressive species on small birds in a fragmented landscape. Journal of Applied Ecology 58, 825–835. doi:10.1111/1365-2664.13838
- Williams, J., and Price, R. J. (2011). Impacts of red meat production on biodiversity in Australia: A review and comparison with alternative protein production industries. Animal Production Science 50, 723–747. doi:10.1071/AN09132
- Wintle, B. A., Kujala, H., Whitehead, A., Cameron, A., Veloz, S., Kukkula, A., Moilanen, A., et al. (2019). Global synthesis of conservation studies reveals the importance of small patches for biodiversity. Proceedings of the National Academy of Sciences 116, 909–914. doi:10.1073/pnas.1813051115
- Yong, D. L., Barton, P. S., Ikin, K., Evans, M. J., Crane, M., Okada, S., Cunningham, S. A., et al. (2018). Cross-taxonomic surrogates for biodiversity conservation in human-modified landscapes – A multi-taxa approach. Biological Conservation 224, 336–346. doi:10.1016/j.biocon.2018.06.008
- Zanette, L., Doyle, P., and Tremont, S. M. (2000). Food shortage in small fragments: Evidence from an area-sensitive passerine. Ecology 81, 1654–1666. doi:10.1890/0012-9658(2000)081[1654:FSISFE]2.0.CO;2
