ABSTRACT
Australia’s Red Goshawk (Erythrotriorchis radiatus) is a taxonomically distinct raptor endemic to the tropics and sub-tropics of eastern and northern Australia, and the Australian mainland’s rarest bird of prey. Classified as Vulnerable when legislation was first enacted in 1992, the species’ status and distribution remain unclear, and it is possibly declining based on limited surveys. However, no comprehensive analysis of its range-wide population trends has ever been undertaken, creating a knowledge gap which potentially delays urgent conservation management. Here, we bridge that knowledge gap. We compile a comprehensive dataset of 1,679 occurrence records spanning the species’ historical range, develop a novel method that overcomes reporting biases centred around nest locations, then identify population trends between 1978 and 2020 at national, state, and regional scales. Our results suggest that the species has declined significantly across eastern Australia and is likely locally extinct in many regions. We estimate the Red Goshawk has disappeared from 34% of its breeding range over the last four decades, and probably persists at extremely low density, if at all, over an additional 29.7% of its breeding range. These results demonstrate the species’ declining population trajectory at multiple scales for the first time and provide further evidence for its up listing under Australian federal legislation to Endangered, using IUCN Red List criterion C2a(ii): small population size and decline. We recommend population surveys and monitoring coupled with targeted research to better understand population trajectories and determine which threats are driving this unique species’ decline.
Introduction
Raptors are disproportionately threatened and declining worldwide when compared to other bird orders (McClure & Rolek, Citation2018). Raptors generally occur at low population densities, have large ranges, and are often found in remote and rugged places (Bildstein and Bird Citation2007). These factors ensure raptor research is challenging and may explain why many species are under-studied, and the conservation outlook of many species unknown (Buechley et al. Citation2019). As top-order predators and scavengers, raptors play a keystone role in shaping their environment. Their predatory habits can regulate and affect the distribution of prey populations (Abramsky et al. Citation2002; Ydenberg et al. Citation2017) and influence the structure of species assemblages (Ogada et al. Citation2012; Buechley and Şekercioğlu Citation2016). Raptors also serve as bio-indicators of wider environmental health (Espín et al. Citation2016; Thompson et al. Citation2021) and high biodiversity areas (Sergio et al. Citation2006), and may provide early warning of ecosystem-wide problems. For example, the detection of organochloride pesticide induced egg-shell thinning in raptors in the 1960s famously alerted scientists to widespread chemical pollution and resulting avian declines (Hickey Citation1969; Ratcliffe Citation1970).
Australia’s Red Goshawk (Erythrotriorchis radiatus) is a taxonomically distinct raptor endemic to the tropics and sub-tropics of eastern and northern Australia. One of only two species of its genus, it exhibits among the highest rates of reverse-size sexual-dimorphism of any raptor in the world, with females 1.9 times heavier than males (mean mass 1125 g cf. 597 g, respectively) (Ligouri et al. Citation2020; MacColl et al. Citation2022). The species feeds almost exclusively on other birds, with a preference for kookaburras (Dacelo) and parrots including lorikeet (Loriini) and cockatoo (Cacatuidae) species (Aumann and Baker-Gabb Citation1991). Long considered the Australian mainland’s rarest bird of prey (Slater Citation1978; Cupper and Cupper Citation1981; Hollands Citation2003), it was listed as Vulnerable under the Endangered Species Protection Act 1992 (CitationCth), a status it maintained when included on the inaugural Environment Protection and Biodiversity (EPBC) Act 1999 (CitationCth) List of Threatened Fauna in 2000. The legislative status of the species has remained unchanged since (Australian Department of Agriculture Water and the Environment Citation2022). However, following a review of research suggesting local declines, particularly in the southeast of the species’ range (Debus Citation1993; Seaton Citation2014), the Red Goshawk was recently up listed to Endangered on the IUCN Red List (MacColl et al. Citation2021). However, this recent review potentially overestimated the population at 900 to 1400 mature individuals, as it assumed a constant population spread across most of the species’ historical range; subsequent research suggests local extirpations may be more widespread than first thought (Noske and Briggs Citation2021). The results of these regional studies highlight the need for a range-wide analysis of population trends and range patterns, in an effort to quantify its population status and current range.
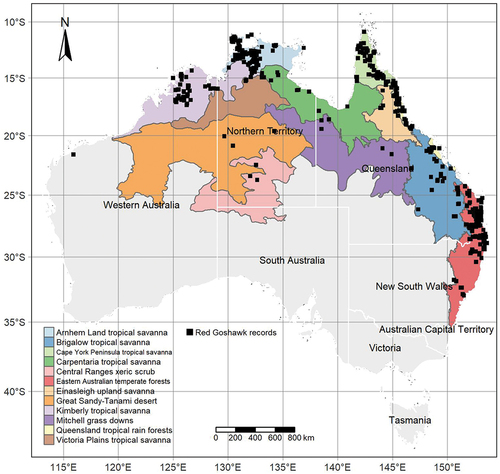
The Red Goshawk’s historical range covered much of the eastern and northern parts of the continent, where it was typically found within a few hundred kilometres of the coast. It was one of the first raptors encountered by Europeans, when a specimen was found nailed to an early settler’s hut at Botany Bay around 1790 (Debus et al. Citation1993). It has primarily been found in the tall woodland and open forest ecosystems along the coastal fringe of eastern and northern Australia. The known breeding range once extended from northern New South Wales to the Kimberley region of Western Australia (Marchant and Higgins Citation1993). Occasional records from the semi-arid and arid interior ensures its total geographic range remains far wider and includes parts of central and western Australia (Hall Citation1974; Aumann Citation1999). However, these environments are not currently considered to form part of the breeding range (Debus et al. Citation1993; Marchant and Higgins Citation1993) despite the presence of some questionable historical records suggesting breeding may occur there (Whitlock Citation1924; Debus et al. Citation1993). It is likely that these regions only support seasonal visitors and dispersing juveniles (MacColl et al. Citation2021). Although it has a broad distribution, the details of this distribution remain poorly known when compared to other Australian raptors. For example, it has the lowest reporting rate of any Australian raptor found in continent-scale census data (Blakers et al. Citation1984; Baker-Gabb and Steele Citation1999; Barrett et al. Citation2003). There is limited area-specific population information, and a significant knowledge gap around the species’ population status and trajectory outside a few well-monitored parts of its range, particularly the south-east (e.g. Czechura et al. Citation2011; Seaton Citation2014). Without this information, policy makers and managers cannot make informed decisions to support the species’ long-term conservation, including appropriate designation of its conservation status and extinction risk (Mace et al. Citation2008) or identification of important areas and populations for conservation planning (Cruz et al. Citation2021).
Here, we conduct the first comprehensive evaluation of the Red Goshawk’s status and distribution across its range, a critical first step in assessing its true extinction risk and need for conservation attention. We compile a comprehensive dataset of all Red Goshawk occurrence records spanning the species’ range. We analyse these data for patterns which might serve as indicators of population trends in the species’ range and status at national, state, and regional scales. Using multiple datasets, we employ a novel method that overcomes issues of data quality that arise when combining datasets. We discuss the implications of these results for the species’ conservation outlook and identify remaining knowledge gaps that require further research.
Methods
Data collation
Red Goshawk occurrence records were obtained from numerous government and non-government sources and merged into one consolidated dataset. Key variables were extracted for all records from these source databases including: date, year, observer, count, locality, latitude, longitude, sighting notes, and source database. Observations completely lacking either spatial or temporal information were removed. Based on the temporal distribution of the data, and the most recent year complete with all available records across all the source databases, our analysis was confined to the period 1978 to 2020. The year 1978 was chosen as there was a clear increase in the frequency of records in that year; data before that year were limited and inconsistent in quality.
There were numerous records duplicated between the source databases. We also discovered that data from about 2009 onwards contained a high reporting bias to single localities known to support nesting birds. Both duplicate records, and the multiple records associated with the same nesting pair, often contained varying spatial locations, creating a cluster of records around a sighting location. This clustering seemed to occur for two reasons. First, records of some threatened species, especially when breeding, are often ‘buffered’ (i.e. a general rather than precise location is allocated by the database so as not to reveal information that may lead to unnecessary disturbance). However, because buffering policies differ between databases, records for the same geographic location can be assigned different geographic locations, including the same records duplicated between the source databases. Second, for sites where there are repeated observations (such as at nest sites), duplication can occur when different observers provide a different location, or approximate location, for the same nest, often with varying degrees of precision, which leads to a cluster. In at least six locations this pattern of clustering was observed around a known nest site.
To minimise the bias these records create in our consolidated dataset, it was essential to standardise our data to avoid inflating reporting trends. We applied a filter to select records containing unique latitude, longitude, and year of observation values in order to standardise our data to one record per year per location. However, given the clustered nature of records spread around these nest locations, coupled with an otherwise widely distributed pattern of records, we had to determine a biologically appropriate spatial scale from which to standardise these records over the entire dataset. Most raptors are territorial, with territory size positively correlated with the level of agility and body mass of their prey (Newton Citation1979; Martínez-Hesterkamp et al. Citation2018). The spacing of territories is influenced by the availability and distribution of suitable nest sites (Both and Visser Citation2003), resulting in a typically non-random, predictable spacing of breeding territories across a landscape (Nilsson et al. Citation1982). Furthermore, Red Goshawks fit the model of territoriality in raptors with medium-large sized birds their common prey, and having rather specific nesting requirements including tall tree stands in proximity to water (Aumann and Baker-Gabb Citation1991; Czechura et al. Citation2011). For these reasons, multiple records in an area surrounding an active nest are very likely to only represent the same resident breeding individuals and their offspring. To avoid over-reporting of these same individuals and stratify our analysis so it aligns with how populations are likely to be dispersed, we chose to standardise our data over 0.1 decimal degree cells, which is equivalent to an area of approximately 11.1 km2. While inter-pair distances of between 10 and 20 km apart have been used to estimate population size across the species range (Garnett et al. Citation2012), higher densities have been reported in northern Australia, with inter-pair distances of between 5 and 8 km recorded (n = 3) (MacColl Citation2022b). Therefore, we believe a 0.1 decimal degree standard cell size is a reasonable, and biologically relevant unit of stratification from which to standardise our reporting data.
To standardise the data in this way, all latitude and longitude values in the consolidated dataset were converted to decimal degrees, then rounded down to 1 decimal place. This coalesced the location of all records to the nearest 0.1 decimal degree intersect. We then applied a filter to select records containing unique latitude, longitude, and year of observation values. This filter removed duplicate records leaving only one record for each unique latitude, longitude, and year combination over a 0.1 decimal degree grid cell area (Appendix S1).
All remaining observations in the filtered dataset were then annotated by respective State or Territory (Australian Bureau of Statistics Citation2021) and ecoregion (Olson et al. Citation2001) in order to stratify our analysis geographically. Ecoregions combine common landscape-scale habitat types such as tropical savanna or rainforest ecosystems. The ecoregion system was chosen because it was spatially broad enough to group our limited data at a regional level and is used in many similar ecological analyses around the world (e.g. Schmitt et al. Citation2009; Saura et al. Citation2017; Escobar-Luján et al. Citation2022). This maximised our ability to produce interpretable population trends at a scale smaller than state, whilst still retaining a biogeographically relevant unit of stratification.
Data analysis
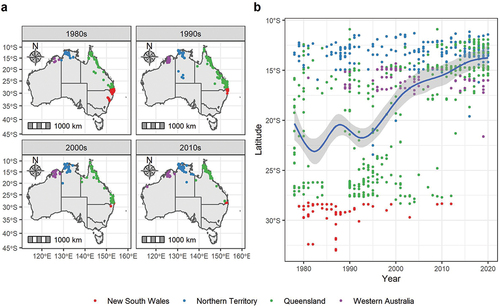
Reporting trends were produced at the national and state scale by plotting the number of observations per year between 1978 and 2020. We also plotted the records spatially onto time-series maps at a national scale stratified across four decades from the 1980s to the 2010s. This allowed us to analyse spatial patterns in reporting and assess any changes in the species’ range over time. Qualitatively, these results suggested a northward range contraction over time. To further investigate this, we used the latitude value of all records over time in a flexible regression model using a natural spline basis with three degrees of freedom. We also fitted a generalised additive model (GAM) where the degree of smoothness is not fixed in advance but estimated by generalised cross validation. This trend allowed us to quantify any northward contraction in the species’ range over time.
Finally, population trends were modelled at the regional scale by fitting a Poisson log-linear mixed effects model with random intercepts and slopes for ecoregion. We modelled the log mean number of records per annum and transformed it back to a frequency scale. The predicted means from this model were used to plot population trend lines along with 95% confidence intervals across the ecoregions comprising the species range between 1978 and 2020.
All analyses were performed in R (R Core Team Citation2021) with plots produced using the packages ggplot2 (Wickham Citation2016) and patchwork (Pedersen Citation2020). All maps were produced using the packages sf (Pebesma Citation2018) and tmap (Tennekes Citation2018). Population models were produced using the glmmTMB package (Brooks et al. Citation2017).
Results
Data compilation and filtering
We compiled a total of 1,679 Red Goshawk occurrence records from the following sources: Atlas of Living Australia (n = 836) (Atlas of Living Australia Citation2022), Australian Wildlife Conservancy (n = 3), Birdlife Australia (n = 348) (Birdlife Australia Citation2021), New South Wales Bird Atlassers (n = 1) (New South Wales Bird Atlassers Inc., Citation2021), Mr. George Swann (n = 51), Red Goshawk Recovery Team including State and Territory representatives (n = 264), and the Red Goshawk Research Project (n = 176) (). The data filtering reduced the total number of records from 1,465 to 742 in the consolidated 1978 to 2020 dataset. This process saw a noticeable decrease in reporting trends between the unfiltered and filtered datasets (Appendix S2), particularly over the last decade when reporting biases to single localities (i.e. nest sites) appeared highest. Of the 723 records removed, 9.8% were from the 1980s, 17.7% were from the 1990s, 25.3% were from 2000s, and 42% were from the 2010s. By modifying the contemporary data more so than the historical data, our method demonstrates that records for this species have become more concentrated in recent times.
National and state scale trends
The reporting trend at the national scale shows some variability through time although the highest number of annual records occurred during the last 5 years of the study period (2016 to 2020) (). There are apparent lulls in reporting during the mid-1980s and early 2000s. The peak in annual observations occurred in 1990, but this appears to be an outlier over the 42 year period and occurred primarily due to two overlapping survey efforts (Aumann and Baker-Gabb Citation1991; Czechura and Czechura Citation1994).
Figure 2. Red Goshawk reporting trends between 1978 – 2020. a) National scale with bars in-filled proportionately by State records. b) New South Wales. c) Queensland. d) Northern Territory. e) Western Australia.
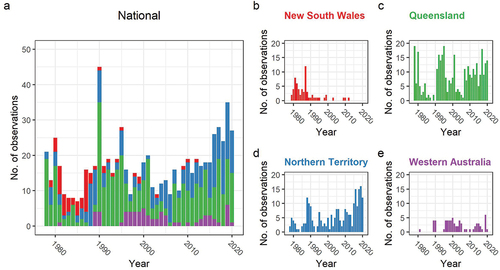
It appears New South Wales has suffered a dramatic loss over a short period time. During the 1980s, it represented a high proportion of the reporting data (). Records from this state decreased dramatically thereafter with annual records largely ceasing by the mid-1990s, with only a handful of sporadic records since 2000 (). The Queensland reporting trend has shown variability through time, with a consistent positive reporting trend since around 2007 ().
In contrast, records from the Northern Territory have proportionally increased at the national scale and absolutely increased at the state scale particularly over the past five years (). From 2016 to 2020, the Northern Territory recorded its highest reporting period, with the next highest occurring in the late 1980s when a targeted research program was underway there (Aumann and Baker-Gabb Citation1991). The number of records from Western Australia have historically been low with consistently fewer than five observations per annum and long periods with no records at all (). However, it appears the species has been more consistently reported since 1995 albeit at low numbers.
We see a decline in the spatial distribution of records by decade () with the species’ range showing a clear trend towards records from higher latitudes over the study period (). This northward shift begins around the late 1990s and has continued unabated, with reporting of the species now essentially limited to northern Australia. This trend shows an ongoing decline culminating in the 2010s, from when there are significantly fewer records throughout eastern Australia compared to earlier decades. Records from the Northern Territory and Western Australia have remained consistently within the northern parts of those two states where the species is more commonly known to occur. However, outlier records from the interior of the Northern Territory and the far west of Western Australia have also been periodically reported.
Ecoregion scale trends
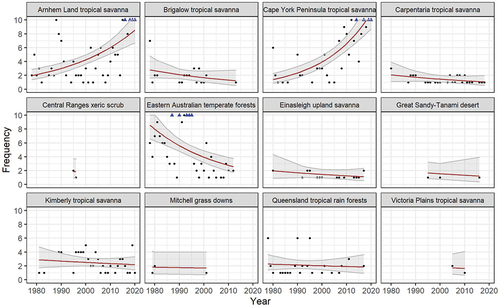
We found that population trends from most ecoregions across the species range reveal a pattern of decline, particularly across eastern Australia (). They show catastrophic declines in the Eastern Australian Temperate Forests and the adjoining Brigalow Tropical Savanna ecoregion where population trends have plummeted over recent decades. Declines are also evident though less pronounced throughout the Queensland Tropical Rainforests, Einasleigh Uplands Savanna, Carpentaria Tropical Savanna, and Kimberley Tropical Savanna ecoregions. In contrast, the Cape York Peninsula and Arnhem Land Tropical Savanna ecoregions both show increasing trendlines. Trends from more inland regions including the Mitchell Grass Downs, Victoria Plains Tropical Savanna, and Great Sandy-Tanami Desert ecoregions are difficult to discern due to limited data availability. The Pilbara Shrublands and Southeast Australia Temperate Savanna ecoregions each contained just a single record of this species and so were removed from the final analyses. Based on ecoregions where the species was known to breed, this analysis suggests the Red Goshawk has disappeared from 34% of its former range, represented by the Eastern Australian Temperate Forests and Brigalow Tropical Savanna ecoregions, as well as the Shoalwater Bay and Mackay/Eungella areas of the Queensland Tropical Rainforests ecoregion. There have been significant declines, and the species likely persists only at extremely low population densities, if at all, over an additional 29.7% of its former breeding range, including the Carpentaria Tropical Savanna, Einasleigh Upland Savanna, and the Wet Tropics area of the Queensland Tropical Rainforests ecoregion.
Figure 4. A Poisson log-linear mixed effects model using the frequency of Red Goshawk reports each year between 1978 – 2020, with the mean (red line) illustrating the population trend, and the shaded area illustrating the 95% confidence interval. Y scales are fixed on a 0 – 10 range with frequencies >10 indicated by blue triangles.
Discussion
This research presents the most comprehensive national assessment of the Red Goshawk’s status and range to date, and clearly shows significant population decline throughout most of the species’ eastern Australian range. The species has disappeared from 34% of its breeding range, and probably persists at extremely low density, if at all, over an additional 29.7% of its breeding range. These catastrophic population declines have occurred over large areas, leaving the species’ status in a perilous state across a number of regions including many not previously considered under threat (e.g. Carpentaria and Einasleigh Tropical Savannas). This trend is most pronounced throughout the species’ range in New South Wales and central and southern Queensland, where contemporary records now appear limited to only the northern-most parts of the latter state. This range collapse is more extensive than recent research has suggested and has resulted in the likely extirpation of breeding populations at regional and state scales. The New South Wales population is almost certainly extinct, and the Queensland population appears to have been lost from more than half its range in the last decade.
Range and status
Widespread and precipitous decline of the Red Goshawk population throughout eastern Australia is clear, with population trends for the Eastern Australian Temperate Forests, and Brigalow Tropical Savanna ecoregions, both in coastal and sub-coastal eastern Australia, falling significantly. It appears likely the species is already extirpated from these regions, if it is not persisting at critically low levels. Local surveys to date support this interpretation; the species is thought to be extirpated from the Rockhampton and Eungella regions, both on the coast of north-eastern Australia (Leach, Daly and Burwell Citation2020, Noske and Briggs Citation2021). However, observations were made in these regions during targeted surveys in 1999 by Czechura et al. (Citation2011), leaving the possibility of targeted search effort detecting remnant populations despite the declining population trend. Such inferences would be better supported with targeted survey effort in suitable and potentially overlooked parts of these large regions, and any remaining isolated populations should be prioritised for immediate conservation action.
Population trends from the far southeast of the species’ range, including the Eastern Australian Temperate Forests, are likely to represent the species’ true regional status. This area encompasses the south-east Queensland and northern New South Wales regions, which supports some of the highest bird watching search effort in Australia. For example, the Brisbane area has the highest number of eBird checklists recorded nationally (eBird Citation2022b). Although recreational bird watching is not as effective as targeted survey effort, the absence of any records over a prolonged period of time when compared to the consistency of earlier reporting, strongly suggests the species is now locally extinct.
Population trends from the northeast and northwest of the species’ range – the Queensland Tropical Rainforests, Einasleigh Upland Savanna, Carpentaria Tropical Savanna, and Kimberley Tropical Savanna ecoregions – also showed declines, although these were only gradual. The lack of records and general survey effort from these areas means it is difficult to be confident in the direction of these trends. These potential declines across the more intact parts of the species’ northern range are concerning though, especially given the losses observed elsewhere.
Our analyses confirm that the species’ stronghold is now far northern Australia, an area now critically important to its conservation. Specifically, the Cape York Peninsula and Arnhem Land Tropical Savanna ecoregions are the only remaining areas to show positive population trends for this species. In fact, reporting trends have increased here, but we attribute this largely to better knowledge of breeding sites and subsequent visitation by bird watchers as the Red Goshawk is a highly desirable, yet difficult bird to see. The emergence of online citizen science projects such as eBird (eBird Citation2022a) has also increased the likelihood and capacity of record capture which likely further contributes to the higher rates of contemporary reporting. These trends could indicate a stable Red Goshawk population, as does the existence of a well-monitored population on the Tiwi Islands, part of the Arnhem Land Tropical Savanna, with an apparently stable fledging rate (MacColl et al. Citation2021). However, it is also possible that declines could be occurring that are undetected due to the focus on just a handful of nest sites. Further research is required to be satisfied of the species’ current status across northern Australia, which now appears integral to its conservation, with a significant proportion of the extant breeding population likely supported here. Some regions in northern Australia remain data deficient including the Einasleigh Uplands Savanna and the Carpentaria Tropical Savanna where breeding populations may persist but for which there is no available information.
The emergence of reliable records from the semi-arid and arid zones is a fascinating development given 97.9% of our data is located along Australia’s coastal fringe. There is a paucity of information regarding the species’ status and use of these environments. Central Australia and the Pilbara are regions now known to support the species at certain times of the year, with the latter occurrence extending the known species range more than 500 km south from the Kimberley region in far north-western Australia. Both adult and juvenile birds have been recorded in these environments, and while the timing and nature of these detections suggest birds are passing through, whether this constitutes part of the breeding range or is only used seasonally, remains unknown.
Conservation implications
Our findings support calls for the Red Goshawk’s Australian conservation status to be up listed from Vulnerable to Endangered under the EPBC Act 1999 (MacColl et al. Citation2021). The IUCN recently adopted this status change, formally recognising the Red Goshawk as an Endangered species globally (BirdLife International Citation2022). Due to its small population size (250–2500 mature individuals), single interconnected population (MacColl et al. Citation2021), and evidence of a declining population trend, the species was eligible for up listing under criteria C2a(ii). This research has shown that declining population trend is more severe than first thought, further supporting this status change. As the IUCN listing criteria mirrors the system used under Australian law, and given the Red Goshawk is endemic to Australia, these two classifications should align.
The reasons for the Red Goshawk’s decline are unclear. If these results accurately reflect the loss of this species over such an immense scale, and with relative rapidity, then it must be considered among the greatest range reductions of any mainland Australian bird species. While it is possible eastern Australia was never the species’ stronghold, the vast area of range loss still suggests a significant proportion of the total population has disappeared, even allowing its occurrence at very low densities. Similarly dramatic declines have been observed in the woodland bird communities of eastern Australia, including species that historically co-occurred with the Red Goshawk, and in some cases still do. The southern Star Finch (Neochmia ruficauda ruficauda) and Paradise Parrot (Psephotellus pulcherrimus) are both now considered extinct from eastern Australia, while the southern Black-throated Finch (Poephila cincta cincta) and southern Squatter Pigeon (Geophaps scripta scripta) have undergone significant population declines and range contractions (Buiosi et al. Citation2021; Garnett and Todd Citation2021; Olsen and Garnett Citation2021; Ward et al. Citation2021). These declines are thought to be the result of land clearing (Fraser, Simmonds, Kutt, & Maron, Citation2019), a threatening process that could also have impacted the Red Goshawk, either directly, or through secondary impacts such as prey availability. On Cape York Peninsula, the Gouldian Finch (Erythrura gouldiae), Golden-shouldered Parrot (Psephotellus chrysopterygius) and Buff-breasted Button-quail (Turnix olivii) are all woodland species that have undergone significant declines (Franklin Citation1999; Golden-shouldered Parrot Recovery Team Citation2021; Webster et al. Citation2021). Rather than outright habitat loss, the declines in these northern species are thought to be the result of habitat change due to altered fire regimes and associated changes in vegetation composition and structure. It is not clear whether these same processes are affecting the Red Goshawk. However, the rapid contraction in the southeast of the species’ range, and potentially ongoing declines in the north, suggest that whatever threatening process is driving these declines is ongoing.
Research recommendations
The conservation of this species now appears heavily reliant, if not dependent, upon northern Australia. The precautionary principle dictates that ongoing population monitoring should be undertaken so that population trends can be tracked, such as reporting trends, territory occupancy, and breeding performance. Such information can provide early warning of changing trajectories so that conservation action can be proactively applied. Species lacking this type of data are much more likely to become extinct (Borgelt et al. Citation2022). Knowledge of breeding territories and respective nest sites will be required in order to build a network of monitoring sites. Targeted surveys and reporting of observations by citizen scientists will have an important role to play.
Ecoregions suspected of supporting breeding populations but for which we have no information (e.g. Einasleigh Uplands Savanna and Carpentaria Savanna Tropical Savanna) should be targeted so questions regarding the species’ current range and breeding population can be addressed. In-fill surveys of the Brigalow Tropical Savanna and Queensland Tropical Rainforests will also help clarify our current assessment suggesting the species’ likely extirpation. This information will improve our assessment of extinction risk and contribute to more effective conservation planning.
The most pressing research needed is determining what the underlying causes are driving this species’ decline. The spatial and temporal scale of this decline suggests the threats are wide-ranging and pervading. Given most declines have been centred on eastern Australia, habitat loss is a likely driver, because this region is less intact and more degraded compared to other parts of the species’ range (Beyer et al. Citation2020). However, ∼91% of suitable habitat was found to still be available in the species’ former range, and 73% available in its current range (Ward et al. Citation2022). Although our understanding of habitat requirements and what constitutes ‘suitable habitat’ requires further work, these findings suggest habitat loss is not the primary driver, but perhaps compounded by habitat change. In either circumstance, this research does reinforce the need to maintain as much good quality habitat as possible in the species’ strongholds on Cape York Peninsula and in the Arnhem Land Tropical Savannas. Regulators considering developments in these regions should be keenly aware of the impact landscape scale habitat loss could have on this species.
Woodland thickening has been implicated in the decline of grassland specialists such as the Golden-shouldered Parrot (Golden-shouldered Parrot Recovery Team Citation2021). Whilst not a grassland species, Red Goshawks require a level of openness within the woodland ecosystems they inhabit in order to effectively navigate and hunt (Debus and Czechura Citation1988). As a long-winged raptor, and largest Australian ‘hawk’, a densely vegetated under-storey could potentially impact their hunting success and make prey less available to them. Eastern Australia is heavily wooded, and the greatest declines have been observed within the Eastern Australian Temperate Forests ecoregion. Determining whether woodland thickening and other environmental change has occurred, how it relates to various land-use regimes (i.e. habitat fragmentation, fire history, grazing pressure, etc.), and what effect these degradations can have on prey populations and their availability to Red Goshawks, is an obvious and prudent focus for research at both currently and formerly occupied sites.
Another pervasive threat may be a pathogen or contamination-related issue. It is emerging that many non-parrot species, including raptors, are susceptible to Beak and Feather Disease Virus (BFDV) (Amery-Gale et al., Citation2017; Sarker et al. Citation2016; Raidal and Peters Citation2018) with the virus detected in 23 of 50 species tested (Amery-Gale et al., Citation2017). In parrots, chronic cases can lead to significant feather dystrophy, skin lesions, and beak and feet deformities, though most hosts show no symptoms (Raidal and Peters Citation2018; Martens et al. Citation2020). Acute cases are most fatal to nestlings and fledglings with susceptibility apparently age related (Wylie and Pass Citation1987; Raidal and Peters Citation2018). A dead nestling Red Goshawk tested positive to BFDV in 2019 and preliminary surveillance has also detected the virus in two living adult females (MacColl Citation2022a). Both birds appear unaffected and have successfully produced young over the last 1–2 breeding seasons. Further investigation is needed to understand how this virus may impact the species, particularly as BFDV is endemic, and has probably been present in the dietary species of Red Goshawks for some time. Further surveillance of BFDV and other potential sources of disease and contamination is recommended targeting a wider sample of Red Goshawk populations.
Conclusion
The Red Goshawk has apparently undergone a significant range collapse over at least the past four decades. Having all but disappeared from eastern Australia, the species’ breeding range has contracted to northern Australia, a region always important for the Red Goshawk, but which is now critical for its conservation. These declines have occurred over a relatively short time span, which is alarming given the large geographical scale at which they have occurred. These declines suggest up-listing the species’ conservation status under federal legislation to Endangered is warranted. More alarming is that the threats which have driven this devastating decline are unclear and are probably ongoing. To help avoid further declines, research is urgently needed to establish what threats are driving the widespread and precipitous decline of this endemic raptor, and how those threats can be managed, to arrest and hopefully reverse this ongoing range collapse. Targeted surveys and proactive population monitoring are also required to better understand the actual range and status of the population and its regional trajectories, and to ensure that any efforts to save the species are effective.
Supplemental Material
Download JPEG Image (151.5 KB)Supplemental Material
Download JPEG Image (191.3 KB)Acknowledgments
The authors would like to acknowledge all those people who contributed their records of Red Goshawks to the databases used in this analysis. The authors would like to particularly acknowledge the works of Greg Czechura, Rod Hobson, David Stewart, and George Swann whose individual efforts have resulted in a disproportionate number of the available records on this species. We also thank Bill Venables for his statistical expertise and assistance in conducting the analyses. Finally, we’d like to acknowledge the works of Stephen Debus, Tom Aumann, and David Baker-Gabb who have contributed much to our current state of knowledge on this rare and difficult species.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed at https://doi.org/10.1080/01584197.2023.2172735
References
- Abramsky, Z., Rosenzweig, M. L., and Subach, A. (2002). The costs of apprehensive foraging. Ecology (Durham) 83(5), 1330–1340. doi:10.1890/0012-9658(2002)083[1330:TCOAF]2.0.CO2
- Amery-Gale, J., Marenda, M. S., Owens, J., Eden, P. A., Browning, G. F., and Devlin, J. M. (2017). A high prevalence of beak and feather disease virus in non-psittacine Australian birds. Journal of Medical Microbiology 66(7), 1005–1013. doi:10.1099/jmm.0.000516
- Atlas of Living Australia (2022). ‘Atlas of living Australia occurrence records download.’ Available at https://doi.org/10.26197/ala.62a9c9c6-298b-4a5b-b1d4-86e5ecd9fc90 [Verified 3rd August 2022].
- Aumann, T. (1999). Riparian raptor assemblages in the Australian arid zone. Doctor of Philosophy Doctoral thesis, Australian National University.
- Aumann, T., and Baker-Gabb, D. J. (1991). The ecology and status of the Red Goshawk in Northern Australia. RAOU Report No. 75. Royal Australasian Ornithologists Union.
- Australian Bureau of Statistics (2021). ‘States and territories - 2021 - shapefile.’ Available at https://www.abs.gov.au/statistics/standards/australian-statistical-geography-standard-asgs-edition-3/jul2021-jun2026/access-and-downloads/digital-boundary-files
- Australian Department of Agriculture Water and the Environment (2022). ‘EPBC ACT list of threatened species [electronic resource].’ (Department of Environment of Agriculture, Water, and the Environment: Canberra, A.C.T.) Available at https://www.environment.gov.au/cgi-bin/sprat/public/publicthreatenedlist.pl
- Baker-Gabb, D. J., and Steele, W. K. (1999). ‘Relative Abundance, Distribution and Seasonal Movements of Australian Falconiformes 1986–1990.’ (Birds Australia: Hawthorn East, Vic.)
- Barrett, G., Silcocks, A., Barry, S., Cunningham, R., and Poulter, R. (2003). ‘The New Atlas of Australian Birds.’ (Royal Australasian Ornothologists Union/Birds Australia: Hawthorn East, Vic.)
- Beyer, H. L., Venter, O., Grantham, H. S., and Watson, J. E. M. (2020). Substantial losses in ecoregion intactness highlight urgency of globally coordinated action. Conservation Letters 13(2), e12692. doi:10.1111/conl.12692
- Bildstein, K. L., and Bird, D. M. (2007). ‘Raptor Research and Management Techniques.’ (Hancock House: Surrey, B.C.)
- Birdlife Australia (2021). ‘Birdata occurrence dataset for Red Goshawk (Erythrotriorchis radiatus). [Verified 4th March 2021].
- BirdLife International (2022). ‘Erythrotriorchus radiatus. The IUCN red list of threatened species: E.T22695699A40347607.’ Available at https://dx.doi.org/10.2305/IUCN.UK.2012-1.RLTS.T22695699A40347607.en. [Verified 15 August 2022].
- Blakers, M., Davies, S. J. J. F., and Reilly, P. N. (1984). ‘The Atlas of Australian Birds.’ (Melbourne University Press: Melbourne, Vic.)
- Borgelt, J., Dorber, M., Høiberg, M. A., and Verones, F. (2022). More than half of data deficient species predicted to be threatened by extinction. Communications Biology 5(1), 679. doi:10.1038/s42003-022-03638-9
- Both, C., and Visser, M. E. (2003). Density dependence, territoriality, and divisibility of resources: From optimality models to population processes. The American Naturalist 161(2), 326–336. doi:10.1086/346098
- Brooks, M. E., Kristensen, K., Benthem, K. J. V., Magnusson, A., Berg, C. W., Nielsen, A., and Bolker, B. M. (2017). glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R Journal 9(2), 378. doi:10.32614/RJ-2017-066
- Buechley, E. R., Santangeli, A., Girardello, M., Montague, H. C. N. C., Oleyar, D., Christopher, J. W. M., and Şekercioğlu, Ç. H. (2019). Global raptor research and conservation priorities: Tropical raptors fall prey to knowledge gaps. Diversity and Distributions 25(6), 856–869. doi:10.1111/ddi.12901
- Buechley, E. R., and Şekercioğlu, Ç. H. (2016). The avian scavenger crisis: Looming extinctions, trophic cascades, and loss of critical ecosystem functions. Biological Conservation 198, 220–228. doi:10.1016/j.biocon.2016.04.001
- Buiosi, P. A., Vanderduys, E. P., Grice, A. C., and Reside, A. E. (2021). Southern Black-throated Finch Poephila cincta cincta. In ‘In the Action Plan of Australian Birds 2020‘. (Eds S. T. Garnett and G. B. Baker.) pp. 786–789. (CSIRO Publishing: Melbourne, VIC.)
- Cruz, C., Santulli-Sanzo, G., and Ceballos, G. (2021). Global patterns of raptor distribution and protected areas optimal selection to reduce the extinction crises. Proceedings of the National Academy of Sciences - PNAS 118(37), 1. doi:10.1073/pnas.2018203118
- Cupper, J., and Cupper, L. (1981). ‘Hawks in Focus: A Study of Australia’s Birds of Prey.’ (Jaclin Enterprises: Mildura, Australia.)
- Czechura, G. V., and Czechura, R. E. (1994). Observations of an aerial display by Red Goshawks. Australian Bird Watcher 15, 325–327.
- Czechura, G. V., Hobson, R., and Stewart, D. A. (2011). Distribution, status and habitat of the Red Goshawk Erythrotriorchis radiatus in Queensland. Corella 35, 3–10.
- Debus, S. J. S., and Czechura, G. V. (1988). The Red Goshawk Erythrotriorchus radiatus: A review. Australian Bird Watcher 12(6), 175–199.
- Debus, S. J. S., McAllan, I. A. W., and Mead, D. A. (1993). Museum specimens of the red goshawk ‘Erythrotriorchis radiatus’. I. annotated list of specimens. The Sunbird: Journal of the Queensland Ornithological Society 23(1), 5–28.
- eBird (2022a). ‘eBird: An online database for bird distribution and abundance [web application].’ Available at http://www.ebird.org
- eBird (2022b). ‘eBird: Number of checklists by Queensland region [web application].’ Available at https://ebird.org/region/AU-QLD/regions?yr=all&m= [Verified 13th August 2022].
- Endangered Species Protection Act 1992 (Cth). http://classic.austlii.edu.au/au/legis/cth/num_act/espa1992282/s15.html
- Environmental Protection and Biodiversity Conservation Act 1999 (Cth). https://www.legislation.gov.au/Details/C2021C00182
- Escobar-Luján, J., Castaño-Quintero, S. M., Villalobos, F., Lira-Noriega, A., Chiappa-Carrara, X., and Yañez-Arenas, C. (2022). Current and future geographic patterns of bird diversity dimensions of the Yucatan Peninsula and their representativeness in natural protected areas. Neotropical Biodiversity 8(1), 242–252. doi:10.1080/23766808.2022.2087282
- Espín, S., García-Fernández, A. J., Herzke, D., Shore, R. F., van Hattum, B., Martínez-López, E., and van den Brink, N. W. (2016). Tracking pan-continental trends in environmental contamination using sentinel raptors-what types of samples should we use? Ecotoxicology (London, England) 25(4), 777–801. doi:10.1007/s10646-016-1636-8
- Franklin, D. C. (1999). Evidence of disarray amongst granivorous bird assemblages in the savannas of northern Australia, a region of sparse human settlement. Biological Conservation 90(1), 53–68. doi:10.1016/S0006-3207(99)00010-5
- Fraser, H., Simmonds, J. S., Kutt, A. S., Maron, M., and Strubbe, D. (2019). Systematic definition of threatened fauna communities is critical to their conservation. Diversity and Distributions 25(3), 462–477. doi:10.1111/ddi.12875
- Garnett, S. T., Dutson, G., and Szabo, J. (2012). ‘The Action Plan for Australian Birds 2010.’ (CSIRO Publishing: Victoria.)
- Garnett, S. T., and Todd, M. (2021). Southern star finch neochmia ruficauda ruficauda. In ‘The Action Plan for Australian Birds 2020.’ (Eds S. T. Garnett and G. B. Baker.) pp. 785–786. (CSIRO Publishing: Melbourne, VIC.)
- Golden-shouldered Parrot Recovery Team. (2021). Golden-shouldered Parrot Psephotellus chrysopterygius. In ‘The Action Plan for Australian Birds 2020.’ (Eds S. T. Garnett and G. B. Baker.) pp. 419–421. (CSIRO Publising: Melbourne VIC.)
- Hall, B. P. (1974). ‘Birds of the Harold Hall Australian Expeditions, 1962–1970: A Report on the Collections Made for the British Museum (Natural History)/edited by B. P. Hall.’ (The Museum: London.)
- Hickey, J. J. (1969). ‘Peregrine Falcon Populations: Their Biology and Decline.’ (University of Wisconsin Press: Madison.)
- Hollands, D. (2003). ‘Eagles, Hawks and Falcons of Australia. 2nd ed.’ (Bloomings Books: Hawthorn, Vic.)
- Leach, E., Daly, G., and Burwell, C. (2020). The avifauna of the Eungella region, central coastal Queensland. Australia Proceedings of the Royal Society of Queensland 125, 117–138.
- Ligouri, J., Watson, J. L., Nicoletti, F., and Oleyar, D. (2020). ‘In-hand Guide to Diurnal North American Raptors.’ (HawkWatch International: Salt Lake City, Utah.)
- MacColl, C. (2022a). Beak and Feather Disease Virus (BFDV) rates in Red Goshawk’s. Unpublished raw data.
- MacColl, C. (2022b). Breeding densities of Red Goshawk’s in Northern Australia. Unpublished raw data.
- MacColl, C., Ryan, S., Murphy, S., Tiwi Islands Council Rangers, Aumann, T., Debus, S. J. S., and Garnett, S. T. (2021). Red Goshawk Erythrotriorchis radiatus. In ‘The Action Plan for Australian Birds 2020.’ (Eds S. Garnett and G. B. Baker.) pp. 374–377. (CSIRO Publishing: Melbourne.)
- MacColl, C., Seaton, R., Stewart, D., Leseberg, N. P., Murphy, S., and Watson, J. E. M. (2022). The home range, habitat use, and seasonal movements of the Red Goshawk in Northern Australia. Unpublished manuscript.
- Mace, G. M., Collar, N. J., Gaston, K. J., Hilton-Taylor, C., Akcakaya, H. R., Leader-Williams, N., and Stuart, S. N. (2008). Quantification of extinction risk: IUCN’s system for classifying threatened species. Conservation Biology 22(6), 1424–1442. doi:10.1111/j.1523-1739.2008.01044.x
- Marchant, S., and Higgins, P. J. (1993). ‘Handbook of Australian, New Zealand, and Antarctic Birds. Vol. 2: Raptors to Lapwings.’ (Melbourne: Oxford University Press.)
- Martens, J. M., Stokes, H. S., Berg, M. L., Walder, K., Raidal, S. R., Magrath, M. J. L., and Bennett, A. T. D. (2020). Beak and feather disease virus (BFDV) prevalence, load and excretion in seven species of wild caught common Australian parrots. PLoS ONE 15(7), e0235406–e0235406. doi:10.1371/journal.pone.0235406
- Martínez-Hesterkamp, S., Rebollo, S., Kennedy, P. L., Pérez-Camacho, L., García-Salgado, G., and Morales-Castilla, I. (2018). Territoriality in diurnal raptors: Relative roles of recent evolution, diet and nest site. Biological Journal of the Linnean Society 124(1), 126–137. doi:10.1093/biolinnean/bly020
- McClure, C. J. W., Westrip, J. R. S., Johnson, J. A., Schulwitz, S. E., Virani, M. Z., Davies, R., and Butchart, S. H. M. (2018). State of the world's raptors: Distributions, threats, and conservation recommendations. Biological conservation 227, 390–402. doi:10.1016/j.biocon.2018.08.012
- New South Wales Bird Atlassers Inc (2021). ‘New South Wales Bird Atlassers (NSWBA) occcurrence dataset for Red Goshawk (Erythrotriorchis radiatus).’ [Verified 19th March 2021].
- Newton, I. (1979). ‘Population Ecology of Raptors.’ 1st ed. (Bloomsbury Publishing (UK): London.)
- Nilsson, I., Nilsson, S., and Sylven, M. (1982). Diet choice, resource depression, and the regular nest spacing of birds of prey. Biological Journal of the Linnean Society 18, 1–9. doi:10.1111/j.1095-8312.1982.tb02030.x
- Noske, R. A., and Briggs, A. (2021). Species loss and decline among birds of coastal central Queensland over 130 years. Pacific Conservation Biology. doi:10.1071/PC20081
- Ogada, D. L., Keesing, F., and Virani, M. Z. (2012). Dropping dead: Causes and consequences of vulture population declines worldwide. Annals of the New York Academy of Sciences 1249, 57–71. doi:10.1111/j.1749-6632.2011.06293.x
- Olsen, P., and Garnett, S. T. (2021). Paradise parrot psephotellus pulcherrimus. In ‘The Action Plan for Australian Birds 2020.’ (Eds S. T. Garnett and G. B. Baker.) pp. 430–431. (CSIRO Publishing: Melbourne, VIC.)
- Olson, D. M., Dinerstein, E., Wikramanayake, E. D., Burgess, N. D., Powell, G. V. N., Underwood, E. C., and Kassem, K. R. (2001). Terrestrial ecoregions of the world: A new map of life on earth: A new global map of terrestrial ecoregions provides an innovative tool for conserving biodiversity. BioScience 51(11), 933–938. doi:10.1641/0006-3568(2001)051[0933:TEOTWA]2.0.CO;2
- Pebesma, E. (2018). Simple Features for R: Standardized support for spatial vector data. The R Journal 10, 439–446. doi:10.32614/RJ-2018-009
- Pedersen, T. L. (2020). ‘patchwork: The composer of plots.’ R package version 1.1.1. Available at https://CRAN.R-project.org/package=patchwork
- Raidal, S. R., and Peters, A. (2018). Psittacine beak and feather disease: Ecology and implications for conservation. Emu - Austral Ornithology 118(1), 80–93. doi:10.1080/01584197.2017.1387029
- Ratcliffe, D. A. (1970). Changes attributable to pesticides in egg breakage frequency and eggshell thickness in some British Birds. Journal of Applied Ecology 7(1), 67–115. doi:10.2307/2401613
- R Core Team (2021). ‘R: A language and environment for statistical computing.’ (R Foundation for Statistical Computing: Vienna, Austria.) Available at https://www.R-project.org/
- Sarker, S., Lloyd, C., Forwood, J., and Raidal, S. R. (2016). Forensic genetic evidence of beak and feather disease virus infection in a powerful Owl, Ninox Strenua. Emu - Austral Ornithology 116(1), 71–74. doi:10.1071/MU15063
- Saura, S., Bastin, L., Battistella, L., Mandrici, A., and Dubois, G. (2017). Protected areas in the world’s ecoregions: How well connected are they? Ecological Indicators 76, 144–158. doi:10.1016/j.ecolind.2016.12.047
- Schmitt, C. B., Burgess, N. D., Coad, L., Belokurov, A., Besançon, C., Boisrobert, L., and Winkel, G. (2009). Global analysis of the protection status of the world’s forests. Biological Conservation 142(10), 2122–2130. doi:10.1016/j.biocon.2009.04.012
- Seaton, R. (2014). Surveys for the Red Goshawk (‘Erythrotriorchis radiatus’) in South East Queensland. The Sunbird: Journal of the Queensland Ornithological Society 44(2), 52–59.
- Sergio, F., Newton, I., Marchesi, L., and Pedrini, P. (2006). Ecologically justified charisma: Preservation of top predators delivers biodiversity conservation. Journal of Applied Ecology 43(6), 1049–1055. doi:10.1111/j.1365-2664.2006.01218.x
- Slater, P. (1978). ‘Rare and Vanishing Australian Birds.’ (Rigby: Adelaide.)
- Tennekes, M. (2018). tmap: Thematic Maps in R. Journal of Statistical Software 84(6), 1–39. doi:10.18637/jss.v084.i06
- Thompson, L. J., Krüger, S. C., Coverdale, B. M., Shaffer, L. J., Ottinger, M. A., Davies, J. P., and Bowerman, W. W. (2021). Assessing African vultures as biomonitors and Umbrella species. Frontiers in Conservation Science 2. doi:10.3389/fcosc.2021.729025
- Ward, M., Reside, A. E., and Garnett, S. T. (2021). Geophaps scripta scripta. In ‘The Action Plan of Australian Birds 2020.‘ (Eds S. T. Garnett and G. B. Baker.) pp. 44–47. (CSIRO Publishing: Melbourne, VIC.)
- Ward, M., Watson, J. E. M., Possingham, H. P., Garnett, S. T., Maron, M., Rhodes, J. R., and Simmonds, J. S. (2022). Creating past habitat maps to quantify local extirpation of Australian threatened birds. Environmental Research Letters 17(2), 024032. doi:10.1088/1748-9326/ac4f8b
- Webster, P., Mathieson, M. T., Smith, G. C., and Garnett, S. T. (2021). Buff-breasted Button-quail Turnix olivii. In ‘The Action Plan for Australian Birds 2020.‘ (Eds S.T. Garnett and G.B. Baker.) pp. 314–316. (CSIRO Publishing: Melbourne, VIC.)
- Whitlock, F. L. (1924). Journey to Central Australia in search of the Night Parrot. Emu - Austral Ornithology 23(4), 248–281. doi:10.1071/MU923248
- Wickham, H. (2016). ‘Ggplot2: Elegant Graphics for Data Analysis.’ 2nd ed. (Springer: Switzerland.)
- Wylie, S. L., and Pass, D. A. (1987). Experimental reproduction of psittacine beak and feather disease/French Moult. Avian Pathology 16(2), 269–281. doi:10.1080/03079458708436374
- Ydenberg, R. C., Barrett, J., Lank, D. B., Xu, C., and Faber, M. (2017). The redistribution of non-breeding dunlins in response to the post-DDT recovery of falcons. Oecologia 183(4), 1101–1110. doi:10.1007/s00442-017-3835-2