ABSTRACT
Captive breeding programs are an increasingly important tool for species’ conservation efforts, but not all species reproduce well in captivity. Identifying factors that affect the reproductive success of captive populations is crucial to improving the performance and management of conservation-breeding programs, both by providing individuals for release and informing decision making. We examined breeding records collected from the long-running conservation-breeding program for the critically endangered Orange-bellied Parrot Neophema chrysogaster over an 11-year period. We examined egg hatching rate, nestling survival rate, and offspring sex ratio in response to a wide range of variables related to characteristics of individual birds, breeding events, and the captive environment. The hatch rate of eggs was higher in first clutches compared to second clutches and was lower than the wild population. The survival rate of nestlings through to fledging was variable between years but became higher and more consistent over the last five years of the study period. Variation in brood sex ratio was not related to any of the potential explanatory variables that we examined. This is one of the first studies to examine reproductive data in a long-running conservation-breeding program and shows that many common metrics do not explain reproductive variation. Our approach provides a framework for managers to investigate factors affecting reproductive success in conservation breeding programs more broadly.
Introduction
Species have been bred in captivity for many reasons (D’Elia Citation2010), but captive breeding programs are increasingly being implemented with the explicit goal of assisting the recovery of wild populations (Cohn Citation1988). Such programs might contribute to species’ recovery actions by breeding animals for release to supplement wild populations, facilitating education/outreach initiatives, providing opportunities for research, and/or maintaining insurance populations against extinction (Saint Jalme Citation2002). While captive breeding programs have undoubtedly saved some species from extinction (Butchart et al. Citation2006), these programs can be difficult to implement, time consuming, and resource intensive (D’Elia Citation2010; Hoffman et al. Citation2010; Collar and Butchart Citation2014). Furthermore, there is no guarantee of success, as many programs are limited by animals reproducing less successfully in captive environments compared to the wild (Lees and Wilcken Citation2009; Mason Citation2010; Farquharson et al. Citation2018).
Optimising the reproductive success of captive animals is crucial to maximise the number of individuals available for release, minimise stress to the animals, and utilise available resources most efficiently (Mason Citation2010; Canessa et al. Citation2016b; Tripovich et al. Citation2021). However, there is often considerable uncertainty about the factors that drive variation in reproductive success in captivity. There are several reasons for this, including data deficiencies that hinder statistical analysis (Conde et al. Citation2019), resource limitations to study the issue (Fa et al. Citation2014), and the difficulties of breeding some species in captivity (D’Elia Citation2010). Overcoming these limitations and optimising the reproductive success of captive animals based on robust statistical evidence is an elusive goal of many captive breeding programs.
Understanding the sources of variation in the breeding output of captive animals is important for optimising conditions to improve their reproductive success (Griffith et al. Citation2017). Intrinsic traits like age (Ricklefs et al. Citation2003), experience (Imlay et al. Citation2017), genetic heterozygosity (Rabier et al. Citation2021), and personality (McCowan et al. Citation2014) have all been linked to variation in reproductive success in captive animals. There is also strong evidence that a captive environment itself can influence reproductive output. Wild birds brought into captivity have shown immediate changes to incubation behaviours (Gilby et al. Citation2013) and critical hormonal responses (Dickens and Bentley Citation2014; Jensen et al. Citation2019). Environmental variables including enclosure size (Ali et al. Citation2016), outdoor access (Dickens and Bentley Citation2014; Griffith et al. Citation2017) and autonomy of mate choice (Massa et al. Citation1996) can all influence individual stress responses, activity levels, and/or social behaviours, which can affect reproductive outputs (Clubb et al. Citation2015).
While optimal reproductive success is often important to achieving conservation goals (Cohn Citation1988; Collar and Butchart Citation2014; Slade et al. Citation2014), disentangling variables contributing to reproductive variation in captive environments is difficult. Confounding variables and resource constraints can make designing experiments to understand variation challenging or impractical. Furthermore, conservation breeding programs usually involve small, and/or vulnerable populations (Martin et al. Citation2012), for which few data have been collected, inherently limiting the statistical rigour of quantitative analyses. For these reasons, correlational studies of accumulated records provide a starting point to narrow the range of variables that could be later investigated in small, targeted experiments. While correlational studies have limitations, they can help identify factors that contribute to variation in reproductive success for captive populations (Canessa et al. Citation2016a; Griffith et al. Citation2017).
We use a correlational approach to identify predictors of breeding productivity in the critically endangered Orange-bellied Parrot Neophema chrysogaster, making use of data collected from a long-running captive breeding program. The Orange-bellied Parrot is a small (~45 g) parrot endemic to south-eastern Australia that breeds in southwest Tasmania and migrates to the south-eastern coast of Australia during the austral winter (Brown and Wilson Citation1980; Higgins Citation1999). Despite decades of interventions, ongoing population decline has reduced the current breeding range to a single location and Orange-bellied Parrots remain critically endangered (DELWP Citation2016; Stojanovic et al. Citation2017; Geyle et al. Citation2018). The remaining wild population is closely monitored: each individual bird is uniquely identifiable via coloured leg bands, nesting boxes and supplemental food are provided, and prescribed burning is undertaken to improve habitat and foraging opportunities (Stojanovic et al. Citation2020).
As part of recovery efforts, a captive breed-for-release program to supplement the declining wild population has existed since 1986 (Smales et al. Citation2000; Morrison et al. Citation2020). The captive population of Orange-bellied Parrots is closely managed, and all breeding attempts are carefully monitored. Thus, detailed demographic information is available and comprehensive husbandry records are maintained (Morrison et al. Citation2020). These long-term and comprehensive records have allowed us to investigate the effects of a range of common metrics on reproductive outcomes. Whilst many conservation breeding programs suffer from inherently low population sizes or data deficiency, this is not the case for the Orange-bellied Parrot, making this an unusually informative model system.
We first compiled data on three components of Orange-bellied Parrot reproductive success (hatch rate, nestling survival rate, and offspring sex ratio) for all breeding attempts from two captive breeding institutions over eleven breeding seasons. We then investigated how these measures of reproductive success related to (i) intrinsic differences among individual parrots, (ii) characteristics of each breeding event, and (iii) variation in the captive environment. Finally, we discuss how our observations of the drivers of reproductive success in captive Orange-bellied Parrots shed light on the intricacies of captive breeding programs that aim to support threatened wild populations.
Methods
Background: captive breeding program
A captive breeding program for the Orange-bellied Parrot was established by the Tasmanian Government (NRE) in 1986 when ten parrots were collected from the wild (Smales et al. Citation2000; Morrison et al. Citation2020). Further collections were made in subsequent decades, and at present, the captive population comprises more than four hundred birds spread between five breeding institutions across Australia (BirdLife Australia Citation2018; Morrison et al. Citation2020; Pritchard et al. Citation2021). The Orange-bellied Parrot captive breeding program is managed through a studbook and mean kinship strategy to maximise genetic heterozygosity (Morrison et al. Citation2020). Breeding birds are paired in early spring (Aug-Oct), and usually comprise one male and one female housed together in a separate enclosure. Enclosure and nest box design vary slightly between institutions but are based on recommendations outlined in the species’ husbandry manual (Gowland and Everaardt Citation2015; DELWP Citation2016).
The intensive management of the captive Orange-bellied Parrot population means information is collected on all breeding attempts and routine husbandry actions. We compiled these data from the two largest and longest running captive breeding institutions (Zoos Victoria, ZV, and the Department of Natural Resources and Environment Tasmania, NRE; (Smales et al. Citation2000; BirdLife Australia Citation2018)) for every recorded Orange-bellied Parrot breeding attempt from the 2011/12–2021/22 breeding seasons. While we were not able to incorporate data from all five captive breeding institutions, these records can be considered a good representative sample as ZV and NRE have historically held the largest populations of captive Orange-bellied Parrots (BirdLife Australia Citation2018). We considered any birds paired by an institution for breeding as a breeding record.
Defining the response variables
We identified three response variables because they each represented a different component of reproductive output:
Hatch rate of eggs - Hatching failure in birds has been associated with a wide array of factors (Clubb et al. Citation2015) including; inbreeding depression (Jamieson Citation2011; Rabier et al. Citation2021), parental age (Ricklefs et al. Citation2003; Griffith et al. Citation2017), stress hormone levels (Khan et al. Citation2016), social environment (Bolund et al. Citation2010; Clark et al. Citation2012; Hemmings et al. Citation2012), lack of mate choice (Massa et al. Citation1996), and inadequate enclosure designs (Dickens and Bentley Citation2014; Malek and Haim Citation2019). We defined hatch rate as the proportion of eggs that hatched in each clutch.
Nestling survival rate - Nestling survival can be influenced by the parents (e.g. health (Sheridan et al. Citation2004), age (Khan et al. Citation2016), condition (Tollington et al. Citation2018), behaviour (Rabier et al. Citation2021), personality (McCowan et al. Citation2014), experience level (Imlay et al. Citation2017)), or factors related to that breeding attempt, such as timing within the season (Ortiz-Catedral and H. Brunton Citation2008) and clutch/brood size (Smith et al. Citation1989; Dijkstra et al. Citation1990; Saino et al. Citation2018). External factors relating to the environment including enclosure aspect (Griffith et al. Citation2017), nest box design (Larson et al. Citation2016) or level of disturbance (Butler and Dufty Citation2007) also have the potential to impact nestling survival. We defined nestling survival rate as the proportion of hatchlings in a brood that survived through to fledging.
Offspring sex ratio – Females of some bird species have some control over the sex ratio of their offspring, often in response to physical condition or environmental stressors (Trivers and Willard Citation1973; Heinsohn et al. Citation1997; Clout et al. Citation2002; Taylor and Parkin Citation2008; Bowers et al. Citation2017). Alternatively, skewed offspring sex ratios in a population can arise from sex-biased mortality of juveniles or nestlings (De Kogel Citation1997; Bowers et al. Citation2011; Heinsohn et al. Citation2021). We defined offspring sex ratio as the proportion of male fledglings in a brood out of the total number of fledglings of known sex.
Defining the predictor variables
Drawing upon some of the research on variables which can affect avian reproduction, we identified three main themes: individual-level variation (e.g. age (Ricklefs et al. Citation2003; Imlay et al. Citation2017); physical condition (Murphy Citation2007); provenance (Gilby et al. Citation2013); attributes of a particular breeding event (e.g. timing within season (Naef-Daenzer et al. Citation2001); brood size (Ortiz-Catedral and Brunton Citation2008)); and the environment (e.g. enclosure size (Ali et al. Citation2016) or disturbance levels (Butler and Dufty Citation2007; Dickens and Bentley Citation2014)). We summarised data from routine husbandry records for variables related to each theme which could influence captive Orange-bellied Parrot reproduction. We selected a total of 19 variables including: basic demographic information for each individual bird (e.g. age, cohort, body mass, and provenance); data for each breeding event (e.g. year, laying dates, clutch number, and clutch size); and information on the breeding environment (e.g. breeding institution, building, proximity to disturbance and aspect). A full list of predictor variables and corresponding definitions are provided in the Supplementary Material.
Statistical analysis
As not all variables of interest were available or applicable for all records, we first undertook exploratory analysis to evaluate if predictor variables that were incomplete over the full dataset were influential in affecting any of the three response variables. In this exploratory step, we fitted generalised linear mixed effect models with a binomial distribution and logit-link function using ‘glmmTMB’ v. 1.1.3 (Brooks et al. Citation2017) to subsets of the data where incomplete predictors were available to evaluate the effects on the three response variables. We fitted each predictor as a fixed effect and included both sire and dam ID as random effects in all models to account for repeat observations of the same individual. We compared these main effects models against a corresponding null model based on ∆AIC> 2 (Burnham and Anderson Citation2002). In all cases, the null model was best supported by the data, so we excluded all incomplete predictor variables from downstream analysis. We further refined the list of predictor variables by excluding the least informative correlated variables from the analysis. We evaluated pairwise correlation between variable using ‘GGally’ v 2.1.2 (Schloerke et al. Citation2021) and excluded variables with a strong correlation (Spearman’s coefficient> 0.8 or < -0.8). Correlations always involved year of breeding attempt, so we only retained year as a variable. We excluded two records of third clutches due to low sample size.
Using the refined list of predictors, we followed the same procedure for each of the three response variables. We fitted three sets of generalised linear mixed effect models (corresponding to hatch rate, nestling survival rate, and offspring sex ratio) with a beta-binomial (hatch rate) or binomial (survival rate, sex ratio) distribution and logit-link function. We fitted predictor variables as fixed effects, and again included both sire and dam ID as random effects in all models to account for repeated observations of the same individuals. We compared the main effects models for each predictor both against one another and a corresponding null model based on ΔAIC> 2. We excluded an interactive term of year*institution due to models failing to converge. We used the model select function in ‘MuMIn’ v. 1.46.0 (Barton Citation2020) to rank models, and evaluated model fit using ‘DHARMa’ v.0.4.5 (Hartig Citation2022). We estimated the effect sizes for different variables using ‘emmeans’ v. 1.7.5 (Lenth Citation2021) and visualised them using ‘ggplot’ v. 3.3.6 (Wickham Citation2016). All analysis was conducted in R v. 4.2.1 (R Core Team Citation2021).
Results
We compiled 722 breeding records of captive Orange-bellied Parrots between the 2011/12 and 2021/22 breeding seasons from two captive-breeding institutions. The data set included detailed information for 521 different individuals (272 dams and 249 sires). Most records (n = 628) involved a nesting attempt being initiated by the pair (defined by females laying > 1 egg), with an overall participation rate of 87.0% of birds that were paired for breeding. Females produced a mean of 1.22 clutches annually, with an average clutch size of 4.74 eggs (range 1–11). The overall hatching rate of eggs was 50.5%, with a mean brood size of 3.29 nestlings (range 1–7). Mean survival rate of nestlings through to fledging was 75.5%, with 1126 fledglings produced by these breeding institutions over the study period: 550 males, 526 females, and 50 of unknown sex; an overall sex ratio of 51.1% male.
We provide the top five models based on AIC values for each response variable in . For the hatch rate of eggs, we found equivalent support (ΔAIC< 2) for the model with the main effect of clutch number and a model that included an interactive effect of clutch number and laying date (). The most parsimonious model included the main effect of clutch number; the effect sizes and confidence intervals from this model are presented in . First clutches had a higher estimated hatch rate (53.5% CI: 49.2–57.8%) compared to second clutches (28.2% CI: 22.2–35.0%).The hatching rate of first clutches was lower for females who went on to lay a second clutch (36.5% CI: 29.9–43.6%) compared to females that only laid one clutch in a season (60.0% CI: 55.4–64.4%).
Figure 1. Model estimates and 95% confidence limits for the preferred model showing the difference in hatch rates of Orange-bellied Parrot eggs laid in captivity between first (53.5% CI: 49.2–57.8%) and second clutches (28.2% CI: 22.2–35.0%).
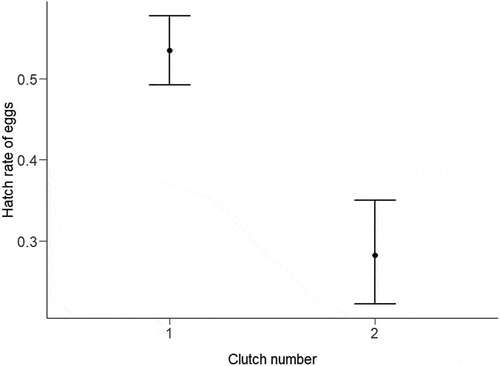
Table 1. Model outputs for the top five generalised mixed effect models for each response variable: hatch rate of eggs, nestling survival rate, and offspring sex ratio. Sire and dam ID have been added as random effects to each model. Models are ranked by AIC values, with the preferred model highlighted in bold.
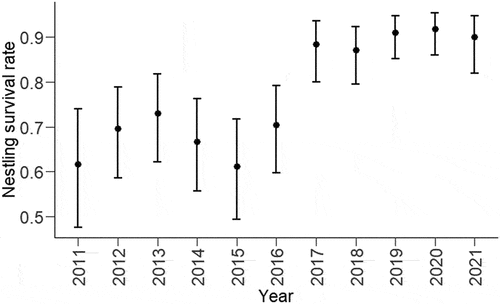
The best supported model for nestling survival rate based on ΔAIC included the effect of year of breeding attempt; effect sizes and confidence intervals estimated from this model are presented in . Nestling survival ranged from a low of 61.1% (CI:49.3–71.7%) in 2015 to a high of 91.8% (CI: 85.9–95.4%) in 2020 (full model estimates are presented in Supplementary Materials S2). For offspring sex ratio, the best supported model based on ΔAIC was the null model () indicating that offspring sex ratios were independent of the predictor variables we considered.
Discussion
Captive breeding programs are a globally important tool for species recovery programs, but many species fail to breed successfully in captive environments (Lees and Wilcken Citation2009). We used the Orange-bellied Parrot captive breeding program as a model to evaluate factors contributing to reproductive success in captivity. The hatch rate of Orange-bellied Parrot eggs was higher in first clutches compared to second clutches (), and the rate of nestling survival through to fledging varied between years (). Offspring sex ratio was not explained by our predictor variables, however the sample sizes for this analysis are small and conclusions should be viewed cautiously. Taken together, our results indicate that many measurable aspects of captive husbandry and individual variation among animals are independent of observed variation in the reproductive success of captive Orange-bellied Parrots.
Second clutches had a lower hatch rate compared to first clutches. This could be a reflection of physiological stress on females who have already laid a first clutch that season (Saino et al. Citation2005; Norte et al. Citation2010; Travers et al. Citation2010), or it could be an artefact of management practices. Pairs of captive parrots are often encouraged to re-nest immediately if their first attempt fails (e.g. by keeping a pair housed together, providing new nesting box(es) or an altered diet) whereas successful pairs are sometimes prevented from laying a second clutch for management reasons (achieved by separating birds or removing access to nesting boxes). As a result of these practices, that second clutches could be disproportionately laid by less fecund pairs. However, we were unable to explicitly test this hypothesis due to confounding effects of management practices which varied between institutions and over time.
The mean hatching rate of eggs in this study was 50.5%, which is comparable to other data from captivity (48%; Penrose Citation2016) but substantially lower than reported hatch rates in the wild population (79.5%; Holdsworth Citation2006); 74.95%, (Troy and Lawrence Citation2022),However, these estimates might not be strictly comparable as second clutches are extremely rare in the wild population (Holdsworth Citation2006; Stojanovic et al. Citation2017), thus the inclusion of second clutches could be lowering the overall estimated hatch rate for the captive population. Nevertheless, even when second clutches are excluded, the hatch rate estimates from only first clutches laid in captivity (53.5%) are still much lower than the wild population.
Captive populations often suffer inbreeding depression or low genetic diversity, which can reduce fertility (Hemmings et al. Citation2012), but Morrison et al. (Citation2020) demonstrated that wild- and captive-bred Orange-bellied Parrots have a similar level of genetic diversity. Thus, lowered hatching success of the captive population could be due to the captive environment itself rather than a trait of individual birds. Clubb et al. (Citation2015) summarised causes of infertility in birds including medical/physical (e.g. inbreeding, age, obesity, disease, malnutrition), environmental (e.g. enclosure design, nest box design, lack of visual cues, disturbance, lack of environmental cues,) or behavioural factors (e.g. pair incompatibility, experience levels, inappropriate social interactions, breeding behaviours or imprinting). These factors could all partly explain our observations, but there are inadequate data to explore these possibilities within the scope of this study. Nevertheless, low hatching success is an area in need of further research to improve captive breeding efficacy of Orange-bellied Parrots.
The rate of nestling survival varied between years, and the higher rates of survival in the last five years matches estimates from the wild population (87.1–96.7%; Holdsworth Citation2006; Stojanovic et al. Citation2017; Troy and Lawrence Citation2022). The observed variation in nestling survival rates could be partially explained by stochastic events. For example, a disease outbreak at one breeding institution in 2016 prompted a review of biosecurity protocols (Stojanovic et al. Citation2017; BirdLife Australia Citation2018). Subsequent changes to biosecurity and husbandry practices could be contributing to the increase in nestling survival from 2017 onwards (). However, this higher and more stable rate of nestling survival in recent years could also be a reflection of increased resources directed into the captive program (BirdLife Australia Citation2018). Unfortunately, factors such as disease outbreaks or changes to husbandry practices (e.g. altered diets) are all confounded with year in our study. Distinguishing between causal factors is often challenging in captive-breeding programs because entire small populations can experience the same variation at the same time. Furthermore, many programs have limited resources to implement experiments and are limited by small sample sizes. Nevertheless, some degree of experimental manipulation or critical review is important for evidence-based decision making (Canessa et al. Citation2019; Fogell et al. Citation2019).
Captive breeding programs are becoming an increasingly important strategy for conservation around the world. These programs represent a substantial investment of scare resources, and they often need to contend with small population sizes, lack of knowledge, and limited resources (Mccleery et al. Citation2007; Fa et al. Citation2011; Collar and Butchart Citation2014). The challenges we faced in this study are typical of captive breeding programs; limited data on specific areas of interest, correlations between variables, and confounding effects of year. However, we can confidently rule out some common intrinsic traits (e.g. mass, age, early life history) as significant drivers of variation in reproductive success for Orange-bellied Parrots in captivity. This is important as individuals that might have previously been excluded from breeding or release events (e.g. very young or old, over- or under-weight, hand-reared) could potentially be included, thus increasing the proportion of the population available for breeding or release. Additionally, our results on hatching rates highlight future areas to focus and direct research to increase reproductive output of the captive population. As many captive breeding programs aimed at species recovery do not have the time, resources, or population sizes for experiments, an approach like the one undertaken here could help programs quantify variation in reproductive success, highlight influential variables, and identify areas to direct further research to improve the efficacy of conservation efforts.
Supplemental Material
Download MS Word (21.9 KB)Acknowledgments
We would like to thank all staff at Zoos Victoria’s Healesville Sanctuary and the Tasmanian Government’s (NRE) Orange-bellied Parrot Tasmanian Program, specifically; Rachael Alderman, Paul Black, Clare Lawrence, Darren Page, Shannon Troy, and Lisa Vassos for making data available and answering questions. Thanks to Dr Teresa Neeman and the ANU BDSI for statistical advice and thanks to two anonymous reviewers whose thoughtful comments improved this manuscript. We acknowledge the traditional custodians of country upon which this research was conducted. Funding was provided to L.T.B. through an Australian Government Research Training Program Scholarship. D.S. received funding from the Australian Government Department of Agriculture, Water, and the Environment.
Disclosure statement
L.T.B. is currently employed with the Tasmanian Government (NRE) Orange-bellied Parrot Tasmanian Program. No other potential conflicts of interest were reported by the authors.
Data availability statement
Data were provided through research agreements with the Department of Natural Resources and Environment Tasmania Orange-bellied Parrot Tasmanian Program (https://nre.tas.gov.au/conservation/threatened-species-and-communities/lists-of-threatened-species/threatened-species-vertebrates/orange-bellied-parrot/the-obp-tasmanian-program), and Zoos Victoria (https://www.zoo.org.au/fighting-extinction/local-threatened-species/orange-bellied-parrot/) and are used with permission.
Supplementary data
Supplemental data for this article can be accessed at https://doi.org/10.1080/01584197.2023.2194541.
References
- Ali, Z., Mushtaq-Ul-Hassan, M., Arshad, M. I., and Gill, A. H. (2016). Effect of spacing on reproductive performance of Indian Peafowl (Pavo cristatus) in captivity. Pakistan Journal of Science 68, 387.
- Barton, K. (2020). MuMIN: Multi-model inference. R Package. version 1.43.17.
- BirdLife Australia. (2018). ‘A Stocktake of Recovery Activities Undertaken for the Orange-Bellied Parrot (Neophema chrysogaster).’ (Department of the Environment and Energy: Carlton, Victoria.)
- Bolund, E., Martin, K., Kempenaers, B., and Forstmeier, W. (2010). Inbreeding depression of sexually selected traits and attractiveness in the zebra finch. Animal Behaviour 79, 947–955. doi:10.1016/j.anbehav.2010.01.014
- Bowers, E. K., Sakaluk, S. K., and Thompson, C. F. (2011). Adaptive sex allocation in relation to hatching synchrony and offspring quality in house wrens. The American Naturalist 177, 617–629. doi:10.1086/659630
- Bowers, E. K., Thompson, C. F., and Sakaluk, S. K. (2017). Maternal natal environment and breeding territory predict the condition and sex ratio of offspring. Evolutionary Biology 44, 11–20. doi:10.1007/s11692-016-9380-9
- Brooks, M. E., Kristensen, K., van Benthem, K. J., Magnusson, A., Berg, C. W., Nielsen, A., and Skaug, H., et al. (2017). glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R Journal 9, 378–400. doi:10.32614/RJ-2017-066
- Brown, P. B., and Wilson, R. I. (1980). ‘A Survey of the Orange-Bellied Parrot (Neophema chrysogaster) in Tasmania, Victoria, and South Australia.’ (The World Wildlife Fund, NPWS Tasmania: Hobart.)
- Burnham, K. P., and Anderson, D. R. (2002). ‘Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach.’ (Springer: New York.)
- Butchart, S. H. M., Stattersfield, A. J., and Collar, N. J. (2006). How many bird extinctions have we prevented? Oryx 40, 266–278. doi:10.1017/S0030605306000950
- Butler, M. W., and Dufty, A. M. (2007). Nestling immunocompetence is affected by captivity but not investigator handling. The Condor 109, 920–928. doi:10.1093/condor/109.4.920
- Canessa, S., Converse, S. J., West, M., Clemann, N., Gillespie, G., McFadden, M., and Silla, A. J., et al. (2016a). Planning for ex situ conservation in the face of uncertainty. Conservation Biology 30, 599–609. doi:10.1111/cobi.12613
- Canessa, S., Guillera-Arroita, G., Lahoz-Monfort, J. J., Southwell, D. M., Armstrong, D. P., Chadès, I., and Lacy, R. C., et al. (2016b). Adaptive management for improving species conservation across the captive-wild spectrum. Biological Conservation 199, 123–131. doi:10.1016/j.biocon.2016.04.026
- Canessa, S., Taylor, G., Clarke, R. H., Ingwersen, D., Vandersteen, J., and Ewen, J. G. (2019). Risk aversion and uncertainty create a conundrum for planning recovery of a critically endangered species. Conservation Science and Practice 2. doi:10.1111/csp2.138
- Clark, J. A., Haseley, A., Van Genderen, G., Hofling, M., and Clum, N. J. (2012). Increasing breeding behaviors in a captive colony of Northern Bald Ibis through conspecific acoustic enrichment. Zoo Biology 31, 71–81. doi:10.1002/zoo.20414
- Clout, M. N., Elliott, G. P., and Robertson, B. C. (2002). Effects of supplementary feeding on the offspring sex ratio of kakapo: A dilemma for the conservation of a polygynous parrot. Biological Conservation 107, 13–18. doi:10.1016/S0006-3207(01)00267-1
- Clubb, S., Velez, J., Garner, M. M., Zaias, J., and Cray, C. (2015). Health and reproductive assessment of selected Puerto Rican Parrots (Amazona vittata) in captivity. Journal of Avian Medicine and Surgery 29, 313–325. doi:10.1647/2013-067
- Cohn, J. P. (1988). Captive breeding for conservation. Bioscience 38, 312. doi:10.2307/1310732
- Collar, N. J., and Butchart, S. H. M. (2014). Conservation breeding and avian diversity: Chances and challenges. International Zoo Yearbook 48, 7–28. doi:10.1111/izy.12039
- Conde, D. A., Staerk, J., Colchero, F., da Silva, R., Scholey, J., Baden, H. M., and Jouvet, L., et al. (2019). Data gaps and opportunities for comparative and conservation biology. Proceedings of the National Academy of Sciences - PNAS 116, 9658–9664. doi:10.1073/pnas.1816367116
- De Kogel, C. H. (1997). Long-term effects of brood size manipulation on morphological development and sex-specific mortality of offspring. The Journal of Animal Ecology 66, 167–178. doi:10.2307/6019
- D’Elia, J. (2010). Evolution of avian conservation breeding with insights for addressing the current extinction crisis. Journal of Fish and Wildlife Management 1, 189–210. doi:10.3996/062010-JFWM-017
- DELWP. (2016.) ‘National Recovery Plan for the Orange-bellied Parrot Neophema chrysogaster.’ (Department of Enviornment Land Water and Planning: Canberra, Australia.)
- Dickens, M. J., and Bentley, G. E. (2014). Stress, captivity, and reproduction in a wild bird species. Hormones and Behavior 66, 685–693. doi:10.1016/j.yhbeh.2014.09.011
- Dijkstra, C., Bult, A., Bijlsma, S., Daan, S., Meijer, T., and Zijlstra, M. (1990). Brood size manipulations in the kestrel (Falco tinnunculus): Effects on offspring and parent survival. The Journal of Animal Ecology 59, 269–285. doi:10.2307/5172
- Fa, J. E., Funk, S. M., and O’connell, D. (2011). ‘Zoo Conservation Biology.’ (Cambridge University Press: Cambridge.)
- Fa, J. E., Gusset, M., Flesness, N., and Conde, D. A. (2014). Zoos have yet to unveil their full conservation potential. Animal Conservation 17, 97–100. doi:10.1111/acv.12115
- Farquharson, K. A., Hogg, C. J., and Grueber, C. E. (2018). A meta-analysis of birth-origin effects on reproduction in diverse captive environments. Nature Communications 9, 1055. doi:10.1038/s41467-018-03500-9
- Fogell, D. J., Groombridge, J. J., Tollington, S., Canessa, S., Henshaw, S., Zuel, N., and Jones, C. G., et al. (2019). Hygiene and biosecurity protocols reduce infection prevalence but do not improve fledging success in an endangered parrot. Scientific Reports 9, 4779. doi:10.1038/s41598-019-41323-w
- Geyle, H. M., Woinarski, J. C. Z., Baker, G. B., Dickman, C. R., Dutson, G., Fisher, D. O., and Ford, H., et al. (2018). Quantifying extinction risk and forecasting the number of impending Australian bird and mammal extinctions. Pacific Conservation Biology 24, 157. doi:10.1071/PC18006
- Gilby, A. J., Mainwaring, M. C., and Griffith, S. C. (2013). Incubation behaviour and hatching synchrony differ in wild and captive populations of the zebra finch. Animal Behaviour 85, 1329–1334. doi:10.1016/j.anbehav.2013.03.023
- Gowland, D. J., and Everaardt, A. (2015). Orange-Bellied Parrot (OBP) Neophema chrysogaster husbandry guidelines.
- Griffith, S. C., Crino, O. L., Andrew, S. C., Nomano, F. Y., Adkins-Regan, E., Alonso-Alvarez, C., and Bailey, I. E., et al. (2017). Variation in reproductive success across captive populations: Methodological differences, potential biases and opportunities. Ethology 123, 1–29. doi:10.1111/eth.12576
- Hartig, F. (2022). DHARMa: Residual diagnostics for hierarchical (multi-level/mixed) regression models. R package. version 0.4.5.
- Heinsohn, R., Au, J., Kokko, H., Webb, M. H., Deans, R. M., Crates, R., and Stojanovic, D. (2021). Can an introduced predator select for adaptive sex allocation? Proceedings of the Royal Society of London Series B: Biological Sciences 288, 20210093. doi:10.1098/rspb.2021.0093
- Heinsohn, R., Legge, S., and Barry, S. (1997). Extreme bias in sex allocation in Eclectus parrots. Proceedings of the Royal Society of London Series B: Biological Sciences 264, 1325. doi:10.1098/rspb.1997.0183
- Hemmings, N., West, M., and Birkhead, T. R. (2012). Causes of hatching failure in endangered birds. Biology Letters 8, 964–967. doi:10.1098/rsbl.2012.0655
- Higgins, P. J. (Ed.). (1999). ‘Handbook of Australian, New Zealand and Antarctic Birds. Volume 4: Parrots to Dollarbird.’ (Oxford University Press: Melbourne.)
- Hoffman, M., Hilton-Taylor, C., Böhm, M., Chanson, J., Cox, N. A., Darwall, W. R. T., and Carpenter, K. E., et al. (2010). The impact of conservation on the status of the world’s vertebrates. Science (American Association for the Advancement of Science) 330, 1503–1509. doi:10.1126/science.1194442
- Holdsworth, M. C. (2006). Reproductive success and demography of the Orange-bellied Parrot Neophema chrysogaster. Masters Thesis, University of Tasmania.
- Imlay, T. L., Steiner, J. C., and Bird, D. M. (2017). Age and experience affect the reproductive success of captive Loggerhead Shrike (Lanius ludovicianus) subspecies. Canadian Journal of Zoology 95, 547–554. doi:10.1139/cjz-2015-0169
- Jamieson, I. G. (2011). Founder effects, inbreeding, and loss of genetic diversity in four avian reintroduction programs. Conservation Biology 25, 115–123. doi:10.1111/j.1523-1739.2010.01574.x
- Jensen, T., Jamieson, S. E., Castro, I., Gartrell, B., Cockrem, J. F., and Durrant, B. (2019). Serum prolactin and testosterone levels in captive and wild brown kiwi (Apteryx mantelli) during the prebreeding, breeding, and incubation periods. Zoo Biology 38, 316–320. doi:10.1002/zoo.21484
- Khan, N., Peters, R. A., and Robert, K. (2016). Compensating for a stressful start: Maternal corticosterone, offspring survival, and size at fledging in the Zebra Finch, Taeniopygia guttata. Emu - Austral Ornithology 116, 262–272. doi:10.1071/MU15095
- Larson, E. R., Eastwood, J. R., Buchanan, K. L., Bennett, A. T. D., and Berg, M. L. (2016). How does nest-box temperature affect nestling growth rate and breeding success in a parrot? Emu - Austral Ornithology 115, 247–255. doi:10.1071/MU14081
- Lees, C. M., and Wilcken, J. (2009). Sustaining the ark: The challenges faced by zoos in maintaining viable populations. International Zoo Yearbook 43, 6–18. doi:10.1111/j.1748-1090.2008.00066.x
- Lenth, R. V. (2021). Emmeans: Estimated marginal means, aka least-squares means. R Package. version 1.5.5-1.
- Malek, I., and Haim, A. (2019). Bright artificial light at night is associated with increased body mass, poor reproductive success and compromised disease tolerance in Australian budgerigars (Melopsittacus undulatus). Integrative Zoology 14, 589–603. doi:10.1111/1749-4877.12409
- Martin, T. G., Nally, S., Burbidge, A. A., Arnall, S., Garnett, S. T., Hayward, M. W., and Lumsden, L. F., et al. (2012). Acting fast helps avoid extinction. Conservation Letters 5, 274–280. doi:10.1111/j.1755-263X.2012.00239.x
- Mason, G. J. (2010). Species differences in responses to captivity: Stress, welfare and the comparative method. Trends in Ecology & Evolution 25, 713–721. doi:10.1016/j.tree.2010.08.011
- Massa, R., Galanti, V., and Bottoni, L. (1996). Mate choice and reproductive success in the domesticated budgerigar, Melopsittacus undulatus. Italian Journal of Zoology 63, 243–246. doi:10.1080/11250009609356140
- Mccleery, R. A., Lopez, R. R., and Silvy, N. J. (2007). Transferring research to endangered species management. The Journal of Wildlife Management 71, 2134–2141. doi:10.2193/2007-169
- McCowan, L. S. C., Rollins, L. A., and Griffith, S. C. (2014). Personality in captivity: More exploratory males reproduce better in an aviary population. Behavioural Processes 107, 150–157. doi:10.1016/j.beproc.2014.08.020
- Morrison, C. E., Johnson, R. N., Grueber, C. E., and Hogg, C. J. (2020). Genetic impacts of conservation management actions in a critically endangered parrot species. Conservation Genetics 21, 869–877. doi:10.1007/s10592-020-01292-4
- Murphy, M. T. (2007). Lifetime reproductive success of female Eastern Kingbirds (Tyrannus tyrannus): Influence of lifespan, nest predation, and body size. The Auk 124, 1010–1022. doi:10.1093/auk/124.3.1010
- Naef-Daenzer, B., Widmer, F., and Nuber, M. (2001). Differential post-fledging survival of great and coal tits in relation to their condition and fledging date. The Journal of Animal Ecology 70, 730–738. doi:10.1046/j.0021-8790.2001.00533.x
- Norte, A. C., Ramos, J. A., Sampaio, H. L., Sousa, J. P., and Sheldon, B. C. (2010). Physiological condition and breeding performance of the great tit. The Condor 112, 79–86. doi:10.1525/cond.2010.080071
- Ortiz-Catedral, L., and Brunton, D. H. (2008). Clutch parameters and reproductive success of a translocated population of red-crowned parakeet (Cyanoramphus novaezelandiae). Australian Journal of Zoology 56, 389–393. doi:10.1071/ZO08069
- Penrose, K. (2016). Behaviour and reproduction of captive and wild-origin Orange-bellied Parrots (Neophema chrysogaster) in the conservation breeding program. Masters of Science, University of Melbourne.
- Pritchard, R. A., Kelly, E. L., Biggs, J. R., Everaardt, A. N., Loyn, R., Magrath, M. J. L., and Menkhorst, P., et al. (2021). Identifying cost‐effective recovery actions for a critically endangered species. Conservation Science and Practice 4. doi:10.1111/csp2.546
- Rabier, R., Lesobre, L., and Robert, A. (2021). Reproductive performance in houbara bustard is affected by the combined effects of age, inbreeding and number of generations in captivity. Scientific Reports 11, 7813. doi:10.1038/s41598-021-87436-z
- R Core Team. (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing. version 4.2.1.
- Ricklefs, R., Scheuerlein, A., and Cohen, A. (2003). Age-related patterns of fertility in captive populations of birds and mammals. Experimental Gerontology 38, 741–745. doi:10.1016/S0531-5565(03)00101-3
- Saino, N., Ambrosini, R., Rubolini, D., Romano, M., Caprioli, M., Romano, A., and Parolini, M. (2018). Carry-over effects of brood size on morphology, reproduction, and lifespan in barn swallows. Behavioral Ecology and Sociobiology 72. doi:10.1007/s00265-018-2446-1
- Saino, N., Romano, M., Ferrari, R. P., Martinelli, R., and Moller, A. P. (2005). Stressed mothers lay eggs with high corticosterone levels which produce low-quality offspring. The Journal of Experimental Zoology 303, 998–1006. doi:10.1002/jez.a.224
- Saint Jalme, M. (2002). Endangered avian species captive propagation: An overview of functions and techniques. Avian and Poultry Biology Reviews 13, 187–202. doi:10.3184/147020602783698557
- Schloerke, B., Cook, D., Larmarange, J., Briatte, F., Marbach, M., Thoen, E., Elberg A., et al. (2021). GGally: Extension to ‘ggplot2’. R package. version 2.1.2.
- Sheridan, J. A., Beissinger, S. R., and Hughes, C. R. (2004). Weak association between measures of health and reproductive success in green-rumped parrotlets (Forpus passerinus) in Venezuela. The Auk 121, 717–725. doi:10.1642/0004-8038(2004)121[0717:WABMOH]2.0.CO;2
- Slade, B., Parrott, M. L., Paproth, A., Magrath, M. J. L., Gillespie, G. R., and Jessop, T. S. (2014). Assortative mating among animals of captive and wild origin following experimental conservation releases. Biology Letters 10, 20140656. doi:10.1098/rsbl.2014.0656
- Smales, I., Brown, P., Menkhorst, P., Holdsworth, M., and Holz, P. (2000). Contribution of captive management of Orange‐bellied Parrots to the recovery programme for the species in Australia. International Zoo Yearbook 37, 171–178. doi:10.1111/j.1748-1090.2000.tb00719.x
- Smith, H. G., Kallander, H., and Nilsson, J. A. (1989). The trade-off between offspring number and quality in the great tit Parus major. The Journal of Animal Ecology 58, 383–401. doi:10.2307/4837
- Stojanovic, D., Alves, F., Cook, H., Crates, R., Heinsohn, R., Peters, A., and Rayner, L., et al. (2017). Further knowledge and urgent action required to save Orange-bellied Parrots from extinction. Emu - Austral Ornithology 118, 126–134. doi:10.1080/01584197.2017.1394165
- Stojanovic, D., Young, C., Troy, S., and Heinsohn, R. (2020). Evaluation of intervention aimed at improving reproductive success in Orange‐bellied Parrots Neophema chrysogaster: Lessons, barriers and successes. Ecological Management & Restoration 21, 205–210. doi:10.1111/emr.12422
- Taylor, T. D., and Parkin, D. T. (2008). Sex ratios observed in 80 species of parrots. Journal of Zoology 276, 89–94. doi:10.1111/j.1469-7998.2008.00476.x
- Tollington, S., Ewen, J. G., Newton, J., McGill, R. A. R., Smith, D., Henshaw, A., and Fogell, D. J., et al. (2018). Individual consumption of supplemental food as a predictor of reproductive performance and viral infection intensity. The Journal of Applied Ecology 56, 594–603. doi:10.1111/1365-2664.13303
- Travers, M., Clinchy, M., Zanette, L., Boonstra, R., and Williams, T. D. (2010). Indirect predator effects on clutch size and the cost of egg production. Ecology Letters 13, 980–988. doi:10.1111/j.1461-0248.2010.01488.x
- Tripovich, J. S., Popovic, G., Elphinstone, A., Ingwersen, D., Johnson, G., Schmelitschek, E., and Wilkin, D., et al. (2021). Born to be wild: Evaluating the zoo-based regent honeyeater breed for release program to optimise individual success and conservation outcomes in the wild. Frontiers in Conservation Science 2. doi:10.3389/fcosc.2021.669563
- Trivers, R. L., and Willard, D. E. (1973). Natural selection of parental ability to vary the sex ratio of offspring. Science 179, 90–92. doi:10.1126/science.179.4068.90
- Troy, S., and Lawrence, C. (2022). Report on the Melaleuca wild population 2021/22. Department of Natural Resources and Environment Tasmania.
- Wickham, H. (2016). GGplot2: Elegant graphics for data analysis. R package. version 3.3.6.