ABSTRACT
Understanding the population dynamics of endangered species is crucial to their conservation. Stochastic population models can be used to explore factors involved in population change, contributing to the understanding of a species’ population dynamics. Norfolk Island Green Parrots Cyanoramphus cookii have undergone significant population fluctuations in the last 50 years. Since 2013, most nestlings hatched in managed, predator-proofed nest sites have been individually marked. These nests have been considered the primary source of population growth. Yet, in 2021, most adult birds were unmarked, raising the question of whether unmarked parrots have been entering the population through undetected breeding in natural nests, and to what extent. We modelled Green Parrot population growth between 2013 and 2021 using stochastic population models in VORTEX to explore the potential dynamics involved in the observed population growth. Basic models involving breeding only in managed nests produced population estimates between 158 and 266, whereas more complex models that included breeding in unmanaged nests, and accounted for the large proportion of unmarked birds, produced population estimates between 360 and 1,041. We conclude that natural nests may have played a significant role in the population growth since 2013. If this is the case, broad-scale predator control may be largely responsible. Furthermore, our study shows how population models may be used to infer underlying demographic processes and inform conservation strategies, even in instances of data scarcity. Our method can be applied to other threatened species, and may prove particularly useful for small populations whose population dynamics remain unclear.
Introduction
Population dynamics underpin the persistence and extinction of species. Of particular importance to conservation ecologists is the ability to understand and predict population fluctuations in the face of environmental change, resulting in intensive effort directed at population modelling (Lande et al. Citation2003). However, population changes are a product of multiple environmental and demographic processes, all of which are subject to stochasticity (Lande et al. Citation2003; Melbourne and Hastings Citation2008). If models do not effectively incorporate stochasticity into demographic simulations, they may fail to account for the full range of possible population outcomes (Lande et al. Citation2003). To accommodate environmental and demographic stochasticity, theoretical ecologists have mostly turned to stochastic population models such as population viability analyses when modelling population change (Melbourne and Hastings Citation2008).
In conservation biology, stochastic population models are often used to quantify extinction risk, understand the possible impacts of environmental pressures and inform management practices (Ovaskainen and Meerson Citation2010; Heinsohn et al. Citation2015, Citation2022). This may be especially useful when it is difficult to observe directly these processes due to a lack of effective monitoring (Heinsohn et al. Citation2022). Given their inherent flexibility, these models can also be used to hindcast the response of wildlife populations to past environmental change. By doing so, practitioners can explore the factors and interactions that may have contributed to past population growth or decline in situations where field data are unavailable. For example, population models have been used to explore the dynamics involved in the extinction of Steller’s Sea Cow Hydrodamalis gigas in the Bering Sea (Turvey and Risley Citation2006), the extinction of the Thylacine Thylacinus cynocephalus in Tasmania (Prowse et al. Citation2013) and the history of Brown Bears Ursus arctos in Ireland (Leonard et al. Citation2013). Modelling past population trajectories may provide insight into a species’ population dynamics and inform better management of future populations.
The Norfolk Island Green Parrot Cyanoramphus cookii (hereafter ‘Green Parrot’) is a prime example of a cryptic, threatened species for which obtaining accurate population data is difficult (Department of Climate Change, Energy, the Environment and Water Citation2023). Green Parrots are often inconspicuous in their breeding behaviour and are known to breed year-round in the dense sub-tropical forests on Norfolk Island (Hicks and Greenwood Citation1989; Hicks and Preece Citation1991; Hill Citation2002). The single population of the species has experienced significant decline since European settlement (Hicks and Greenwood Citation1989; Hill Citation2002; Ortiz-Catedral et al. Citation2018). This has been driven by extensive habitat clearance, persecution, predation (by introduced Cats Felis catus and rats Rattus spp.) and introduced competitors (Hicks and Preece Citation1991; Hill Citation2002). Since the 1980s, the population has been managed within Norfolk Island National Park, with a particular focus on pest control (both predators and competitors) and the predator-proofing, maintenance and monitoring of known and potential Green Parrot nests (hereafter ‘managed nests’) (Hicks and Greenwood Citation1989; Hicks and Preece Citation1991; Hill Citation2002). Despite the population recovering from possibly fewer than 17 birds in the 1980s (Hermes et al. Citation1986) to approximately 160 birds in 2001 (Hill Citation2002), it apparently remains unstable, with an estimated 46–92 birds reported in 2013 (Ortiz-Catedral Citation2013). Since 2013, four successive annual population estimates made using distance sampling suggest recovery to 438 ± 168 S.E. by 2017 (Skirrow Citation2018). However, monthly monitoring of managed nests from 2013 to 2017 detected only 240 fledglings (Parks Australia, unpub.), which does not account for the rapid population growth suggested by the surveys. High rates of banding at these managed nest sites are also not reflected in the contemporary population, leading us to suspect that Green Parrots have been breeding undetected in natural nest sites.
We used stochastic population models to hindcast the most probable population dynamics of Green Parrots. While Green Parrots benefit from managed nests (Ortiz-Catedral et al. Citation2018), other interventions such as predator control also are implemented concurrently, and the large proportion of unmarked birds suggests these interventions are enabling some successful breeding outside managed nests. We use eight years of nest monitoring data and surveys of marked birds in the 2021 population to inform simulations of population growth for the Green Parrot since the latest population slump in 2013. We investigate several scenarios to explain observed changes in population size and the high proportion of unmarked birds observed in 2021 by varying potential mortality rates, initial population size and the availability of natural nest sites. We compare the results of our models and evaluate the efficacy of our approach as a tool for understanding population dynamics in this endangered species.
Materials and methods
Study area and species
Norfolk Island is a small, isolated island territory located in the South Pacific Ocean, approximately 1400 km off the east coast of Australia. The Mount Pitt Section of Norfolk Island National Park comprises approximately 460 hectares of remnant sub-tropical forest in the northern half of the island (Director of National Parks Citation2010). This is the largest tract of natural vegetation on Norfolk Island and is considered the stronghold of the island’s endemic forest birds, including the Green Parrot (Director of National Parks Citation2010). Since the 1980s national park staff have been establishing managed nest sites for the Green Parrot by modifying existing natural nests and creating artificial nests to increase nest availability and limit access by introduced mammalian predators (Hicks and Greenwood Citation1989; Hill Citation2002; Ortiz-Catedral et al. Citation2018). These managed nests are heterogeneous and have experienced varying levels of use by breeding birds (Gautschi et al. Citation2022). Despite some ongoing management, a population slump was recorded in 2013 (Ortiz-Catedral Citation2013). Subsequently, the intensity of both predator control and nest-site monitoring and maintenance was increased (for details see Ortiz-Catedral et al. Citation2018). In total, 85 managed nest sites were available between 2013 and 2020, with 71 still in useable condition by 2021 (Gautschi et al. Citation2022).
Data collection
Since 2013, staff from Norfolk Island National Park have conducted monthly checks of parrot nesting at managed nest sites. Although Green Parrots are known to breed occasionally in natural sites, when discovered their nests have typically been incorporated into the managed nest cohort and made predator-proof (Hicks and Greenwood Citation1989; Hill Citation2002; Ortiz-Catedral et al. Citation2018). Active nests at managed sites were visited frequently to record brood size and nest fate and to measure and band nestlings. Most birds were fitted with two bands, including a numbered, colour band. Between 2013 and 2020, the fledging of over 400 Green Parrots was recorded, including more than 300 banded birds (Parks Australia, unpub.). From these data, the number of known fledglings per active nest site per year can be determined (recorded by estimated hatch date), with reasonable confidence, in addition to the average number of managed nest sites used each year. Nests were assumed to be successful when they had advanced nestlings on the last visit and the nests were then observed to be empty without signs of predation or failure. Only data from 2014 to 2020 were used here to determine demographic statistics, as only a partial year of nesting was monitored in 2013.
To estimate the proportion of the population marked with leg bands in the Norfolk Island National Park, we conducted two field surveys in March and May 2021. One of us (DG) walked public paths, management trails, and went off-track in areas known to be frequented by Green Parrots. We adapted the mark-resighting methodology used for Green-rumped Parrotlets Forpus passerinus (Casagrande and Beissinger Citation1997). Both surveys were conducted over five days and covered 34 km and 30 km from 12–16 March and 10–14 May, respectively. Birds were counted if the observer confirmed whether they were marked using binoculars or a digital camera with a 300 mm lens. Among the birds counted, only one marked bird was confirmed to have been observed in both March and May. Because there is no way of knowing which other birds were included in both surveys, despite the possibility of double counting, all birds from both surveys were included when calculating the combined ratio of marked to unmarked birds. This follows the logic that all birds (whether marked or unmarked) were equally likely to be observed in both surveys and therefore the observed ratio should remain consistent.
Stochastic population models
We used VORTEX v10.5.5 software (Lacy and Pollak Citation2021) to simulate the growth of the Green Parrot population between 2013 and 2021. We harnessed the program’s ability to incorporate stochastic demographic events (Lacy Citation1993) to explore how mortality rates and dependence on managed nests might explain the observed proportion of unmarked birds in our study system. We modelled the Green Parrot population both as a population that used only managed nests (referred to as a ‘single population model’ hereafter), and as a population that could use both managed and unmanaged nests (referred to as a ‘metapopulation model’ hereafter). The metapopulation models represented the system as two connected sub-populations, comprising marked birds hatched in managed nests and unmarked birds hatched in natural nests. The metapopulation models assumed that the two sub-populations, though entirely sympatric and free to intermingle socially, remained largely separate when breeding. Thus, in those models, pairs that occupied managed nest sites preferentially reoccupied these sites and dispersed to the natural population only when these managed sites were occupied. Parrots often show nest site fidelity (Heinsohn et al. Citation2003; Saunders et al. Citation2018) and nest site fidelity has been observed in Green Parrots (Hicks and Preece Citation1991). We also know that the number of managed nests used each year was fairly consistent from 2014 to 2020 (21 ± 1.5 S.D.), despite population growth. If females saturated the managed nests, the model allowed them to spill over into natural nests (if available) as determined by a formula in VORTEX (Supplementary Material, Formula S1), but all birds hatched in natural nests bred in natural nests. Breeding was limited in each sub-population by the number of available nest sites using a further VORTEX formula (Supplementary Material, Formula S2). The metapopulation model enabled us to differentiate mortality and breeding rates due to different predation pressure in managed/natural nests.
We selected demographic and environmental rates for our models based on knowledge of Green Parrots and related taxa (, Table S1). We assumed long-term monogamy, which has been observed in Green Parrots (D. Gautschi, unpub.) and is typical of many parrot species (Toft and Wright Citation2015), and an age of first reproduction of 2.12 (Bird et al. Citation2020). Green Parrots can nest multiple times in a year (Hicks and Greenwood Citation1989; Hill Citation2002), so we calculated fecundity as the number of fledglings produced per year per nest (2.74 ± 2.12 S.D., Parks Australia, unpub.). To avoid constraining population growth over the study period, carrying capacity was set at an arbitrarily high value of 5,000 ± 500 S.D. for each population (10,000 total). No information is available on inbreeding depression in Green Parrots, so we used the default setting of 6.29 lethal equivalents due to recessive alleles (O’Grady et al. Citation2006). The environmental variation (EV) correlation between reproduction and survival was kept at the default level (0.5). Sensitivity testing exploring a range of possible values indicated that this did not have a significant impact on population size (Supplementary Material, Table S3 and Method S1). In the metapopulation models, we set the EV correlation among populations at 1 because they are sympatric. All model scenarios were run with 1,000 iterations.
Table 1. Life-history parameters used for stochastic modelling of the Norfolk Island Green Parrot population. EV represents environmental variation. NA indicates where a value is not applicable.
We used a mortality rate of 20% per annum, estimated for the genus by Bird et al. (Citation2020). We used the same mortality rate for first year birds, second year birds and adult birds as age-specific mortality rates are unknown for the species. Sensitivity testing indicated that adult mortality and juvenile mortality rates had an equivalent impact on population size (Supplementary Material, Table S3). We tested explicitly the implications of a 5% increase and decrease in this modelled estimate of mortality. We also created scenarios in which both increased breeding female mortality and decreased reproductive output occurred in the natural population, to represent potentially heightened predation in natural nests. In these scenarios we increased mortality for breeding aged females in the natural population by 5% and decreased reproductive output by 25%. This was achieved by multiplying the yearly output of all managed nest sites recorded between 2014 and 2020 by 0.75 and recalculating the mean and standard deviation (2.06 ± 1.59). Maximum lifespan was set to 21, the age at which fewer than 1% of birds would still be alive according to a 20% yearly mortality rate (maximum lifespan is uncertain).
We chose the initial population sizes for 2013 based on the upper, middle and lower estimates from Ortiz-Catedral (Citation2013). We assumed that the population in 2013 was divided equally between managed and natural nesters (i.e. 34 marked birds and 34 unmarked). While a male-biased adult sex ratio was inferred from observations in 2013 (Ortiz-Catedral Citation2013), we chose to use an even sex ratio for the 2013 population as visual assessments have not been validated using molecular techniques. In addition, an even sex ratio at birth was observed across nestlings sampled at nest sites between 2015 and 2022 (D. Gautschi, unpub.). However, models in which female mortality was higher due to increased predation led to scenarios where the adult sex ratio was male-biased. This is similar to other parrot species in the wild, such as Eclectus Parrots Eclectus roratus and Swift Parrots Lathamus discolor (Heinsohn and Legge Citation2003; Heinsohn et al. Citation2019), in which the sex ratio at hatching is equal but sex-biased mortality leads to biased adult sex ratios.
The number of natural nest sites available for Green Parrot breeding is not known. Sensitivity testing indicated that the number of natural nests available would have a significant impact on population size (Supplementary Material, Table S3). Therefore, based on our understanding of the study site, we assumed 100 natural nest sites were available per year, but also modelled scenarios with half or double this number.
To convert the managed population estimate produced in VORTEX to a marked population estimate, we needed to account for three factors that should influence the number of marked birds available for detection: (i) dispersal from the managed population to the natural population; (ii) missed marking opportunities in managed nests; and (iii) band attrition. We corrected the managed population estimate for each of these factors to derive a realistic marked population size, as follows ().
Figure 1. Process used to convert Norfolk Island Green Parrot managed population and natural population estimates produced by VORTEX into marked and unmarked population estimates.
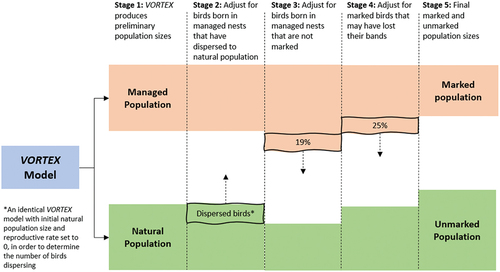
Firstly, in our models, we set dispersal to be one-way from the managed population to the natural population. Because managed birds were marked, it follows that birds dispersing to breed elsewhere were also marked. Therefore, a subset of the natural population must have been marked, even though they produced unmarked offspring. Thus, the total number of marked birds in the metapopulation was the sum of the managed population plus the subset of naturally-breeding marked birds. To estimate the number of marked birds in the natural population, we ran a second stochastic simulation in VORTEX for each scenario (M1–M45, Table S2) to estimate the number of marked natural breeders that survived to 2021. These birds were then added to the managed population size to derive a marked population size.
Secondly, to address missed marking opportunities, we calculated that 19.02% of birds in the managed nests were reported to have fledged prior to banding between 2013 and 2020 (Parks Australia, unpub.). We subtracted this percentage from the marked population and added the corresponding number to the unmarked population.
Thirdly, some attrition of colour bands has been observed in the Green Parrot population but the rate at which this occurs is unknown (D. Gautschi, unpub.). To ensure that the population size and the importance of natural nesting was not overestimated as a result, 25% band attrition (presumed to be the likely upper value) was applied.
Results
Mark-resighting surveys
One hundred and nine Norfolk Island Green Parrots were observed across the two band resighting surveys. Of these, the banding status of 68 birds could be determined. In the March survey 3/29 (10.34%) birds were marked, while in the May survey 9/39 (23.08%) birds were marked; equating to a mean 17.65% of the population marked.
Population models
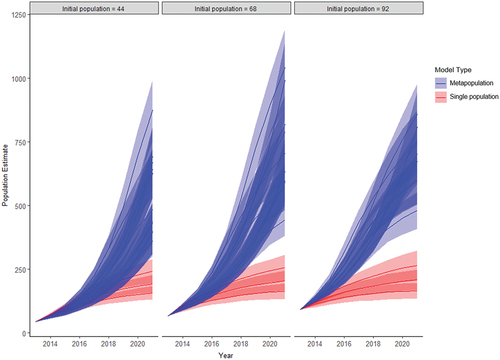
The single population model produced a median population estimate of 206 ± 38.5 S.D. for 2021 (Range 158–266, ).
Table 2. Results of the single population models showing Norfolk Island Green Parrot population estimates for combinations of mortality rates and initial population size.
Thirty-one of 45 metapopulation models produced a comparable (±5%) proportion of marked birds in the 2021 population to the proportion observed in the field (17.65%) (). These models had a median population estimate of 635 ± 113.3 S.D. (Range 360–1,041). The full range of model scenarios aimed at testing the combinations of variables is presented in Table S2.
Table 3. Metapopulation models leading to a percentage of Norfolk Island Green Parrots with bands within 5% of the estimate for 2021 (17.65%) with 25% band loss. All instances for which natural breeding females have a different mortality rate to the rest of the population also incorporate a 25% reduction in reproductive output from natural nest sites.
The 2021 population estimates produced by the single population model (158–266) were consistently lower than the population estimates produced by the metapopulation model with a comparable proportion of unmarked birds to that observed in 2021 (360–1,041). Population trajectories illustrating the differences in the range of estimates the models produced are shown in .
Figure 2. Yearly Norfolk Island Green Parrot population estimates produced by all single population models and metapopulation models with a comparable proportion of marked birds in the population (±5%) to that observed in the wild population in 2021 (17.65%). Ribbons indicate standard deviation of the estimates.
Discussion
An understanding of the nature and cause of population fluctuations is a fundamental part of conservation biology (Lande et al. Citation2003). The Norfolk Island Green Parrot has experienced drastic population fluctuations in the last 50 years in which numbers have dropped to fewer than 100 individuals on more than one occasion (Hicks and Greenwood Citation1989; Ortiz-Catedral Citation2013). Distance sampling estimates indicate the population rapidly increased in size from 46–92 in 2013 (Ortiz-Catedral Citation2013) to 438 ± 168 S.E. in 2017 (Skirrow Citation2018). Based on rudimentary calculations, this increase was not possible if the parrots bred only in managed nest sites. Furthermore, 81% of birds hatched in managed nests were marked, so the rarity of marked birds in the 2021 population (17.65%) also suggests managed nests were not the only breeding habitat used. These observations combined necessitated further exploration of the population dynamics at play. We created several population models, varying potential mortality rates, initial population size and the availability of natural nest sites. Below we discuss the important insights into population dynamics and wildlife management that can be gained by the application of stochastic population models, and the assumptions made in our approach.
Annual population estimates derived from the single population models, which limited breeding to managed nests, failed to show the rapid increase in population size observed by Skirrow (Citation2018). Metapopulation models, which allowed for breeding in natural nests, produced consistently larger population estimates that were able to both account for the large proportion of unmarked birds observed in 2021 and a rapid increase in population size since 2013. These results suggest that frequent undetected natural nesting could explain the proportion of parrots with bands observed in 2021. As Green Parrots are capable of successful reproduction in tree hollows as well as ground cavities formed by root decay all year round (Hicks and Preece Citation1991; Hill Citation2002), the necessary level of additional recruitment to the population is possible. If our model-based findings are reflective of the real world, they suggest that broadscale predator control may yield greater benefit at a population level than predator-proofing individual nest sites, particularly considering the uncertainty regarding suitable nest design for Green Parrots (Gautschi et al. Citation2022).
Although our models suggest that many birds must breed in natural sites, we made several necessary assumptions that should be considered when interpreting these findings. We assumed that no nesting in managed nests was missed or misreported between 2013 and 2020, that half of the population in 2013 was marked, that the initial sex ratio was 50:50, and that no more than 25% band attrition occurred. Our approach, summarising nesting success on an annual basis, necessitated taking initial population sizes from mid-2013 (Ortiz-Catedral Citation2013), despite our model beginning from the start of 2013.
Our surveys for the percentage of birds with bands observed in 2021 did not allow the construction of confidence intervals and assumed that all parrots were equally likely to be encountered. This assumption was tested for Green-rumped Parrotlets by Casagrande and Beissinger (Citation1997) who used a similar method and found it to be reliable. However, a substantial surveying effort would also be required to increase the confidence of this percentage (Casagrande and Beissinger Citation1997). Nonetheless, our observation that 17.65% of sighted birds had bands was further supported by mist netting efforts in 2021 and 2022, which found that nine of 51 (17.65%) captured birds had at least one leg band (D. Gautschi, unpub.).
When not known for Green Parrots, we used demographic values from the genus or family. These included using a mortality rate of 20% for both sexes as a guide, based on the hierarchical extrapolation for the genus (Bird et al. Citation2020), and experimenting with 5% variations to this figure. While members of the Cyanoramphus genus have been documented breeding at a young age (Ortiz-Catedral et al. Citation2010), in the absence of studies on the age of first breeding for Green Parrots we used an age of first breeding of 2, based on the modelled value of 2.12 years produced by Bird et al. (Citation2020).
Sensitivity testing indicated that adult male, adult female and juvenile mortality all had a significant but equivalent impact on population size. We kept juvenile (first year and second year) mortality rates the same as those for adults based on a lack of age-specific mortality data, but modelled scenarios in which females breeding in natural nests faced a 5% increase in mortality and 25% reduction in output in an attempt to account for increased predation risks at these nests. While natural nests have not been studied and therefore mortality and reproductive rates are unknown (Hill Citation2002), heightened predation risk is likely to be faced by birds in these nests as they are less protected from rats and cats (Hicks and Greenwood Citation1989; Ortiz-Catedral Citation2013). Both the 5% mortality increase, and 25% reproductive output decrease were arbitrary but nonetheless produced plausible population trajectories in keeping with other estimates.
Our metapopulation models assumed high hollow fidelity between seasons, which has been observed in many parrot species (Heinsohn et al. Citation2003; Saunders et al. Citation2018) including Green Parrots (Hicks and Preece Citation1991, D. Gautschi, unpub.). Our models further assumed that any records of multiple breeding attempts within a year at a single nest site involved a single pair. This may not always be the case, and it is also possible that some birds that normally use natural nests sometimes secure a managed nest site. In either case, nestlings were marked only in managed nests, regardless of the status of the parents, so all offspring would be accurately reflected as part of either the managed (marked) or natural (unmarked) population. Movement of adults from the natural population to the managed population would have only a minor impact on final population size. Our metapopulation models also assumed that the number of natural nest sites remains constant over time. We know that this is not strictly the case for managed nest sites, as the number of nests used between 2014 and 2020 was 21 ± 1.5 S.D. Similarly, the number of natural sites available for nesting is likely to vary over time due to progressive development and decay of sites.
The limitations discussed above highlight that the quantitative aspects of our analysis (e.g. final population estimate) should be treated with caution. However, even in cases of data sparsity, population models can still be of great value (Brook et al. Citation2002), particularly when guided by clear research questions (Chaudhary and Oli Citation2020). The main value of our method lies in an enhanced understanding of the likely drivers of Green Parrot population growth including the indication that a large proportion of the population may be breeding in unmodified natural nests, which previously have largely been considered inadequate to support breeding due to their exposure to introduced predators (Hicks and Greenwood Citation1989; Hicks and Preece Citation1991; Hill Citation2002; Ortiz-Catedral Citation2013). Our models suggest that natural nests may have played a significant role in the population recovery since 2013. This has implications for the management of the species. Currently, a major focus for Green Parrot management is placed on providing managed nest sites for breeding, with general predator control and habitat restoration used to support all native species on the island. Our models suggest that Green Parrots are capable of breeding successfully without predator proofing of nests under the recent regime of predator control. Managed nest sites may be a crucial conservation tool, especially when a population reaches critically low levels (Hicks and Greenwood Citation1989; Ortiz-Catedral et al. Citation2018), but our models also suggest that natural nests have high value if broad-scale control of introduced rats and cats is maintained. Further research into life history and monitoring of natural nest sites will be essential in establishing any difference in mortality and reproductive rates between these sites and managed sites. Our method can also be adapted to compare the effect of different conservation approaches moving forward, and help to inform the best management decisions (Heinsohn et al. Citation2022).
For threatened species around the world, imperfect monitoring and sparse data can be a hindrance to proper assessment of the efficacy of different management actions (Fraser et al. Citation2022). In this study, we show how by combining stochastic population models and field observations, important insights into the population dynamics of a threatened bird can be attained, whilst also inferring the importance of management actions used to support them. Our method is not limited to the Norfolk Island Green Parrot, and can be applied to any species for which population dynamics are poorly understood. This may prove particularly useful for small populations, in which the impact of ongoing management is difficult to measure.
Author contributions
DG and RH conceived and designed the research; DG conducted the surveys; MW collected long-term data; DG and RH analysed the data; DG, RH, DS, NM, LO, PO, RC and MW wrote and edited the manuscript.
Supplemental Material
Download MS Word (28.1 KB)Supplemental Material
Download MS Word (28.1 KB)Acknowledgments
Thanks to staff at Norfolk Island National Park for providing the nesting and banding records used for this study and to all park staff, past and present, involved in the collection of these data.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed at https://doi.org/10.1080/01584197.2023.2267606.
Additional information
Funding
References
- Bird, J. P., Martin, R., Akçakaya, H. R., Gilroy, J., Burfield, I. J., Garnett, S. T., and Symes, A., et al. (2020). Generation lengths of the world’s birds and their implications for extinction risk. Conservation Biology 34(5), 1252–1261. doi:10.1111/cobi.13486
- Brook, B. W., Burgman, M. A., Akçakaya, H. R., O’Grady, J. J., and Frankham, R. (2002). Critiques of PVA ask the wrong questions: Throwing the heuristic baby out with the numerical bath water. Conservation Biology 16(1), 262–263. doi:10.1046/j.1523-1739.2002.01426.x
- Casagrande, D. G., and Beissinger, S. R. (1997). Evaluation of four methods for estimating parrot population size. The Condor 99(2), 445–457. doi:10.2307/1369951
- Chaudhary, V., and Oli, M. K. (2020). A critical appraisal of population viability analysis. Conservation Biology 34(1), 26–40. doi:10.1111/cobi.13414
- Department of Climate Change, Energy, the Environment and Water. (2023). ‘Cyanoramphus Cookii in Species Profile and Threats Database.’ (Department of Climate Change, Energy, the Environment and Water: Canberra.)
- Director of National Parks. (2010). ‘Norfolk Island Region Threatened Species Recovery Plan.’ (Department of the Environment, Water, Heritage and the Arts: Canberra.)
- Fraser, H., Legge, S. M., Garnett, S. T., Geyle, H., Silcock, J., Nou, T., and Collingwood, T., et al. (2022). Application of expert elicitation to estimate population trajectories for species prioritized in Australia’s first threatened species strategy. Biological Conservation 274, 109731. doi:10.1016/j.biocon.2022.109731
- Gautschi, D., Heinsohn, R., Crates, R., Macgregor, N. A., Wilson, M., and Stojanovic, D. (2022). Utilization of modified and artificial nests by endemic and introduced parrots on Norfolk Island. Restoration Ecology 30(5), e13586. doi:10.1111/rec.13586
- Heinsohn, R., Lacy, R., Elphinstone, A., Ingwersen, D., Pitcher, B. J., Roderick, M., and Schmelitschek, E., et al. (2022). Population viability in data deficient nomadic species: What it will take to save regent honeyeaters from extinction. Biological Conservation 266, 109430. doi:10.1016/j.biocon.2021.109430
- Heinsohn, R., and Legge, S. (2003). Breeding biology of the reverse-dichromatic, co-operative parrot Eclectus roratus. Journal of Zoology 259(2), 197–208. doi:10.1017/S0952836902003138
- Heinsohn, R., Murphy, S., and Legge, S. (2003). Overlap and competition for nest holes among eclectus parrots, palm cockatoos and sulphur-crested cockatoos. Australian Journal of Zoology 51(1), 81–94. doi:10.1071/ZO02003
- Heinsohn, R., Olah, G., Webb, M., Peakall, R., Stojanovic, D. (2019). Sex ratio bias and shared paternity reduce individual fitness and population viability in a critically endangered parrot. Journal of Animal Ecology 88(4), 502–510. doi:10.1111/1365-2656.12922
- Heinsohn, R., Webb, M., Lacy, R., Terauds, A., Alderman, R., and Stojanovic, D. (2015). A severe predator-induced population decline predicted for endangered, migratory swift parrots (Lathamus discolor). Biological Conservation 186, 75–82. doi:10.1016/j.biocon.2015.03.006
- Hermes, N., Evans, O., and Evans, B. (1986). Norfolk Island birds: A review 1985. Notornis 33, 141–149.
- Hicks, J., and Greenwood, D. (1989). Rescuing Norfolk Island’s parrot. Birds International 1, 34–47.
- Hicks, J., and Preece, M. (1991). Green parrot. 1991 recovery plan. Unpublished Report, Australian Parks and Wildlife Service, Canberra.
- Hill, R. (2002). ‘Recovery Plan for the Norfolk Island Green Parrot (Cyanoramphus novaezelandiae cookii).’ (Natural Resource Management, Environment Australia: Canberra.)
- Lacy, R. C. (1993). VORTEX: A computer simulation model for population viability analysis. Wildlife Research 20(1), 45–65. doi:10.1071/WR9930045
- Lacy, R. C., and Pollak, J. P. (2021). ‘Vortex: A Stochastic Simulation of the Extinction Process.’ (Chicago Zoological Society: Brookfield, IL.)
- Lande, R., Engen, S., and Saether, B. (2003). ‘Stochastic Population Dynamics in Ecology and Conservation.’ (Oxford University Press: Oxford, UK.)
- Leonard, S. A., Risley, C. L., and Turvey, S. T. (2013). Could brown bears (Ursus arctos) have survived in Ireland during the last glacial maximum? Biology Letters 9(4), 20130281. doi:10.1098/rsbl.2013.0281
- Melbourne, B. A., and Hastings, A. (2008). Extinction risk depends strongly on factors contributing to stochasticity. Nature 454, 100–103. doi:10.1038/nature06922
- O’Grady, J. J., Brook, B. W., Reed, D. H., Ballou, J. D., Tonkyn, D. W., and Frankham, R. (2006). Realistic levels of inbreeding depression strongly affect extinction risk in wild populations. Biological Conservation 133(1), 42–51. doi:10.1016/j.biocon.2006.05.016
- Ortiz-Catedral, L. (2013). ‘The Population and Status of Green Parrot (Tasman Parakeet) Cyanoramphus cookii on Norfolk Island 2013.’ (Institute of Natural and Mathematical Sciences, Massey University: Auckland, New Zealand.)
- Ortiz-Catedral, L., Kearvell, J. C., Hauber, M. E., and Brunton, D. H. (2010). Breeding biology of the critically endangered malherbe’s parakeet on Maud Island, New Zealand, following the release of captive-bred individuals. Australian Journal of Zoology 57(6), 433–439. doi:10.1071/ZO09098
- Ortiz-Catedral, L., Nias, R., Fitzsimons, J., Vine, S., and Christian, M. (2018). Back from the brink–again: The decline and recovery of the Norfolk Island green parrot. In ‘Recovering Australian Threatened Species: A Book of Hope.’ (Eds S. Garnett, J. Woinarski, D. Lindenmayer, and P. Latch.) pp. 105–114. (CSIRO Publishing: Clayton South, Australia.)
- Ovaskainen, O., and Meerson, B. (2010). Stochastic models of population extinction. Trends in Ecology & Evolution 25(11), 643–652. doi:10.1016/j.tree.2010.07.009
- Prowse, T. A. A., Johnson, C. N., Lacy, R. C., Bradshaw, C. J. A., Pollak, J. P., Watts, M. J., Brook, B. W. (2013). No need for disease: Testing extinction hypotheses for the thylacine using multi-species metamodels. Journal of Animal Ecology 82(2), 355–364. doi:10.1111/1365-2656.12029
- Saunders, D., White, N., Dawson, R., and Mawson, P. (2018). Breeding site fidelity, and breeding pair infidelity in the endangered Carnaby’s Cockatoo Calyptorhynchus latirostris. Nature Conservation 27, 59–74. doi:10.3897/natureconservation.27.27243
- Skirrow, M. J. A. (2018). Estimating the population size of two critically endangered South Pacific parakeets: The Tasman Parakeet and Malherbe’s Parakeet. Thesis, Massey University, New Zealand.
- Toft, C. A., and Wright, T. F. (2015). ‘Parrots of the Wild: A Natural History of the World’s Most Captivating Birds.’ (University of California Press: Oakland, CA.)
- Turvey, S. T., and Risley, C. L. (2006). Modelling the extinction of Steller’s sea cow. Biology Letters 2(1), 94–97. doi:10.1098/rsbl.2005.0415
