ABSTRACT
Vocalisations are a key component of communication for species that employ crypsis. The Eastern Ground Parrot (Pezoporus wallicus wallicus) is one such species that lives within dense heathland habitats in eastern Australia. Passive acoustic monitoring is now a common method used for avian monitoring and conservation programmes. For implementation of this method to be successful, we require a minimum level of understanding of the environmental and behavioural factors that may influence vocal activity. We tested how Eastern Ground Parrot call rate and chorus duration fluctuated across key temporal, environmental and meteorological variations over one year using passive acoustic monitoring from one site. Season, moon phase and days since rainfall predicted vocal activity of the birds at this site. Our results demonstrate the importance of considering the behavioural response of species to environmental conditions to account for potential bias in the interpretation of passive acoustic monitoring data.
Introduction
Avian vocalisations are important behaviours for reproduction, foraging, territory maintenance and predator awareness/avoidance (Gil and Gahr Citation2002; Magrath et al. Citation2015). Passive acoustic monitoring (PAM) devices can utilise the ubiquity of avian vocalisations by collecting consistent and repeatable acoustic datasets with relatively low labour input across long time periods (Sugai et al. Citation2019). Data obtained using these methods can inform researchers on population metrics, vocal activity and associated behaviours of vocal species (Pérez-Granados and Traba Citation2021; Ross et al. Citation2023).
In the wild, the transmission of acoustic signals can depend on habitat, climatic conditions and the surrounding soundscape (Darras et al. Citation2016; Hutschenreiter et al. Citation2023). For example, rain impedes acoustic transmission of Tawny Owl Strix aluco calls (Lengagne and Slater Citation2002), and other vocalisations have been found to be masked by heterospecific acoustic signals, anthropogenic noise or abiotic natural noise (Pijanowski et al. Citation2011; Depraetere et al. Citation2012; Bedoya et al. Citation2017). Increased transmission potential is a common explanation for why studies tend to focus on what is termed the ‘chorus’: a period of increased acoustic signalling across many taxa usually occurring around sunrise (dawn chorus) and/or sunset (dusk chorus; Brown and Handford Citation2003; Catchpole and Slater Citation2008; Sugai et al. Citation2019).
Vocal activity itself may also be predicted by environmental conditions. Climatic variation may contribute to changes in metabolic energy demands, impacting availability of food and/or thermoregulation requirements (Berg et al. Citation2005; Gillooly and Ophir Citation2010; Ophir et al. Citation2010). Meteorological circumstances such as rainfall were found to impact call rate of two non-passerine avian species, where both had increased vocal activity around periods of rainfall in the dry season despite decreased transmission, potentially due to the increased food resource it provides (Pérez-Granados and Schuchmann Citation2021). Interestingly, the amount of light available at night can affect the circadian rhythm and the vocal behaviours of some avian species. For example, natural light variability based on moon phase likely influences reproductive behaviours of the diurnal avian species Willie Wagtail Rhipidura leucophrys (Dickerson et al. Citation2020), although these patterns are far from universal (Digby et al. Citation2014).
Acoustic signals are the main form of communication for Eastern Ground Parrots (EGP: Pezoporus wallicus wallicus), a cryptic bird living within dense vegetation consisting of heathland and sedgeland, typically below 2 metres in height (McFarland Citation1988, Citation1991a, Citation1991c). Fire history and rainfall may influence resource availability within coastal heathland habitats for this species (McFarland Citation1988, Citation1991a). Clarifying its status is challenging because observations of individuals within their natural habitat is near impossible, but a recent assessment (Oliver et al. Citation2021) highlighted a decreasing population trend for EGP and classified the species as near threatened (McFarland Citation1991b).
EGP are known to chorus immediately before sunrise and shortly after sunset with low sunlight, making these time periods the most effective for monitoring populations (McFarland Citation1991b). Previous studies have suggested that longer calling events correspond with full moons, but this correlation has not yet been statistically verified (McFarland Citation1991b). Further information such as seasonal temporal variations in vocal activity are otherwise poorly understood for EGPs, yet are likely important considerations as additional context for future assessment and monitoring (Ross et al. Citation2023).
The aim of this study was to quantify vocal outputs of a population of EGPs during dawn and dusk chorus periods and investigate potential correlative environmental conditions that could influence call rate and chorus duration in this species. Understanding the rate and extent of vocal activity across the year could improve monitoring programmes by allowing optimised deployment periods of automated acoustic recorders for EGP.
Methods
Study site
The study was completed in a patch of subtropical wallum heathland habitat within Noosa National Park, Sunshine Coast, Queensland (−26.473S, 153.083E). The patch size is roughly 147 hectares, with an estimated occupancy of 5–25 birds calculated in 2004 (Chan and Mudie Citation2004, ‘Woodland Drive’ site). Movement within this patch is unknown and previous studies suggest the birds may utilise different areas seasonally, depending on the vegetation structure and floristic diversity (McFarland Citation1991a).
Data collection
Field data were collected over six deployment periods of approximately three weeks each, between July 2018 and May 2019 (Supplementary Table S1) with one Passive Acoustic Recorder (Frontier Labs, model: BAR, https://frontierlabs.com.au/). Recordings were timed to capture the dawn and dusk period each day, with a recording duration of 100 minutes concluding at sunrise and 100 minutes commencing at sunset to capture chorus periods as described by McFarland (Citation1991b). The recorder was fitted with an omnidirectional microphone (total gain 50 dB) and programmed to collect 16bit acoustic samples (sampling rate 44.1 kHz; frequency response 100 Hz−12 kHz), tailored to recording EGP vocalisations (frequency range 26 kHz; Chan and Mudie Citation2004). The recorder was mounted to a star picket 1.1 m above ground, with an estimated radius of acoustic detection for EGP between 90 and 150 m (Thomas et al. Citation2020).
Acoustic analysis
A random number generator arranged the order for analysing each recording, aimed at keeping the analyst blind to the date and time of recordings (Bluff Citation2016). EGP calls were identified by comparing the structure of call notes and syllables to those of previous research (Chan and Mudie Citation2004). Raven Pro v1.5 (Cornell Lab of Ornithology, Ithaca, NY, https://ravensoundsoftware.com/software/raven-pro), was used by author LP to visually identify and annotate all calls on a spectrogram (an example can be found in Supplementary Figure S1). The response variables extracted included the call rate (calls per hour of recording) and the duration of each chorus (difference in seconds between the first call start and last call end time). Recordings were excluded from the study where calls were less detectable due to poor weather conditions, masking from excessive background noise or technical errors (Depraetere et al. Citation2012; Priyadarshani et al. Citation2018). This resulted in the use of 195 recordings (316.8 hours, which includes 131.6 dawn and 185.2 dusk hours) out of the 385 (604.3 hours) recording sampling effort (Supplementary Table S1).
Predictor variables
Meteorological data were collected using daily weather information from the closest (10 km) weather station (Tewantin; Bureau of Meteorology Citation2019). The predictor variables obtained included: (i) number of days since rainfall; (ii) mean daily temperature (degrees Celsius); (iii) moon phase (days to/from full moon, where full moon = 0 and new moon = 15). Temporal variables associated with each recorded chorus were also collected, including season (based on solar equinox and solstice dates) and time of day (dawn or dusk).
Statistical analysis
All statistical analyses were run using R version 4.2.1 (R Core Team Citation2022). Covariance of predictor variables was examined using correlation plots. Where predictor variables were shown to be correlated (R2 ≥ 0.50), only one variable was retained in the dataset to reduce the potential model bias (Freckleton Citation2002). Mean temperature was correlated with season (Supplementary Table S2), thus only season was retained as a predictor variable, as it was determined to be likely the most biologically significant for vocal behaviour. Days since rainfall was kept in the model even though recordings with heavy rainfall were discarded, since rainfall could affect resource availability and thus vocal behaviour. Response variables (call rate, chorus duration) were individually tested for normality, and both showed positively skewed distributions. Data were log-transformed for call rate and square root-transformed for duration to establish normal distributions.
A generalised linear model (GLM) with Gaussian distribution and identity link function was run for each response variable (above). The maximal models were then simplified using a backwards stepwise selection process and compared using Akaike’s Information Criterion (AIC; Symonds and Moussalli Citation2011). Time of day did not interact with any other variables in the call rate model, thus it was included as an additive predictor without interaction in this model.
Results
Time of day was a significant predictor of EGP call rate, where call rate was ~ 80% higher at dusk compared to dawn (back-transformed coefficient estimate = 182.5; ). The mean value for call rate at dawn was 15.6 calls per hour, while dusk was 51.9 calls per hour (Supplementary Figure S2). Multiple interactive effects were also identified as affecting both call rate and chorus duration ().
Table 1. ANOVA results on the minimum adequate generalised linear model for call rate and chorus duration of Eastern Ground Parrots in Noosa National Park, SE Queensland, with degrees of freedom (df), F values and p values. Significant predictors are indicated in bold.
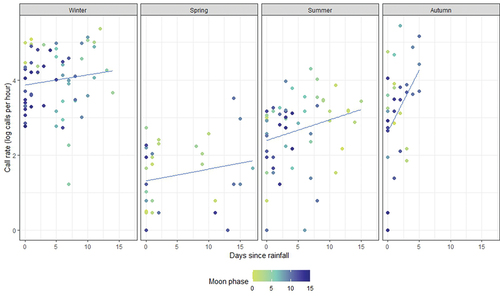
Generally, parrots called at a higher rate in winter, and at the lowest rate in spring, although across all seasons we observed an increase in call rate with days since rainfall, most pronounced in autumn (). In autumn, rainfall appeared much more important than moon phase in predicting call rate. Notably, autumn analysed recordings had a maximum of five days since rain. Most seasons showed a tendency for higher call rates during full moon periods (Supplementary Figure S3). There was also an interaction between days since rainfall and moon phase, which was most apparent in summer (, ).
Figure 1. Call rate (calls per hour, log-transformed) of Eastern Ground Parrots across season in Noosa National Park, SE Queensland, showing the relationship with days since rainfall. Moon phase is represented by the darkness of the datapoints, where full moon = 0 (lightest datapoints) and new moon = 15 (darkest datapoints).
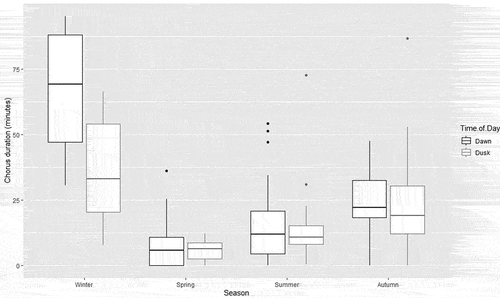
Generally, chorus periods lasted approximately the same length of time at dawn and dusk in the same recording period with an average duration of 30.2 minutes at dawn and 22.7 minutes at dusk (Supplementary Table S3). Winter choruses averaged longer than chorus periods at other times of year, with spring having the lowest average duration (). Meteorological patterns of longer chorus duration were less significant than with call rate, but similarly associated with full moons and recent rain in winter and autumn. The opposite of this trend was shown in summer and spring (, Supplementary Figure S4).
Figure 2. Chorus duration (time spent calling in minutes) of Eastern Ground Parrots in Noosa National Park, SE Queensland, across season and time of day. The box plot demonstrates the dawn chorus in winter was longer than dusk, although most seasons showed minimal difference between the median lines.
Discussion
EGP vocal activity was significantly influenced by a variety of environmental and temporal interactions at our study site. Specifically, season, moon phase and time since rainfall interacted to predict both call rate and chorus duration. Dusk choruses in general showed the highest call rates, and, seasonally, we observed the most vocal activity during the winter months. This is consistent with previous literature for EGPs, yet remains interesting as the breeding season is estimated to occur from August to November (spring/summer) (Chan and Mudie Citation2004). Thus, higher calling rates may be a feature of pre-reproductive season (i.e. mate selection) rather than reproduction itself (Gibbs and Wenny Citation1993).
Many species alter vocal output across the year, increasing vocalisations before or during the breeding season to attract or communicate with mates and young (Topp and Mennill Citation2008). In the congeneric Night Parrot Pezoporus occidentalis, breeding events follow rainfall episodes rather than abiding by seasonality (Murphy et al. Citation2017). Days since rainfall was identified as a contributing factor to both call rate and chorus duration, indicating that it may be important to understanding EGP’s vocal behaviour. Further research into vocalisation types and their respective functions (including reproduction, foraging, territorial or predator awareness/avoidance), together with research into variability between sites, would help shed light on driving behavioural factors for these patterns.
Increased calling activity during fuller moon phases may be associated with elevated light levels, a trend that has been observed in other species (Dickerson et al. Citation2020). Various hypotheses have been put forth for correlations between bird vocal behaviours and moonlight, but the drivers may vary between species. Adaptive reasons for increased calling during full moons might be associated with predator behaviour (Schmidt and Belinsky Citation2013), or may be a physiological and behavioural response to elevated levels of light at night (Digby et al. Citation2014). We strongly encourage more experimental research into this field more broadly to test these hypotheses (La Citation2012).
Deployment of acoustic recorders or human aural census studies for occupancy should be conducted at times that increase the chances of detection (Depraetere et al. Citation2012). Our limited study demonstrates that call rate, and thus detectability, is influenced by interactions between season, moon phase and rainfall patterns. Prediction of the best timeframes for detection is somewhat difficult, but the long deployment capability of some PAM recorders allows for an excess of data collection which can account for subsampling to exclude unfavourable periods. Season, time of day and moon phase are cyclical and predictable, even if rainfall is less so, and our data suggests periods to efficiently target EGPs with acoustic surveys. This study indicates that surveying the dusk chorus also increases the likelihood of call detection, providing the best indication of occupancy within a study area.
Supplemental Material
Download MS Excel (10.6 KB)Supplemental Material
Download MS Excel (11.4 KB)Supplemental Material
Download MS Excel (12.6 KB)Supplemental Material
Download MS Word (144.2 KB)Supplemental Material
Download MS Word (86.3 KB)Supplemental Material
Download MS Word (65.7 KB)Supplemental Material
Download MS Word (118.4 KB)Acknowledgements
We acknowledge the Gubbi Gubbi peoples, the traditional custodians of the lands of Noosa, of Elders past, present and emerging, on which this work was conducted. Many thanks to Dr Scott Burnett for discussions and advice during the initial stages of this project, Alina Zwar (Wildwise Environmental Services Pty Ltd) and Dr Ed Meyer for assistance with field data collection. We thank Future-plus Environmental Pty Ltd for logistical support, Jo Zadkovich (Queensland Parks Wildlife Service [QPWS]) and Harry Hines (QPWS) for facilitating access to the study area and ongoing liaison. The fieldwork (acoustic data collection) was conducted within QPWS’s conservation estate under scientific purposes permit (number: PTU18-001058), and Queensland animal ethics approval (number: CA2017/06/1074).
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary data
Supplemental data for this article can be accessed at https://doi.org/10.1080/01584197.2023.2269575.
References
- Bedoya, C., Isaza, C., Daza, J. M., and López, J. D. (2017). Automatic identification of rainfall in acoustic recordings. Ecological Indicators 75, 95–100. doi:10.1016/j.ecolind.2016.12.018
- Berg, M., Beintema, N., Welbergen, J., and Komdeur, J. (2005). Singing as a handicap: The effects of food availability and weather on song output in the Australian reed warbler Acrocephalus australis. Journal of Avian Biology 36(2), 102–109. doi:10.1111/j.0908-8857.2005.03285.x
- Bluff, L. A. (2016). Ground Parrots and fire in east Gippsland, Victoria: Habitat occupancy modelling from automated sound recordings. Emu 116(4), 402–410. doi:10.1071/MU16014
- Brown, T., and Handford, P. (2003). Why birds sing at dawn: The role of consistent song transmission. Ibis 145, 120–129. doi:10.1046/j.1474-919X.2003.00130.x
- Bureau of Meteorology (2019). ‘Climate Data Online: Tewantin RSL Park Station 040908.’ (Australian Government.) Available at http://www.bom.gov.au/climate/data/index.shtml [Verified 8 July 2019].
- Catchpole, C. K. and Slater, P. J. B. (2008). ’Bird Song: Biological Themes and Variations.’ 2nd ed. (Cambridge University Press: Cambridge.)
- Chan, K., and Mudie, D. (2004). Variation in vocalisations of the Ground Parrot at its northern range. Australian Journal of Zoology 52(2), 147–158. doi:10.1071/ZO03009
- Darras, K., Pütz, P., Fahrurrozi, Rembold, K., and Tscharntke, T. (2016). Measuring sound detection spaces for acoustic animal sampling and monitoring. Biological Conservation 201, 29–37. doi:10.1016/j.biocon.2016.06.021
- Depraetere, M., Pavoine, S., Jiguet, F., Gasc, A., Duvail, S., and Sueur, J. (2012). Monitoring animal diversity using acoustic indices: Implementation in a temperate woodland. Ecological Indicators 13(1), 46–54. doi:10.1016/j.ecolind.2011.05.006
- Dickerson, A., Hall, M., and Jones, T. (2020). The effect of variation in moonlight on nocturnal song of a diurnal bird species. Behavioral Ecology and Sociobiology 74(9), 109. doi:10.1007/s00265-020-02888-z
- Digby, A., Towsey, M., Bell, B. D., and Teal, P. D. (2014). Temporal and environmental influences on the vocal behaviour of a nocturnal bird. Journal of Avian Biology 45(6), 591–599. doi:10.1111/jav.00411
- Freckleton, R. (2002). On the misuse of residuals in ecology: Regression of residuals vs. multiple regression. Journal of Animal Ecology 71(3), 542–545. doi:10.1046/j.1365-2656.2002.00618.x
- Gibbs, J. P., and Wenny, D. G. (1993). Song output as a population estimator: Effect of male pairing status. Journal of Field Ornithology 64(3), 316–322.
- Gil, D., and Gahr, M. (2002). The honesty of bird song: Multiple constraints for multiple traits. Trends in Ecology and Evolution 17(3), 133–141. doi:10.1016/S0169-5347(02)02410-2
- Gillooly, J. F., and Ophir, A. G. (2010). The energetic basis of acoustic communication. Proceedings Biological Sciences 277(1686), 1325–1331. doi:10.1098/rspb.2009.2134
- Hutschenreiter, A., Sosa-López, J. R., González-García, F., and Aureli, F. (2023). Evaluating factors affecting species detection using passive acoustic monitoring in neotropical forests: A playback experiment. Bioacoustics 1–19. doi:10.1080/09524622.2023.2246413
- La, V. T. (2012). Diurnal and nocturnal birds vocalise at night: A review. The Condor 114(2), 245–257.
- Lengagne, T., and Slater, P. J. (2002). The effects of rain on acoustic communication: Tawny owls have good reason for calling less in wet weather. Proceedings Biological Sciences 269(1505), 2121–2125. doi:10.1098/rspb.2002.2115
- Magrath, R. D., Haff, T. M., Fallow, P. M., and Radford, A. N. (2015). Eavesdropping on heterospecific alarm calls: From mechanisms to consequences. Biological Reviews 90(2), 560–586. doi:10.1111/brv.12122
- McFarland, D. C. (1988). Fire and the vegetation composition and structure of subtropical heathlands in South-Eastern Queensland. Australian Journal of Botany 36, 533–546. doi:10.1071/BT9880533
- McFarland, D. C. (1991a). The biology of the Ground Parrot, Pezoporus wallicus, in Queensland. I. Microhabitat use, activity cycle and diet. Wildlife Research 18(2), 169–184. doi:10.1071/WR9910169
- McFarland, D. C. (1991b). The biology of the Ground Parrot, Pezoporus wallicus, in Queensland. II. Spacing, calling and breeding behaviour. Wildlife Research 18(2), 185–197. doi:10.1071/WR9910185
- McFarland, D. C. (1991c). The biology of the Ground Parrot, Pezoporus wallicus, in Queensland. III. Distribution and abundance. Wildlife Research 18(2), 199–213. doi:10.1071/WR9910199
- Murphy, S. A., Austin, J. J., Murphy, R. K., Silcock, J., Joseph, L., Garnett, S. T., and Leseberg, N. P., et al. (2017). Observations on breeding Night Parrots (Pezoporus occidentalis) in western Queensland. Emu 117(2), 107–113. doi:10.1080/01584197.2017.1292404
- Oliver, D., Bain, D., Bluff, L., Smith, G., Hines, H., Andren, M., and Aland, K., et al. (2021). Eastern Ground Parrot Pezoporus wallicus wallicus. In ‘The Action Plan for Australian Birds 2020.’ (Eds S. T. Garnett and G. B. Baker.) pp. 440–444. (CSIRO Publishing: Melbourne.)
- Ophir, A. G., Schrader, S. B., and Gillooly, J. F. (2010). Energetic cost of calling: General constraints and species-specific differences. Journal of Evolutionary Biology 23(7), 1564–1569. doi:10.1111/j.1420-9101.2010.02005.x
- Pérez-Granados, C., and Schuchmann, K. L. (2021). Seasonal climate impacts on vocal activity in two neotropical nonpasserines. Diversity 13(7), 319. doi:10.3390/d13070319
- Pérez-Granados, C., and Traba, J. (2021). Estimating bird density using passive acoustic monitoring: A review of methods and suggestions for further research. Ibis 163(3), 765–783. doi:10.1111/ibi.12944
- Pijanowski, B. C., Farina, A., Gage, S. H., Dumyahn, S. L., and Krause, B. L. (2011). What is soundscape ecology? An introduction and overview of an emerging new science. Landscape Ecology 26(9), 1213–1232. doi:10.1007/s10980-011-9600-8
- Priyadarshani, N., Marsland, S., and Castro, I. (2018). Automated birdsong recognition in complex acoustic environments: A review. Journal of Avian Biology 49(5), jav–01447. doi:10.1111/jav.01447
- R Core Team (2022). ’R: A Language and Environment for Statistical Computing.’ (R Foundation for Statistical Computing: Vienna, Austria.) https://www.R-project.org/
- Ross, S. J., O’Connell, D. P., Deichmann, J. L., Desjonquѐres, C., Gasc, A., Philips, J. N., and Sethi, S. S., et al. (2023). Passive acoustic monitoring provides a fresh perspective on fundamental ecological questions. Functional Ecology 37, 959–975. doi:10.1111/1365-2435.14275
- Schmidt, K. A., and Belinsky, K. L. (2013). Voices in the dark: Predation risk by owls influences dusk singing in a diurnal passerine. Behavioural Ecology and Sociobiology 67(11), 1837–1843. doi:10.1007/s00265-013-1593-7
- Sugai, L. S. M., Silva, T. S. F., Ribeiro, J. W., and Llusia, D. (2019). Terrestrial passive acoustic monitoring: Review and perspectives. BioScience 69(1), 15–25. doi:10.1093/biosci/biy147
- Symonds, M. R. E., and Moussalli, A. (2011). A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behavioural Ecology and Sociobiology 65(1), 13–21. doi:10.1007/s00265-010-1037-6
- Thomas, A., Speldewinde, P., Roberts, J. D., Burbidge, A. H., and Comer, S. (2020). If a bird calls, will we detect it? Factors that can influence the detectability of calls on automated recording units in field conditions. Emu 120(3), 239–248. doi:10.1080/01584197.2020.1787848
- Topp, S. M., and Mennill, D. J. (2008). Seasonal variation in the duetting behaviour of rufous-and-white wrens (Thryothorus rufalbus). Behavioral Ecology and Sociobiology 62(7), 1107–1117. doi:10.1007/s00265-007-0538-4
