ABSTRACT
The distribution of the resources needed by females for reproduction affects their dispersion in space and time, which in turn influences the number of females a male can defend. When males provide parental care they in turn become a resource for females, and influence where females choose to breed. Combined, these factors explain a preponderance of monogamous and (to a lesser extent) polygynous mating systems in birds. However, less frequently observed mating systems in birds remain harder to explain. Here, we analyse the home ranges of male Eclectus parrots (Eclectus roratus) to investigate how their use of space facilitates an unusual polygynandrous mating system (where multiple males mate with multiple females). Female Eclectus parrots in the rainforests of northern Queensland, Australia, have limited movements. They remain at their nest trees for most of the year and rely on multiple wide-ranging males to bring them their food. We used light aircraft and VHF transmitters to track the movements of four male Eclectus parrots during the breeding season. We combined data on home range size with molecular analyses of a larger sample of breeding birds. Our analyses demonstrate that male home ranges overlap with each other and encompass multiple (3–14) trees with nesting females. We show that some males divide their time between, and gain reproductive success with, females up to 7 km apart. Our tracking and molecular data offer insights into how polygynandrous mating systems relate to space use among males and females.
Introduction
Mating systems reflect the outcomes of the behaviour of individuals of both sexes competing to maximise their reproductive success. A major determinant of mating system type is the dispersion of males and females in space and time (Emlen and Oring Citation1977; Davies et al. Citation2012). In species without male parental care, female dispersion is often determined by the dispersion of resources they need, which in turn affects how males position themselves so they can defend as many females as possible. Polygamy in these situations is more likely when resources, and therefore females, have patchy distributions allowing males to defend more than one female (Ims Citation1987; Warner Citation1990). Alternatively, when males do provide parental care, they become a resource for females and the mating system then reflects the extent of male parental care necessary for raising offspring (Lack Citation1968). Monogamy may be obligate, or females may have the option to mate polygynously when males have high quality territories and the benefits of sharing a male outweigh the costs (Orians Citation1969; Pribil and Searcy Citation2001). Variable mating systems within a species may arise when there is conflict between the sexes over which strategies maximise reproductive success (Davies et al. Citation1995).
Polyandry and polygynandry are of special interest because they are the rarest mating systems among birds. Polyandry occurs when a single female mates with two or more males during a breeding season (Oring Citation1986), and may be extended to polygynandry when the same males mate with additional females (Koenig and Stacey Citation1990; Briske et al. Citation1998). Polyandry falls into two distinct categories. Classical polyandry occurs when the females mate sequentially with single males who then care for the clutch alone, whereas cooperative polyandry occurs when multiple males sire and care for the clutch together (Faaborg and Patterson Citation1981; Oring Citation1986). When use of space differs between the sexes, mating systems may vary within a species depending on the extent of overlap of male and female home ranges. In these cases, males compete vigorously for mating success, and shared paternity may be under female control (Hartley and Davies Citation1994; Davies et al. Citation1995).
In this paper, we analyse the home ranges of male Eclectus parrots to investigate how their use of space is linked to an unusual polygynandrous mating system. Eclectus parrots have striking reversed plumage dichromatism (red and blue females, green males) (Forshaw Citation2002; Heinsohn et al. Citation2005), but are not sex role reversed as is generally the case for reverse dichromatic birds (Eens and Pinxten Citation2000; Heinsohn Citation2008). The bright colouration of females functions in intra-specific competition for scarce nest hollows in emergent rainforest trees (Heinsohn et al. Citation2005), which they guard aggressively for as long as 11 months each year. Female Eclectus parrots have retained the role of incubating eggs and protecting young. The males outnumber females by two to one and compete aggressively for access to them (Heinsohn and Legge Citation2003). Throughout this period, females are provided with all of their food by the males who forage for fruit. Molecular analyses have shown that the mating system includes monogamy, cooperative polyandry with a surprisingly large number of males (up to seven) attending and feeding each female, and polygynandry where the same males attend females at different nest trees (Heinsohn et al. Citation2007). The males who attend the same female are not related and instead compete for direct mating privileges.
Here, we demonstrate the behavioural ecological basis behind the unusual mating system described via genetic analyses in this species (Heinsohn et al. Citation2007). We overcame major logistical difficulties to catch and track the movements of wide-ranging male Eclectus parrots during the breeding season in the rainforests of Cape York Peninsula in northern Australia, and use this data in conjunction with genetic analyses. We demonstrate how major differences in space use between the sexes facilitates the highly variable mating system including monogamy, polyandry, and polygynandry in this species.
Methods
Study site and field methods
We studied a population of the subspecies E.r. macgillivrayi at Kutini-Payamu (previously Iron Range) National Park on Cape York Peninsula in far north Queensland, Australia (12°45′S, 143°17′E) from August 1997 until March 2008. The national park is part of a lowland rainforest of approximately 500 km2. E.r. macgillivrayi are approximately 35–42 cm in length and weigh from 540–605 g (unpublished data). They are frugivores and typically nest during the dry season on Cape York Peninsula (from July to November), although some females who lay a second clutch may fledge their last young as late as February (Heinsohn and Legge Citation2003). They nest in hollows in emergent rainforest trees 15–30 m above the ground. We located the trees used in our study using the calls made by females from their hollows during nesting, by males as they fly towards the nest tree where they tend females, and the further vocalisations males make when they fight each other for access to the females (Heinsohn and Legge Citation2003). Aerial and ground-based surveys have revealed that nest hollows are a limited resource and that nest trees occur at a density of approximately one per square kilometre (Legge et al. Citation2004). Our study comprised 33 nest trees with 45 nest hollows over approximately 65 km2. Most nest trees had one hollow (n = 28) but some had two (n = 4) or three hollows (n = 3). The nearest nest trees were 50 m apart, and the furthest were 10.1 km apart (Heinsohn et al. Citation2007).
Nest trees were climbed using single rope techniques between five and 11 times each breeding season to establish the breeding status of the female, to gain a small (10–70 µl) blood sample from each nestling, and to band nestlings (when approximately 6 weeks old), and establish the success of the brood. Feathers left in the hollow by adult females were also collected for DNA extraction. For further description of field methods and nest tree distribution, see Heinsohn (Citation2008) and Heinsohn et al. (Citation2011).
Radio-telemetry, home range and male time budgets
The capture of Eclectus parrots was time- and labour-intensive (2 days to clear each canopy gap for mist nets plus approximately six mist-netting mornings per captured bird). Four adult male Eclectus parrots were captured in October or November 2001 when nests in the area were active. These were banded and fitted with tail mounted radio transmitters (Holohil, model RI-2C, 10 g) glued and sewn to the two central tail feathers (Kenward Citation2001). Transmitters were less than 1.9% of the birds’ weight (range = 540–605 g). Due to a lack of roads or tracks in most of the study area, radio-tracking was done by light aircraft (a Cessna Superhawk 172N) to determine home range, and subsequently by foot (when possible) for behavioural observations. Tracking from the light aircraft was done using two antennae, one attached to each wing strut. This allowed the tracker (S. Legge) to direct the pilot (R. Heinsohn) towards the bird according to the relative strength of the right and left signal. Tests with dummy transmitters showed that individuals were reliably located from the air at 160 m above ground level to within 300 m. Signals could be detected up to 10 km from higher altitudes (up to 1,000 m) and all four individuals were always located on tracking flights. Locations were recorded on a Garmin GPS. Sequential fixes of an individual were separated by at least 8 hours to minimise autocorrelation of the data. Radio-tracking flights to locate each bird were typically undertaken once or twice daily, early (between 07:30 and 08:30) and late in the day (between 17:00 and 18:30) to take advantage of calm flying conditions. Date range of tracking and number of fixes obtained for each male are shown in . Tracking on foot was done using a Yagi 3-stage antenna connected to an ICOM-R10 receiver.
Table 1. Estimates of minimum convex polygon (MCP), home range (95% isopleth), and core range (50% isopleth), and number of nests in each. N = number of radio-tracking fixes.
Home ranges of the four tracked males were calculated in Rstudio (Team Citation2020) using the ‘adehabitatHR’ package (Calenge Citation2006). We used epsg 4326 and WGS84 data. We first calculated the minimum convex polygon for each individual using 100% of bird relocations to assess the areas visited. We examined whether we had sufficient data by assessing the stability of home ranges at different percentages of relocations (Stark et al. Citation2017). We then calculated kernel utilisation distributions for each individual using the function ‘kernelUD’ with Epanechnikov kernels selected. We considered the 95% isopleth to be a good estimate of birds’ home ranges, and a 50% isopleth to represent their core ranges (Stark et al. Citation2017).
We confirmed that males spent time close to multiple nests, and were not simply in the vicinity of multiple nests by chance, using additional radio-tracking from the ground. We recorded their presence and absence at six nest trees we frequently visited in the area of greatest overlap of their home ranges. This entailed opportunistic scanning of the area around each nest tree upon each nest visit by the researchers using a hand-held Yagi 3-stage aerial. Signal detection ranges were up to 500 m under these conditions but we only recorded a male as present at the nest tree if the signal was determined to be coming from within 50 m of the nest tree itself. This was done by determining the direction of the signal from multiple angles so that it was clear the signal was coming from the vicinity of the nest tree and not away from it. Scans were conducted from 4–46 visits to these nest trees between 21 October and 26 November 2001.
Molecular techniques
We used previous molecular analyses of the Eclectus parrot mating system to infer the distances between the nests where individual males fathered nestlings at different nests within the same breeding season. We analysed the distribution of paternity based on DNA from 310 Eclectus parrots sampled over eight breeding seasons, including 18 adult females (nine from blood samples, nine from feathers left in nests), 14 adult males (11 from blood samples, three from feathers from birds found dead), and 278 nestlings (all from blood samples). The nestlings were from 99 broods with two nestlings and 80 broods with one nestling. All samples were genotyped at nine polymorphic microsatellite loci including one sex-linked locus. The difficulty of capturing large canopy-dwelling parrots meant that the proportion of the adult male breeding population captured was insufficient to apply likelihood methods of paternity assignment. Instead we assessed maternity directly by exclusion, and rates of shared paternity between nests indirectly by kinship analyses of nestlings (Heinsohn et al. Citation2007).
Results
Male movements
The radio-tracking data confirmed that male Eclectus parrots have extensive and overlapping home ranges that encompass many nest trees. Analyses showed that measures of home range were still increasing as the number of data points increased (Supplementary Figure S1). We use the 50% and 95% isopleths as estimates of the core and home ranges but recognise that these may be underestimates. The mean 50% and 95% isopleths and minimum convex polygon (MCP) for the four tracked males are shown in . Known nest trees varied from zero to three in the individuals’ core ranges, three to 14 in the 95% isopleth, and three to 17 in the MCP (). One of the tracked birds had most of its range outside of the known study area, so that the number of nest trees in its range was probably underestimated (bird 491, ). The MCPs and 95% isopleths of all four males overlapped extensively (). Three out of the four tracked males had home ranges comprising a single area (as defined by the 95% isopleth, ) whereas one male’s home range was split into two areas ().
Figure 1. Home ranges of four radio-tracked Eclectus parrot males showing the minimum convex polygon (MCP, straight edges), 95% isopleth (grey area), and 50% isopleth (smaller shaded area) for (a) individual 491; (b) individual 531; (c) individual 511; and (d) individual 451. MCP overlap for males is shown in (e) and 95% isopleths in (f). Crosses denote known Eclectus parrot nest trees. Scale in km is shown in the bottom right panel. Location of the study area in northern Queensland, Australia is shown as an inset map in (a).
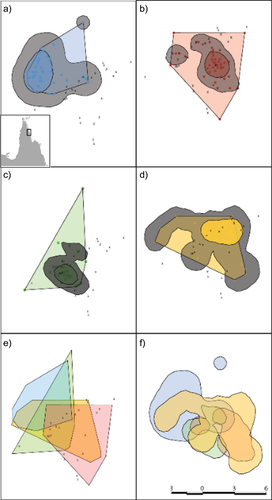
Radio-tracking from the ground at a sample of six nests in the area where the male ranges overlapped confirmed that individual males were often in close proximity to nest trees (within 50 m). One male was found at only one of the nest trees (male 511, Smugglers Tree) on over half of our nest visits. The other three males were detected at more than one nest tree. Males 531 and 491 were present at two nest trees, whereas male 451 visited four nest trees (Supplementary Table S1). The proportion of our visits to these nest trees that males were present ranged from 12.5–57.1% suggesting that they commonly visited these sites. Our long-term observations of male Eclectus parrots at nest trees has also shown that they stay for long periods in the vicinity of the tree between foraging forays (Heinsohn and Legge Citation2003).
In 2001, 24 nestlings were sampled for DNA at the above six nest trees. Using exclusion (see Heinsohn et al. Citation2007) most of these nestlings were shown not to be offspring of any of the radio-tracked males. One male (531) was a likely father of three nestlings (at two nests) but these were all at nests outside his core area. Two nestlings were at a tree within his 95% isopleth (Pisa), and the third was at a nest tree outside his 95% isopleth (Smugglers).
Frequency and spatial dimensions of polygynandrous mating
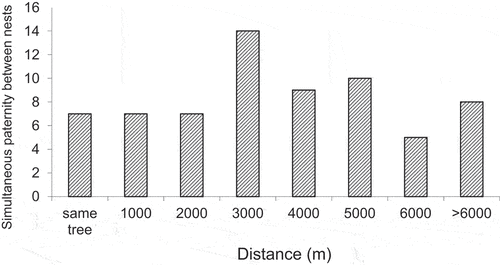
Heinsohn et al. (Citation2007) used a large sample of nestlings and kinship analysis to estimate the frequency of monogamous, polyandrous, and polygynandrous mating over 8 years of study. Knowledge of nest locations combined with the kinship analysis also enabled estimation of the distances between the nests where the same male fathered nestlings. We plot these data for the first time to illustrate the frequency and distances involved. Females in the study area overlapped temporally in their breeding efforts including laying their first clutches within a few weeks of each other, and females with hollows in the same tree were similarly synchronous (Heinsohn and Legge Citation2003; Heinsohn et al. Citation2007). In seven cases, males gained paternity at two different nests in the same tree and in 56 cases they gained paternity at nests in different trees. The mean distance apart of nest trees where the same male gained paternity was 3503 m ± 260 SE (maximum = 7,226 m). shows that there was no clear bias towards nest trees that were close together, and that distances between trees where a male fathered offspring were roughly evenly distributed up to the maximum recorded distance.
Figure 2. Number of instances when individual offspring in synchronous nests had shared paternity (179 broods), with distance between the nests. Data are grouped in 1000m bins and are from Heinsohn et al. (Citation2007).
Discussion
Parrots are difficult to capture and their destructive abilities make it challenging to design transmitters that stay on the birds (Meyers Citation1996). Due to these constraints, knowledge of parrot movements remains limited. However, VHF, GPS and Argos satellite tracking studies have confirmed aspects of within and between season movements for some species (Snyder et al. Citation1994; Salinas-Melgoza and Renton Citation2005; Stojanovic et al. Citation2021). Studies of individual movements of parrots in relation to space use and how this reflects mating strategies have been especially rare. We were able to determine the daily movements of four male Eclectus parrots during the breeding season using VHF transmitters attached to the birds’ tails and tracked from light aircraft. We combined these spatial data with molecular information to give an unusually detailed picture of how and where males of a large parrot species spend their time when courting multiple mates. Even though our estimates of home range size were probably conservative we nonetheless found that the tracked males had broadly overlapping home ranges and that they spent time with females at nests up to 3.5 km apart. This was supported by additional analyses of molecular data that confirmed males could father offspring at nests separated by over 7 km. Our analyses confirm, on an unusually large spatial scale (where individuals range over thousands of hectares), how differences in space use by the sexes influence mating systems.
The disparity in space use by male and female Eclectus parrots is extreme. Females stay at their nest hollows for most of the year, and are entirely dependent on males to bring them food (Heinsohn and Legge Citation2003). By contrast, our data confirm that the males have large (3,667–6,575 ha) overlapping home ranges, and each male’s range encompasses the nests of many females (range = 3–14). Male Eclectus parrots compete aggressively for access to breeding females but do not defend territories as such (Heinsohn and Legge Citation2003). This differs from most cooperatively polyandrous species in which males benefit from group-territoriality to the extent that the long-term benefits outweigh the costs of sharing paternity (Faaborg and Bednarz Citation1990; Koenig and Stacey Citation1990; Jamieson et al. Citation1994; Sherman Citation1995). Our long-term data (over 8 years) have shown that males often do not receive paternity at the nests where they attend females suggesting they invest their efforts for longer term gains. Some were shown to only gain paternity occasionally, for example one male fathered two offspring at the same nest hollow 7 years apart (Heinsohn et al. Citation2007). Proximity of nests was not a determinant of the likelihood of male paternity with multiple females. This differs from patterns of extra-pair paternity often described for birds (Schlicht et al. Citation2015) and suggests that mating opportunities are determined by the social environment over larger scales (Maldonado-Chaparro et al. Citation2018). The long-term data showed that males gained paternity at roughly equal likelihood at nests up to 7 km apart. The variability of the mating system of Eclectus parrots shows similarities to that of Alpine Accentors Prunella collaris (Davies et al. Citation1995), in which the males do not defend territories but have overlapping home ranges that may encompass the ranges of multiple females. Like accentors, Eclectus parrot males are probably constrained by having to travel each day to obtain fruit for the females they attend, and the areas they traverse are likely to be too big to defend. Female Eclectus parrots have been observed mating with multiple males in quick succession, and high rates of multiple paternity have also been detected molecularly (Heinsohn et al. Citation2007). This suggests they may use the strategy seen in female accentors of using copulations to encourage multiple males to attend them (Davies et al. Citation1995; Hartley et al. Citation1995). Some males also realise the potential for multiple mates afforded by their home ranges overlapping with several nests, as many in this study gained paternity at more than one nest tree. Our ground-based radio-tracking data confirmed that males could spend considerable time at up to four nest trees, although there were other nests at which they were not recorded. Further analysis of the molecular data from Heinsohn et al. (Citation2007) showed that they can gain paternity at nests both close together and several kilometres (maximum 7,226 m) apart.
Polygynandry is a rare and intriguing mating system among birds and our tracking and molecular data offer unusual insights into how it relates to space use among males and females. We have already established that females are unusually sedentary and spend much of the year defending a nest hollow, and are dependent on multiple males to bring them food (Heinsohn and Legge Citation2003; Heinsohn Citation2008). In this study, we overcame major logistical difficulties to demonstrate the strategies males use given the distribution of the sedentary females. Males split their time between, and gain reproductive success with, some females in their range even if some of the nests they attend are surprisingly far apart. This suggests they assess which females afford them the greatest chance of reproductive success and focus their attention on these few accordingly even if they are several kilometres apart.
Supplemental Material
Download MS Word (33.2 KB)Acknowledgements
We thank N. Langmore for helpful comments on the manuscript, M. Hall, S. Murphy, C. Blackman and A. Nathan for help with fieldwork, D. Ebert for advice with genetic analysis and P. and E. Huybers, M. Blackman and K. Goetze for generous logistical support. Our research was funded by an Australian Research Council QEII Fellowship, two ARC Discovery grants, the National Geographic Society and the Winifred Violet Scott Foundation. This research was conducted under license from the ANU Animal Ethics Committee (Permit No: C.R.E.35.04).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets are available from the authors on request.
Supplemental data
Supplemental data for this article can be accessed at https://doi.org/10.1080/01584197.2024.2368006
References
- Briske, J. V., Montgomerie, R., Oldmaa, T., and Boag, P. T. (1998). Paternity and paternal care in the polygynandrous Smith’s longspur. Behavioral Ecology and Sociobiology 43(3), 181–190. doi:10.1007/s002650050479
- Calenge, C. (2006). The package “adehabitat” for the R software: A tool for the analysis of space and habitat use by animals. Ecological Modelling 197(3–4), 516–519. doi:10.1016/j.ecolmodel.2006.03.017
- Davies, N. B., Hartley, I. R., Hatchwell, B. J., Desrochers, A., Skeer, J., and Nebel, D. (1995). The polygynandrous mating system of the alpine accentor, Prunella collaris. I. Ecological causes and reproductive conflicts. Animal Behaviour 49(3), 769–788. doi:10.1016/0003-3472(95)80209-6
- Davies, N. B., Krebs, J. R., and West, S. A. (2012). ‘An Introduction to Behavioural Ecology,’ 4th edn. (Wiley-Blackwell: West Susses, U.K.)
- Eens, M., and Pinxten, R. (2000). Sex-role reversal in vertebrates: behavioral and endocrinological accounts. Behavioural Processes 51(1–3), 135–147. doi:10.1016/S0376-6357(00)00124-8
- Emlen, S. T., and Oring, L. W. (1977). Ecology, sexual selection, and the evolution of mating systems. Science 197(4300), 215–223. doi:10.1126/science.327542
- Faaborg, J., and Bednarz, J. C. (1990). Galapogos and Harris’ hawks: divergent causes of sociality in two raptors. In ‘Cooperative Breeding in Birds: Long-Term Studies of Ecology and Behavior.’ (Eds P. B. Stacey and W. D. Koenig) pp. 359–383. (Cambride University Press: Cambridge.)
- Faaborg, J., and Patterson, C. B. (1981). The characteristics and occurrence of cooperative polyandry. Ibis 123(4), 477–484. doi:10.1111/j.1474-1919X.1981.tb04051.x
- Forshaw, J. (2002). ‘Australian Parrots,’ 3rd (revised) pp. 500–509. (Lansdowne Press: Melbourne.)
- Hartley, I. R., and Davies, N. B. (1994). Limits to cooperative polyandry in birds. Proceedings of the Royal Society of London, Series B 257, 67–73.
- Hartley, I. R., Davies, N. B., Hatchwell, B. J., Desrochers, A., Nebel, D., and Burke, T. (1995). The polygynandrous mating system of the alpine accentor, Prunella collaris. 2. Multiple paternity and multiple effort. Animal Behaviour 49(3), 789–803. doi:10.1016/0003-3472(95)80210-X
- Heinsohn, R. (2008). The ecological basis of unusual sex roles in reverse-dichromatic eclectus parrots. Animal Behaviour 76(1), 97–103. doi:10.1016/j.anbehav.2008.01.013
- Heinsohn, R., Ebert, D., Legge, S., and Peakall, R. (2007). Genetic evidence for cooperative polyandry in reverse dichromatic Eclectus parrots. Animal Behaviour 74(4), 1047–1054. doi:10.1016/j.anbehav.2007.01.026
- Heinsohn, R., Langmore, N. E., Cockburn, A., and Kokko, H. (2011). Adaptive secondary sex ratio adjustments via sex-specific infanticide in a bird. Current Biology 21(20), 1744–1747. doi:10.1016/j.cub.2011.08.064
- Heinsohn, R., and Legge, S. (2003). Breeding biology of the reverse-dichromatic, co-operative parrot Eclectus roratus. Journal of Zoology 259(2), 197–208. doi:10.1017/S0952836902003138
- Heinsohn, R., Legge, S., and Endler, J. A. (2005). Extreme reversed sexual dichromatism in a bird without sex role reversal. Science 309(5734), 617–619. doi:10.1126/science.1112774
- Ims, R. A. (1987). Responses in spatial organization and behaviour to manipulations of the food resource in the vole Clethrionomys rufocanus. The Journal of Animal Ecology 56(2), 585–596. doi:10.2307/5070
- Jamieson, I. G., Quinn, J. S., Rose, P. A., and White, B. N. (1994). Shared paternity among non-relatives is the result of an egalitarian mating system in a communally breeding bird, the pukeko. Proceedings of the Royal Society of London Series B 257, 271–277.
- Kenward, R. E. (2001). ‘A Manual for Wildlife Tagging.’ (Academic Press: London.)
- Koenig, W. D., and Stacey, P. B. (1990). Acorn woodpeckers: Group-living and food storage under contrasting ecological conditions. In ‘Cooperative Breeding in Birds: Long-Term Studies of Ecology and Behavior.’ (Eds P. B. Stacey & W. D. Keonig.) pp. 413–453. (Cambridge University Press: Cambridge, U.K.)
- Lack, D. (1968). ‘Ecological Adaptations for Breeding in Birds.’ (Methuen and Co., Ltd.: London.)
- Legge, S., Heinsohn, R., and Garnett, S. (2004). Availability of nest hollows and breeding population size of eclectus parrots, Eclectus roratus, on Cape York Peninsula, Australia. Wildlife Research 31, 149–161.
- Maldonado-Chaparro, A. A., Montiglio, P., Forstmeier, W., Kempenaers, B., and Farine, D. R. (2018). Linking the fine-scale social environment to mating decisions: A future direction for the study of extra-pair paternity. Biological Reviews 93(3), 1558–1577. doi:10.1111/brv.12408
- Meyers, J. M. (1996). Evaluation of 3 radio transmitters and collar designs for Amazona. Wildlife Society Bulletin 24, 15–20.
- Orians, G. H. (1969). On the evolution of mating systems in birds and mammals. The American Naturalist 103(934), 589–603. doi:10.1086/282628
- Oring, L. W. (1986). Avian polyandry. In ‘Avian Biology.’ (Ed. R. J. Johnston.) pp. 309–351. (Plenum: New York.)
- Pribil, S., and Searcy, W. A. (2001). Experimental confirmation of the polygyny threshold model for red–winged blackbirds. Proceedings of the Royal Society B 268(1476), 1643–1646. doi:10.1098/rspb.2001.1720
- Salinas-Melgoza, A. A. R. K., and Renton, K. (2005). Seasonal variation in activity patterns of juvenile Lilac-crowned Parrots in tropical dry forest. The Wilson Bulletin 117(3), 291–295. doi:10.1676/04-096.1
- Schlicht, L., Valcu, M., and Kempenaers, B. (2015). Spatial patterns of extra-pair paternity: beyond paternity gains and losses. Journal Fo Animal Ecology 84, 518–531. doi:10.1111/1365-2656.12293
- Sherman, P. T. (1995). Social organization of cooperatively polyandrous white-winged trumpeters (Psophia leucoptera) in Peru. The Auk 112(2), 296–309. doi:10.2307/4088718
- Snyder, N. F. R., Koenig, S. E., Koschmann, J. A., Snyder, H. A., and Johnson, T. B. (1994). Thick-billed Parrot releases in Arizona. The Condor 96(4), 845–862. doi:10.2307/1369097
- Stark, D. J., Vaughan, I. P., Ramirez Saldivar, D. A., Nathan, S. K. S. S., Goossens, B., and Yue, B.-S. (2017). Evaluating methods for estimating home ranges using GPS collars: A comparison using proboscis monkeys (Nasalis larvatus). PLoS One 12(3), e0174891. doi:10.1371/journal.pone.0174891
- Stojanovic, D., McEvoy, J., Alves, F., Rayner, L., Heinsohn, R., Saunders, D., and Webb, M. (2021). Parental care does not compensate for the effects of bad years on reproductive success of a vagile bird. Journal of Zoology 314(4), 256–265. doi:10.1111/jzo.12888
- Team, R. C. (2020). ‘R: A Language and Environment for Statistical Computing.’ (R Foundation for Statistical Computing: Vienna, Austria). https://www.R-project.org/
- Warner, R. R. (1990). Male versus female influences on mating site detrmination in a coral reef fish. Animal Behaviour 39(3), 540–548. doi:10.1016/S0003-3472(05)80420-8
