Abstract
Multiple strategies have been used in the National Health System (NHS) in England to reduce inappropriate antibiotic prescribing and consumption in order to tackle antimicrobial resistance. These strategies have included, among others, restricting dispensing, introduction of prescribing guidelines, use of clinical audit, and performance reviews as well as strategies aimed at changing the prescribing behaviour of clinicians. However, behavioural interventions have had limited effect in optimising doctors’ antibiotic prescribing practices. This study examines the determinants of decision-making for antibiotic prescribing in hospitals in the NHS. A system dynamics model was constructed to capture structural and behavioural influences to simulate doctors’ prescribing practices. Data from the literature, patient records, healthcare professional interviews and survey responses were used to parameterise the model. The scenario simulation shows maximum improvements in guideline compliance are achieved when compliance among senior staff is increased, combined with fast laboratory turnaround of blood cultures, and microbiologist review. Improving guideline compliance of junior staff alone has limited impact. This first use of system dynamics modelling to study antibiotic prescribing decision-making demonstrates the applicability of the methodology for design and evaluation of future policies and interventions.
Introduction
Antibiotic resistance and prescribing management
Antimicrobial resistance (AMR) happens when medicines used to prevent and treat infections caused by microorganisms (such as bacteria, fungi, viruses, and parasites) became ineffective (World Health Organization, Citation2018). Antibiotic resistance (ABR) is a subset of AMR, specifically meaning the resistance developed in bacterial pathogens, occurs when bacteria are able to survive and grow in the presence of one or more antibiotics and continue to cause infection (Bush et al., Citation2011). ABR creates poor individual patient health outcomes, a burden on health systems and an economic burden on countries (de Kraker et al., Citation2011). Inappropriate use of antibiotics, especially broad-spectrum antibiotics, is one of the major drivers of ABR and contributes to the emergence of hard-to-treat infections (Holmes et al., Citation2016; Public Health England, Citation2018). Inappropriate antibiotic use includes underuse, overuse and misuse of antibiotics. Underuse, when antibiotics are needed but not given. Overuse, when antibiotics are used to treat non-bacterial infections. Misuse, when the wrong types are used for a given bacterial infection, or when antibiotics exceed the required duration, or administered by incorrect routes (oral versus intravenous) (Spivak et al., Citation2016). While there has been a 13.2% reduction in antibiotic prescriptions in primary health care across the National health System (NHS) in England between 2008-12 (Public Health England, 2018), specifically, the progress in hospitals has been poor, with no evidence of sustained reduction in total antibiotic prescribing (Public Health England, 2018). The United Kingdom (UK) has set a target to reduce antimicrobial use in humans by 15% by 2024 (Department of Health and Social Care, Citation2019). Various policies and interventions aimed at healthcare professionals and patients have been promoted to improve appropriate use of antimicrobials and to preserve their future effectiveness (Ramsay et al., Citation2003; Zarb et al., Citation2011). These include the use of prescribing guidelines and education programmes, restriction to dispensing certain antimicrobials, timely de-escalation of therapy when sufficient information is available to do so, and the use of different drug combinations to treat infections (Ramsay et al., Citation2003). In hospitals, over the last decade, the policy focus has shifted from structural and procedural interventions to behavioural interventions to optimise antibiotic prescribing, improve individual patient care, reduce hospital expenditure and decelerate the development of AMR (Tonkin-Crine et al., Citation2015). Such a shift in policy focus is supported by the research evidence, which shows that behavioural interventions, such as education programmes and the use of prescribing guidelines, have more sustained effect on prescribing in comparison to structural interventions, such as restricting dispensing (MacDougall & Polk, Citation2005).
Factors influencing antibiotic prescribing: Literature review
We undertook a review of the published literature in order to identify the factors influencing doctors’ antibiotic prescribing decision-making in hospitals in the UK. We searched the PubMed database for literature published from January 2000 to March 2019. Additionally, we searched for grey literature using the Institute for Scientific Information (ISI) Web of Knowledge. We identified 110 studies through PubMed and grey literature search; 16 studies met our inclusion criteria. We employed a hierarchical multi-level framework to categorise factors of influence, identified from the rapid literature review, including both barriers and facilitators, as individual, organisational, and system level factors based on where they arose in a health system (Robert & Fulop, Citation2014), presented in . Some influence factors cannot be solely attributed to one level, which motivated the use of systems thinking to examine these barriers and facilitators.
Table 1. Influence factors affecting prescribing behaviour categorised using a hierarchical framework.
The challenges to successful and sustained implementation of behavioural interventions and programmes are documented in the literature. However, there are no studies which provide a detailed process analysis and seek to understand the interactions among different policy initiatives and interventions. There is a paucity of knowledge on why certain interventions appear to be more effective than others in influencing prescribing behaviour, and how interventions work in synergy or in opposition to influence prescribing behaviour. An analysis of factors influencing prescribing behaviour, their interaction and the magnitude of the effects produced by these interactions is critical in order to inform the selection of policy alternatives and to shape an optimal “mix” of policy interventions (Kyratsis et al., Citation2019).
In this paper, we addressed the following research question: How do multiple and concurrent influences on prescribing decision-making processes influence prescribing in hospitals? Our objective was to understand and analyse these influences to develop a model that could predict the effects of policy interventions when introduced individually, sequentially or synchronously. To answer our research question and to achieve our objective, we developed a system dynamics (SD) model to simulate healthcare professional’s antibiotic prescribing decisions when treating hospital inpatients. First, we reviewed the published literature to identify factors influencing antibiotic prescribing decision-making in NHS hospitals in England. Second, we analysed data from survey and interviews with clinicians in NHS Trust hospitals in England to construct a SD model which conceptualised the antibiotic prescribing process in hospital medical wards. Third, we parameterised the model using routinely collected hospital data, and validated the model through multiple expert discussion panels and validation tests. Finally, we used the SD model under various scenarios, to predict prescribing outcomes, measured as the percentage of decisions in line with NHS antibiotic prescribing guidelines.
Rationale for selecting system dynamics
The results from the literature review suggested that antibiotic prescribing decision-making processes are influenced by complex, interlinked cultural, structural and procedural factors operating at multiple levels across health systems. To design interventions for maximum impact on improved prescribing behaviours, it is important to systematically analyse the causal relationships between prescribing behaviour, the cultural and structural determinants driving these behaviours, the context within which these behaviours happen, and to predict how prescribing decisions change as an outcome of the synergic impact.
In addition to observed clinical features of patients, factors influencing antibiotic prescribing decision-making processes included structural and cultural determinants, such as prescribing guideline accessibility, hospital budgets to implement antimicrobial stewardship (AMS) interventions, the provision of information to clinicians on the pathogens causing the infection (Ashiru-Oredope et al., 2012; Charani et al., Citation2011, Citation2013; Davey et al., Citation2013; Thakkar et al., 2011), and team level cultures (Charani et al., Citation2019). However, extant research has not explored the value of using a systems lens for this problem of complex causal mechanisms, the role of various determinants and context.
Application of a hierarchical framework to categorise influence factors at individual, organisational and health system levels has been an important initial step in providing a systems perspective. However, such frameworks have not been applied to develop a systems thinking tool or a systems dynamic model to explore the factors influencing prescribing behaviour.
Further, to date, published studies investigating sub-optimal prescribing behaviour have typically provided a description and/or an explanation of the behaviours observed but do not adequately examine or explain cause-effect relationships. This earlier research has exhausted its utility for informing targeted policies, as causality is either over-simplified or based on qualitative analysis alone with limitations for scale up. The current literature on complexity in healthcare is not well developed empirically and theoretically (Greenhalgh et al., Citation2010). A paradigm shift is urgently required if the translation from research evidence to routine healthcare practice policy is to be achieved (Greenhalgh & Papoutsi, Citation2018; Moore et al., Citation2015). Therefore, we embraced the philosophical shift from the conventional linear cause-effect perspective to a system perspective (Ahmad, Sim, et al., Citation2019). We conceptualised causality as multiple interacting influences giving rise to a particular outcome, none of the influence factors can be said to have a fixed “effect size” (Moore et al., Citation2015). In addition, the assumptions we made in this study differed from earlier research which considered doctors as perfectly rational decision makers. We assume here that doctors are not able to make optimal prescribing decisions due to “bounded rationality.” The theory of bounded rationality takes into account the cognitive limitations of decision makers in both knowledge and computational capacity, as well as the limitation in the time available to make a decision (Rawson et al., Citation2019; Simon, Citation1991). Human decision makers are not able to think in “feedback loops,” due to the limited nature of human information-processing capabilities, especially in the presence of multiple interlinked feedback loops, as they do not realise that their own behaviour has an impact on the whole system and the feedback on itself (Senge, Citation1990; J. D. Sterman, Citation1987). When decisions need to be made with incomplete knowledge (pathogen information) and in limited time (prescriptions are needed to treat the patient as early as possible, and sometimes without diagnostic testing), analytical tools are required to prevent sub-optimal decisions.
Analytic tools from the discipline of systems thinking help researchers gain insights in underlying structures of a complex situation (National Institute for Health Research (NIHR) Research Design Service: East Midlands, Citation2017). System Dynamics (SD), a systems thinking approach, has demonstrated its capability to solve problems in health management when employed to simulate population flows, predict health-seeking behaviours, support health decision-making, test health policy alternatives, and evaluate effectiveness of health interventions (Ahmad, Zhu, et al., Citation2019; Barton et al., Citation2004; Günal & Pidd, Citation2010; Lane & Husemann, Citation2008). SD conceptualises systems as networks of feedback loops. Non-linear relationships and time delays within systems are drivers of dynamic and complex system behaviour, and the source of policy resistance whether intentional or as a result of an unintended consequence (Forrester, Citation1994; Lebcir, Citation2006; J. Sterman, Citation2002). SD takes into account the cognitive limitations of decision-makers in both knowledge and computational capacity and allows researchers to incorporate emotional variables when managing human behaviour. However, despite its attractiveness as a method, to date, SD has not been utilised to investigate antibiotic prescribing behaviour.
The UK government has set an objective for health institutions to report by 2024 on the percentage of prescriptions supported by a diagnostic test or decision support tool (Department of Health and Social Care, Citation2019). Adopting systems thinking approaches, such as SD, will enhance decision-making by predicting intervention impact on health systems in future decision support strategies, which has not been achieved using traditional clinical decision supports that solely focus on individual-level outcomes.
In this study, we developed a SD model by systematically considering factors which influence prescribing behaviour of doctors, to enable us to model and simulate doctors’ antibiotic prescribing decision-making processes, in order to predict prescribing outcomes in hospitals.
Research methods
We followed the established protocol for a SD approach to develop the model (Martinez-Moyano & Richardson, Citation2013; J. D. Sterman, Citation1992). We followed seven steps: conceptualising the problems to be addressed, defining model boundaries to set the context in which the problem will be addressed, determining model variables, mapping the causal relationships between variables qualitatively using causal loop diagrams (CLDs), formulating model variables quantitatively using source data and stock and flow diagrams (SFDs), validating the model, and simulating the model to provide estimates of doctors’ antibiotic prescribing outcomes under various “what if” scenarios.
Model conceptualisation
In order to understand how antibiotics were prescribed to treat hospital inpatients, we re-analysed secondary data from (i) a survey with 109 Foundation Year doctors from 5 NHS Trust teaching hospitals in the NHS England to study their knowledge and attitudes in antibiotic prescribing (Gharbi et al., Citation2016), and (ii) semi-structured interviews with 3 specialist registrar and 8 consultants from 3 NHS Trust teaching hospitals to assess how “empiric” decisions were made when treating patients with infections in medical wards and the barriers perceived by these doctors to make optimal prescribing decisions (Rawson et al., Citation2016).
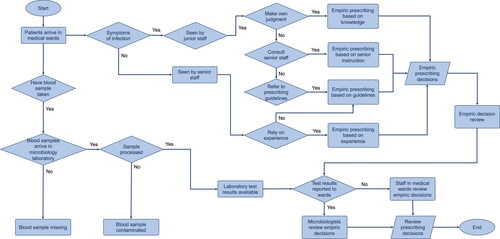
We constructed a flow chart to capture the typical process of clinicians prescribing antibiotics, which is divided into empiric and review stages. Clinicians who can prescribe antibiotics in medical wards include senior staff, which refers to specialist registrars and consultants, and junior staff (Foundation Year doctors, core trainees, and nurses). When patients with symptoms of infections are admitted in medical wards, clinicians make empiric prescribing decisions without information of pathogens that caused the infection. Patients have blood samples taken for microbiology laboratory testing to identify the pathogens. When the test results become available, the empiric decisions are reviewed by microbiologists. However, for those patients who have not had blood samples taken, or have blood samples missing or contaminated, the empiric decisions made for them will are reviewed by clinicians in medical wards (instead of microbiologists). The review decision can be to stop antibiotic treatment, to escalate to a different treatment regime by increasing dosage or frequency or switching from oral to intravenous route, to de-escalate to a treatment regime by increasing dosage or frequency or switching from oral to intravenous route, or to switch to a different type of antibiotic medication. The flow chart of the antibiotic decision-making processes is presented in .
Qualitative description of the decision-making processes
We identified a series of individual-level factors with direct impact on clinicians’ antibiotic prescribing practices through the literature review, the survey, and interview data analysis. Key individual-level factors which emerged from these analyses include:
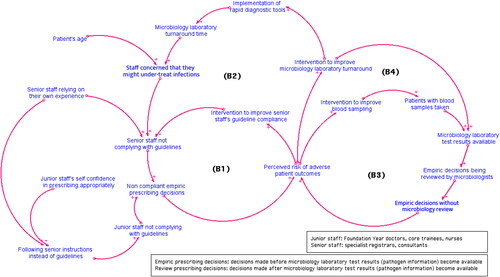
Clinician’s seniority: senior staff tend to rely on their own experience; while junior staff tend to consult senior staff or refer to prescribing guidelines. However, when senior staff’s decisions contradict the prescribing guidelines, junior doctors always followed senior staff’s instructions (the culture of deference and conflict avoidance) (Charani et al., Citation2013).
Patient’s age: elderly patients in general present with more complicated clinical symptoms and illnesses. In order to cover all possible infections, especially when pathogen information is not available, clinicians tend to prescribe antibiotics with broader spectrum than the ones recommended in the guidelines.
Microbiology laboratory turnaround time: the time lag caused by the process of transferring patient’s blood samples, performing tests to identify pathogens and susceptibility (i.e., which antibiotics the pathogens are resistant to), and varies from less than 24 hours to longer than 72 hours in NHS Trust hospitals. The shorter the turnaround time, the earlier the clinicians can make precise prescribing decisions based on pathogen information. In addition, improving blood sampling practice quality, so that all patients have blood samples taken and all of these are tested would allow microbiologists to provide expert input when empiric decisions are reviewed.
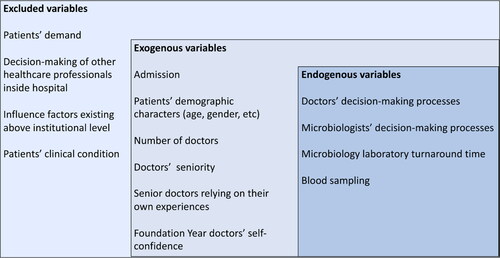
To explicitly visualise the causal relationships between these factors and clinicians’ tendency to comply with prescribing guidelines, we developed a causal loop diagram (CLD) which provides a qualitative description of the SD model (). The model boundary chart is provided in Appendix 1 to present exogenous variables, endogenous variables, and the variables excluded in this model.
Simulation model to predict level of prescribing guideline compliance
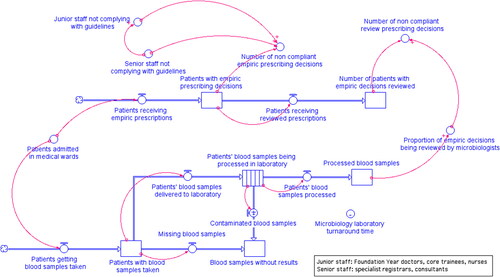
We used a “stock and flow” methodology to develop a simulation model representing the progression of doctors prescribing decisions when treating patients with antibiotics in medical wards. In our model, stock levels correspond to the number of patients with prescribing decisions made at different stages. Flows represent the number of patients going through prescribing stages per unit of time. The flow rates are expressed by mathematical equations.
In addition, we included a microbiology laboratory sub-system to simulate the process of patients’ blood samples being tested for pathogen information in the microbiology laboratory. The patients’ blood samples arrive in the microbiology laboratory and are processed to determine the pathogen. The time before the pathogen information becomes available is determined by the average laboratory turnaround time. When blood culture results become available, the patients’ progress from empiric stage to review stage with empiric decisions having been reviewed by microbiologists. The mathematical equations quantitatively describing the flow rates, the initial conditions of the stocks, and the average microbiology laboratory turnaround time of each blood sample being processed, were calculated using the source data.
We used the software iThink® (iSee Systems, Citation2005) to construct and simulate the model ().
The core of the model is the Number_of_non_ compliant_empiric_prescribing_decisions determined by the percentage of senior staff and junior staff who did not comply with prescribing guidelines, and the Number_of_non_compliant_review_prescribing_decisions determined by the proportion of empiric decisions reviewed by microbiologists. Model parameter calculation is discussed in detail in the section Model parameterisation using source data.
We made a series of assumptions listed below when modelling the system behaviour ().
Table 2. Assumptions made to simplify the system behaviour.
Model parameterisation using routine data from hospitals
In order to formulate the variables quantitatively, we used routinely collected hospital data on prescriptions for treating all patients with Escherichia coli (E. coli) bacteraemia admitted in 3 NHS Trust teaching hospitals during March to August 2016. A total of 150 patients were admitted, 127 patients had empiric prescribing decisions made by a staff member in medical wards, and 1792 prescribing decisions were made during their stay in the medical wards of 17 different specialties.
Three main parameters were estimated to formulate the model using the source data:
Guideline compliance: we performed binomial logistic regression analysis to calculate the odds ratio of prescribing decisions in compliance with prescribing guideline to test the association between level of compliance and clinician’s seniority, with specialty and hospital adjusted as confounders. The adjusted odds ratio for clinicians at different specialties were predicted for Foundation Year 1 doctors (1.00, reference category), Foundation Year 2 doctors (1.88, 95% CI: 1.13 to 2.63, p-value: 0.12), core trainees (1.72, 95% CI: 0.10 to 30.48, p-value: 0.71), nurses (1.01, 95% CI: 1.01 to 1.02, p-value: 0.05), specialist registrars (0.71, 95% CI: 0.35 to 0.98, p-value: 0.18), and consultants (0.29, 95% CI: 0.17 to 0.41, p-value: 0.07), which indicated decreased guideline compliance associated with higher seniority. The odds ratios were used to provide point estimation of the proportions of junior and senior staff not following prescribing guidelines (Junior_staff_not_complying_with_guidelines and Senior_staff_not_complying_with_guidelines in ). The total amount of non-compliant empiric decisions (Number_of_non_compliant_ empiric_prescribing_decisions) is calculated by multiplying Patients_with_empiric_prescribing_ decisions and the proportions of non-compliance.
Microbiology laboratory turnaround time: based on the length of turnaround time documented in the source data, the probability of a blood sample being processed in one day is approximated using Poisson distribution with λ = 0.82. The transit time (Microbiology_laboratory_turnaround_time) in the conveyer stock in is determined by the mean processing time of the Poisson distribution: t = 1/λ = 1.22 days.
Number of empiric decisions reviewed: an empiric decision was assumed to have been reviewed by microbiologists if the laboratory test result was available. In the source data, 10.2% of patients admitted did not have blood sample taken, and 6.6% of delivered blood samples did not have test results provided. Therefore, the value of the parameter Proportion_of_empiric_decisions_being_reviewed_by_ microbiologists is calculated as
(1-10.2%) × (1-0.66%) = 84.14%.
All parameter values of the variables included in the simulation model are listed in Supplementary Material 1.
Model validation
The model was subjected to a full set of validation tests. Model validation has been depicted as a parallel activity, overlapping the other model development stages. The validation tests include structure-verification test, parameter-verification test, extreme condition test, boundary-adequacy test, dimensional-consistency test, behaviour reproduction test, and behaviour sensitivity analysis (Barlas, Citation1996; Barlas & Carpenter, Citation1990).
First, six panel workshops were conducted to provide expert opinion to verify the model structure and parameters. The experts attending the workshops included front-line staff and managers from the local NHS Trust teaching hospitals, with extensive expertise in infectious diseases and AMS management in UK settings, health economics modellers from Public Health England (PHE), infectious diseases modellers and SD modellers. Second, the model passed the extreme condition test, boundary-adequacy test, and dimensional-consistency tests performed. We then performed behaviour reproduction tests performed by simulating the model to check its ability to replicate past real world observed data. The model predicted 92.9% of empiric decisions to be in line with prescribing guidelines. The estimation was compared to the real-life data, which is (i) 91.9% reported in the 2017 annual statistics collected from the same NHS Trust teaching hospitals, and (ii) 93.0% in ESPAUR 2014 report sampling the guideline compliance in 99 NHS Trusts. Based on these results, the validity of the model was approved by the expert panel.
Policy analysis and results
Interventions and scenarios
The model was simulated to predict doctors’ prescribing decisions in 100 days for 1000 patients admitted in medical wards under each scenario. At empiric stage, the system behaviour was measured the percentage of compliant empiric decisions under the scenarios of which the microbiology laboratory turnaround time was shortened (Scenario1-3); and the guideline compliance level among senior staff (specialist registrars and consultants) was increased with and without influencing doctors with lower seniority (Scenario 4-10). After review stage, the system behaviour was measured by the percentage of empiric decisions reviewed by microbiologists under the scenarios which the availability of microbiology laboratory test results varied (Scenario 11-12)) (). A symbol “-” in indicates that the value is unchanged from the baseline scenario.
Table 3. ‘What if’ scenarios simulated using the SD model.
The simulation results suggested that the decision-making of senior staff in medical wards played a pivotal role in guideline compliance. The guideline compliance level under the scenario of improving senior practice and shortening microbiology turnaround time (99.4% under Scenario 10) only increased slightly compared to the scenario of improving senior practice alone (99.3% under Scenario 9).
The findings provide quantitative evidence for hospital managers to make decisions in which interventions are to be implemented. Interventions such as adopting rapid diagnostic tools or point-of-care testing to shorten turnaround time can certainly guide rational use of antibiotics. However, enhancing senior staff’s, especially specialist registrars’ prescribing practice is predicted to have stronger impact on overall prescribing practices compared to improving microbiology laboratory turnaround. The model results suggest a pivotal role is played by specialist registrars. Our panel of clinician experts provided interpretation of these results, that specialist registrars in general make more empiric decisions than other senior staff. In our source data, specialist registrars made the most empiric decisions (37.8%) compared to other medical staff (19.7% for junior staff).
Discussion
SD has existed for well over sixty years (Forrester, Citation1961), but only has gained widespread popularity within the last 20 years as a specialised method in public health and healthcare. SD, as a collaborative approach, enables better understanding of complex systems and allows dynamic behaviour to be projected using computer simulation. It is predicted that, with “what if” scenario testing, SD can be used as a tool for persuasion and tactical evaluation, and can inform population-level public policy design in healthcare (Dangerfield, Citation1999; Richardson, Citation1999). However, despite the effort made in applying SD to various healthcare issues, the growing body of SD literature remains as fragmented learning experiences generated from different case studies in healthcare.
There is no established theory to describe the fundamental assumptions made to describe health systems and intelligent agents within the systems from a complex systems’ perspective, and what the best practice of SD modelling looks like in simulation for healthcare. In most recent OR literature, the importance of engaging experts in model development and validation stages was recognised (Robinson, Citation2019). Simulation in healthcare is unique (Tako & Robinson, Citation2015). Three key factors contribute to impactful application of OR in healthcare, including expert engagement, use of contextually relevant data, and communication and feedback of simulation findings (Bradley et al., Citation2017).
A growing body of research has applied behavioural science to establish doctors’ mental models and interpret their behaviour when prescribing antibiotics. Here the approach has been explanatory rather than theoretically based (Manchanda & Honka, Citation2005; Theodorou et al., Citation2009). Those studies which have employed theoretical models, such as reasoned action theory, planned behaviour theory, and the theoretical domains framework (TDF) (Gallan, Citation2004; Godin et al., Citation2008; Rashidian et al., Citation2006), make an underlying assumption, that doctors are perfectly rational when making prescribing decisions; these assumptions are disputable (Murshid & Mohaidin, Citation2017; Theodorou et al., Citation2009; Vancelik et al., Citation2007). Prescribing decision-making processes are heavily (and rationally) weighted towards the avoidance of tangible, immediate and short-term risks (i.e., adverse patient outcomes), at the cost of the potential catastrophic, but abstract and uncertain future outcome (i.e., emergence and spread of AMR) (Krockow et al., Citation2019). Prescribing decision-making processes are dynamic and not always rational, influenced by multiple interconnected factors, and embedded in complex healthcare systems. A different theoretical model is required to develop a better understanding of doctors’ decision making and help support the outcome of optimal antibiotic use.
In this study, we formulated the SD model using empirical data from the UK, where optimising antibiotic use is a national priority. Stakeholders were involved in multiple stages of model development to enhance model credibility and to disseminate policy implications. We demonstrated that SD is a suitable analytical tool to investigate complex healthcare management issues because of its technical strengths and its fundamental philosophical roots of model validation (Barlas & Carpenter, Citation1990). SD enables quantitative prediction of complex system behaviour where multiple influences occur and takes into consideration the cognitive limitations of the decision makers inside the system.
There are four limitations of this study, which provide scope for future research. First, patients were assumed to passively accept treatment and disassociated from decision-making processes. Second, the soft variable “fear of under-treating” was not simulated quantitatively. Third, the time elements were not included in the model structure. The omitted variables associated with time include delay in doctors’ behaviour change in response to environmental change, and the variability of speed of patients progressing within the modelled system.
In future, we plan to expand the model by including more influence factors, such as patient knowledge and empowerment to understand treatment decisions better (Rawson et al., Citation2018), soft variables that measure doctors’ emotions, and time lag in doctors’ behaviour change, so that the model is useful in different settings within the NHS. We will collect additional primary data, and incorporate adequate discrete scaling methods, to define behavioural “rules” for individual doctors so that these emotional variables can be simulated quantitatively.
Our study is the first to successfully apply SD modelling to the important global challenge of AMR, to explain factors which interact to influence doctors’ behaviour. Notwithstanding limitations, the findings of this study have important policy implications for prescribing behaviour management in hospitals in the UK and beyond.
Conclusions
System dynamics models can effectively capture structural and behavioural influences that influence prescribing to simulate doctors’ prescribing practices – which is an important factor in the development of antimicrobial resistance that has major health and economic consequences worldwide.
Antibiotic prescribing practices measured by guideline adherence can be achieved by improving senior doctors’ decision-making, reducing turnaround time of blood culture to less than 24 h for all patients, and providing microbiology laboratory test results for all patients. The application of systems thinking methodologies embracing complexity and context dependence in behaviour management, as demonstrated here, has the potential for operational, implementation and health systems research relevant for decision making to address major global health challenges.
Supplemental Material
Download MS Word (20.2 KB)Acknowledgements
This research was funded by the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Healthcare Associated Infections and Antimicrobial Resistance at Imperial College London in partnership with Public Health England (PHE), in collaboration with, Imperial Healthcare Partners, University of Cambridge and University of Warwick. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research, the Department of Health and Social Care or Public Health England. Dr Nina Zhu, Dr Raheelah Ahmad, Dr Reda Lebcir, and Prof Alison Holmes gratefully acknowledge the support of ESRC as part of the Antimicrobial Cross Council initiative supported by the seven UK research councils, and also the support of the Global Challenges Research Fund. Co-author Prof Alison Holmes is a National Institute for Health Research (NIHR) Senior Investigator. Co-author Dr Raheelah Ahmad is supported by a National Institute for Health Research (NIHR) Fellowship in knowledge mobilisation at the NIHR HPRU in Healthcare Associated Infection and Antimicrobial Resistance. The grant number is KMRF-2015-04-007.
We thank our colleagues: Dr Esmita Charani, Dr Timothy Miles Rawson, Dr Gabriel Birgand from NHS Trust teaching hospitals who provided insight and expertise that helped develop and validate the model. We thank the modellers from the Modelling and Economics Unit, National Infections Service, PHE, including Dr Esther van Kleef, Dr Timo Smieszek, and Dr Koen Pouwels for assistance with model validation and scenario testing.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ahmad, R., Sim, F., & Mackie, P. (2019). Navigating complexity: Harnessing appropriate methodologies. Public Health, 174, A1–A2. https://doi.org/https://doi.org/10.1016/j.puhe.2019.08.001
- Ahmad, R., Zhu, N. J., Lebcir, R. M., & Atun, R. (2019). How the health-seeking behaviour of pregnant women affects neonatal outcomes: Findings of system dynamics modelling in Pakistan. BMJ Global Health, 4(2), e001242. https://doi.org/https://doi.org/10.1136/bmjgh-2018-001242
- Ashiru-Oredope, D., Hopkins, S., on behalf of the English Surveillance Programme for Antimicrobial Utilization and Resistance Oversight Group. (2013). Antimicrobial stewardship: English Surveillance Programme for Antimicrobial Utilization and Resistance (ESPAUR). Journal of Antimicrobial Chemotherapy, 68(11), 2421–2423. https://doi.org/https://doi.org/10.1093/jac/dkt363
- Ashiru-Oredope, D., Sharland, M., Charani, E., McNulty, C., Cooke, J, & on behalf of ARHAI Antimicrobial Stewardship Group. (2012). Improving the quality of antibiotic prescribing in the NHS by developing a new Antimicrobial Stewardship Programme: Start Smart–Then Focus. Journal of Antimicrobial Chemotherapy, 67(suppl 1), i51–i63. https://doi.org/https://doi.org/10.1093/jac/dks202
- Bal, A. M., & Gould, I. M. (2011). Antibiotic stewardship: Overcoming implementation barriers. Current Opinion in Infectious Diseases, 24(4), 357–362. https://doi.org/https://doi.org/10.1097/QCO.0b013e3283483262
- Barlas, Y. (1996). Formal aspects of model validity and validation in system dynamics. System Dynamics Review, 12(3), 183–210. https://doi.org/https://doi.org/10.1002/(SICI)1099-1727(199623)12:3<183::AID-SDR103>3.0.CO;2-4
- Barlas, Y., & Carpenter, S. (1990). Philosophical roots of model validation: Two paradigms. System Dynamics Review, 6(2), 148–166. https://doi.org/https://doi.org/10.1002/sdr.4260060203
- Barton, P., Bryan, S., & Robinson, S. (2004). Modelling in the economic evaluation of health care: Selecting the appropriate approach. Journal of Health Services Research & Policy, 9(2), 110–118. https://doi.org/https://doi.org/10.1258/135581904322987535
- Bradley, B. D., Jung, T., Tandon-Verma, A., Khoury, B., Chan, T. C. Y., & Cheng, Y.-L. (2017). Operations research in global health: A scoping review with a focus on the themes of health equity and impact. Health Research Policy and Systems, 15(1), 32. https://doi.org/https://doi.org/10.1186/s12961-017-0187-7
- Bush, K., Courvalin, P., Dantas, G., Davies, J., Eisenstein, B., Huovinen, P., Jacoby, G. A., Kishony, R., Kreiswirth, B. N., Kutter, E., Lerner, S. A., Levy, S., Lewis, K., Lomovskaya, O., Miller, J. H., Mobashery, S., Piddock, L. J. V., Projan, S., Thomas, C. M., … Zgurskaya, H. I. (2011). Tackling antibiotic resistance. Nature Reviews Microbiology, 9(12), 894–896. https://doi.org/https://doi.org/10.1038/nrmicro2693
- Chambers, A., MacFarlane, S., Zvonar, R., Evans, G., Moore, J. E., Langford, B. J., Augustin, A., Cooper, S., Quirk, J., McCreight, L., & Garber, G. (2019). A recipe for antimicrobial stewardship success: Using intervention mapping to develop a program to reduce antibiotic overuse in long-term care. Infection Control & Hospital Epidemiology, 40(1), 24–31. https://doi.org/https://doi.org/10.1017/ice.2018.281
- Charani, E., Ahmad, R., Rawson, T. M., Castro-Sanchèz, E., Tarrant, C., & Holmes, A. H. (2019). The differences in antibiotic decision-making between acute surgical and acute medical teams: An ethnographic study of culture and team dynamics. Clinical Infectious Diseases, 69(1), 12–20. https://doi.org/https://doi.org/10.1093/cid/ciy844
- Charani, E., Castro-Sanchez, E., Sevdalis, N., Kyratsis, Y., Drumright, L., Shah, N., & Holmes, A. (2013). Understanding the determinants of antimicrobial prescribing within hospitals: The role of "prescribing etiquette". Clinical Infectious Diseases, 57(2), 188–196. https://doi.org/https://doi.org/10.1093/cid/cit212
- Charani, E., Edwards, R., Sevdalis, N., Alexandrou, B., Sibley, E., Mullett, D., Franklin, B. D., & Holmes, A. (2011). Behavior change strategies to influence antimicrobial prescribing in acute care: A systematic review. Clinical Infectious Diseases, 53(7), 651–662. https://doi.org/https://doi.org/10.1093/cid/cir445
- Charani, E., Smith, I., Skodvin, B., Perozziello, A., Lucet, J.-C., Lescure, F.-X., Birgand, G., Poda, A., Ahmad, R., Singh, S., & Holmes, A. H. (2019). Investigating the cultural and contextual determinants of antimicrobial stewardship programmes across low-, middle- and high-income countries—A qualitative study. PLoS One, 14(1), e0209847. https://doi.org/https://doi.org/10.1371/journal.pone.0209847
- Cooke, J., Alexander, K., Charani, E., Hand, K., Hills, T., Howard, P., Jamieson, C., Lawson, W., Richardson, J., & Wade, P. (2010). Antimicrobial stewardship: An evidence-based, antimicrobial self-assessment toolkit (ASAT) for acute hospitals. Journal of Antimicrobial Chemotherapy, 65(12), 2669–2673. https://doi.org/https://doi.org/10.1093/jac/dkq367
- Dangerfield, B. C. (1999). System dynamics applications to European health care issues. Journal of the Operational Research Society, 50(4), 345–353. https://doi.org/https://doi.org/10.1057/palgrave.jors.2600729
- Davey, P., Brown, E., Charani, E., Fenelon, L., Gould, I. M., Holmes, A., Ramsay C. R., Wiffen, P. J., & Wilcox, M. (2013). Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane database of systematic reviews, (4), Art. No.: CD003543. http://doi.org/10.1002/14651858.CD003543.pub3.
- de Kraker, M. E. A., Davey, P. G., Grundmann, H, & on behalf of the BURDEN study group. (2011). Mortality and hospital stay associated with resistant Staphylococcus aureus and Escherichia coli bacteremia: Estimating the burden of antibiotic resistance in Europe. PLoS Medicine, 8(10), e1001104. https://doi.org/https://doi.org/10.1371/journal.pmed.1001104
- Department of Health and Social Care. (2019). Tackling antimicrobial resistance 2019 – 2024: The UK’s five-year national action plan. Retrieved January 19, 2020 from https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/784894/UK_AMR_5_year_national_action_plan.pdf
- Forrester, J. W. (1961). Industrial dynamics. Massachusetts Institute of Technology Press. https://books.google.co.uk/books/about/Industrial_Dynamics.html?id=4CgzAAAAM AAJ&redir_esc=y
- Forrester, J. W. (1994). System dynamics, systems thinking, and soft OR. System Dynamics Review, 10(2-3), 245–256. https://doi.org/https://doi.org/10.1002/sdr.4260100211
- Gallan, A. S. (2004). Factors that influence physicians’ prescribing of pharmaceuticals: A literature review. Journal of Pharmaceutical Marketing & Management, 16(4), 3–46. https://doi.org/https://doi.org/10.3109/J058v16n04_02
- Gharbi, M., Moore, L. S. P., Castro-Sánchez, E., Spanoudaki, E., Grady, C., Holmes, A. H., & Drumright, L. N. (2016). A needs assessment study for optimising prescribing practice in secondary care junior doctors: The Antibiotic Prescribing Education among Doctors (APED). BMC Infectious Diseases, 16(1), 456. https://doi.org/https://doi.org/10.1186/s12879-016-1800-z
- Godin, G., Bélanger-Gravel, A., Eccles, M., & Grimshaw, J. (2008). Healthcare professionals' intentions and behaviours: A systematic review of studies based on social cognitive theories. Implementation Science: IS, 3(1), 36. https://doi.org/https://doi.org/10.1186/1748-5908-3-36
- Greenhalgh, T., & Papoutsi, C. (2018). Studying complexity in health services research: Desperately seeking an overdue paradigm shift. BMC Medicine, 16(1), 95. https://doi.org/https://doi.org/10.1186/s12916-018-1089-4
- Greenhalgh, T., Plsek, P., Wilson, T., Fraser, S., & Holt, T. (2010). Response to 'The appropriation of complexity theory in health care'.Journal of Health Services Research & Policy, 15(2), 115–117. https://doi.org/https://doi.org/10.1258/jhsrp.2010.009158
- Günal, M. M., & Pidd, M. (2010). Discrete event simulation for performance modelling in health care: A review of the literature. Journal of Simulation, 4(1), 42–51. https://doi.org/https://doi.org/10.1057/jos.2009.25
- Holmes, A. H., Moore, L. S. P., Sundsfjord, A., Steinbakk, M., Regmi, S., Karkey, A., Guerin, P. J., & Piddock, L. J. V. (2016). Understanding the mechanisms and drivers of antimicrobial resistance. The Lancet, 387(10014), 176–187. https://doi.org/https://doi.org/10.1016/S0140-6736(15)00473-0
- Hulscher, M. E., Grol, R. P., & van der Meer, J. W. (2010). Antibiotic prescribing in hospitals: A social and behavioural scientific approach. The Lancet Infectious Diseases, 10(3), 167–175. https://doi.org/https://doi.org/10.1016/S1473-3099(10)70027-X
- iSee Systems. (2005). iThink® Software V9.0.2. Retrieved January 19, 2020 from https://www.iseesystems.com/store/products/ithink.aspx
- Krockow, E. M., Colman, A. M., Chattoe-Brown, E., Jenkins, D. R., Perera, N., Mehtar, S., & Tarrant, C. (2019). Balancing the risks to individual and society: A systematic review and synthesis of qualitative research on antibiotic prescribing behaviour in hospitals. The Journal of Hospital Infection, 101(4), 428–439. https://doi.org/https://doi.org/10.1016/j.jhin.2018.08.007
- Kyratsis, Y., Ahmad, R., Iwami, M., Castro-Sánchez, E., Atun, R., & Holmes, A. H. (2019). A multilevel neo-institutional analysis of infection prevention and control in English hospitals: Coerced safety culture change? Sociology of Health and Illness, 41(6), 1138–1158. https://doi.org/10.1111/1467-9566.12897
- Lane, D. C., & Husemann, E. (2008). System dynamics mapping of acute patient flows. Journal of the Operational Research Society , 59(2), 213–224. https://doi.org/https://doi.org/10.1057/palgrave.jors.2602498
- Lebcir, R. M. (2006). Health care management: The contribution of systems thinking. The Business School. University of Hertfordshire College Lane (May), 1–33.
- MacDougall, C., & Polk, R. E. (2005). Antimicrobial stewardship programs in health care systems. Clinical Microbiology Reviews, 18(4), 638–656. https://doi.org/https://doi.org/10.1128/CMR.18.4.638-656.2005
- Manchanda, P., & Honka, E. (2005). The effects and role of direct-to-physician marketing in the pharmaceutical industry: An integrative review. Yale Journal of Health Policy, Law, and Ethics, 5(2), 785–822. http://www.ncbi.nlm.nih.gov/pubmed/16052900
- Martinez-Moyano, I. J., & Richardson, G. P. (2013). Best practices in system dynamics modeling. System Dynamics Review, 29(2), 102–123. https://doi.org/https://doi.org/10.1002/sdr.1495
- McNulty, C. A. M., Cookson, B. D., & Lewis, M. A. O. (2012). Education of healthcare professionals and the public. Journal of Antimicrobial Chemotherapy, 67(suppl 1), i11–i18. https://doi.org/https://doi.org/10.1093/jac/dks199
- Moore, G. F., Audrey, S., Barker, M., Bond, L., Bonell, C., Hardeman, W., Moore, L., O’Cathain, A., Tinati, T., Wight, D., & Baird, J. (2015). Process evaluation of complex interventions: Medical Research Council guidance. BMJ, 350, h1258. https://doi.org/https://doi.org/10.1136/bmj.h1258
- Murshid, M. A., & Mohaidin, Z. (2017). Models and theories of prescribing decisions: A review and suggested a new model. Pharmacy Practice, 15(2), 990. https://doi.org/https://doi.org/10.18549/PharmPract.2017.02.990
- National Institute for Health Research (NIHR) Research Design Service: East Midlands. (2017). Highlight notice: Complex health and care needs in older people. Retrieved January 19, 2020 from https://www.nihr.ac.uk/funding-and-support/documents/themed-calls/ComplexHealthNeedsHighlightNoticeFINALVERSION.pdf
- Public Health England. (2018). English surveillance programme for antimicrobial utilisation and resistance (ESPAUR) Report 2018. Public Health England, 1–143. www.facebook.com/PublicHealthEngland%0Awww.gov.uk/phe%5Cnwww.facebook.com/PublicHealthEngland
- Ramsay, C., Brown, E., Hartman, G., & Davey, P. (2003). Room for improvement: A systematic review of the quality of evaluations of interventions to improve hospital antibiotic prescribing. The Journal of Antimicrobial Chemotherapy, 52(5), 764–771. https://doi.org/https://doi.org/10.1093/jac/dkg460
- Rashidian, A., Miles, J., Russell, D., & Russell, I. (2006). Sample size for regression analyses of theory of planned behaviour studies: Case of prescribing in general practice. British Journal of Health Psychology, 11(Pt 4), 581–593. https://doi.org/https://doi.org/10.1348/135910705X66043
- Rawson, T. M., Ahmad, R., Toumazou, C., Georgiou, P., & Holmes, A. H. (2019). Artificial intelligence can improve decision-making in infection management. Nature Human Behaviour, 3(6), 543–545. https://doi.org/https://doi.org/10.1038/s41562-019-0583-9
- Rawson, T. M., Charani, E., Moore, L. S. P., Hernandez, B., Castro-Sánchez, E., Herrero, P., Georgiou, P., & Holmes, A. H. (2016). Mapping the decision pathways of acute infection management in secondary care among UK medical physicians: A qualitative study. BMC Medicine, 14(1), 1–10. https://doi.org/https://doi.org/10.1186/s12916-016-0751-y
- Rawson, T. M., Moore, L. S. P., Castro-Sanchez, E., Charani, E., Hernandez, B., Alividza, V., Husson, F., Toumazou, C., Ahmad, R., Georgiou, P., & Holmes, A. H. (2018). Development of a patient-centred intervention to improve knowledge and understanding of antibiotic therapy in secondary care. Antimicrobial Resistance and Infection Control, 7(1), 43. https://doi.org/https://doi.org/10.1186/s13756-018-0333-1
- Richardson, G. P. (1999). Reflections for the future of system dynamics. Journal of the Operational Research Society , 50(4), 440–449. https://doi.org/https://doi.org/10.1057/palgrave.jors.2600749
- Robert, G., Fulop, N. (2014). The role of context in successful improvement. London. https://www.health.org.uk/sites/default/files/PerspectivesOnContextRobertFulop TheRoleOfContextInSuccessfulImprovement.pdf
- Robinson, S. (2019). Conceptual modelling for simulation: Progress and grand challenges. Journal of Simulation, 14(1), 1–20. https://www.tandfonline.com/doi/full/10.1080/17477778.2019.1604466
- Rzewuska, M., Charani, E., Clarkson, J. E., Davey, P. G., Duncan, E. M., Francis, J. J., Gillies, K., Kern, W. V., Lorencatto, F., Marwick, C. A., McEwen, J., Möhler, R., Morris, A. M., Ramsay, C. R., Rogers Van Katwyk, S., Skodvin, B., Smith, I., Suh, K. N., Grimshaw, J. M, & Joint Programming Initiative on Antimicrobial Resistance (JPIAMR) Working Group on Behavioural Approaches to Antibiotic Stewardship Programs. (2019). Prioritizing research areas for antibiotic stewardship programmes in hospitals: A behavioural perspective consensus paper. Clinical Microbiology and Infection, 25(2), 163–168. https://doi.org/https://doi.org/10.1016/j.cmi.2018.08.020
- Senge, P. M. (1990). The Fifth Discipline: The art and practice of the learning organization. Currency.
- Simon, H. A. (1991). Bounded rationality and organizational learning. Organization Science, 2(1), 125–134. https://doi.org/https://doi.org/10.1287/orsc.2.1.125
- Skodvin, B., Aase, K., Charani, E., Holmes, A., & Smith, I. (2015). An antimicrobial stewardship program initiative: A qualitative study on prescribing practices among hospital doctors. Antimicrobial Resistance and Infection Control, 4(1), 24. https://doi.org/https://doi.org/10.1186/s13756-015-0065-4
- Spivak, E. S., Cosgrove, S. E., & Srinivasan, A. (2016). Measuring appropriate antimicrobial use: Attempts at opening the black box. Clinical Infectious Diseases, 63(12), 1639–1644. https://doi.org/https://doi.org/10.1093/cid/ciw658
- Sterman, J. (2002). System dynamics: Systems thinking and modeling for a complex world. Retrieved January 19, 2020 from https://dspace.mit.edu/handle/1721.1/102741
- Sterman, J. D. (1987). Systems simulation. Expectation formation in behavioral simulation models. Behavioral Science, 32(3), 190–211. https://doi.org/https://doi.org/10.1002/bs.3830320304
- Sterman, J. D. (1992). System dynamics modeling for project management. Retrieved January 19, 2020 from http://web.mit.edu/jsterman/www/SDG/project.pdf
- Tako, A. A., & Robinson, S. (2015). Is simulation in health different? Journal of the Operational Research Society, 66(4), 602–614. https://doi.org/https://doi.org/10.1057/jors.2014.25
- Thakkar, K., Gilchrist, M., Dickinson, E., Benn, J., Franklin, B. D., Jacklin, A., … Anti-Infective Policy Implementation Group. (2011). A quality improvement programme to increase compliance with an anti-infective prescribing policy. Journal of Antimicrobial Chemotherapy, 66(8), 1916–1920. https://doi.org/https://doi.org/10.1093/jac/dkr207
- Theodorou, M., Tsiantou, V., Pavlakis, A., Maniadakis, N., Fragoulakis, V., Pavi, E., & Kyriopoulos, J. (2009). Factors influencing prescribing behaviour of physicians in Greece and Cyprus: Results from a questionnaire based survey. BMC Health Services Research, 9(1), 150. https://doi.org/https://doi.org/10.1186/1472-6963-9-150
- Tonkin-Crine, S., Walker, A. S., & Butler, C. C. (2015). Contribution of behavioural science to antibiotic stewardship. BMJ, 350(jun25 8), h3413. https://doi.org/https://doi.org/10.1136/bmj.h3413
- Vancelik, S., Beyhun, N. E., Acemoglu, H., & Calikoglu, O. (2007). Impact of pharmaceutical promotion on prescribing decisions of general practitioners in Eastern Turkey. BMC Public Health, 7(1), 122. https://doi.org/https://doi.org/10.1186/1471-2458-7-122
- Wickens, H. J., Farrell, S., Ashiru-Oredope, D. A. I., Jacklin, A., Holmes, A., Cooke, J., … Antimicrobial Stewardship Group of Department of Health Advisory Committee on Antimicrobial Resistance and Health Care Associated Infections (ASG-ARHAI). (2013). The increasing role of pharmacists in antimicrobial stewardship in English hospitals. Journal of Antimicrobial Chemotherapy, 68(11), 2675–2681. https://doi.org/https://doi.org/10.1093/jac/dkt241
- World Health Organization. (2018). Antibiotic resistance. World Health Organization Fact Sheet: Antibiotic resistance. Retrieved January 19, 2020, from https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance
- Zarb, P., Amadeo, B., Muller, A., Drapier, N., Vankerckhoven, V., Davey, P., Goossens, H., Metz-Gercek, S., Jansens, H., Markova, B., Kontemeniotou, C., Andrasevic, A., Vlcek, J., Frimodt-Moller, N., Mitt, P., Lyytikainen, O., Bertrand, X., de With, K., Antoniadou, A., … on behalf of the ESAC-3 Hospital Care Subproject Group. (2011). Identification of targets for quality improvement in antimicrobial prescribing: The web-based ESAC point prevalence survey 2009. The Journal of Antimicrobial Chemotherapy, 66(2), 443–449. https://doi.org/https://doi.org/10.1093/jac/dkq430