ABSTRACT
Objectives
To identify predictors of functional outcome in patients with an anterior circulation large-vessel occlusion (LVO) in a setting of long transfer distances.
Methods
Outcomes of LVO patients transferred for an endovascular thrombectomy (EVT) from North Karelia Central Hospital to Kuopio University Hospital between January 2018 and October 2019 were analysed using retrospective patient chart review.
Results
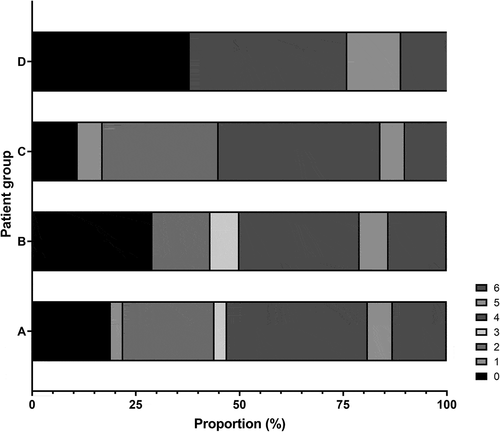
Three months after the stroke, modified Rankin Scale (mRS) was 0–2 in 20 of the 41 transferred patients. They were younger (66.7 vs. 74.2 years, p = 0.032) and had less severe stroke symptoms (National Institutes of Health Stroke Scale, NIHSS, 11.5 vs. 16.5, p = 0.029) than those with mRS 3–6. They also had the occlusion less often in M1 and more often in M2. EVT was performed in 32 patients (no differences between those treated with EVT and those not treated with EVT). Their median age was 73.0 years (interquartile range 65.5, 79.8; range 32–86; 25% over 80), mean NIHSS score 14.0 (standard deviation 5.9) and mRS eventually 0–2 in 44%. Only NIHSS was associated with mRS (OR = 1.16; p = 0.016) in the EVT-treated patients. mRS was 0 in 38% of all EVT-treated octogenarians but 4–6 in 83% of those with an internal carotid artery and/or M1 occlusion.
Discussion
Outcomes depended on stroke severity, age and vessel of occlusion. Prognosis was worse if the occlusion included M1, especially in octogenarians. Mothership and Drip-n-ship strategies should be compared in patients from remote locations stratified by stroke severity and patient age.
Introduction
Since 2015, mechanical clot removal with endovascular thrombectomy (EVT) has been the standard treatment method in large-vessel occlusions (LVO) [Citation1,Citation2]. Unfortunately, this treatment is not available at all centers that provide acute stroke care with intravenous thrombolysis (IVT) but only in comprehensive stroke centres (CSC). Since both IVT and EVT should be administered as early as possible for best effectiveness and no certain method to recognize an LVO without imaging exists [Citation2–4] it is unclear if and which patients should be transferred directly to a CSC if an IVT-capable primary stroke centre (PSC) is closer [Citation2]. Moreover, the publication of DAWN and DEFUSE III trials in January 2018 led to considerably more patients being eligible for EVT as the time window was expanded to within 24 hours of last seen well [Citation5,Citation6] and patient selection for EVT now often requires specific expertise and use of advanced imaging methods, resources that are routinely available only in CSCs. The recent RACECAT trial showed that a direct transfer of patients with stroke and suspected LVO to a CSC did not improve outcomes in settings where the median time difference between the mothership and ‘drip and ship’ paradigms is less than 60 minutes [Citation7]. More data are needed to improve pathway modeling for different environments and situations [Citation2,Citation8].
In North Karelia Central Hospital (NKCH), a PSC, IVT results are generally good but many patients with an LVO are left with significant disability [Citation9] indicating an unmet treatment need. Anterior circulation ischemic stroke patients transferred from NKCH to the CSC, Kuopio University Hospital (KUH) were therefore investigated to identify predictors of functional outcome that could help in designing more efficient patient-specific care pathways in a setting of long transfer distances. Particular interest was paid to octogenarians due to the uncertainty concerning treatment strategies in these patients [Citation10–12].
Materials and methods
Data collection and study site
All patients with an anterior circulation ischemic stroke transferred from NKCH to KUH for a possible EVT between January 2018 and October 2019 were identified from monthly billing data from KUH to NKCH by the author. Electronic medical charts were scrutinised for data. Unfortunately, the information on exactly when each patient had arrived at NKCH or KUH had been insufficiently recorded (although in most cases the door-to-needle (DNT) time for IVT appeared to have been 15–25 minutes). Treatment times were consequently assessed only using onset-to-treatment time (OTT). LVO was defined as an occlusion in the internal carotid artery, the M1 or M2 branches of the middle cerebral artery that correlated with the clinical symptoms. The procedure has been described previously, although it is currently performed routinely with the stent retriever (Anne-Mari Kantanen, personal communication).15 Precise recanalisation data, such as Thrombolysis in cerebral infarction (TICI) scale, was not available but recanalisation results had been otherwise described and was scored here as none, partial or complete.
During the study period NKCH stroke treatment protocol was to consider all previously functionally independent patients with an LVO for an EVT regardless of age and to consult KUH (by telephone and transferring the CT images electronically) whether the patient should be sent to KUH for advanced imaging and possible EVT. The patient was required to reach KUH in 24 hours of witnessed symptom onset, or last seen well, to be eligible. Patients considered eligible for EVT were transported to KUH immediately after the decision was made with the same ambulance they arrived with and the results of a possible IVT were not waited for.
Hyperacute stroke imaging is usually available only with CT (native and angiography) at NKCH. Perfusion CT imaging was used sporadically from the summer of 2019 but did not become routinely available during the study period so all transferred patients went through perfusion imaging at KUH where the final decision on whether to proceed to EVT was made. NKCH is 1.5 hours away from KUH by ambulance. NKCH serves the North Karelia province with an area of c. 21,500 km2 and a population c. 167,000.
Statistical methods
The distribution of continuous variables was assessed with Shapiro-Wilk and Kolmogorov-Smirnov tests and histograms which were used to choose either Mann-Whitney U test or independent samples t-test to analyse patient characteristics and means and standard deviations or medians and interquartile ranges to present the results.
Ordinal regression analysis was used to analyse possible correlations between different variables and mRS outcomes. Good functional outcome was defined as modified Rankin Scale score 0–2 three months after the stroke. Statistical significance was inferred at a P-value < 0.05. Analyses were conducted using IBM SPSS Statistics for Windows, Version 27.0 (Armonk, NY: IBM Corp).
Results
In all, 41 patients had been transferred to KUH for a possible EVT. Endovascular treatment was deferred in nine patients since the clot had dissolved or moved beyond M2 during transfer in eight and one had a large infarct core with no penumbra left upon arrival at KUH. There was no difference in age (p = 0.75), NIHSS at NKCH (p = 0.63), the proportion of patients treated with IVT (p = 1.00) or the proportion of patients with a good outcome (p = 0.28) between the patients who eventually did not need EVT and those who did.
Patients with a good functional outcome were younger, had lower NIHSS scores at NKCH and their culprit vessel was less often M1 and more often M2. There was no difference in sex distribution or the proportion of patients treated with IVT between the groups ().
Table 1. Patient and stroke characteristics by outcome group. CI, confidence interval; ICA, internal carotid artery; IVT, intravenous thrombolysis; M1 and M2, branches of the middle cerebral artery; NIHSS, National Institutes of Health Stroke Scale; mRS, modified Rankin Scale
Treatment and outcomes in the EVT group
The median age of the EVT-treated patients was 73.0 (IQR 65.5, 79.8; range 32–86) years with 21 (65%) being over 70 and eight (25%) over 80 years of age. There was no difference in age between sexes (p = 0.87). All had been functionally independent before symptom onset with pre-stroke mRS of 1–2 in three patients (one ICA and two M1 occlusions) and 0 in the rest (91%). The majority of occlusions were in M1 (). Mean NIHSS at NKCH was 14.0 (SD 5.9) with no difference between sexes (p = 0.28).
Table 2. Characteristics and outcomes by vessel of occlusion of the patients who were treated with endovascular thrombectomy. Age is presented as mean and standard deviation or median and interquartile range, as appropriate. ICA, internal carotid artery; IVT, intravenous thrombolysis administered; OTT, onset-to-treatment time to thrombectomy (presented as mean and standard deviation or median and interquartile range); M1 and M2, branches of the middle cerebral artery; MRS, modified Rankin scale (presented as median and interquartile range); NIHSS, National Institutes of Health Stroke Scale score (presented as mean and standard deviation)
IVT had been given to 66% (p = 0.71 for difference between sexes) yielding partial recanalisation in 24% of them and none in the rest. The precise time of symptom onset known in 50% yielding a mean OTT to IVT of 139 minutes (95% CI 98–180) in men and 80 minutes (95% CI 50–108) in women (p = 0.016). Median OTT to EVT was 290 (IQR 195; 308) minutes (p = 0.056 for difference between sexes). Complete recanalisation was achieved with EVT in 59% and no recanalisation in 19%.
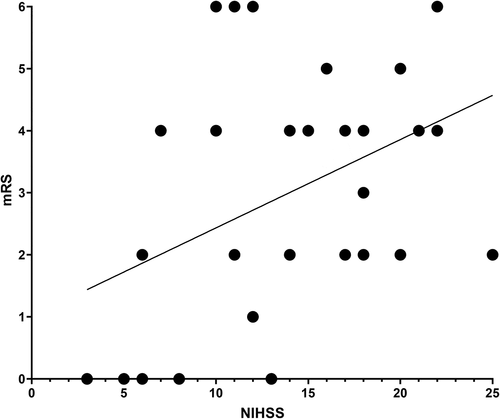
Three months after EVT, mRS was 0–2 in 44% and 13% were dead (). In univariate regression analyses, modified Rankin Scale score at three months was associated only with NIHSS at NKCH (OR = 1.16; p = 0.016), not with patient age (p = 0.40), sex (p = 0.76), pre-stroke mRS (p = 0.56), having received IVT (p = 0.48), OTT to IVT (p = 0.087) or OTT to EVT (p = 0.72). Good outcome was observed in 43% of men and 44% of women (p = 0.63; ). No patient with an mRS of 5–6 had an NIHSS score lower than 10 ().
Octogenarians
Of the nine patients aged 80 or more (66% women) five (56 %) had been given IVT. In one case, the clot moved to M4 en route to KUH and EVT was deferred (mRS 6 at three months) whereas the remaining eight were treated endovascularly. The occlusion was in ICA and/or M1 in six patients, of whom one (17%) of them reached mRS 0 (NIHSS 8, partial recanalization with IVT), one (17%) had died and the score was 4–5 in the rest (67%). Two patients had M2 occlusions (IVT not given to either) and they both reached mRS 0.
Discussion
In this analysis of patients transferred from a single peripheral PSC for endovascular treatment of anterior circulation large vessel occlusion, good functional outcome was associated with less severe stroke symptoms and lower patient age. Outcomes were poorer when the occlusion involved the M1 segment of middle cerebral artery, especially when the patient was an octogenarian.
Previous data from this hospital showed that, before EVT became far more widely applicable in clinical practice in the beginning of 2018, outcomes of patients with LVOs needed improvement [Citation9]. Although it is important to note that the current results are not directly comparable to the previous era because of changes in diagnostic and treatment protocols and criteria, it appears that progress has been made with more active use of EVT. Nevertheless, a good functional outcome of mRS 0–2 was still observed in less than half of the patients indicating that further work is needed to improve these results.
Should patients with a suspected LVO then be taken directly to an EVT-capable CSC even if an IVT-capable PSC is nearer? The recent randomized controlled trials showed no difference between direct EVT and bridging therapy in reaching good functional outcome [Citation13]. This has also been shown with Finnish retrospective data [Citation14]. It therefore appears that, for LVO patients, prioritizing fast access to EVT is desirable but uncertainty concerning the utility of Mothership versus Drip-and-Ship in patients with unknown LVO status and long transfer times remains. Intriguingly, a recent meta-analysis found that patients directly admitted to CSCs had higher chances of achieving a favorable functional outcome than those secondarily transferred to one but in the subgroup analysis restricted to studies exploring bridging therapy there was no confirmed difference (OR, 1.26; 95% CI, 0.98 to 1.63) [Citation15]. Furthermore, data from the SITS-ISTR registry show that LVO patients treated with IVT+EVT reached better functional outcomes even though they had more often been transferred from other hospitals than those patients treated with EVT alone [Citation16]. Fresh telestroke data also show that IVT at spoke hospitals is beneficial in LVO [Citation17]. Uncertainty, therefore, remains and it appears possible that specific predefined transfer protocols and care pathways need to be identified for specific patient groups. The current sample size is too small to provide definitive answers, but these data suggest some areas of interest for subsequent studies. M1 occlusions had the worst prognosis in this study and prioritizing these for a direct transfer to a CSC in similar settings should be investigated further. The Finnish Prehospital Stroke Scale could provide the method for identifying these patients [Citation18].
Older patients also appear more vulnerable and might benefit from more direct access to EVT and patient age should be considered in designing further investigations. Studies on the benefits of EVT in octogenarians have reported conflicting results and it seems that the possible benefit is smaller than in younger patients [Citation10–12,Citation19,Citation20]. In the current study, a good functional outcome of mRS 0–2 was observed in 38% of the EVT-treated octogenarians. This was a higher proportion than the previously reported 27% for octogenarians treated with EVT at KUH in 2009–2015 and the 27–30% of a recent meta-analysis suggesting that the treatment may have become more effective [Citation10,Citation19]. However, the prognosis of octogenarians with the occlusion in ICA and/or M1 was bleak with the three-month mRS being 4–6 in all but one who recanalized with IVT and it is not readily apparent that these patients benefited from the transfer to EVT. Possible benefits of a mothership strategy in older patients and especially octogenarians with moderate to severe strokes should therefore be investigated, at least where transfer times are long.
In the current data, IVT treatment was not associated with improved outcome which may be associated with brain lost during the long transfer time in those patients who did not respond to thrombolysis. However, the sample size is small and patients who recanalized during transport to KUH were excluded from the EVT analyses. Indeed, in 22% of all the IVT-treated patients identified in this study the clot had dissolved by the time they had reached KUH, a proportion clearly higher than the 10% reported in a recent meta-analysis of clinical trials [Citation21]. It should be noted that the proportion of transferred patients who eventually received EVT was one fifth both in the entire cohort indicating that the need for EVT was not reduced with IVT.
The main limitation of this study was its retrospective nature and therefore the main caveats include the problems of missing data and variation between clinicians in their habits of recording. The greatest problem is the missing data on intrahospital delays, both at NKCH and KUH since these are important for a pathway analysis [Citation22–24]. Likewise, precise revascularisation data such as a thrombolysis in cerebral ischemia (TICI) scores were unavailable. The sample size is also rather modest so the differences between the prevalence of the occlusion of specific vessels was, for example, not statistically analysed and should be interpreted with great caution. Nevertheless, these results reflect the reality in peripheral centers and should primarily be considered as clues for designing further studies, which should also consider helicopter transportation strategies in remote settings to reduce treatment delays and make the distance between the CSC and the PSC less important [Citation25,Citation26].
In conclusion, anterior circulation LVO treatment outcomes still need to be improved for patients primarily taken to a PSC. Larger studies are needed to evaluate patient transfer protocols, with special attention paid to individual patient characteristics, especially age and stroke severity. Identification of M1 occlusions in the field to guide transfer decisions appears a valuable target.
Availability of data and material
Access to data is regulated by Finnish law and Siun Sote. Those fulfilling the requirements for viewing confidential data as required by Finnish law and Siun Sote are able to access the data. Legal basis for processing of personal data is public interest and scientific research (EU General Data Protection Regulation 2016/679 (GDPR), Article 6(1)(e) and Article 9(2)(j); Data Protection Act, Sections 4 and 6).
Ethics approval
The study was approved by the registry keeper, Siun sote. Since no contact with patients was involved, ethical board approval was not stipulated or sought.
Acknowledgments
The author would like to thank professor Pekka Jäkälä and adjunct professor Jori Ruuskanen for their valuable comments on the first versions of the manuscript, adjunct professor Ville Kytö for statistical advice and dr. Anne-Mari Kantanen for information of procedures at KUH.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11–20.
- Turc G, Bhogal P, Fischer U, et al. European Stroke Organisation (ESO) - European Society for Minimally Invasive Neurological Therapy (ESMINT) Guidelines on Mechanical Thrombectomy in Acute Ischaemic StrokeEndorsed by Stroke Alliance for Europe (SAFE). Eur Stroke J. 2019;4(1):6–12.
- Jahan R, Saver JL, Schwamm LH, et al. Association Between Time to Treatment With Endovascular Reperfusion Therapy and Outcomes in Patients With Acute Ischemic Stroke Treated in Clinical Practice. JAMA. 2019;322(3):252–263.
- Saver JL, Fonarow GC, Smith EE, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA. 2013;309:2480–2488.
- Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N Engl J Med. 2018;378(1):11–21.
- Albers GW, Marks MP, Kemp S, et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N Engl J Med. 2018;378(8):708–718.
- Desai SM, Leslie-Mazwi TM, Hirsch JA, et al. Optimal transfer paradigm for emergent large vessel occlusion strokes: Recognition to recanalization in the RACECAT trial. J Neurointerv Surg. 2021 Feb;13(2):97–99.
- Ismail M, Armoiry X, Tau N, et al. Mothership versus drip and ship for thrombectomy in patients who had an acute stroke: A systematic review and meta-analysis. J Neurointerv Surg. 2019;11(1):14–19.
- Sipilä JOT. Intravenous thrombolysis in a peripheral primary stroke center without advanced imaging, a retrospective 2016–2017 cohort study. Int J Neurosci. Apr 13 2020;1–5. Online ahead of print.
- Zhao W, Ma P, Zhang P, et al. Mechanical Thrombectomy for Acute Ischemic Stroke in Octogenarians: A Systematic Review and Meta-Analysis. Front Neurol. 2020;10:1355.
- Alawieh A, Starke RM, Chatterjee AR, et al. Outcomes of endovascular thrombectomy in the elderly: A ’real-world’multicenter study. J Neurointerv Surg. 2019;11(6):545–553.
- Alawieh A, Chatterjee A, Feng W, et al. Thrombectomy for acute ischemic stroke in the elderly: A ’real world’ experience. J Neurointerv Surg. 2018;10(12):1209–1217.
- Podlasek A, Dhillon PS, Butt W, et al. Direct mechanical thrombectomy without intravenous thrombolysis versus bridging therapy for acute ischemic stroke: A meta-analysis of randomized controlled trials. Int J Stroke. 2021;16(6):621–631.
- Pienimäki JP, Ollikainen J, Sillanpää N, et al. In-Hospital Intravenous Thrombolysis Offers No Benefit in Mechanical Thrombectomy in Optimized Tertiary Stroke Center Setting. Cardiovasc Intervent Radiol. 2021;44(4):580–586.
- Romoli M, Paciaroni M, Tsivgoulis G, et al. Mothership versus Drip-and-Ship Model for Mechanical Thrombectomy in Acute Stroke: a Systematic Review and Meta-Analysis for Clinical and Radiological Outcomes. J Stroke. 2020 Sep;22(3):317–323.
- Ahmed N, Mazya M, Nunes AP, et al. Safety and Outcomes of Thrombectomy in Ischemic Stroke With vs Without IV Thrombolysis. Neurology. 2021;97(8):e765–e776.
- Regenhardt RW, Rosenthal JA, Awad A, et al. ‘Drip-and-ship’ intravenous thrombolysis and outcomes for large vessel occlusion thrombectomy candidates in a hub-and-spoke telestroke model. J Neurointerv Surg. Jul 29 2021;neurintsurg-2021–017819. Online ahead of print.
- Ollikainen JP, Janhunen HV, Tynkkynen JA, et al. The Finnish Prehospital Stroke Scale Detects Thrombectomy and Thrombolysis Candidates-A Propensity Score-Matched Study. J Stroke Cerebrovasc Dis. 2018;27(3):771–777.
- Karhi S, Nerg O, Miettinen T, et al. Mechanical Thrombectomy of Large Artery Occlusion Is Beneficial in Octogenarians. In Vivo. 2018;32(5):1223–1230.
- Kastrup A, Brunner F, Hildebrandt H, et al. Endovascular therapy versus thrombolysis in patients with large vessel occlusions within the anterior circulation aged ≥80 years. J Neurointerv Surg. 2018;10(11):1053–1056.
- Tsivgoulis G, Katsanos AH, Schellinger PD, et al. Successful Reperfusion With Intravenous Thrombolysis Preceding Mechanical Thrombectomy in Large-Vessel Occlusions. Stroke. 2018;49(1):232–235.
- Holodinsky JK, Williamson TS, Demchuk AM, et al. Modeling Stroke Patient Transport for All Patients With Suspected Large-Vessel Occlusion. JAMA Neurol. 2018;75(12):1477–1486.
- Holodinsky JK, Patel AB, Thornton J, et al. Drip and ship versus direct to endovascular thrombectomy: The impact of treatment times on transport decision-making. Eur Stroke J. 2018;3(2):126–135.
- Harrington RA. Prehospital Phase of Acute Stroke Care: Guideline and Policy Considerations as Science and Evidence Rapidly Evolve. Stroke. 2019;50(7):1637–1639.
- Pappinen J, Miettinen T, Laukkanen-Nevala P, et al. The selection of an optimal transportation strategy in urgent stroke missions: A simulation study. Scand J Trauma Resusc Emerg Med. 2020 Jun 1;28(1):48.
- Coughlan D, McMeekin P, Flynn D, et al. Secondary transfer of emergency stroke patients eligible for mechanical thrombectomy by air in rural England: Economic evaluation and considerations. Emerg Med J. 2021 Jan;38(1):33–39.