ABSTRACT
Objective
This retrospective cohort study aimed to investigate the association of sleep characteristics measured by the Pittsburgh Sleep Quality Index (PSQI) with the vertigo outcome in vertiginous patients with comorbid cardiometabolic diseases.
Methods
Four hundred and thirteen patients with comorbid cardiometabolic diseases who consecutively visiting vertigo and dizziness clinic were enrolled between October 2018 and January 2020 in a tertiary teaching medical center. Regression analyses and stratified analyses were used to explore the relationship between PSQI and vertigo outcome, which was measured by the visual analogue scale (VAS) score.
Results
In the study sample, 73.8% (305/413) were defined as ‘poor sleep’ (PSQI>5). Participants with better recovery tended to have better baseline PSQI global score, PSQI sleep quality, PSQI sleep onset latency, PSQI daytime dysfunction, less severe baseline vertigo symptoms indicated by VAS, Vertigo Symptom Scale (VSS) and Dizziness Handicap Inventory (DHI) scores. Moreover, baseline PSQI global score and PSQI daytime dysfunction score were independently associated with the vertigo VAS scores at the last follow-up.
Conclusion
The present results clearly indicated that poor sleep is common and inversely associated with vertigo outcome in vertiginous patients with co-morbid cardiometabolic diseases. Therefore, sleep deserves greater attention in the total medical care in specific subgroup of vertiginous patients.
1. Introduction
Vertigo and dizziness are common complaints in clinic, which can be caused by different diseases of various etiology and consume substantial healthcare resources [Citation1]. It has been reported that over half of the vertigo attacks are caused by peripheral vestibular disorders, while central disorders account for approximately 25% of the established diagnoses [Citation2]. Compared to cerebrovascular diseases, cardiometabolic diseases have less potential to cause dizziness or vertigo, but are more often noted as common comorbidities of vertiginous diseases. Previous studies are scant concerning the characteristics of vertiginous patients with co-morbid cardiometabolic diseases.
Not only cardiometabolic diseases, but also sleep disturbance, anxiety and depression are closely associated with dizziness or vertigo [Citation3,Citation4]. It has been reported that the altered vascular endothelial function and blood pressure due to insufficient or disrupted sleep increase risk for cardiovascular diseases [Citation5,Citation6]. Moreover, sleep disturbance is known to be linked with the metabolic syndrome related to cardiovascular diseases [Citation7]. Not surprisingly, sleep problems can trigger attacks in over half of migraine sufferers while migraine headache is a major cause of recurrent vertigo [Citation8]. Up to date, however, the relationship between sleep disturbance and the outcome of dizziness or vertigo have not been analyzed in vertiginous patients with co-morbid cardiometabolic diseases.
In this light, we hypothesize that subjective sleep quality measured by the Pittsburgh Sleep Quality Index (PSQI) have the potentials to predict the outcome of dizziness/vertigo in patients with comorbid cardiometabolic diseases. Once an independent association between sleep quality and the outcome of vertiginous diseases is verified, it might prompt vertiginous patients comorbid with cardiometabolic diseases to develop healthy sleep habits more aggressively. Furthermore, it potentially urges physicians to adopt the measures to improve sleep quality properly in vertiginous patients.
2. Materials and methods
2.1. Study population
We have documented clinical profiles for 1605 patients who consecutively visiting vertigo and dizziness clinic in a tertiary teaching medical center, between October 2018 and January 2020. Patients received an online questionnaire [https://www.wjx.cn/m/38173287.aspx] during their first visit, pertaining to medical history, family history, demographics, medications, emotional status, and comorbidities arranged according to physiological systems. In our analysis, we limited the range of cardiometabolic diseases co-morbidities to the more common diseases, such as hypertension, atherosclerosis, arrhythmia, atrial fibrillation, diabetes mellitus, hyperlipidemia and so on. Participants with history of cerebral infarction, transient ischemic attack (TIA) were also eligible. All participants were visited by otolaryngologists and neurologists for detailed evaluation, including medical history, extensively systemic review, audiometry, otolaryngologic and neurologic physical examination. Further diagnostics, encompassing advanced clinical neurophysiology, vestibular function testing and imaging procedures, were performed if considered to be necessary. In addition, all the participants with self-reported history of cardiometabolic diseases or current relevant symptoms such as chest pain, presyncope or syncope were evaluated by cardiologists to exclude cardiogenic vertigo (or dizziness), thus confirming cardiometabolic diseases as co-morbidities of the vertigo syndrome in the present study. Patients with co-morbid cardiometabolic diseases were included if their dizziness and vertigo could be explained by at least one of the following diagnosis: vestibular migraine (VM) [Citation9], Ménière’s disease (MD) [Citation10], vestibular neuritis (VN) [Citation11], persistent postural-perceptual dizziness (PPPD) [Citation12], sudden sensorineural hearing loss with vertigo [Citation13], bilateral vestibulopathy [Citation14], delayed endolymphatic hydrops (DEH) [Citation15], vestibular paroxysmia (VP) [Citation16], Ramsay-Hunt syndrome [Citation17], and undefined nonspecific dizziness. As shown in , participants were excluded if they met any of the following criteria: (1) the symptom of vertigo could be explained by benign paroxysmal positional vertigo (BPPV) alone by the whole follow-up period; (2) patients who had dizziness or vertigo due to malignant central disorders such as cerebellopontine angle tumor and stroke; (3) patients who had missing data on the PSQI questionnaire; (4) patients who lost in the follow-up. BPPV was excluded because most of the cases were successfully treated by the readily available liberatory maneuvers without medication, which is quite different from other vertiginous disease. Therefore, the study sample was etiologically heterogeneous and included patients with vertigo, non-vertigo and mixed (vertigo and non-vertigo) symptoms of dizziness. All the participants were followed through online platform (https://drdanbing.haodf.com/station) and/or telephone call for a median time of 158 days (interquartile range: 91–222 days). This study was approved by the Committee of Medical Ethics. Informed consent was waived because this was a retrospective cohort study with no additional intervention and minimal privacy risks.
2.2. Data availability
All interested and qualified researchers can get access to the data by submitting their requests to the corresponding author.
2.3. Baseline characteristics
Demographic and clinical information were obtained using a uniform questionnaire at the first visit. Height and weight were measured to calculate body mass index (BMI). Education level was classified into four categories: primary school, high school, university education and postgraduate education. Smoking status and alcohol use were categorized as yes or no by self-report.
Cardiometabolic morbidity was defined at baseline as presence of at least one of following disorders by self-report medical history and/or use of relevant medication: hypertension, diabetes mellitus (DM), hyperlipidemia, coronary heart disease, arrhythmia, stroke, and TIA.
Sleep parameters were assessed by subjective report to the PSQI in Chinese version. PSQI is a self-rated questionnaire containing a 0–3 interval scale with seven components (sleep onset latency, sleep duration, sleep difficulty, daytime dysfunction due to sleepiness, sleep efficiency, subjective sleep quality, and use of sleep medication). The higher the score, the worse the sleep quality. A global PSQI score of 5 or greater indicates clinically significant poor sleep with well-validated sensitivity [Citation18]. The Chinese version of PSQI had an overall reliability coefficient of 0.82–0.83 [Citation19].
The severity of dizziness and vertigo were evaluated using the Chinese version of the Dizziness Handicap Inventory (DHI), the Vertigo Symptom Scale (VSS), and Visual Analogue Scale (VAS) of dizziness and vertigo at the study entry. The DHI score was calculated based on a 25-item scale that assesses an individual’s perception of handicap due to dizziness including emotional, functional, and physical burden. The Vertigo Severity Scale (VSS) is an assessment tool to quantify vertigo and dizziness symptoms. It consists of two subscales, the VSS-V for vestibular-balance and VSS-A for autonomic-anxiety [Citation20]. Both Chinese DHI and VSS have good internal consistency with Cronbach alpha coefficient > 0.7, and 0.886, respectively. The test-retest reliability was also very good in both scales with an intraclass correlation coefficient (ICC) 0.64 to 0.87 in Chinese DHI and 0.965 to 0.992 in Chinese VSS [Citation21,Citation22]. The VAS, widely used in pain evaluation, proved to be valid for assessing vertigo/dizziness symptoms [Citation23].
The Hospital Anxiety and Depression Scale (HADS) is a self-report instrument that is helpful for screening general psychological distress in vertiginous patients [Citation24]. It consists of two subscales, i.e. the Anxiety subscale (HADS-A) and the Depression subscale (HADS-D) [Citation25].
The above scales were administered as part of a baseline assessment battery prior to treatment initiation.
2.4. Treatment
The treatment for vertigo or dizziness and underlying diseases were based upon the recommendation from consensus documents and guidelines [Citation16,Citation26,Citation27]. The most common primary diagnosis in this study is VM, and the second most common is MD. We used flunarizine hydrochloride and Tianshu capsule as the first-line medication for VM. Though not mention in the guideline, Tianshu capsule has a very long history in China in prophylactic treatment of headache and shows better compliance [Citation28], For MD and DEH patients, betahistine is the treatment of choice. VP patients were prescribed with carbamazepine as the first option. Vestibular rehabilitation exercises were introduced to patients in addition to medical treatment or as stand-alone treatment option, if no contraindications existed [Citation29].
2.5. Outcome measure
Patient outcome was established by the record of the last available follow-up, which was measured by the VAS score of dizziness and vertigo. As a large fraction of patients were unable to distinguish between dizziness and vertigo symptoms, we adopted a combination of dizziness and vertigo for history taking and outcome evaluation. The VAS rather than DHI or VSS was chosen because it is the most rapid, user-friendly, and best understood by all the patients from varying background.
2.6. Statistical analysis
We dichotomized all the participants based on the vertigo VAS score at the last follow-up. Continuous values were shown as the mean ± SD and compared by unpaired Student’s t-test for normal distribution, while the non-normal distributed variables were presented as medians (interquartile range) and compared by the Mann-Whitney U-test. The categorical variables were presented as numbers (percentages) and compared through chi-square tests or the Fisher’s exact test. Linear regression models for examining the associations of VAS scores in the last follow-up with baseline PSQI global scores and component scores in vertiginous patients with comorbid cardiometabolic diseases. In addition to crude model, an adjusted model was performed after adjusting for age, sex, BMI. All the analyses were performed using Empower (R) (www.empowerstats.com, X&Ysolutions, inc. Boston MA) and R (http://www.R-project.org). A two-sided p value of < 0.05 was considered to represent statistical significance.
3. Results
3.1. The sleep quality and vertigo outcome of vertiginous patients with comorbid cardiometabolic diseases
We dichotomized the study sample according to the median value of vertigo VAS score at the last follow-up (median VAS score = 2). There were 236 (57.1%) participants had better recovery from vertigo and dizziness (VAS score ≤ 2). In contrast, 12(2.9%) cases reported aggravation in terms of VAS scores, while the severity of vertigo and dizziness remained unchanged in 23 (5.6%) participants.
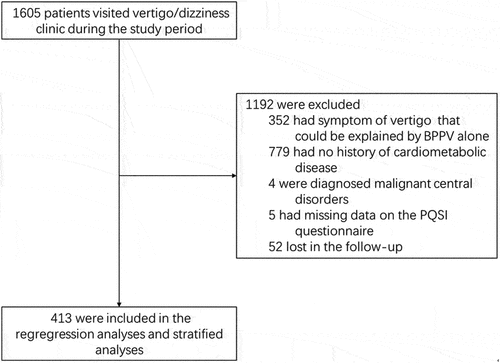
The median PSQI global score at the study entry was 8.0 (interquartile range: 5.0–11.0). Among these participants, 73.8% (305) met the criteria for ‘poor sleep’ which was defined as PSQI>5 [Citation18,Citation30–33]. PSQI global score were significantly higher in the better vertigo outcome group compared to the worse vertigo outcome group (). We also observed that PSQI sleep onset latency and PSQI daytime dysfunction were the major contributors for the differences in baseline PSQI global score among the groups (). Three months after the first visit, 115 participants gave their feedback questionnaire which showed a reduction in PSQI scores in the better vertigo outcome group (). We did not request the PSQI feedback in the latest telephone follow-up.
Figure 2. The number of participants at each PSQI components score in better and worse vertigo outcome groups. P values calculate the difference in the frequency of each PSQI components score between the two groups. The constituent ratios of PSQI sleep onset latency score and PSQI daytime dysfunction score among the three groups were significantly different (P= 0.001 and 0.040, retrospectively).
Figure 3. The median PSQI global scores at the study entry and after 3 months among better and worse vertigo outcome groups.
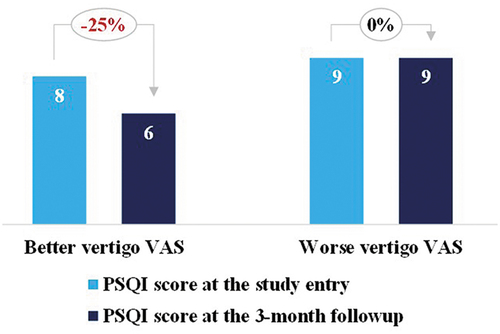
Table 1. Characteristics of participants with better and worse vertigo outcome according to VAS scores.
In addition, participants with better recovery tended to have lower baseline score in the depression and anxiety subscale of HADS (HADS-D, HADS-A), less severe baseline vertigo symptoms indicated by VAS, VSS and DHI scores ().
3.2. Baseline PSQI global score and PSQI daytime dysfunction is independently associated with the VAS scores at the last follow-up
In the unadjusted analysis shown in , the VAS score at the last follow-up increased significantly with growing level of baseline PSQI global scores (β [95% confidence interval {CI}]: 0.1 [0.0, 0.1]). In addition, we investigate the association of vertigo/dizziness outcome with the seven PSQI components that were expressed as categorical variables. The participants groups with PSQI sleep onset latency scored 1–3 and PSQI daytime dysfunction scored 1–3 showed significant higher VAS scores at the last follow-up as compared to their 0-score counterparts. No significant association were seen between the vertigo/dizziness outcome and the other five PSQI components.
Table 2. The crude and adjusted regression coefficient (β) and 95% confidence interval (CI) for the outcome of vertigo and dizziness measured by VAS scores at the last follow-up in relation to PSQI and its components.
When PSQI global score and PSQI daytime dysfunction score was forced into the multivariate logistic-regression model with potential confounders simultaneously, their association with the vertigo outcome remained statistically significant ().
4. Discussion
Sleep problems, vertigo/dizziness and cardiometabolic disorders are highly comorbid conditions. As we known, sleep disturbance exerts deleterious effects on cardiovascular, metabolic, and endocrine systems [Citation34–36]. On the other hand, the relationship between poor sleep and vertigo/dizziness has been the subject of much attention and discussion recently. Poor sleep was found to exert negative impact on symptoms, emotional distress, quality of life, recurrence, and improvements in subjective handicap in patients with vertigo and dizziness [Citation37]. Sleep quality and regulation in vertiginous disease subtypes were also reported [Citation38]. Nevertheless, few studies have investigated whether sleep quality differentially contribute to the vertigo/dizziness outcome in vertiginous individuals with co-morbid cardiometabolic diseases.
In the present study, all the vertiginous participants with co-morbid cardiometabolic diseases were classified into two categories (better vertigo outcome, worse vertigo outcome) according to the VAS score at the last follow-up. Multivariate logistic-regression model with potential confounders were applied to elucidate the crosstalk between vertigo outcome and seven PSQI components. We observed that better vertigo outcome group tended to have better sleep parameters, including baseline PSQI global score, PSQI sleep quality, PSQI sleep onset latency, and PSQI daytime dysfunction. After adjusting for potential confounders, we found PSQI global score and PSQI daytime dysfunction was independently associated with the outcome of vertigo and dizziness assessed by VAS scores in participants with self-reported co-morbid cardiometabolic diseases.This finding is pragmatic and clinically valuable since it supports the idea that sleep quality is a modifiable risk factor to improve the outcome of vertigo and dizziness in patients with self-reported co-morbid cardiometabolic diseases.
Compared to previous works [Citation38–41], our research focused more on the prognostic factors of vertigo outcome. The participants in the present study were confined to vertiginous patient co-morbid with cardiometabolic diseases. The result provided us essential evidences about how important role of sleep quality played in vertigo outcome in such special population. Physicians should consider the high proportion of co-existing sleep disturbance in vertiginous patients with comorbid cardiometabolic diseases, thus granting optimal care. In addition to medication, sleep pattern modification is required for a better outcome.
The underlying mechanisms of sleep disturbance on the vestibular system remains controversial so far. Some studies showed a significant impairment of postural balance or vestibular-ocular reflexes (VOR) after sleep deprivation while others found no change in vestibular function. It is reported that nystagmus and vestibular function abnormality were more common in the moderate-to-severe obstructive sleep apnea syndrome group than their mild counterpart [Citation42,Citation43]. Lin BY and Young YH found the mean asymmetry ratio of the ocular vestibular-evoked myogenic potential (oVEMP) after sleep deprivation (12 h) was significantly higher than that of the normal sleep participants [Citation44]. In contrast, Quarck G et al. indicated that total sleep deprivation (26–29 h) induced a significant increase in VOR gain. Interestingly, some recent evidence shows bi-directional communication between the sleep and vestibular system. Sleep influences the vestibular system and vice versa [Citation45,Citation46].
In the present study, no significant differences were identified between the participants with and without good recovery in terms of characteristic parameters of caloric test, the video head impulse test and vestibular-evoked myogenic potentials (data not shown). Although discrepancies exist in findings, sleep disturbance apparently influences vestibular system somehow. A recent review postulated that vestibular compensation supported by neuroplasticity, which naturally occurs during sleep, could be affected by the sleep quality in vestibular patients [Citation40]. The detailed mechanism and pathological role of sleep problem in the regulation of vertigo and dizziness disorders are yet to be investigated in depth.
Our data suggests that PSQI questionnaire may aid in predicting outcome of vertigo and dizziness. As is known, PSQI is reliable and valid for assessing sleep pattern in vertigo and dizziness patients due to its multidimensional nature. The total score may reflect poor sleep that results from a variety of causes, including insomnia symptoms, sleep apnea, chronic pain, or bad dreams. In addition, PSQI proved to relate to self-report measures of mood and stress [Citation47]. Further evaluation of the causes and effective interventions to reduce insomnia symptoms may have a significant effect on the improvement of vertigo and dizziness.
It has been reported that participants with vertigo symptoms appear to have significantly higher prevalence of hypertension and relevant medication use, which implicates that cardiometabolic diseases may have a connection with vertigo [Citation48]. Meanwhile, our unpublished data showed that no statistical difference was observed between vertigo outcomes and PSQI components in vertiginous patients without comorbid cardiometabolic diseases (data not shown). Thus, we assumed that the comorbidity of cardiometabolic disease may strengthen the adverse influence of sleep disturbances on vertigo outcomes.
Under normal conditions, the healthy endothelium is one of the factors controlling vascular homeostasis by modulating vascular tone and counteracting vascular inflammation [Citation49]. As such, endothelial dysfunction is essential in the progression of atherosclerosis and metabolic disease [Citation50]. Recent discoveries also have identified important roles of the capillary endothelium and microvasculature in regulating metabolic functions under homeostatic and pathological conditions [Citation51]. Meanwhile, vasospasm of the internal auditory artery or its branches could be responsible for the sudden onset of vestibular symptoms [Citation52]. Therefore, we suggested that cardiovascular metabolic diseases may affect the vertigo through endothelial health and vascular homeostasis.
5. Strengths and limitations
Our present work, to our knowledge, was one of the few studies focus on the influence on sleep quality in vertiginous patients with self-reported cardiometabolic diseases. Furthermore, the sample size of this study was larger than that of the previous similar studies, allowing us to perform comparisons between the groups. Meanwhile, our large sample of patients also increases the applicability of our findings to other institutions.
However, there are several drawbacks. First, the research was conducted at a single center in patients consulting in vertigo and dizziness clinic which is affiliated to otolaryngology department. A great part of vertiginous patients, especially those with co-morbid cardiometabolic diseases, have been treated in relevant departments, such as cardiology, neurology, and endocrinology. Therefore, the present finding may not be extrapolated to the general vertiginous patients. Second, we adopted subjective questionnaire for assessment of sleep patterns instead of objective measurements, such as polysomnography (PSG) or actigraphy. It should be noted that PSQI scores and their PSG or actigraphy counterparts are not well correlated [Citation53]. Third, the outcome measure was also subjective assessment. Although subjectivity is always a concern when using self-reported measures, any reporting bias are likely to be similar for all participants and thereby equivalent across the whole study sample. Fourth, the respective design in this study was inherently limited for elucidating cause–effect relationships between PSQI and the outcome of vertigo and dizziness.
6. Perspectives
Studies are needed to determine whether improvements in vertiginous disorders cause improved sleep, or improved sleep leads to improvements in vertigo. In the future endeavors, we will confirm the results of the present study in the prospective longitudinal researches to infer causality. In addition, objective sleep measurement such as polysomnography and actigraph will be utilized. Furthermore, rodent models of vestibular pathology, or modified gravity conditions, will be utilized to further investigate the neurobiological mechanisms of vestibular-sleep interactions.
7. Conclusion
In conclusion, this study suggests that in vertiginous patients with self-reported co-morbid cardiometabolic disease, the contribution of poor sleep quality indicated by PSQI global score to negative outcome of vertigo and dizziness is clinically significant. This finding may urge physicians to pay more attention to sleep problem, which would be beneficial for the vertigo/dizziness outcome in specific population.
Abbreviations
PSQI, the Pittsburgh Sleep Quality Index; VAS, Visual Analogue Scales; DHI, the Dizziness Handicap Inventory; VSS, the Vertigo Symptom Scale; VSS-A, VSS items relating to autonomic anxiety symptoms; VSS-V, VSS items relating to vertigo-balance; HADS, the Hospital Anxiety and Depression Scale; HADS-A, the Anxiety subscale of HADS; HADS-D, the Depression subscale of HADS; VM, vestibular migraine; MD, Ménière’s disease; VN, vestibular neuritis; PPPD, persistent postural-perceptual dizziness; DEH, delayed endolymphatic hydrops; VP, vestibular paroxysmia; BPPV, benign paroxysmal positional vertigo; DM, diabetes mellitus; TIA, transient ischemic attack; ICC, intraclass correlation coefficient; BMI, body mass index; VOR, vestibular-ocular reflexes; oVEMP, ocular vestibular-evoked myogenic potential; PSG, polysomnography.
Authors’ contributions
Dr Dan Bing and Fang Yang had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept, design and drafting of the manuscript: Dan Bing, Fang Yang, Zhihui Du, Guoliang Wang.
Acquisition, analysis, or interpretation of data: Dan Bing, Fang Yang, Guoliang Wang, Zhihui Du, Dan Yan.
Ethics approval and consent to participate
This study was approved by the Committee of Medical Ethics of Tongji Hospital (No.TJ-IRB20200710). Informed consent was waived because this was a retrospective cohort study with no additional intervention and minimal privacy risks.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Strupp M, Brandt T. Diagnosis and treatment of vertigo and dizziness. Dtsch Arztebl Int. 2008;105(10):173–180.
- Brandt T, Dieterich M. The dizzy patient: don’t forget disorders of the central vestibular system. Nat Rev Neurol. 2017;13(6):352–362.
- Neuhauser HK, von Brevern M, Radtke A, et al. Epidemiology of vestibular vertigo: a neurotologic survey of the generalpopulation. Neurology. 2005;65(6):898–904.
- Monzani D, Casolari L, Guidetti G, et al. Psychological distress and disability in patients with vertigo. J Psychosom Res. 2001;50(6):319–323.
- Lanfranchi PA, Pennestri M, Fradette L, et al. Nighttime blood pressure in normotensive subjects with chronic insomnia: implications for cardiovascular risk. Sleep. 2009;32(6):760–766.
- Hall MH, Mulukutla S, Kline CE, et al. Objective sleep duration is prospectively associated with endothelial health. Sleep. 2017;40:1.
- Hall MH, Okun ML, Sowers M, et al. Sleep is associated with the metabolic syndrome in a multi-ethnic cohort ofmidlife women: the SWAN sleep study. Sleep. 2012;35(6):783–790.
- Andreou AP, Edvinsson L. Mechanisms of migraine as a chronic evolutive condition. J Headache Pain. 2019;20(1):117.
- Lempert T, Olesen J, Furman J, et al. Vestibular migraine: diagnostic criteria. J Vestib Res. 2012;22(4):167–172.
- Lopez-Escamez JA, Carey J, Chung W, et al. Diagnostic criteria for meniere’s disease. J Vestib Res. 2015;25(1):1–7.
- Taylor RL, Mcgarvie LA, Reid N, et al. Vestibular neuritis affects both superior and inferior vestibular nerves. Neurology. 2016;87(16):1704–1712.
- Staab JP, Eckhardt-Henn A, Horii A, et al. Diagnostic criteria for persistent postural-perceptual dizziness (PPPD): consensus document of the committee for the classification of vestibulardisorders of the barany society. J Vestib Res. 2017;27(4):191–208.
- Chandrasekhar SS, Tsai DBS, Schwartz SR, et al. Clinical practice guideline: sudden hearing loss (update). Otolaryngol Head Neck Surg. 2019;161(1_suppl):S1–S45.
- Strupp M, Kim J, Murofushi T, et al. Bilateral vestibulopathy: diagnostic criteria consensus document of theclassification committee of the barany society. J Vestib Res. 2017;27(4):177–189.
- Iwasa Y, Tsukada K, Kobayashi M, et al. Bilateral delayed endolymphatic hydrops evaluated by bilateral intratympanicinjection of gadodiamide with 3T-MRI. Plos One. 2018;13(12):e206891.
- Strupp M, Lopez-Escamez JA, Kim J, et al. Vestibular paroxysmia: diagnostic criteria. J Vestib Res. 2016;26(5–6):409–415.
- Uscategui T, Doree C, Chamberlain IJ , et al. Antiviral therapy for Ramsay Hunt syndrome (herpes zoster oticus with facialpalsy) in adults. Cochrane Database Syst Rev. 2008;2008(4):CD006851.
- Buysse DJ, Reynolds CFR, Monk TH, et al. The pittsburgh sleep quality index: a new instrument for psychiatric practice andresearch. Psychiatry Res. 1989;28(2):193–213.
- Tsai P, Wang S, Wang M, et al. Psychometric evaluation of the Chinese version of the Pittsburgh Sleep QualityIndex (CPSQI) in primary insomnia and control subjects. Qual Life Res. 2005;14(8):1943–1952.
- Kondo M, Kiyomizu K, Goto F, et al. Analysis of vestibular-balance symptoms according to symptom duration: dimensionality of the Vertigo Symptom Scale-short form. Health Qual Life Outcomes. 2015;13:4.
- Poon DMY, Chow LCK, Au DKK, et al. Translation of the dizziness handicap inventory into Chinese, validation of it, and evaluation of the quality of life of patients with chronic dizziness. Ann Otol Rhinol Laryngol. 2004;113(12):1006–1011.
- Deng Z, Yuan W, Wang H, et al. Development of Chinese version vertigo symptom scale (VSS): reliability and validity. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2015;44(2):138–144.
- Toupet M, Ferrary E, Grayeli AB. Visual analog scale to assess vertigo and dizziness after repositioning maneuvers for benign paroxysmal positional vertigo. J Vestib Res. 2011;21(4):235–241.
- Piker EG, Kaylie DM, Garrison D, et al. Hospital anxiety and depression scale: factor structure, internal consistency and convergent validity in patients with dizziness. Audiol Neurootol. 2015;20(6):394–399.
- Mykletun A, Stordal E, Dahl AA. Hospital Anxiety and Depression (HAD) scale: factor structure, item analyses and internal consistency in a large population. Br J Psychiatry. 2001;179:540–544.
- [Guideline of diagnosis and treatment of Meniere disease. (2017)]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2017;52(3):167–172.
- Stroke and Vertigo Association of Chinese Stroke Association, Vertigo Professional Committee of Neurology Branch of Chineses Physicians Association. Chinese multidisciplinary expert consensus on assessment and management of vestibular migraine. Zhonghua Nei Ke Za Zhi. 2019;58(2):102–107.
- Yu S, Ran Y, Xiao W, et al. Treatment of migraines with Tianshu capsule: a multi-center, double-blind, randomized, placebo-controlled clinical trial. BMC Complement Altern Med. 2019;19(1):370.
- Dunlap PM, Holmberg JM, Whitney SL. Vestibular rehabilitation: advances in peripheral and central vestibular disorders. Curr Opin Neurol. 2019;32(1):137–144.
- Zuraikat FM, Makarem N, Liao M, et al. Measures of poor sleep quality are associated with higher energy intake and poor diet quality in a diverse sample of women from the go red for women strategically focused research network. J Am Heart Assoc. 2020;9(4):e14587.
- Aggarwal B, Makarem N, Shah R, et al. Effects of inadequate sleep on blood pressure and endothelial inflammation in women: findings from the american heart association go red for women strategically focused research network. J Am Heart Assoc. 2018;7:12.
- Bush AL, Armento MEA, Weiss BJ, et al. The pittsburgh sleep quality index in older primary care patients with generalized anxiety disorder: psychometrics and outcomes following cognitive behavioral therapy. Psychiatry Res. 2012;199(1):24–30.
- Bruno RM, Palagini L, Gemignani A, et al. Poor sleep quality and resistant hypertension. Sleep Med. 2013;14(11):1157–1163.
- Imeri L, Opp MR, Strupp M. How (and why) the immune system makes us sleep. Nature reviews. Nat Rev Neurosci. 2009;10(3):199–210.
- Mcalpine CS, Kiss MG, Rattik S, et al. Sleep modulates haematopoiesis and protects against atherosclerosis. Nature. 2019;566(7744):383–387.
- Jackson CL, Redline S, Emmons KM. Sleep as a potential fundamental contributor to disparities in cardiovascular health. Annu Rev Public Health. 2015;36:417–440.
- Sugaya N, Arai M, Goto F. The effect of sleep disturbance in patients with chronic dizziness. Acta Otolaryngol. 2017;137(1):47–52.
- Wu J, Liu C, Yu H, et al. Clinical characteristics of sleep disorders in patients with vestibular migraine. Sleep Breath. 2020;24(4):1383–1388.
- Xue H, Wang B, Meng T, et al. Differences of sleep disorders between vestibular migraine and benign paroxysmal positional vertigo. Front Psychiatry. 2021;12:726038.
- Besnard S, Tighilet B, Chabbert C, et al. The balance of sleep: role of the vestibular sensory system. Sleep Med Rev. 2018;42:220–228.
- Tsai MS, Lee LA, Tsai YT, et al. Sleep apnea and risk of vertigo: a nationwide population-based cohort study. Laryngoscope. 2018;128(3):763–768.
- Kayabasi S, Iriz A, Cayonu M, et al. Vestibular functions were found to be impaired in patients with moderate-to-severe obstructive sleep apnea. Laryngoscope. 2015;125(5):1244–1248.
- Ulusoy B, Gul O, Elsurer C, et al. The relationship between the findings of vestibular evoked myogenic potential sand severity of obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol. 2020;277(1):37–46.
- Lin B, Young Y. Effect of short-duration sleep deprivation on the vestibulo-ocular reflex system evaluated by ocular vestibular-evoked myogenic potential test. Acta Otolaryngol. 2014;134(7):698–703.
- Martin T, Mauvieux B, Bulla J, et al. Vestibular loss disrupts daily rhythm in rats. J Appl Physiol (1985). 2015;118(3):310–318.
- Horowitz SS, Blanchard JH, Morin LP. Intergeniculate leaflet and ventral lateral geniculate nucleus afferent connections: an anatomical substrate for functional input from the vestibulo-visuomotor system. J Comp Neurol. 2004;474(2):227–245.
- Buysse DJ, Hall ML, Strollo PJ, et al. Relationships between the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), and clinical/polysomnographic measures in a community sample. J Clin Sleep Med. 2008;4(6):563–571.
- Ray A, Spankovich C, Bishop CE, et al. Association between cardiometabolic factors and dizziness in African Americans: the jackson heart study. J Am Acad Audiol. 2021;32(3):186–194.
- Winkler G, Lakatos P, Salamon F, et al. Elevated serum TNF-alpha level as a link between endothelial dysfunction and insulin resistance in normotensive obese patients. Diabet Med. 1999;16(3):207–211.
- Soehnlein O, Libby P. Targeting inflammation in atherosclerosis - from experimental insights to the clinic. Nat Rev Drug Discov. 2021;20(8):589–610.
- Pi X, Xie L, Patterson C. Emerging roles of vascular endothelium in metabolic homeostasis. Circ Res. 2018;123(4):477–494.
- Baloh RW. Neurotology of migraine. Headache. 1997;37(10):615–621.
- Grandner MA, Kripke DF, Yoon I, et al. Criterion validity of the Pittsburgh Sleep Quality Index: investigation in anon-clinical sample. Sleep Biol Rhythms. 2006;4(2):129–139.