ABSTRACT
Objective
The association between herpes zoster (HZ) and stroke has been the subject of much previous research. Nevertheless, the connection remains ambiguous. A two-sample Mendelian randomisation study was conducted to explore the potential causal link between HZ and ischaemic stroke, including its subtypes.
Methods
For our MR analysis, we identified genetic instrumental variables related to both HZ and stroke by screening two prominent publicly accessible genome-wide association study databases. The primary approach involved using the inverse variance weighting method. To supplement this, we also employed methods such as MR-Egger regression, the weighted median approach, simple and weighted models. Lastly, to ascertain the stability and reliability of the results, we conducted tests for heterogeneity detection, horizontal pleiotropy assessment, and a leave-one-out analysis;
Results
The genetically predicted HZ did not indicate an association with stroke risk ([OR] 1.041; 95% [CI] 0.958–1.131;p = 0.336). This lack of association also held true for different subtypes of stroke: ischaemic stroke (OR = 1.047, 95% CI = 0.955–1.148, p = 0.323), large vessel stroke (OR = 1.13, 95% CI = 0.90–1.41, p = 0.272), cardioembolic stroke (OR = 1.020, 95% CI = 0.859–1.211, p = 0.816), small vessel stroke (OR = 1.14, 95% CI = 0.93–1.40, p = 0.195), and lacunar stroke (OR = 1.195, 95% CI = 0.967–1.476, p = 0.097).
Conclusion
This MR study showed that not uncover a causal link between herpes zoster and ischaemic stroke. Additional research will be necessary in the future to shed light on the fundamental mechanisms involved.
1. Introduction
Ranking as the second foremost cause of death across the globe, stroke also tops the list of disability inducers [Citation1]. Broadly classified into haemorrhagic and ischaemic types, the latter form, ischaemic stroke (IS), afflicts a majority of patients, making up nearly 80% of all stroke incidents [Citation2]. Mortalities attributed to IS saw a rise from 2.04 million in 1990 to 3.29 million in 2019, and the upward trend is expected to persist, with predictions suggesting a surge to possibly 4.9 million by the year 2030 [Citation3]. Often leaving residual effects, the aftermath of a stroke can detrimentally impact various facets of a patient’s existence, including their professional life, mental health, and overall well-being, thereby exerting substantial pressure on healthcare resources. As the incidence of IS continues to grow, prioritizing preventive measures becomes indispensable. Early detection of risk elements for IS, followed by the formulation of efficacious preventive interventions, is vital to decelerate the disease’s advancement.
Herpes zoster, an acute skin infection, stems from the reactivation of varicella-zoster virus (VZV).Predominantly seen in the elderly or those with weakened immunity, the worldwide rate of herpes zoster has been found to be between 3–5 per 1,000 person-years, exhibiting a rising trend annually. Several studies have established an association between herpes zoster and stroke, emphasizing that VZV reactivation might precipitate stroke [Citation4–8]. A cohort study(The Health Improvement Network) involving over 300,000 individuals demonstrated that herpes zoster (HZ) independently increased the risk of stroke. However, it failed to delineate clearly between ischemic and hemorrhagic subtypes. Moreover, a meta-analysis across the United States, Europe, and Asia indicated a heightened risk of ischaemic stroke for individuals with HZ [Citation9]. In addition, many previous observational studies have also shown that inflammatory factors and factors that promote VZV reactivation, such as aging, diabetes and hypertension [Citation10], play an important role in mediating the relationship between herpes zoster and stroke However, observational studies probing the connection between herpes zoster and stroke may be hindered by lingering confounders or reverse causality, complicating the precise assessment and interpretation of vascular risk. Hence, existing research doesn’t rule out the influence of unexamined or latent confounding elements on the association between herpes zoster and stroke risk. There’s also debate over whether herpes zoster preventive vaccination mitigates stroke occurrence [Citation11,Citation12], indicating an unresolved causal link that necessitates further exploration. Given the extensive prevalence and incidence of both herpes zoster infection and stroke in the general populace, elucidating their relationship bears substantial clinical and public health significance.
Mendelian randomisation (MR) is an approach for evaluating the influence of alterable risk elements on the likelihood of a disease. By employing genetic variation, specifically single nucleotide polymorphisms (SNPs), as an instrumental factor, this method can emulate a randomised controlled experiment. During conception, alleles are assigned at random, as posited by Smith GD [Citation13]. In this context, neither the disease, phenotype, nor any other factors alter the genotype, thereby eliminating confounders and negating reverse causation interference. Consequently, this method offers a more direct illustration of the connection between exposure and the resulting effects.
This investigation is designed to shed light on the authentic cause-and-effect linkage between herpes zoster and the susceptibility to ischaemic stroke, including its various classifications such as large atherosclerotic stroke (LAS), small-vessel stroke (SVS), and cardioembolic stroke (CES), as well as lacunar stroke. By focusing on the orientation of genetic variances, we intend to bypass the challenges of reverse causation and any other elements that may introduce confusion or bias.
2. Materials and methods
2.1. Study design
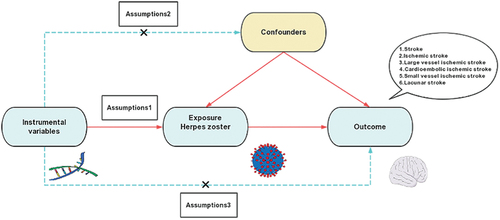
This research is structured as a two-sample Mendelian randomisation (MR) study. The detailed layout of the MR procedure, including the three principal hypotheses put forth, is illustrated in and . To conduct this investigation successfully, three essential hypotheses must be satisfied: First, the instrumental variables (IVs) chosen for genetic variations must have a notable connection with the exposure being examined; Second, these IVs must not bear any significant relationship with potential confounding variables like lipids, hypertension, diabetes mellitus, or BMI; Lastly, the connection between genetic variations and stroke risk must exclusively be mediated through herpes zoster. Since this study was carried out utilizing data from publicly available databases or previously published research, additional ethical approval or informed consent was not required. This is consistent with the ethical guidelines that were adhered to during the original studies from which this data was derived.
Figure 1. Flowchart of the overall design of the Mendelian randomisation analysis. SNP, single nucleotide polymorphisms.
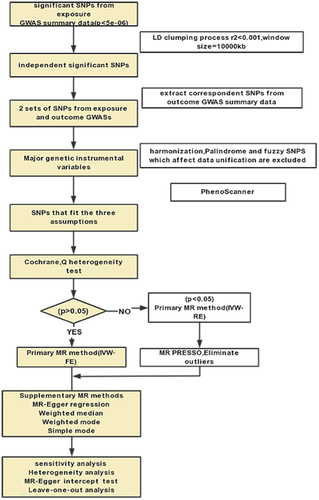
Figure 2. Overview of the study design and hypotheses for the MR design. Hypothesis 1 suggests that the selected instrumental variables (IVs) for genetic variation are significantly associated with the exposure of interest (HZ); hypothesis 2 suggests that the instrumental IVs for genetic variation are not associated with potential confounders; and hypothesis 3 suggests that genetic variation affects the risk of outcome only through the exposure (HZ), and not through other pathways.
2.2. Data sources
The data pertaining to herpes zoster’s genetic information were extracted from an extensive GWAS investigation conducted by the FINNGen consortium. Within this dataset, there are 4,973 cases of herpes zoster and 362,997 unaffected controls, all of European descent, totaling 367,970 subjects. This herpes zoster GWAS information encompasses 20,170,064 SNPs, and the exposure details are accessible in the FinnGen database.
For the stroke aspect, pooled genome-wide association study (GWAS) data were gathered from the MEGASTROKE consortium [Citation14]. This dataset contains information on 40,585 stroke patients and 406,111 controls, all of European ancestry. Among these, there are data for 34,217 ischemic stroke cases. Based on the Acute Stroke Treatment Criteria, The Org 10,172 trial classified this data into three subtypes [Citation15], which are 4,373 cases for large atherosclerotic stroke (LAS), 5,386 cases for small-vessel stroke (SVS), and 7,193 cases for cardioembolic stroke (CES). A separate meta-analysis provided the genetic association data for lacunar stroke (LS), including 225,419 samples from European pedigrees, comprising 6,030 LS cases and 219,389 controls. LS is characterized by small subcortical infarcts due to the obstruction of deep penetrating arteries within the brain. The comprehensive data related to these strokes are made available in the IEU OpenGWAS database (refer to ).
Table 1. Detailed characterisation of GWAS data.
2.3. Selection of Instrumental Variables (IVs)
Utilizing the GWAS summary data, we identified potential genetic instrumental variables (IVs) in line with the three essential hypotheses of our Mendelian randomization (MR) study. Our initial step involved selecting SNPs having a notable association with herpes zoster. These SNPs were identified as IVs based on a genome-wide significance threshold (p < 5 × 10−6). To negate any differential outcomes arising from high linkage disequilibrium (LD), we took the approach of truncating SNPs where R2 = 0.001 and maintained a window size of 10,000 kb. In addition, by using the Steiger filter, we obtained R2.Subsequently, we calculated the F statistic to ensure a robust relationship between the selected SNP and the exposure, thus fully meeting the criteria of our first hypothesis. For data uniformity, palindromes and ambiguous SNPs were omitted as these could compromise allelic consistency [Citation16]. Finally, risk factors associated with stroke in previous studies such as blood lipids, hypertension, diabetes and body mass index were considered to be confounding factors affecting outcomes.To preclude these variables from skewing the results, we employed Phenoscanner (http://www.phenoscanner.medschl.cam.ac.uk/) to verify each SNP, excluding any that correlated with these confounders. Through this meticulous process, we derived the SNPs for the following two-sample MR analyses.
2.4. Statistical analysis
In the present study, we employed an array of methods to examine the causal link between herpes zoster and ischemic stroke, along with its different subtypes. Among these approaches, inverse variance weighting (IVW) was the primary and most prevalent research method, utilized for assuming the validity of all SNPs as instruments of genetic variation. IVW combines Wald estimates of causality for each instrumental variable (IV) to obtain an overall effect estimate of exposure on the outcome [Citation17]. Additional techniques included MR-Egger regression, weighted median, simple model, and weighted model. MR-Egger regression was particularly used to pinpoint unusual instrumental variables and offer consistent estimates of causal impacts that remain unaffected by anomalies [Citation18]. It also has the capacity to identify the occurrence of horizontal pleiotropy, with a p-value of < 0.05 for the intercept symbolizing its presence. In our analysis, horizontal pleiotropy was not considered as influencing the outcome via the exposure under study, where confounders mediate. Further evaluation and handling were performed through the Multiple Residuals and Outliers in Pleiotropy (MR-PRESSO) test [Citation19], a process that excludes multiple outliers before reassessing the causal effects. To detect any heterogeneity, Cochran’s test was employed [Citation20]. We also conducted ‘leave-one-out’ analyses to ascertain if a singular SNP could cause a bias in the effect’s outcome. The MR analyses were executed with R software (version 4.2.3), employing the ‘TwoSampleMR’ and ‘MR-presso’ packages. Additionally, MR power analysis was accomplished using the online calculator in mRnd software (https://shiny.cnsgenomics.com/mRnd/), as outlined in the Supplementary file. Within our two-sample MR, multiple tests were corrected using the Bonferroni method, where a P-value below 0.008 (with p = 0.05/6) symbolizes strong evidence of causal association, and an association between 0.008 and 0.05 is deemed suggestive.
3. Results
3.1. Causal effect of herpes zoster on ischaemic stroke and its subtypes
After a comprehensive and stringent selection process, the SNPs related to herpes zoster were finally included, aligning with the three principal hypotheses formulated in the experimental approach. One particular SNP, identified as rs9266504, was omitted from consideration owing to its correlation with blood pressure levels. The F-statistics for all the included SNPs were observed to be significantly above 10, confirming that there was no distortion arising from feeble instrumental variables (detailed information is available in Table S1-S6). In the course of the Mendelian Randomization (MR) analyses, where Inverse Variance Weighting (IVW) was the primary method, no detectable causal connection was found between the genetically projected herpes zoster and any categories of stroke. This included stroke itself (OR = 1.041, 95% CI = 0.958–1.131, p = 0.336), IS (OR = 1.047, 95% CI = 0.955–1.148, p = 0.323), LAS (OR = 1.13, 95% CI = 0.90–1.41, p = 0.272), CES (OR = 1.020, 95% CI = 0.859–1.211, p = 0.816), SVS (OR = 1.14, 95% CI = 0.93–1.40, p = 0.195), and LS (OR = 1.195, 95% CI = 0.967–1.476, p = 0.097).
Additional corroborating methods, encompassing MR-Egger regression, weighted median, and both simple and weighted modes, endorsed these findings, each lacking statistical significance (refer to ). The absence of horizontal pleiotropy was affirmed by the application of the MR-Egger regression technique (detailed in , ). Concurrently, Cochran Q-tests (encompassing both IVW and MR-Egger) did not disclose any heterogeneity (p > 0.05, as seen in ), and no outliers were discerned in the MR-PRESSO analysis (p > 0.05). Furthermore, leave-one-out evaluations demonstrated that the removal of any individual SNP had no substantial impact on the final outcomes (illustrated in ).
Figure 3. Scatterplot of the genetic association of HZ with stroke and its subtypes. (a) total stroke; (b) ischaemic stroke; (c) large artery stroke; (d) cardioembolic stroke; (e) small vessel stroke; and (f) lacunar stroke. The slope of the straight line indicates the magnitude of causality. Abbreviations: HZ, herpes zoster; SNP, single nucleotide polymorphism; MR, Mendelian randomization.
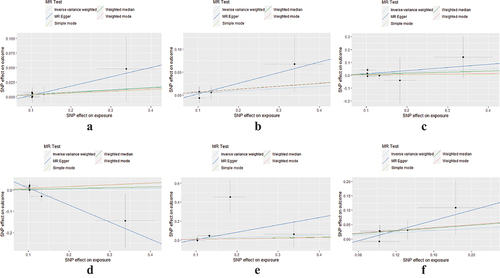
Figure 4. Forest plot for leave-one-out analysis of genetic associations between HZ and stroke and its subtypes. (a) total stroke; (b) ischaemic stroke; (c) large artery stroke; (d) cardioembolic stroke; (e) small vessel stroke; and (f) lacunar stroke. Bars indicate CI. Abbreviations: HZ, herpes zoster; CI, confidence interval.
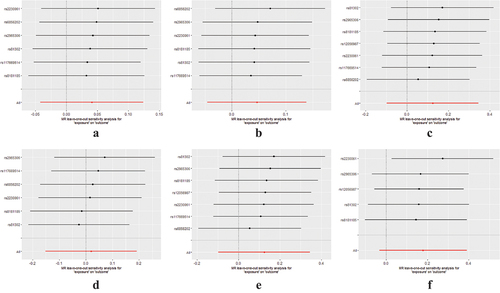
Table 2. Causal association of HZ on stroke and its subtypes assessed by 5 MR analysis methods.
Table 3. Sensitivity analysis of genetically predicted HZ to stroke detailed characterisation of GWAS data.
4. Discussion
4.1. Findings
This research represents the inaugural comprehensive investigation into the causal effects of herpes zoster on various subtypes of ischaemic stroke, namely IS, LAS, SVS, CES, and LS, utilizing data derived from a genome-wide association study (GWAS). The aggregate findings from the Mendelian Randomization analyses conducted on our two sample groups lead to the conclusion that there is no evidence to suggest a causal link between the genetic predisposition to herpes zoster and the likelihood of developing ischaemic stroke.
4.2. Literature comparison
Contrary to our study’s conclusions, there have been multiple observations in recent literature that hint at a link between herpes zoster and ischaemic stroke. In particular, an Asian population-based case-control study [Citation21] reported a discernible escalation in stroke risk within the initial month following herpes zoster infection, with a greater risk associated with herpes zoster affecting the head. While considering potential ethnic disparities, there is a need for corresponding clinical investigations in European cohorts to offset these studies’ constraints. A German population-centric research [Citation22] that evaluated stroke risk post herpes zoster onset paralleled the Asian findings, indicating a surge in stroke incidence of up to 50% within 3 to 4 weeks following herpes zoster initiation. Intriguingly, these two studies diverged in their risk coefficients for haemorrhagic stroke. The former pointed to a more substantial risk of ischaemic stroke relative to haemorrhagic stroke after herpes zoster infection, whereas the latter intimated a diminished rate of ischaemic stroke. The underlying reasons for these discrepancies remain to be elucidated through subsequent studies and fall beyond the scope of the current discussion.
Our analysis reveals an inconclusive stance on whether herpes zoster vaccination or antiviral therapy could lead to a decrease in the risk of IS (ischaemic stroke) development subsequent to HZ (herpes zoster) infection, as per existing studies. Recent investigations have acknowledged that the risk of AIS (acute ischaemic stroke) post-HZ does see a marked escalation; however, this heightened risk seems unaffected by the herpes zoster vaccine and antiviral interventions [Citation23]. Contrarily, research conducted by Leonard H. Calabrese and colleagues posits that immediate antiviral treatment correlated with a diminished incidence of stroke that follows. Numerous studies have identified infection as a critical contributory factor to stroke [Citation24], and the onset of stroke by HZ is likely tied to vasculopathy and systemic inflammation resulting from infection in cerebral arteries. This is considered the primary causation for cerebral arterial damage post-HZ. Evaluating the TOAST classification of stroke etiologies – covering large artery atherosclerotic stroke, small artery occlusive stroke, cardiogenic stroke, other stroke causes, and undetermined stroke origins – the prevalence was found to be notably higher in the elderly compared to younger patients for the first three subtypes [Citation25]. This aligns with the greater occurrence of HZ in older individuals, leading us to surmise that strokes induced by HZ mainly fall within the initial two subcategories. Nevertheless, our clinical and MR studies did not yield corresponding positive findings, indicating a need for a more extensive database in subsequent research.
Comprehensive review of current literature establishes a close association between ischaemic stroke incidence and the duration of herpes zoster (HZ) infection. As per a retrospective cohort research, HZ is a contributing factor for stroke, with elderly HZ sufferers having autoimmune and inflammatory diseases being especially vulnerable. For such individuals, stroke risk post-hospitalization exhibits a time-dependent pattern, showing the highest risk at initial stages and then gradually diminishing. Prompt medical intervention in patients experiencing HZ-associated complications, such as cerebral nerve involvement, can decrease the risk of stroke [Citation26]. Similarly, a different cohort investigation established a temporal relationship between HZ and stroke, suggesting that stroke risk increases for a short period after the HZ infection but not beyond three months. The Mendelian randomization (MR) study, in contrast, used instrumental variables for approximating lifetime exposure to determine the correlation between exposure and outcome [Citation27]. This evidence indicates that the HZ and ischaemic stroke correlation is time-dependent, specifically early in the disease course, while MR studies analyze genetics-based causality and the impact of extended exposure on the outcome [Citation28]. SNPs information is usually collected at a specific time point for an individual or a group, which leads us to hypothesize that the inconsistency between MR study outcomes and clinical research results could be due to the short HZ infection duration not fulfilling the condition for extended exposure. Additionally, the data collection time point might not align with the disease’s timeline. Post-HZ, although complications persist, the infectiousness of the disease lessens, reducing its potential impact on stroke incidence. Hence, late-stage HZ does not correlate with stroke risk, a finding aligning with clinical study results. In summary, according to clinical investigations, HZ onset is age-dependent and linked to stroke based on symptom duration. This factor could explain the negative outcomes of the current study.
4.3.. Mechanism
Contemporary research unequivocally establishes that VZV (Varicella Zoster Virus) is the sole human virus with the ability to replicate in cerebral arteries, and to spread via nerve fibers to the vascular structure. Here, it incites a cascade of inflammatory and thrombotic reactions, culminating in pathological vascular changes, including intimal hyperplasia. Such changes can eventually result in vascular obstruction, cerebral arterial ischemia, and the formation of thrombosis, occlusion, infarction, or aneurysm [Citation29]. There is a growing recognition of infection as a significant stroke precipitant, with inflammatory mechanisms serving as critical contributors, possibly within the framework of underlying processes [Citation4,Citation30]. The mechanisms by which HZ (herpes zoster) influences stroke remain partially obscure. However, some research indicates that HZ patients experience markedly higher interleukin-6 (IL-6) levels compared to healthy subjects, coupled with a rise in inflammation-related oxidative stress [Citation31], and an imbalance in oxidative stress [Citation32]. Notwithstanding the support from human genetic studies for IL-6’s pivotal role in ischaemic stroke pathogenesis, a comprehensive review and meta-analysis unveiled a linear association between IL-6 levels and ischaemic stroke risk. This relationship appeared independent of traditional vascular risk factors, pointing to a higher long-term risk of ischaemic stroke occurrence [Citation33]. Large artery atherosclerotic stroke stands as the most prevalent ischaemic stroke subtype. Our understanding of VZV’s involvement with cerebral arteries, along with alterations in those arteries as predictors for atherosclerotic ischaemic stroke risk, contrasts with our limited knowledge of VZV’s potential role in atherosclerosis development. A key determinant of atherosclerotic ischemic stroke is vascular aging, characterized by oxidative stress. Evidence suggests that elevated oxidative stress, linked to vascular aging, is associated with atherosclerotic ischaemic stroke and that this oxidative stress corresponds with inflammatory responses [Citation34]. Furthermore, based on existing research, we believe that the mechanisms of both may be related to autophagy, which plays a certain role in both HZ infection and stroke. However, further research is needed to confirm this hypothesis. In summation, further investigation is warranted to determine if HZ’s connection with ischaemic stroke and its subtypes may be mediated by oxidative stress and inflammatory reactions. Our research did not establish a causal link between herpes zoster (HZ) and ischemic stroke, including its subtypes. However, conducting a large-scale cohort study in the future is imperative to ascertain the potential impact of herpes zoster on stroke occurrence and its temporal variation. Additionally, actively exploring the potential mediating mechanisms involving inflammatory factors and oxidative stress in the relationship between herpes zoster and stroke is crucial for future research.
4.4. Strengths and limitations
The strengths of our investigation are underlined by the incorporation of extensive GWAS (Genome-Wide Association Studies) data and the application of a judicious Mendelian Randomization (MR) approach, shielding the results from reverse causality, external manipulations, and an array of both discernible and concealed confounding variables. This includes clinical studies’ incapacity to differentiate stroke risks between patients with herpes zoster (HZ) who received systemic antiviral medications and those who remained untreated. The broad spectrum of analyses conducted further solidifies our conclusions. Yet, several potential shortcomings must be acknowledged. Primarily, the genetic instruments utilized originated from subjects of European descent, potentially restricting the generalizability of the conclusions to diverse ethnic backgrounds, due to interactions between genotypes and environmental factors. Secondly, the acquisition of exposure and outcome information from disparate databases might introduce bias, notwithstanding the absence of detectable pleiotropy or heterogeneity in our sensitivity assessments. In addition, due to the comprehensive effect of genetic factors, lifestyle and other factors, it is limited to eliminate the confounding factors that affect the outcome in the process of tool variable screening, which may also lead to bias. Lastly, while the current MR study failed to establish a causal linkage between HZ and stroke, including its various categories, we speculate that the absence of positive findings might stem from the limited validated instrumental variables within the databases, the occurrence of undefined VZV genotypes resulting from variations in VZV genotyping, or unaccounted factors such as the timing and age of disease onset. Further investigation into whether ischaemic stroke preventive measures need to be stratified according to the severity levels of HZ in affected patients is still warranted.
5. Conclusions
Our current Mendelian Randomization (MR) investigation did not manage to uncover substantial evidence supporting a direct cause-and-effect association between herpes zoster (HZ) and ischaemic stroke, encompassing its various categorizations. Looking ahead, there is a clear need to engage in further comprehensive exploration. This will necessitate the utilization of more expansive and detailed databases, combined with the identification of decisive SNPs, to delve deeper into the causal relationship that HZ may have with ischemic stroke and its various subtypes. Such advancements will offer a more definitive validation of our present findings [Citation35].
Author contributions
Conceptualization, X.C. and J.W.; methodology and data collection, Y.W.; Investigation, P.X.; writing – original draft preparation, K.W., P.X. and Y.W.; writing – review and editing, X.J. K.W. and X.P.; Data curation, Y.W. and P.X.; Formal analysis, Y.W.; visualization, J.L. and T.C.; supervision, B.W. and D.Z.; funding acquisition, J.W. All authors have read and agreed to the published version of the manuscript.
Supplemental Material
Download MS Word (32 KB)Acknowledgments
The authors gratefully thank the MEGASTROKE Consortium for providing summary statistics data. All MEGASTROKE Consortium authors are listed online (https://www.megastroke.org/authors.html). The MEGASTROKE project received funding from sources specified at https://www.megastroke.org/acknowledgements.html. The authors thank all the researchers for sharing this data.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/01616412.2024.2363098
Data availability statement
Making data available in publicly accessible repositories.
The data provided in this study are publicly available in the FinnGen Consortium [https://www.finngen.fi/en], the MEGASTROKE Consortium, and the IEU OpenGWAS database [https://gwas.mrcieu.ac.uk/].
Additional information
Funding
References
- Campbell BCV, De Silva DA, Macleod MR, et al. Ischaemic stroke. Nat Rev Dis Primers. 2019;5(1):70. doi: 10.1038/s41572-019-0118-8
- Ajoolabady A, Wang S, Kroemer G, et al. Targeting autophagy in ischemic stroke: from molecular mechanisms to clinical therapeutics. Pharmacol Ther. 2021;225:107848. doi: 10.1016/j.pharmthera.2021.107848
- Feigin VL, Brainin M, Norrving B, et al. World stroke organization (WSO): global stroke fact sheet 2022. Int J Stroke. 2022;17(1):18–29. doi: 10.1177/17474930211065917
- Boehme AK, Esenwa C, Elkind MS. Stroke risk factors, genetics, and prevention. Circ Res. 2017;120(3):472–495. doi: 10.1161/circresaha.116.308398
- Doyle PW, Gibson G, Dolman CL. Herpes zoster ophthalmicus with contralateral hemiplegia: identification of cause. Ann Neurol. 1983;14(1):84–85. doi: 10.1002/ana.410140115
- Powell DR 2nd, Patel S, Franco-Paredes C. Varicella-zoster virus vasculopathy: The growing association between herpes zoster and strokes. Am J Med Sci. 2015;350(3):243–245. doi: 10.1097/maj.0000000000000327
- Reshef E, Greenberg SB, Jankovic J. Herpes zoster ophthalmicus followed by contralateral hemiparesis: report of two cases and review of literature. J Neurol Neurosurg Psychiatry. 1985;48(2):122–127. doi: 10.1136/jnnp.48.2.122
- Verghese A, Sugar AM. Herpes zoster ophthalmicus and granulomatous angiitis. An ill-appreciated cause of stroke. J Am Geriatr Soc. 1986;34(4):309–312. doi: 10.1111/j.1532-5415.1986.tb04227.x
- Lian Y, Zhu Y, Tang F, et al. Herpes zoster and the risk of ischemic and hemorrhagic stroke: a systematic review and meta-analysis. PLOS One. 2017;12(2):e0171182. doi: 10.1371/journal.pone.0171182
- Consensus Workgroup on Herpes Zoster CDA, Diseases NCRCFSAI. Chinese consensus on the diagnosis and management of herpes zoster (2022). Zhonghua Yi Xue Za Zhi. 2022;55(12):1033–1040. doi: 10.35541/cjd.20220608
- Dalli LL, Kim J, Kilkenny MF. Vaccination against herpes zoster and the potential to reduce to the global burden of stroke. Stroke. 2021;52(5):1722–1723. doi: 10.1161/strokeaha.121.034671
- Minassian C, Thomas SL, Smeeth L, et al. Acute cardiovascular events after herpes zoster: a self-controlled case series analysis in vaccinated and unvaccinated older residents of the United States. PLoS Med. 2015;12(12):e1001919. doi: 10.1371/journal.pmed.1001919
- Smith GD, Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32(1):1–22. doi: 10.1093/ije/dyg070
- Malik R, Chauhan G, Traylor M, et al. Publisher correction: multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet. 2019;51(7):1192–1193. doi: 10.1038/s41588-019-0449-0
- Adams HP Jr., Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35–41. doi: 10.1161/01.str.24.1.35
- Cheng W, Liao Y, Mou R, et al. Inflammatory bowel disease and prostate cancer risk: a two-sample Mendelian randomization analysis. Front Immunol. 2023;14:1157313. doi: 10.3389/fimmu.2023.1157313
- Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37(7):658–665. doi: 10.1002/gepi.21758
- Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–525. doi: 10.1093/ije/dyv080
- Verbanck M, Chen CY, Neale B, et al. Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–698. doi: 10.1038/s41588-018-0099-7
- Greco MF, Minelli C, Sheehan NA, et al. Detecting pleiotropy in mendelian randomisation studies with summary data and a continuous outcome. Stat Med. 2015;34(21):2926–2940. doi: 10.1002/sim.6522
- Hosamirudsari H, Rashed P, Afsari F, et al. Correlation between herpes zoster and stroke-a case-control study. J Med Virol. 2018;90(8):1370–1374. doi: 10.1002/jmv.25202
- Schink T, Behr S, Thöne K, et al. Risk of stroke after herpes zoster – evidence from a German self-controlled case-series study. PLOS One. 2016;11(11):e0166554. doi: 10.1371/journal.pone.0166554
- Yang Q, George MG, Chang A, et al. Effect of herpes zoster vaccine and antiviral treatment on risk of ischemic stroke. Neurology. 2020;95(6):e708–e717. doi: 10.1212/wnl.0000000000010028
- Murala S, Nagarajan E, Bollu PC. Infectious causes of stroke. J Stroke Cerebrovasc Dis. 2022;31(4):106274. doi: 10.1016/j.jstrokecerebrovasdis.2021.106274
- Ying L, Xiaomei Z, Libin L, et al. A comparative study on risk factors and etiological classification of stroke in middle-aged and middle-aged adults. Contemp Med. 2020;26(33):34–36. doi: 10.3969/j.issn.1009-4393.2020.33.014
- Calabrese LH, Xie F, Yun H, et al. Herpes zoster and the risk of stroke in patients with autoimmune diseases. Arthritis Rheumatol. 2017;69(2):439–446. doi: 10.1002/art.39855
- Davies NM, Holmes MV, Davey Smith G. Reading mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018;362:k601. doi: 10.1136/bmj.k601
- Zhang X, Liu T, Zhu W, et al. Mendelian randomization and its application in the exploration of the etiology of stroke. Chin J Gerontol. 2022;42(23):5885–5888. doi: 10.3969/j.issn.1005-9202.2022.23.055
- Nagel MA, Gilden D. The relationship between herpes zoster and stroke. Curr Neurol Neurosci Rep. 2015;15(4):16. doi: 10.1007/s11910-015-0534-4
- Emsley HC, Hopkins SJ. Acute ischaemic stroke and infection: recent and emerging concepts. Lancet Neurol. 2008;7(4):341–353. doi: 10.1016/s1474-4422(08)70061-9
- Khazan M, Nasiri S, Riahi SM, et al. Measurement of melatonin, indole-dioxygenase, IL-6, IL-18, ferritin, CRP, and total homocysteine levels during herpes zoster. J Med Virol. 2020;92(8):1253–1259. doi: 10.1002/jmv.25484
- Khazan M, Hedayati M, Robati RM, et al. Impaired oxidative status as a potential predictor in clinical manifestations of herpes zoster. J Med Virol. 2018;90(10):1604–1610. doi: 10.1002/jmv.25204
- Papadopoulos A, Palaiopanos K, Björkbacka H, et al. Circulating interleukin-6 levels and incident ischemic stroke: a systematic review and meta-analysis of prospective studies. Neurology. 2022;98(10):e1002–e1012. doi: 10.1212/wnl.0000000000013274
- Koutsaliaris IK, Moschonas IC, Pechlivani LM, et al. Inflammation, oxidative stress, vascular aging and atherosclerotic ischemic stroke. Curr Med Chem. 2022;29(34):5496–5509. doi: 10.2174/0929867328666210921161711
- Breuer J, Pacou M, Gautier A, et al. Herpes zoster as a risk factor for stroke and TIA: a retrospective cohort study in the UK. Neurology. 2014;83(2):e27–33. doi: 10.1212/wnl.0000000000000584
