Abstract
We previously reported that oral administration of black raspberry powder decreased promoter methylation of tumor suppressor genes in tumors from patients with colorectal cancer. The anthocyanins (ACs) in black raspberries are responsible, at least in part, for their cancer-inhibitory effects. In the present study, we asked if ACs are responsible for the demethylation effects observed in colorectal cancers. Three days of treatment of ACs at 0.5, 5, and 25 μg/ml suppressed activity and protein expression of DNMT1 and DNMT3B in HCT116, Caco2 and SW480 cells. Promoters of CDKN2A, and SFRP2, SFRP5, and WIF1, upstream of Wnt pathway, were demethylated by ACs. mRNA expression of some of these genes was increased. mRNA expression of β-catenin and c-Myc, downstream of Wnt pathway, and cell proliferation were decreased; apoptosis was increased. ACs were taken up into HCT116 cells and were differentially localized with DNMT1 and DNMT3B in the same cells visualized using confocal laser scanning microscopy. Although it was reported that DNMT3B is regulated by c-Myc in mouse lymphoma, DNMT3B did not bind with c-Myc in HCT116 cells. In conclusion, our results suggest that ACs are responsible, at least in part, for the demethylation effects of whole black raspberries in colorectal cancers.
INTRODUCTION
Anthocyanins are the most abundant flavonoid constituents of fruits and vegetables. The conjugated bonds in their structures, which absorb light at about 500 nm, are the basis for the bright red, blue, and purple colors of fruits and vegetables, as well as the autumn foliage of deciduous trees (Citation1). The daily intake of anthocyanins in residents of the United States is estimated to be about 200 mg or about ninefold higher than that of other dietary flavonoids (Citation2). In the United States, the daily intake of anthocyanins is estimated to be at around 180–215 mg/day (Citation3). This is much higher than the daily intake of other polyphenols such as quercetin, kaempferol, myricetin, apigenin, and luteolin, which are at around 23 mg/day (Citation3). Anthocyanins are provided in the diet by foods such as strawberries, raspberries, and blackberries. Consumption of 100 g of berries would provide up to 500 mg of anthocyanins (Citation4). We reported the importance of anthocyanins from black raspberries (BRBs) in the prevention of esophageal tumors in rats induced by N-nitrosomethylbenzylamine (Citation5). Rats were fed the anthocyanin fraction of BRBs containing the same amount of anthocyanins as are present in a diet containing 5% BRBs, a dietary concentration of BRBs that is effective in reducing esophageal cancer. The anthocyanin fraction was nearly equally effective as the 5% BRB diet in the suppression of esophageal tumor development indicating that the anthocyanins are important chemopreventive constituents of BRBs (Citation5).
We recently demonstrated that whole BRB powder, administered orally on a daily basis for an average of approximately 4 wk, led to the protective modulation of multiple epigenetic and cellular biomarkers in human colorectal tumors (Citation6). The methylation of 3 Wnt inhibitors—SFRP2, SFRP5, and WIF1—upstream genes in the Wnt signaling pathway, and PAX6a, a developmental regulator, was modulated in a protective direction by BRBs in normal tissues and in colorectal tumors in patients who received BRB treatment for an average of 4 wk. This was associated with decreased expression of DNA methyltransferase 1 (DNMT1). The components of BRBs that exert demethylation effects however, remain to be elucidated.
DNA methylation in mammalian cells is regulated by a family of highly related DNA methyltransferase enzymes (DNMT1, DNMT3A, and DNMT3B) that mediate the transfer of methyl groups from S-adenosylmethionine to the 5 position of cytosine bases in the dinucleotide sequence CpG (Citation7). DNMT1 functions as the maintenance DNA methyltransferase in mammalian cells and is responsible for accurately replicating genomic DNA methylation patterns during the S phase of the cell cycle (Citation8). In contrast, de novo methylation of DNA is believed to be performed by DNMT3A and DNMT3B enzymes, which possess both maintenance and de novo DNA methylation activities (Citation9). Both groups of enzymes however, have been shown to exhibit some level of both maintenance and de novo methylation in vitro, suggesting that this classification of the DNMTs may be oversimplified (Citation10). Confirming the importance of DNA methylation in tumorigenesis, studies have shown all 3 DNMTs to be overly expressed in several tumor types, including tumors of the colon and rectum, bladder, and kidney (Citation11). When DNMT1 and DNMT3B are knocked out in colon cancer cell lines, methylation of tumor suppressor genes such as CDKN2A is almost entirely eliminated and the genes are reexpressed (Citation12). Inhibition of DNMTs, therefore, may lead to demethylation and reactivation of the silenced genes. Aberrant methylation of tumor suppressor genes by DNMTs may be a promising target for both chemoprevention and chemotherapy, as DNMT inhibitors are being developed as potential therapeutic agents for cancer (Citation13).
The goal of this study was to investigate whether BRB-derived anthocyanins (ACs) are responsible for the demethylation effects of whole BRBs in tumors from colorectal cancer patients. Our results suggest that ACs demethylate tumor suppressor genes through inhibition of DNMT1 and DNMT3B in human colon cancer cells.
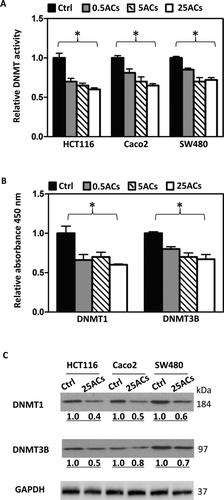
FIG. 1 Black raspberry-derived anthocyanins (ACs) suppress DNMT1 and DNMT3B. A: Total DNMT activity in nuclear extracts from human colon cancer cells, HCT116, Caco2, and SW480, treated with ACs at 0.5, 5, and 25 μg/ml for 3 days was decreased. B: In a cell free in vitro inhibition assay, ACs inhibit DNMT1 and DNMT3B activities. C: Three days treatment with 25 μg/ml ACs decrease protein expression of DNMT1 and DNMT3B in nuclear extracts from all 3 lines. * P < 0.05.
MATERIALS AND METHODS
Chemicals
Naturstoff reagent A (Sigma-Aldrich, Milwaukee, WI) was freshly dissolved in ethanol at a concentration of 5% and diluted in PBS for application. Anthocyanin-enriched extract was prepared as described (Citation5). Briefly, BRBs were incubated with hexane and the residue from the hexane extract was then incubated with ethanol/H2O:80/20. A polystyrene-based resin was activated and was added to the ethanol/H2O mixture to absorb anthocyanins. The mixture was then applied to a glass column containing a glass frit. The column was washed using H2O to remove sugar and then ethanol was used to elute anthocyanin. The fraction was concentrated using a centrifugal vacuum concentrator (Citation5). The tar-like anthocyanin-enriched extract was used in the study. The 4 major anthocyanins in this extract and their concentrations are as follows: cyanidin-3-O-glucoside (23.40 μg/mg), cyanidin-3-O-rutinoside (96.91 μg/mg), cyanidin-3-O-xylosylrutinoside (29.02 μg/mg) and cyanidin-3-O-sambubioside (14.65 μg/mg). Total anthocyanin content in the anthocyanin-enriched extract is 163.97 μg/mg.
Cell Culture and Treatment
HCT116, Caco2, and SW480 cells were treated with BRB-derived ACs at 0.5, 5, and 25 μg/ml for 3 days for DNMT activity, and DNMT1 and DNMT3B inhibition assays. We noted that 25 μg/ml ACs produced the strongest inhibitory effect in these assays ( and ), therefore, unless stated otherwise, 25 μg/ml ACs was the dose used for subsequent assays (i.e., Western blot, real-time PCR, methylation analysis, cell proliferation, and apoptosis). ACs are water-soluble and were added freshly to cell culture medium every day.
Total DNMT Activity, DNMT1 and DNMT3B Inhibition Assays, and Western Blot Analysis
Total DNMT activity of nuclear extracts from HCT116, Caco2, and SW480 cells was measured using EpiQuik DNMT activity/inhibition assay kit (Epigentek, Brooklyn, NY). Inhibition of DNMT1 and DNMT3B by ACs was performed using EpiQuik DNA Methyltransferase 1 and 3B Activity/Inhibitor Screening Assay Kit, respectively (Epigentek, Brooklyn, NY). The nuclear extracts were used for Western blot analysis. DNMT1 (Santa Cruz Biotechnology, Santa Cruz, CA), DNMT3B (Santa Cruz Biotechnology, Santa Cruz, CA) and GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA) antibodies were used for identification of their respective proteins.
Real-time PCR
Human colon cancer cell lines HCT116, Caco2, and SW480 were purchased from the American Type Culture Collection (ATCC, Manassas, VA) and were cultured in McCoy's 5A medium supplemented with 10% fetal bovine serum in a humidified incubator at 37°C and in an atmosphere of 5% CO2. All 3 lines were treated with ACs as described above and cells were harvested for mRNA extraction. Real-time PCR primers for β-catenin and c-Myc were purchased from SABiosciences (Frederick, MD). Primers for CDKN2A, SFRP2, SFRP5, and WIF1 are as follows: CDKN2A forward 5′-CTGAGGAG CTGGGCCATC-3′, reverse 5′-GTTGTGGCCCTGTAGGAC CT-3′; SFRP2 forward 5′-GGTATGTGAAGCCTGCAAAA A-3′, reverse 5′-CCGATTTCTTCAGGTCCCTT-3′; SFRP5 forward 5′-GCGCCCAGTGTGAGATG-3′, reverse 5′-TTGG TGTCCTTGCGCTTC-3′; WIF1 forward 5′-GAACCTGCCAT GAACCCAA-3′, reverse 5′-CAGATGTCGGAGTTCACCAG AT-3′.
Nonradioactive Cell Proliferation and Capase-3/7 Activity Assays
HCT116, Caco2, and SW480 cells were treated with ACs as described above. Cell proliferation was quantified using the CellTiter 96TM AQueous assay (Promega, Madison, WI). Caspase-3/7 activity was quantified using Apo-ONE® homogenous caspase-3/7 assay according to the manufacturer's instructions (Promega, Madison, WI).
DNA Methylation of Candidate Genes and LINE1 Repetitive Element
To determine methylation levels of candidate genes, CDKN2A, SFRP2, SFRP5, and WIF1, in HCT116, Caco2 and SW480 cells were treated with ACs as described above. The MassARRAY assay was used to detect methylated CpG sites in sequencing reactions (Citation14). Briefly, bisulfite-converted DNA was amplified with primers (primer sequences are given in Supplemental Table 1), PCR products were spotted on a 384-pad SpectroCHIP (Sequenom, San Diego, CA), and were spectrally acquisited on a MassARRAY analyzer. Methylation data of individual units (1-4 CpG sites per unit) were generated by EpiTyper software (Sequenom, San Diego, CA).
For LINE1 methylation analysis, bisulfite-converted DNA was amplified and sequenced using PyroMark LINE1 kit (Qiagen, Valencia, CA) that contains PCR primers and a sequencing primer provided by the company. PCR cycling conditions were 95°C (30 sec), 50°C (30 sec), and 72°C (30 sec) for 35 cycles. The PCR product was purified and methylation was quantified using the PSQ HS 96 Pyrosequencing System (Pyrosequencing Inc, Westborough, MA).
Confocal Laser Scanning Microscopy (CLSM)
Because ACs produced the strongest inhibitory effects on DNMT activity and on protein expression of DNMT1 and DNMT3B in HCT116 cells, this cell line was chosen for visualization of uptake of ACs. HCT116 cells were treated with 25 μg/ml ACs in the presence of Naturstoff reagent A, a fluorescent dye, (2-Aminoethyl-diphenylborinate; Sigma-Aldrich, Milwaukee, WI) for 4 h and 1 day. Cells were washed using 1X PBS and fixed in 4% paraformaldehyde for 10 min at room temperature. They were then washed with 0.1% Triton X-100 in 1X PBS for 10 min and blocked with 1% BSA/0.1% Triton X-100 in 1X PBS for 30 min. After blocking, cells were incubated with primary antibody to DNMT1 or DNMT3B (IMGENEX, San Diego, CA), 1:100 dilution in 1% BSA/0.1% Triton X-100 in 1X PBS, for 1 h at room temperature. Cells were further incubated with secondary antibody, FITC-goat antimouse IgG (Santa Cruz), 1:200 dilution in 1% BSA/0.1% Triton X-100 in 1X PBS, for 30 min at room temperature. Finally, cells were mounted using ProLong® Gold antifade reagent with DAPI (Invitrogen, Carlsbad, CA). Images were captured using Zeiss Confocal Fluorescence Microscope (LSM 510).
Immunoprecipitation
HCT 116 cells were treated with 5 or 25 μg/ml ACs for 3 days. Cells were lysed by RIPA buffer [50 mM TrisHCl pH7.4, 150 mM NaCl, 2 mM EDTA, 1% NP-40, 0.1% SDS, supplemented with protease and phosphatase inhibitor cocktails (Roche, Indianapolis, IN)]. An equal amount of each protein lysate was incubated in IP buffer [20 mM TrisHCl pH7.4, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, supplemented with protease and phosphatase inhibitor cocktails (Roche) with anti-DNMT1, anti-DNMT3B (Santa Cruz Biotechnology, Santa Cruz, CA), or anti-c-Myc (Cell Signaling, Danvers, MA) polyclonal antibodies for overnight at 4°C, followed by incubation with 25 μl of protein A or G-Agarose beads (Roche) for 2 hours at 4°C]. The immune complexes were washed 3 times with IP buffer and analyzed by SDS-PAGE.
Statistical Analysis
Total DNMT activity, ACs inhibition of DNMT1 and DNMT3B activities, DNA methylation changes, mRNA expression, cell proliferation, and apoptosis were compared using analysis of variance and an unpaired t-test. A P value <0.05 was considered to be statistically significant.
RESULTS
BRB-Derived Anthocyanins (ACs) Inhibit Activity of DNA Methyltransferases
HCT116, Caco2, and SW480 cells were treated with ACs at 0.5, 5, and 25 μg/ml for 3 days and total DNMT activity in the nuclear extracts was measured. As shown in , ACs at all 3 doses significantly decreased total DNMT activity in all 3 cell lines. Using EpiQuik™ DNMT1 and DNMT3B activity/inhibitor screening assay kits that screen DNMT1 and DNMT3B inhibitors, respectively, ACs at 0.5, 5, and 25 μg/ml significantly inhibited the activity of DNMT1 and DNMT3B (). The most inhibitory dose on total DNMT activity and on DNMT1 and DNMT3B activities was 25 μg/ml. Nuclear extracts from HCT116, Caco2, and SW480 cells treated with 25 μg/ml ACs were used to determine the protein expression of DNMT1 and DNMT3B. Results show that ACs decrease expression of both proteins in all 3 cell lines ().
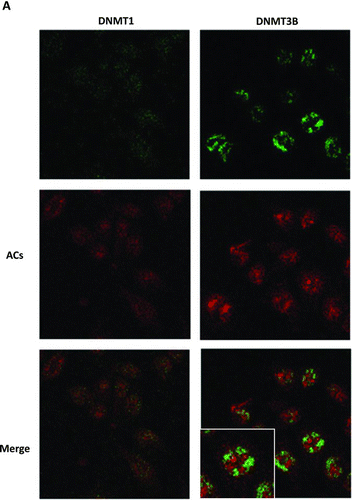
We then asked how do ACs inhibit DNMT1 and DNMT3B. Do they enter cells? Visualization of cellular uptake of the cyanidin component of the anthocyanin molecule using Naturstoff reagent A in human keratinocytes has been reported (Citation15). Therefore, we used Naturstoff reagent A to visualize the uptake of ACs into HCT116 cells because the major ACs present in BRB have a cyanidin nucleus. As shown in , after 1 day of treatment, ACs (red color) were localized in both the cytoplasm and nucleus. These same cells were also stained for DNMT1 or DNMT3B that are localized mainly in the nucleus (green color). Where the red and green are merged, the color is yellow due to colocalization of ACs and DNMT1 or DNMT3B. There are only few yellow spots in the cells, and the red, staining for ACs, and green, staining for DNMT1 or DNMT3B, are differentially distributed in the cells. Treatment of HCT116 cells with ACs for 4 h shows the same staining pattern (data not shown). In the absence of ACs, no fluorescence was detected in Naturstoff reagent A treated cells (data not shown).
FIG. 2 Cellular uptake of anthocyanins (ACs) and their colocalization with DNMT proteins in HCT116 cells. A: ACs differentially localize with DNMT1 and DNMT3B in HCT116 cells. B: ACs inhibit c-Myc mRNA expression in HCT116, Caco2 and SW480 cells. C: c-Myc and DNMT3B do not bind with one another in HCT116 cells. (Continued)
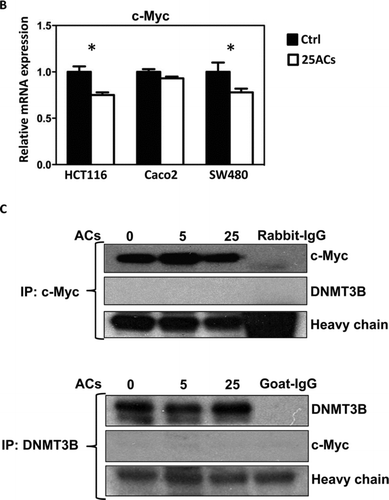
It has been reported that c-Myc regulates DNMT3B in mouse lymphoma cells (Citation16). In the present study, we found that ACs inhibit c-Myc mRNA expression in HCT116, Caco2, and SW480 cells () and asked whether this leads to suppression of DNMT3B. Although ACs decrease both c-Myc and DNMT3B in HCT116 cells, immunoprecipitation experiments revealed that c-Myc does not bind to DNMT3B in these cells. Therefore, it is unlikely that c-Myc directly regulates DNMT3B in HCT116 cells ().
BRB-Derived ACs Demethylate Promoters of Tumor Suppressor Genes and Increase Their mRNA Expression
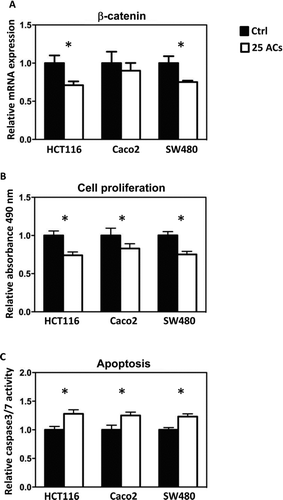
Methylation of promoter sequences of tumor suppressor genes was quantified using MassARRAY, and LINE-1 repetitive elements were measured using Pyrosequencing. The map of CpG sites in the promoter regions and numbers of CpG sites covered are given in Supplemental . Results show that AC-induced promoter demethylation is cell line dependent. For example, methylation of CDKN2A, a cell cycle regulator, is decreased by ACs only in HCT116 and SW480 cells (); methylation of SFRP5, a Wnt pathway negative regulator, is decreased by ACs only in Caco2 cells (). Interestingly, methylation of SFRP2 and WIF1, the Wnt pathway negative regulators, is decreased by ACs in all 3 lines (). Genome-wide methylation, measured by LINE1 repetitive element, is not altered by ACs (). Although promoter methylation of CDKN2A is decreased in HCT116 and SW480 cells by ACs, AC treatment does not change mRNA expression of CDKN2A in these cells or in Caco2 cells (). mRNA expression of SFRP5 is significantly increased by ACs in HCT116 cells (). Interestingly, mRNA expression of SFRP2 is significantly increased in all 3 lines by ACs (). We then asked if genes downstream of the Wnt pathway are altered by ACs and found that mRNA expression of β-catenin is decreased by ACs in HCT116 and SW480 cells (). In addition, 25 μg/ml ACs significantly suppresses cell proliferation and induces apoptosis in all 3 lines ( and ).
DISCUSSION
Our results show the colocalization of ACs with DNMT1 and DNMT3B in HCT116 cells after 1 day of treatment () suggesting the possibility that ACs directly suppress DNMT1 and DNMT3B. However, the binding of ACs to DNMTs may be a minor event because there were only a few yellowish spots in the cells (); the majority of ACs and DNMTs were differentially localized in AC-treated cells. However, like other polyphenols (e.g., ellagic acid) (Citation17), ACs may be pumped out and retaken up into cells quickly. Our present studies capturing images only at 4 h and 1 day after AC treatment may not capture this dynamic phenomenon and underestimate actual AC/DNMT interactions. Future experiments using real-time imaging of ACs are necessary for assessing overall AC/DNMT interactions.
There are 2 prominent theories of how DNA methylation patterns are maintained by the DNMT family (Citation18). The first was published 30 yr ago by Riggs (Citation19) and Holliday and Pugh (Citation20). They suggested that methylation patterns are established by de novo enzymes, for example, DNMT3A and DNMT3B, and DNMT1 is mostly responsible for copying the patterns (Citation19,Citation20). There are no overlapping functions between these 2 types of enzymes. The other theory proposed that the maintenance of DNA methylation relies not only on the recognition of hemimethylated DNA by DNMT1 but also on the localization of the de novo enzymes, DNMT3A and DNMT3B, to specific chromatin regions that contain methylated DNA (Citation18). Continual cooperation between the DNMTs is necessary to maintain DNA methylation patterns after DNA leaves the replication fork (Citation18). In addition, DNA in a nucleosome bearing specific histone modifications might be differentially susceptible to methylation (Citation18).
FIG. 3 Differential responses of human colon cancer cells to anthocyanins (ACs)-induced demethylation. ACs (25 μg/ml, 3 days) demethylate the promoter sequence of CDKN2A in HCT116 and SW480 cells (A), of SFRP5 in Caco2 cells (B), and of SFRP2 (C) and WIF1 (D) in HCT116, Caco2, and SW480 cells. ACs do not induce genome-wide methylation changes in all 3 lines as measured by the LINE-1 repetitive element (E). Ctrl = control. * P < 0.05.
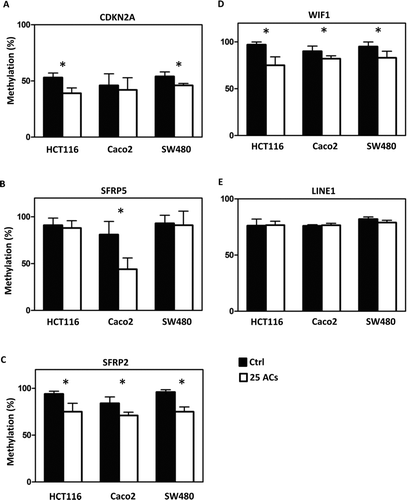
FIG. 4 Effects of anthocyanins (ACs) on mRNA expression of tumor suppressor genes. ACs do not alter the mRNA expression of CDKN2A in HCT116, Caco2, and SW480 cells (A). ACs increase the mRNA expression of SFRP5 in HCT116 cells (B) and of SFRP2 in all 3 cell lines (C). ACs increase WIF1 mRNA expression in HCT116 and SW480 cells (D). * P < 0.05.
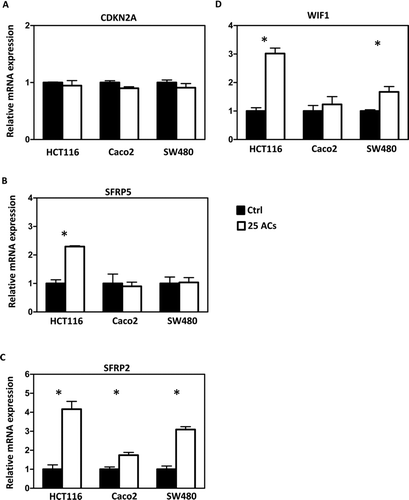
FIG. 5 Anthocyanins (ACs) decreased the mRNA expression of β-catenin in HCT116 and SW480 cells (A). This is accompanied by decreased cell proliferation (B) and increased apoptosis (C). * P < 0.05.
Little is known about the regulators of DNMT1 and DNMT3B. Our recent review summarized the known factors/pathways that directly regulate these enzymes (Citation21). For example, p53 mutation is associated with high expression of DNMT1 in cultured lung cancer cells as well as in tumors from lung cancer patients (Citation22). c-Myc is thought to modulate DNMT3B in a mouse mediastinal lymphoma model (Citation16). We found that ACs decrease c-Myc mRNA expression in HCT116 and SW480 cells (). However, c-Myc did not bind to DNMT3B () and p53 did not bind to DNMT1 in HCT116 cells (data not shown). It is likely, therefore, that DNMTs are regulated differently in different cell types.
The 3 colorectal cancer cell lines responded differently to AC-induced DNA demethylation. Moreover, the methylation of different genes in the same cell line varied after treatment with ACs (). Interestingly, similar effects were observed in HCT116 cells following treatment with the demethylating drug, 5-aza-deoxycytidine (5-aza-dC); TIMP-3 was more readily demethylated than p16 (Citation23). The molecular basis for this selective demethylating effect of 5-aza-dC remains to be elucidated. With respect to the ACs, we suspect that their ability to methylate specific CpG sequences determines their effectiveness in demethylating genes. However, as for 5-aza-dC, the selectivity of anthocyanin-induced demethylation in cultured cells requires further investigation. It is interesting to note that the degree of demethylation and the degree of increase in mRNA expression for a given tumor suppressor gene is not linear in AC-treated cells ( and ). Given the fact that ACs increase expression of tumor suppressor genes suggesting that they might have effects on other epigenetic regulations (e.g., chromatin remodeling and/or histone modification). Studies are ongoing, therefore, to determine if ACs have effects on histone modification and/or chromatin remodeling.
In conclusion, we found that anthocyanins derived from BRBs are taken up into the cytoplasm and nucleus of cultured human colon cancer cells. They demethylate tumor suppressor genes through inhibition of DNMT1 and DNMT3B. Based on the fluorescence staining results, ACs may inhibit DNMTs through direct as well as indirect target(s). Future studies aimed on identifying these targets are warranted.
ACKNOWLEDGMENTS
This work was supported by NCI R01 CA148818 to L.-S. Wang. The authors thank Dr. Stephen Hecht for isolating anthocyanins from black raspberries.
REFERENCES
- Wang , L-S and Stoner , G D . 2008 . Anthocyanins and their role in cancer prevention . Cancer Lett , 269 : 281 – 290 .
- Hertog , M GL , Hollman , P CH , Katan , M B and Kromhout , D . 1993 . Intake of potentially anticarcinogenic flavonoids and their determinants in adults in the Netherlands . Nutr Cancer , 20 : 21 – 29 .
- Mazza , G J . 2007 . Anthocyanins and heart health . Ann Ist Supper Sanita , 43 : 369 – 374 .
- Cao , G and Prior , R L . 1999 . Anthocyanins are detected in human plasma after oral administration of an elderberry extract . Clinical Chemistry , 45 : 574 – 576 .
- Wang , L-S , Hecht , S S , Carmella , S G , Yu , N Larue , B . 2009 . Anthocyanins in black raspberries prevent esophageal tumors in rats . Cancer Prev Res , 2 : 84 – 93 .
- Wang , L-S , Arnold , M , Huang , Y-W , Sardo , C Seguin , C . 2011 . Modulation of genetic and epigenetic biomarkers of colorectal cancer in humans by black raspberries: a phase i pilot study . Clin Cancer Res , 17 : 598 – 610 .
- Herman , J G and Baylin , S B . 2003 . Gene silencing in cancer in association with promoter hypermethylation . N Engl J Med , 349 : 2042 – 2054 .
- Bestor , T H . 2000 . The DNA methyltransferases of mammals . Hum Mol Genet , 9 : 2395 – 2402 .
- Okano , M , Xie , S and Li , E . 1998 . Cloning and characterization of a family of novel mammalian DNA (cytosine-5) methyltransferases . Nat Genet , 19 : 219 – 220 .
- Pradhan , S , Bacolla , A , Wells , R D and Roberts , R J . 1999 . Recombinant human DNA (cytosine-5) methyltransferase . J Biol Chem , 274 : 33002 – 33010 .
- Robertson , K D , Uzvolgyi , E , Liang , G , Talmadge , C Sumegi , J . 1999 . The human DNA methyltransferases (DNMTs) 1, 3a and 3b: coordinate mRNA expression in normal tissues and overexpression in tumors . Nucleic Acids Res , 27 : 2291 – 2298 .
- Rhee , I , Bachman , K E , Park , B H , Jair , K-W Yen , R-WC . 2002 . DNMT1 and DNMT3b cooperate to silence genes in human cancer cells . Nature , 416 : 552 – 556 .
- Issa , J-PJ . 2007 . DNA Methylation as a Therapeutic Target in Cancer . Clin Cancer Res , 13 : 1634 – 1637 .
- Huang , Y-W , Liu , J C , Deatherage , D E , Luo , J Mutch , D G . 2009 . Epigenetic repression of microRNA-129-2 leads to overexpression of SOX4 oncogene in endometrial cancer . Cancer Res , 69 : 9038 – 9046 .
- Ernst , I MA , Wagner , A E , Lipinski , S , Skrbek , S Ruefer , C E . 2010 . Cellular uptake, stability, visualization by ‘Naturstoff reagent A’, and multidrug resistance protein 1 gene-regulatory activity of cyanidin in human keratinocytes . Pharmacol Res , 61 : 253 – 258 .
- Shah , M Y , Vasanthakumar , A , Barnes , N Y , Figueroa , M E Kamp , A . 2010 . DNMT3B7, a truncated DNMT3B isoform expressed in human tumors, disrupts embryonic development and accelerates lymphomagenesis . Cancer Res , 70 : 5840 – 5850 .
- Whitley , A C , Stoner , G D , Darby , M V and Walle , T . 2003 . Intestinal epithelial cell accumulation of the cancer preventive polyphenol ellagic acid—extensive binding to protein and DNA . Biochemical Pharmacology , 66 : 907 – 915 .
- Jones , P A and Liang , G . 2009 . Rethinking how DNA methylation patterns are maintained . Nat Rev Genet , 10 : 805 – 811 .
- Riggs , A D . 1975 . X inactivation, differentiation, and DNA methylation . Cytogenet Cell Genet , 14 : 9 – 25 .
- Holliday , R and Pugh , J E . 1975 . DNA modification mechanisms and gene activity during development . Science , 187 : 226 – 232 .
- Huang , Y-W , Kuo , C-T , Stoner , K , Huang , T HY and Wang , L-S . 2011 . An overview of epigenetics and chemoprevention . FEBS Lett , 585 : 2129 – 2136 .
- Lin , R-K , Wu , C-Y , Chang , J-W , Juan , L-J Hsu , H-S . 2010 . Dysregulation of p53/Sp1 Control leads to DNA methyltransferase-1 overexpression in lung cancer . Cancer Res , 70 : 5807 – 5817 .
- Rhee , I , Jair , K-W , Yen , R-WC , Lengauer , C Herman , J G . 2000 . CpG methylation is maintained in human cancer cells lacking DNMT1 . Nature , 404 : 1003 – 1007 .