ABSTRACT
We investigated whether alcohol and dietary folate intakes were associated with promoter methylation in clear-cell renal cell carcinoma (ccRCC). The Netherlands Cohort Study with a case-cohort design included 120,852 subjects aged 55–69 yr in 1986. Diet was measured with a food-frequency questionnaire. After 20.3 yr of follow-up, paraffin-embedded tumor blocks were collected. Methylation-specific polymerase chain reaction (MSP) was used to analyze promoter methylation of 11 genes. ccRCC cases were classified into low (0–19% of the genes), intermediate (20–39%), and high (40%+) methylation. Multivariable Cox regression analyses were conducted, stratified according to methylation, including 3980 subcohort members and 297 ccRCC cases. Increasing alcohol intake was associated with decreased ccRCC risk, but was not statistically significant; multivariable adjusted hazard ratio (HR) for ≥30 g alcohol/day versus 0 g/day was 0.78 [95% confidence interval (CI): 0.48–1.24], and P-value for trend was 0.46. In strata according to methylation index, no significant heterogeneity was observed. Dietary folate intake was not associated with ccRCC risk. There was no significant heterogeneity between strata according to methylation index. There was no effect modification of alcohol and dietary folate intake on ccRCC risk, nor in strata according to methylation index. Our findings do not support the hypothesis that alcohol and dietary folate intakes are involved in ccRCC.
Introduction
Cigarette smoking, excess body weight, a diagnosis of hypertension, and familial cancer syndromes are established risk factors of renal cell cancer (RCC) Citation(1). Recent evidence suggests that alcohol consumption may be inversely associated with RCC risk, although the mechanism explaining the inverse association has not been established yet Citation(1–4).
One of the suggested mechanisms that may link alcohol to cancer is DNA methylation Citation(5). Aberrant promoter CpG island hypermethylation, in short, promoter methylation, has been observed in many cancers, including RCC Citation(6,7). It has been suggested that there is a specific subtype of cancer that is characterized by extensive promoter methylation of tumor suppressor and DNA repair genes and referred to as the CpG island methylator phenotype (CIMP) Citation(8). CIMP has been investigated extensively in colorectal cancer, but may also exist in other cancers, such as RCC Citation(8,9). However, no definition for CIMP in RCC has been established so far.
An adequate folate metabolism is necessary for maintaining DNA methylation and a high intake of alcohol is associated with folate reduction by different mechanisms. High alcohol intake decreases the absorption of folate from the intestinal lumen Citation(10,11). Alcohol may also increase urinary excretion of folate and influences proteins involved in folate metabolism Citation(10,11). Heavy abusers of alcohol often have a diet with a deficient folate intake Citation(10). The combination of high alcohol intake and low dietary intake of folate may be specifically the cause for adverse effects of folate deficiency Citation(10–13).
In a previous analysis, we investigated whether alcohol intake was associated with promoter methylation in the von Hippel-Lindau (VHL) gene in RCC Citation(14). Alcohol intake was inversely associated with the RCC subtype without VHL promoter methylation; however, the number of RCC cases with VHL promoter methylation was too low for reliable point estimates.
If high alcohol and low folate intakes are associated with promoter methylation in RCC, the association of these dietary exposures with RCC risk might differ by molecular subtypes of RCC defined by promoter methylation status. The overall association of alcohol with RCC risk is inverse, but this might not be true in the subgroup of RCC characterized by a high promoter methylation status. We therefore investigated whether associations between alcohol and dietary folate intake are different in RCC subgroups defined by levels of promoter methylation of CpG islands in a specifically selected gene panel in a large prospective study.
Materials and Methods
Study Design and Study Population
The Netherlands Cohort Study on Diet and Cancer (NLCS) is a prospective cohort study that was initiated in 1986 and included 120,852 subjects aged 55–69 yr at baseline Citation(15). The NLCS was designed as a case-cohort study for efficiency in questionnaire processing and follow-up. Cases were derived from the entire cohort, whereas a subcohort of 5,000 subjects was randomly sampled at baseline to estimate person years at risk for the entire cohort Citation(15). Subcohort members were regularly followed up for vital status information, whereas all cohort members were followed up for cancer occurrence using record linkage with the Netherlands Cancer Registry and the Netherlands Pathology Registry (PALGA) Citation(16). Only one male subcohort member was lost to follow-up. The completeness of cancer follow-up by the Netherlands Cancer Registry and PALGA is estimated to be over 96% Citation(17). Cases and subcohort members who reported prevalent cancer (excluding skin cancer) at baseline were excluded. A unique population-based collection of DNA isolated from formalin-fixed paraffin-embedded (FFPE) tissues of RCC cases is nested within the NLCS. Initially, this collection of DNA material included only cases from the first 11.3 yr of follow-up Citation(18), yet recently the collection was expanded to 20.3 yr of follow-up.
The NLCS has been approved by the institutional review boards of the TNO Quality of Life Research Institute (Zeist, the Netherlands) and Maastricht University (Maastricht, the Netherlands). All cohort members consented to participate in the study by completing and returning the self-administered questionnaire.
Tissue Collection and DNA Isolation
A total of 608 RCC cases were identified within the NLCS during 20.3 yr of follow-up (between 1986 and 2006). Only histologically confirmed epithelial RCC cases (n = 568) were eligible for the collection of FFPE tumor tissues from ∼50 pathology laboratories throughout the Netherlands (see ). Tumor blocks of 454 out of 568 eligible cases (80%) could be retrieved and adjacent normal renal parenchyma tissues were collected of 314 cases (55%). The RCC cases were classified according to the World Health Organization (WHO) Classification of Tumors of 2004 Citation(19) by two experienced urogenital pathologists (C.A. Hulsbergen-van de Kaa and M.M.L.L. Baldewijns). 81% of the cases were classified as clear-cell RCC (ccRCC) Citation(19).
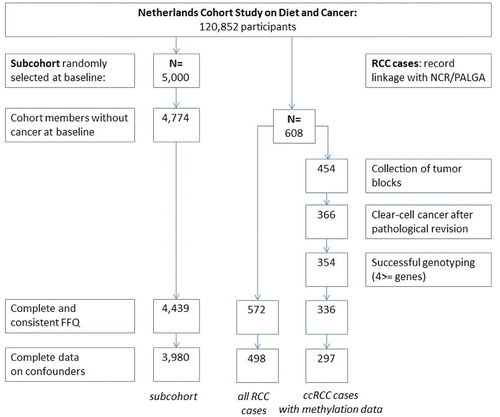
Figure 1. Flowchart of available subcohort members and renal cell cancer cases after 20.3 yr of follow-up, Netherlands Cohort Study on Diet and Cancer, 1986–2006. Notes: ccRCC, clear-cell renal cell cancer; FFQ, food-frequency questionnaire; NCR, Netherlands Cancer Registry; PALGA, Netherlands Pathology Registry; RCC, renal cell cancer.
Methods used for DNA isolation of FFPE tissues from RCC cases identified during the first 11.3 yr of follow-up included in the initial collection have been described previously Citation(18). For the recently added RCC cases, vital tissue areas were dissected before DNA isolation. DNA was isolated using the QIAamp DNA Mini Kit (Qiagen), according to manufacturer's instructions.
Dietary Assessment
All NLCS subjects returned a mailed, self-administered, baseline questionnaire, including a 150-item, semiquantitative food-frequency questionnaire (FFQ), which concentrated on habitual consumption of foods and beverages in the year before the start of the study.
Consumption of alcoholic beverages was addressed by questions on beer, red wine, white wine, sherry, other fortified wines, liqueur, and liquor. Respondents who consumed alcoholic beverages less than once a month were considered nonusers. Four items from the questionnaire (i.e., red wine, white wine, sherry, and liqueur) were combined into one wine variable, since these items were substantially correlated Citation(20). Mean daily alcohol consumption was calculated using the Dutch Food Composition table Citation(21). Based on a pilot study, standard glass sizes were defined as 200 ml for beer, 105 ml for wine, 80 ml for sherry, and 45 ml for both liqueur and liquor, corresponding to 8, 10, 11, 7, and 13 g of alcohol, respectively.
Mean daily intakes of other nutrients were calculated by multiplying the frequencies and portion sizes of all food items and their tabulated nutrient contents from the Dutch Food Consumption table from 1986 Citation(21). Folate data were derived from a validated liquid chromatography trienzyme method Citation(22) used to analyze the 125 most important Dutch foods contributing to folate intake Citation(23). Although the FFQ contained questions on the use of dietary supplements, the use of folic acid was not accounted for in the analyses, as dietary supplements in general did not contain folic acid in the Netherlands in the late 1980s. Subjects with incomplete or inconsistent dietary questionnaires were excluded Citation(24). The FFQ ranked individuals adequately according to dietary intakes when compared to 9-day dietary records Citation(24) and reflected nutrient intakes for at least 5 yr Citation(25).
Gene Selection and Promoter Methylation
To date, no definition for CIMP in ccRCC has been established. Therefore, we carefully selected CpG islands of interest in ccRCC as a measure for promoter methylation. Besides the VHL gene Citation(14), genes were selected exhibiting 1) promoter CpG island methylation (–2000 to +500 bp from transcription start site) in ccRCC cell lines (SKRC1, SKRC10, SKRC52, and SKRC59) and not in a normal kidney cell line (HK-2) in a genome-wide methylation screen (MBD-affinity massive parallel sequencing) Citation(26) and 2) cancer-specific downregulation among ccRCC in publicly available expression data from the Cancer Genome Atlas. Using this selection strategy, we selected 11 genes as candidate markers: cysteine dioxygenase type 1 (CDO1); follistatin (FST); frizzled class receptor 10 (FZD10); gremlin 1, DAN family BMP antagonist (GREM1); ladinin 1 (LAD1); neurofilament, heavy polypeptide (NEFH); neuralized E3 ubiquitin protein ligase 1 (NEURL); Ras-specific guanine nucleotide-releasing factor 2 (RASGFR2); stratifin (SFN); secreted frizzled-related protein 1 (SFRP1); and VHL.
Promoter methylation of the CpG islands was analyzed using nested methylation-specific polymerase chain reaction (MSP), as previously described elsewhere Citation(27–29). MSP primer design was based on the MBD-affinity massive parallel sequencing data. Primer sequences and MSP conditions are shown in supplemental Table S1. From 45 cases, MSP analysis was repeated in order to measure reproducibility. After excluding missing values due to genotyping failures, only one discrepancy in one gene was recorded (99.7% reproducibility).
A sum score representing promoter methylation was calculated combining promoter methylation in all 11 individual genes dividing the ccRCC cases into three categories that were approximately the same size: those with low (0–19% of the genes), intermediate (20–39%), and high (40% or more) methylated tumors.
Statistical Analysis
All analyses were conducted in Stata version 11 (Stata Corp., College Station, TX, USA). Associations between alcohol and folate intake and RCC risk were performed for total RCC and for ccRCC. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using Cox proportional hazards analyses adjusted for the case-cohort design Citation(30). The proportional hazards assumption was tested using the scaled Schoenfeld residuals and by introducing time-covariate interactions into the models. The proportional hazards assumption in the analyses using the time-on-study timescale for the variable age was not fulfilled. Therefore, analyses for all models were carried out using age as the timescale. Analyses were performed for a simple model (adjusted for sex and year of birth) and a multivariable adjusted model, including a priori selected potential confounders: year of birth (1916–1920, 1921–1925, and 1926–1931), sex (male/female), total energy intake (kcal/day), BMI (kg/m2), cigarette smoking (status, intensity, and duration), self-reported doctors' diagnosis of hypertension, and/or use of antihypertensive medication (yes/no). In a previous publication, we investigated the association between alcohol intake and RCC risk after 11.3 yr of follow-up Citation(14). Current analyses were split into <11.3 and ≥11.3 yr of follow-up, to compare the results with the previous publication Citation(14).
P-values for interaction were assessed by including cross-product terms in the models and performing a Wald test. Because of the limited number of cases in stratified interaction analyses, alcohol and folate intakes were each categorized into two categories (referred to as high vs. low intake); alcohol intake was categorized into <15 and ≥15 g/day intake and folate intake into higher and lower than median intake in the subcohort (median intake in males 212.07 µg/day and in females 188.71 µg/day).
Tests for heterogeneity were performed to evaluate differences across tumors with different promoter methylation profiles, using an adapted version of the competing risks procedure in Stata developed for the case-cohort design, as described previously Citation(31,32).
All tests were two sided. A P-value of <0.05 was considered statistically significant.
Results
After exclusion of cohort members with incomplete and/or inconsistent FFQs or with missing data on confounders, 3,980 subcohort members and 498 RCC cases were available for analysis (). For 297 ccRCC cases, promoter methylation could be genotyped of at least four genes.
Of the 297 ccRCC cases, MSP analyses of gene promoter regions were successful for 91% (RASGRF2) to 99% (NEURL). Proportions of promoter methylation per investigated gene are shown in . The proportion of promoter methylation was on average 36%, but varied from 8% (VHL) to 97% (SFN). In 112 cases, less than 20% of the 11 investigated genes were methylated (referred to as low methylation), in 81 cases between 20% and 40% of the genes were methylated (intermediate methylation), and in 104 cases more than 40% of the genes were methylated (high methylation).
Table 1. Descriptive data on methylation in clear-cell renal cell cancer (ccRCC) cases, Netherlands cohort study on diet and cancer, 1986–2006.
The proportion of nonusers of alcohol was slightly higher in the subcohort (24%) than in the total group of RCC cases (22%) (). Mean alcohol intake in users was similar in subcohort and RCC cases (15 g/day). The intake of folate was also slightly lower in the subcohort than in RCC cases (212 vs. 219 µg/day). RCC cases were more often males, had a slightly higher BMI, were more often smokers, and had more often a diagnosis of hypertension or diabetes at baseline than subcohort members.
Table 2. Baseline characteristics of subcohort members and renal cell cancer cases according to the methylation status of selected genes, Netherlands cohort study on diet and cancer, 1986–2006.
Multivariable adjusted HRs for categories of alcohol intake and RCC risk were all lower than 1, but not statistically significant (). The multivariable adjusted HR for subjects with 30+ g/day alcohol intake was 0.79 (95% CI: 0.54–1.16) compared to nonusers, and the P-value for trend was 0.27. When analyses were restricted to ccRCC, multivariable HRs were comparable.
Table 3. Multivariable hazard ratios for the association between alcohol and folate intakes and the risk of renal cell cancer, Netherlands Cohort Study on Diet and Cancer, 1986–2006
When ccRCC cases were stratified according to methylation status (), heterogeneity of associations between alcohol intake and ccRCC risk was not statistically significant (P-value for heterogeneity, 0.23). HRs for 30+ g/day alcohol intake compared to nonusers for ccRCC with low, intermediate, and high methylation were 1.29 (95% CI: 0.65–2.58; P-value for trend: 0.97), 0.46 (95% CI: 0.17–1.25; P-value for trend: 0.25), and 0.55 (95% CI: 0.24–1.27; P-value for trend: 0.76), respectively.
Table 4. Multivariable hazard ratios for the association between alcohol and folate intake and the risk of clear-cell renal cell cancer according to molecular subtypes, Netherlands Cohort Study on Diet and Cancer, 1986–2006
There was no heterogeneity with respect to the association between alcohol intake from beer, wine, and liquor, and the risk of ccRCC, overall or stratified according to methylation (supplemental Table S2).
Multivariable adjusted HRs of dietary folate intake and RCC risk were all close to 1 (). In ccRCC, the multivariable HR for subjects with the highest quintile of folate intake was 1.07 (95% CI: 0.70–1.64) compared to the lowest quintile (P-value for trend: 0.59). In addition, there was no indication for heterogeneity according to methylation status (; P-value for heterogeneity: 0.84). The HRs for the highest compared to the lowest quintile of dietary folate intake for ccRCC with low, intermediate, and high methylation were 1.09 (95% CI: 0.56–2.13; P-value for trend: 0.77), 1.15 (95% CI: 0.52–2.57; P-value for trend: 0.74), and 0.93 (95% CI: 0.45–1.93; P-value for trend, 0.72), respectively.
In interaction analyses on the combined effect of alcohol and folate intake (), we observed that the multivariable HR of ccRCC risk for high-alcohol/low-folate consumers did not increase compared to low-alcohol/high-folate consumers: HR 0.87 (95% CI: 0.56–1.35, P-value for interaction: 0.08). HRs for high-alcohol/low-folate consumers compared to low-alcohol/high-folate consumers for ccRCC risk stratified according to low, intermediate, and high methylation were 0.73 (; 95% CI: 0.36–1.46; P-value for interaction: 0.32), 0.89 (95% CI: 0.39–2.04; P-value for interaction: 0.47), and 1.09 (95% CI: 0.51–2.33; P-value for interaction: 0.16), respectively.
When the analyses of alcohol intake and RCC risk were split according to follow-up duration (<11.3 and ≥11.3 yr of follow-up), heterogeneity was observed (). During the first 11.3 yr of follow-up, HRs of alcohol consumers were inversely associated with risk of RCC overall and ccRCC, compared to nonusers of alcohol. Analyses, using data from 11.3 until 20.3 yr of follow-up, showed that alcohol consumption was positively associated with risk of RCC overall and ccRCC. In ccRCC, HRs for >15 g/day alcohol intake were 0.59 (95% CI: 0.39–0.91; P-value for trend: 0.02) and 1.91 (95% CI: 1.02–3.57; P-value for trend: 0.04) for the first 11.3 yr and remaining follow-up years, respectively. There was no heterogeneity in the association between dietary folate intake and RCC risk according to the period of follow-up (data not shown).
Table 5. Multivariable hazard ratios for the association between alcohol and folate intake and the risk of (clear-cell) renal cell cancer according to the duration of follow-up, Netherlands Cohort Study on Diet and Cancer, 1986–2006
Discussion
In this large prospective cohort study, alcohol intake was associated with a decreased RCC risk, although not statistically significant, and folate intake was not associated with RCC risk. There was no heterogeneity between alcohol or folate intake and ccRCC risk stratified according to categories of the methylation score.
Most prospective studies that investigated the association between alcohol intake and RCC risk have observed an inverse association with a clear dose-response effect Citation(3,4,14,33,34). Data from the NLCS were included in two previous publications with a shorter follow-up Citation(4,14). In the analysis of the Pooling Project of Prospective Studies on Diet and Cancer Citation(4), 7.3 yr of follow-up was used, and in another analysis Citation(14), 11.3 yr of follow-up was used. In the current analysis, with a longer follow-up, the inverse association was weaker. When the analysis was split according to the duration of follow-up, heterogeneity was observed. Alcohol consumption was associated with a decreased risk during the first 11.3 yr of follow-up and with an increased risk after 11.3 yr of follow-up. This heterogeneity may be explained by the fact that alcohol intake was measured only once at baseline and that lifestyle, including alcohol intake, of subjects may have changed during follow-up. Alternatively, the latency period of the inverse association between alcohol intake and the occurrence of RCC could be short (i.e., ∼<10 yr). It is also possible that the decreased HRs in the first period and the increased HRs in the second period of follow-up are due to chance.
Several studies have investigated the association between folate intake and RCC risk. A hospital-based case-control study, with a limited number of RCC cases, observed a statistically significant inverse association between folate intake and RCC risk Citation(35). Other studies, including two case-control studies Citation(36,37) and a previous publication from the NLCS with shorter follow-up Citation(38), did not observe an association between folate intake and RCC risk, although most point estimates were lower than one. In a nested case-control study in a cohort of Finnish smokers, serum levels of folate were not statistically significantly associated with RCC risk Citation(39). However, subjects with the lowest levels of serum folate were at an increased risk compared to subjects with higher serum folate levels Citation(39). In the hospital-based case-control study, in which questionnaires were used, subjects with the lowest dietary intake of folate were also at increased risk Citation(36). In a large Central and Eastern European hospital-based case-control study, polymorphisms in genes involved in folate metabolism [methylenetetrahydrofolate reductase (MTHFR) and solute carrier family 19 (folate transporter), member 1 (SLC19A1)] were associated with increased risk of RCC, especially in subjects with the lowest tertile of vegetable intake, an important source of folate Citation(40,41). The observations from these studies may be explained by a threshold effect in which only subjects with the lowest intake of folate have an increased risk. However, in the current study, no association (including no indication of a threshold effect) between dietary folate intake and RCC risk was observed.
Folate supplementation may have effects that are different from those in physiological ranges. In randomized trials, folate supplementation was not associated with a decreased risk of cancer Citation(42), but was associated with an increased risk of colorectal adenomas Citation(43). Supraphysiological levels of folate have been associated with hypermethylation in in vitro and in vivo studies Citation(44,45). However, we could not study whether folate supplementation was associated with promoter hypermethylation in ccRCC, as dietary supplements in the Netherlands in the late 1980s did not contain folate.
The aim of this study was to investigate whether alcohol and folate intake play a role of promoter methylation in genes frequently methylated in ccRCC. It has been suggested that there is a specific subtype of cancer that is characterized by extensive promoter methylation of tumor suppressor and DNA repair genes and referred to as CpG island methylator phenotype (CIMP) Citation(8). CIMP has been investigated extensively in colorectal cancer, but may also exist in other cancers, such as ccRCC Citation(8,9). Since no definition for CIMP in ccRCC has been established so far, we used a methylation index of CpG islands frequently methylated in ccRCC as a measure for general promoter methylation. Although we recognize that the Infinium 450K platform would be a more comprehensive way to analyze promoter methylation of CpG islands in ccRCC, our selection strategy of particular genes of interest is much more efficient in large epidemiological studies.
Different cancer phenotypes may be associated with different pathways of carcinogenesis, including etiology. However, the current study did not uncover such a pathway, because no heterogeneity was observed in associations between alcohol and folate intake and different molecular subtypes of ccRCC defined by the methylation index of selected genes inactivated by promoter methylation in ccRCC. There are several explanations for the absence of heterogeneity. First, the subjects of the NLCS reported moderate alcohol consumption, and less than 10% of the subcohort reported a consumption of more than 30 g of alcohol per day. It is conceivable that only a high level of alcohol intake is associated with promoter methylation and that the subgroup of high-alcohol consumers in the current study is too small to demonstrate this. Second, since there is no clear definition of CIMP in ccRCC or in other cancers, we developed a methylation index to define subgroups of ccRCC defined by promoter methylation. Our definition of “high methylation” included ccRCC cases with promoter methylation of more than 40% of the 11 selected genes. This subgroup constituted 35% of the ccRCC cases in our series. In the study of Arai et al., 14 out of 104 cases (13.5%) were characterized by high promoter methylation, using DNA methylation levels of 801 CpG islands Citation(9). More research is needed to confirm whether CIMP exists in ccRCC and what panel of markers can be used to classify CIMP accurately. Third, risk estimates may be attenuated due to random misclassification of dietary intakes, caused by a single baseline measurement and a long follow-up, even though intakes were rather stable during the first 5 yr of follow-up Citation(25). Furthermore, correlations between estimates of dietary folate intake using questionnaires and folate level measures in serum or erythrocytes have been reported to be low to moderate Citation(46). Finally, only dietary folate intake was investigated. Other nutrients, such as choline, vitamin B, and methionine, are also involved in folate metabolism Citation(13,47) and might influence adequate methylation status as well.
The current study also has several strengths. Loss to follow-up was very limited and cancer follow-up was very complete (>96%) Citation(17). Furthermore, we collected tumor samples from ∼80% of the cases. This, combined with the prospective study design, made selection and information bias unlikely. The study is among the largest prospective studies on RCC and, so far, the only prospective study including FFPE tumor tissues.
In conclusion, in this study we did not observe an association between alcohol, folate intake, and promoter CpG island hypermethylation in ccRCC using the methylation index as a tool to define methylation-specific subgroups ccRCC. Therefore, this study does not support the hypothesis that alcohol and folate intakes are involved in ccRCC carcinogenesis through promoter methylation of our methylation index.
Supplemental_table_2.docx
Download MS Word (17.8 KB)Supplemental Table 1
Download MS Word (16.5 KB)Acknowledgment
The authors are indebted to the subjects of this study and further wish to thank the Netherlands Cancer Registry, the Dutch Pathology Registry (PALGA), and all the pathology laboratories that provided tissue samples. They are grateful to Kim van Straeten and Kim Wouters, laboratory technicians, and Zheng Feng at the Department of Pathology, Maastricht University Medical Centre, for all their efforts in the laboratory. Finally, they would like to thank Dr. R. Alexandra Goldbohm for her contributions in the design of the NLCS; Dr. Christine Hulsbergen-van de Kaa, Kjeld van Houwelingen, and Dr. Boukje van Dijk for their work on the initial series of cases from the first 11.3 yr of follow-up; Dr. Arnold Kester for statistical advice; Sacha van de Crommert, Jolanda Nelissen, Jacqueline Spronck, Henny Brants, Conny de Zwart, Marijke Moll, and Annemie Pisters for their assistance with data entry and data management; and Harry van Montfort, Ton van Moergastel, Ellen Dutman, Ralph Meijer, and Ruud Schmeitz for programming assistance.
The results published here are in part based on data generated by the TCGA Research Network: http://cancergenome.nih.gov/.
Funding
The Netherlands Cancer Society (UM 2009–4536).
References
- Chow WH, Dong LM, Devesa SS: Epidemiology and risk factors for kidney cancer. Nat Rev Urol 7, 245–257, 2010. doi: nrurol.2010.46 [pii]/10.1038/nrurol.2010.46 [doi]
- Song DY, Song S, Song Y, and Lee JE: Alcohol intake and renal cell cancer risk: a meta-analysis. Br J Cancer 106, 1881–1890, 2012. doi: 10.1038/bjc.2012.136
- Bellocco R, Pasquali E, Rota M, Bagnardi V, Tramacere I, et al.: Alcohol drinking and risk of renal cell carcinoma: Results of a meta-analysis. Ann Oncol 23, 2235–2244, 2012. doi: 10.1093/annonc/mds022
- Lee JE, Hunter DJ, Spiegelman D, Adami HO, Albanes D, et al.: Alcohol intake and renal cell cancer in a pooled analysis of 12 prospective studies. J Natl Cancer Inst 99, 801–810, 2007.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans: Alcohol consumption and ethyl carbamate. IARC Monogr Eval Carcinog Risks Hum 96, 3–1383, 2010.
- Esteller M: Epigenetics in cancer. N Engl J Med 358, 1148–1159, 2008. doi: 10.1056/NEJMra072067
- Baldewijns MM, van Vlodrop IJ, Schouten LJ, Soetekouw PM, de Bruine AP, et al.: Genetics and epigenetics of renal cell cancer. Biochim Biophys Acta 1785, 133–155, 2008.
- Hughes LA, Melotte V, de Schrijver J, de Maat M, Smit VT, et al.: The CpG island methylator phenotype: what's in a name? Cancer Res 73, 5858–5868, 2013. doi: 10.1158/0008-5472.CAN-12-4306
- Arai E, Chiku S, Mori T, Gotoh M, Nakagawa T, et al.: Single-CpG-resolution methylome analysis identifies clinicopathologically aggressive CpG island methylator phenotype clear cell renal cell carcinomas. Carcinogenesis 33, 1487–1493, 2012. doi: 10.1093/carcin/bgs177
- Mason JB, and Choi SW: Effects of alcohol on folate metabolism: implications for carcinogenesis. Alcohol 35, 235–241, 2005.
- McKay JA, and Mathers JC: Diet induced epigenetic changes and their implications for health. Acta Physiol (Oxf) 202, 103–118, 2011. doi: 10.1111/j.1748-1716.2011.02278.x
- Supic G, Jagodic M, and Magic Z: Epigenetics: a new link between nutrition and cancer. Nutr Cancer 65, 781–792, 2013. doi: 10.1080/01635581.2013.805794
- Varela-Rey M, Woodhoo A, Martinez-Chantar ML, Mato JM, and Lu SC: Alcohol, DNA methylation, and cancer. Alcohol Res 35, 25–35, 2013.
- Schouten LJ, van Dijk BA, Oosterwijk E, van Engeland M, Hulsbergen-van de Kaa CA, et al.: Alcohol consumption and mutations or promoter hypermethylation of the von Hippel-Lindau gene in renal cell carcinoma. Cancer Epidemiol Biomarkers Prev 17, 3543–3550, 2008.
- Van den Brandt PA, Goldbohm RA, Van't Veer P, Volovics A, Hermus RJ, et al.: A large-scale prospective cohort study on diet and cancer in The Netherlands. J Clin Epidemiol 43, 285–295, 1990.
- Van den Brandt PA, Schouten LJ, Goldbohm RA, Dorant E, and Hunen PM: Development of a record linkage protocol for use in the Dutch Cancer Registry for Epidemiological Research. Int J Epidemiol 19, 553–558, 1990.
- Goldbohm RA, Van den Brandt PA, and Dorant E: Estimation of the coverage of Dutch municipalities by cancer registries and PALGA based on hospital discharge data. Tijdschr Soc Gezondheidsz 72, 80–84, 1994.
- van Houwelingen KP, van Dijk BA, Hulsbergen-van de Kaa CA, Schouten LJ, Gorissen HJ, et al.: Prevalence of von Hippel-Lindau gene mutations in sporadic renal cell carcinoma: Results from The Netherlands cohort study. BMC Cancer 5, 57, 2005.
- Eble J, Sauter G, Epstein J, and Sesterhenn I. World Health Organization Classification of Tumours. Pathology and Genetics. Tumours of the Urinary System and Male Genital Organs. Lyon: IARC Press, 2004.
- Goldbohm RA, Van den Brandt PA, Van't Veer P, Dorant E, Sturmans F, et al.: Prospective study on alcohol consumption and the risk of cancer of the colon and rectum in the Netherlands. Cancer Causes Control 5, 95–104, 1994.
- Nevo Tabel: Dutch Food Composition Table 1986–1987; Nederlands Voedingsstoffenbestand 1986–1987. The Hague, The Netherlands: Voorlichtingsbureau voor de Voeding, 1986.
- Konings EJ: A validated liquid chromatographic method for determining folates in vegetables, milk powder, liver, and flour. J AOAC Int 82, 119–127, 1999.
- Konings EJ, Roomans HH, Dorant E, Goldbohm RA, Saris WH, et al.: Folate intake of the Dutch population according to newly established liquid chromatography data for foods. Am J Clin Nutr 73, 765–776, 2001.
- Goldbohm RA, van den Brandt PA, Brants HA, van't Veer P, Al M, et al.: Validation of a dietary questionnaire used in a large-scale prospective cohort study on diet and cancer. Eur J Clin Nutr 48, 253–265, 1994.
- Goldbohm RA, van't Veer P, van den Brandt PA, van't Hof MA, Brants HA, et al.: Reproducibility of a food frequency questionnaire and stability of dietary habits determined from five annually repeated measurements. Eur J Clin Nutr 49, 420–429, 1995.
- van Vlodrop IJ, Baldewijns MM, Smits KM, Schouten LJ, van Neste L, et al.: Prognostic significance of Gremlin1 (GREM1) promoter CpG island hypermethylation in clear cell renal cell carcinoma. Am J Pathol 176, 575–584, 2010. doi: 10.2353/ajpath.2010.090442
- Derks S, Lentjes MH, Hellebrekers DM, de Bruine AP, Herman JG, et al.: Methylation-specific PCR unraveled. Cell Oncol 26, 291–299, 2004.
- Herman JG, Graff JR, Myohanen S, Nelkin BD, and Baylin SB: Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci U S A 93, 9821–9826, 1996.
- van Engeland M, Roemen GM, Brink M, Pachen MM, Weijenberg MP, et al.: K-ras mutations and RASSF1A promoter methylation in colorectal cancer. Oncogene 21, 3792–3795, 2002.
- Barlow WE: Robust variance estimation for the case-cohort design. Biometrics 50, 1064–1072, 1994.
- de Vogel S, Bongaerts BW, Wouters KA, Kester AD, Schouten LJ, et al.: Associations of dietary methyl donor intake with MLH1 promoter hypermethylation and related molecular phenotypes in sporadic colorectal cancer. Carcinogenesis 29, 1765–1773, 2008.
- Wacholder S, Gail MH, Pee D, Brookmeyer R: Alternative variance and efficiency calculations for the case-cohort design. Biometrika 76, 117–123, 1989.
- Lew JQ, Chow WH, Hollenbeck AR, Schatzkin A, and Park Y: Alcohol consumption and risk of renal cell cancer: the NIH-AARP diet and health study. Br J Cancer 104, 537–541, 2011. doi: 10.1038/sj.bjc.6606089
- Allen NE, Beral V, Casabonne D, Kan SW, Reeves GK, et al.: Moderate alcohol intake and cancer incidence in women. J Natl Cancer Inst 101, 296–305, 2009. doi: 10.1093/jnci/djn514
- Aune D, Deneo-Pellegrini H, Ronco AL, Boffetta P, Acosta G, et al.: Dietary folate intake and the risk of 11 types of cancer: a case-control study in Uruguay. Ann Oncol 22, 444–451, 2011. doi: 10.1093/annonc/mdq356
- Bosetti C, Scotti L, Maso LD, Talamini R, Montella M, et al.: Micronutrients and the risk of renal cell cancer: a case-control study from Italy. Int J Cancer 120, 892–896, 2007. doi: 10.1002/ijc.22374
- Brock KE, Ke L, Gridley G, Chiu BC, Ershow AG, et al.: Fruit, vegetables, fibre and micronutrients and risk of US renal cell carcinoma. Br J Nutr 108, 1077–1085, 2012. doi: 10.1017/S0007114511006489
- van Dijk BA, Schouten LJ, Oosterwijk E, Hulsbergen-van de Kaa CA, Kiemeney LA, et al.: Carotenoid and vitamin intake, von Hippel-Lindau gene mutations and sporadic renal cell carcinoma. Cancer Causes Control 19, 125–134, 2008.
- Gibson TM, Weinstein SJ, Mayne ST, Pfeiffer RM, Selhub J, et al.: A prospective study of one-carbon metabolism biomarkers and risk of renal cell carcinoma. Cancer Causes Control 21, 1061–1069, 2010. doi: 10.1007/s10552-010-9534-5
- Moore LE, Hung R, Karami S, Boffetta P, Berndt S, et al.: Folate metabolism genes, vegetable intake and renal cancer risk in central Europe. Int J Cancer 122, 1710–1715, 2008.
- Gibson TM, Brennan P, Han S, Karami S, Zaridze D, et al.: Comprehensive evaluation of one-carbon metabolism pathway gene variants and renal cell cancer risk. PLoS One 6, e26165, 2011. doi: 10.1371/journal.pone.0026165
- Qin X, Cui Y, Shen L, Sun N, Zhang Y, et al.: Folic acid supplementation and cancer risk: a meta-analysis of randomized controlled trials. Int J Cancer 133, 1033–1041, 2013. doi: 10.1002/ijc.28038
- Cole BF, Baron JA, Sandler RS, Haile RW, Ahnen DJ, et al.: Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA 297, 2351–2359, 2007. doi: 10.1001/jama.297.21.2351
- Wallace K, Grau MV, Levine AJ, Shen L, Hamdan R, et al.: Association between folate levels and CpG Island hypermethylation in normal colorectal mucosa. Cancer Prev Res (Phila) 3, 1552–1564, 2010. doi: 10.1158/1940-6207.CAPR-10-0047
- Charles MA, Johnson IT, and Belshaw NJ: Supra-physiological folic acid concentrations induce aberrant DNA methylation in normal human cells in vitro. Epigenetics 7, 689–694, 2012. doi: 10.4161/epi.20461
- van de Rest O, Durga J, Verhoef P, Melse-Boonstra A, and Brants HA: Validation of a food frequency questionnaire to assess folate intake of Dutch elderly people. Br J Nutr 98, 1014–1020, 2007. doi: 10.1017/S0007114507747827
- Anderson OS, Sant KE, and Dolinoy DC: Nutrition and epigenetics: an interplay of dietary methyl donors, one-carbon metabolism and DNA methylation. J Nutr Biochem 23, 853–859, 2012. doi: 10.1016/j.jnutbio.2012.03.003
