ABSTRACT
Regulatory authorities worldwide have found the nonnutritive sweetener, sucralose, to be noncarcinogenic, based on a range of studies. A review of these and other studies found through a comprehensive search of electronic databases, using appropriate key terms, was conducted and results of that review are reported here. An overview of the types of studies relied upon by regulatory agencies to assess carcinogenicity potential is also provided as context. Physiochemical and pharmacokinetic/toxicokinetic studies confirm stability under conditions of use and reveal no metabolites of carcinogenic potential. In vitro and in vivo assays reveal no confirmed genotoxic activity. Long-term carcinogenicity studies in animal models provide no evidence of carcinogenic potential for sucralose. In studies in healthy adults, sucralose was well-tolerated and without evidence of toxicity or other changes that might suggest a potential for carcinogenic effects. In summary, sucralose does not demonstrate carcinogenic activity even when exposure levels are several orders of magnitude greater than the range of anticipated daily ingestion levels.
Introduction
One question that is being repeatedly responded to by health and regulatory authorities is the question of whether nonnutritive sweeteners (NNSs) can cause cancer (Citation1–3). While a wide range of studies are published that describe the safety of sucralose, a comprehensive review of the studies that lead regulators to conclude that sucralose is noncarcinogenic can be useful to the scientific community, including health care professionals and others who have the responsibility to provide sound advice in response to questions related to health and nutrition. This article provides a systematic review, and summarizes the results, of these and other studies through a comprehensive search of electronic databases, using appropriate key terms, including “sucralose,” “carcinogenic,” “metabolism,” and “safety”; using defined selection criteria (i.e., abstracts and case reports not included in the literature retrieved from search); and through critical appraisal of results by experienced independent reviewers Citation(4). The data discussed are both published literature and those required by, and reported to, international regulatory agencies. As context, an overview of the types of studies relied upon by regulatory agencies to assess carcinogenicity potential is also provided.
In reviewing these data, it is important to understand the fundamentals used by food safety and health regulatory agencies in evaluating the safety of new food ingredients. Studies submitted to regulatory agencies undergo initial assessment for the quality and appropriateness of the study design and methodology(ies), taking into account the nature of the study being reported, e.g., whether the study is an in vitro or in vivo study, or a short-term or long-term dosing study, etc. Food safety and public health regulatory agencies evaluate the potential for carcinogenicity based on the quality and reliability of the studies conducted; known/predicted structure–activity relationships (SAR) and chemical characteristics of the substance under investigation; the fate of the substance in conditions of requested use; and outcomes from all types of studies designed to evaluate safety, including in vitro studies, whole animal model studies, and studies in humans. Not all the studies are weighted equally in safety assessments (Citation5–9). A hierarchy exists for evidence needed to allow for the approval of a new food ingredient. At the first level, in silico SARs and in vitro studies, notably genotoxicity and biochemical studies, are required. At the second level, tests for cancer and systemic toxicity in animals are required. Pharmacokinetic data are also recommended. At a third level, outcomes from human tolerance studies can provide additional important information. Human epidemiology studies are not a regulatory requirement in the initial assessment of the safety of sweeteners. However, any study already performed would be expected to be submitted and results would be considered in the safety assessment.
Regulatory guidelines provide the framework for the kinds of studies that should be considered in investigating the overall safety, including carcinogenic potential, of any new food ingredient (Citation9–13). It is important to emphasize that toxicological investigations performed as part of the requirements for the regulation of drugs, pesticides or food additives are not investigations performed with the primary intent of conducting basic research, in which the testing of a hypothesis is the primary objective. Rather, research studies conducted to investigate the safety of a new food ingredient are designed to enable detection of potentially adverse outcomes of the test substance so as to identify risk potential and to safeguard the target population. Research programs recommended by regulatory agencies are designed to maximize the possibility of detecting adverse effects. Study parameters are set to intentionally provoke a measurable response. Importantly, observations are made that cover not only the changes that might be found in the range of expected human exposures but exposures that are significantly (i.e., hundreds to thousands of times) greater. Such high doses are unlikely to be experienced by the target population and are also delivered with a frequency and duration that is seldom experienced in a normal diet (Citation8,Citation14–16).
These toxicologic exposures are expected to produce adverse outcomes at some point and the types of adverse outcomes elicited can allow for important insights into the biochemical nature of the food ingredient, its potential for health effects, and its appropriateness for the intended use. Indeed, regulatory guidances relating to the design of studies required for safety assessments make clear that, toxic effects (observable effect levels) at the maximum tolerated dose are necessary for certain tests to be considered satisfactory (Citation17,Citation18).
Regulatory safety testing protocols have been carefully evaluated and approved over many years of experience by international authorities Citation(19). The Good Laboratory Practices (GLP) methodologies are accepted as the norm for pharmaceutical, food development, and regulatory laboratories that have a responsibility for conducting studies designed to evaluate food ingredient safety (Citation20–23). Few laboratories outside of those routinely conducting food ingredient safety studies for the purpose of regulatory submissions may have the resources available to conduct studies in accordance with GLP requirements.
To maximize the ability to obtain reliable study outcomes, studies in rodents and other laboratory animal species are also typically performed utilizing strains that have been well-characterized and with abundant contemporary historical control data. Diets are carefully produced and constructed to minimize any potential effects that could confound data interpretation; the conditions and density of animal housing are specified and require a testing environment that supports good animal health; and data collection within the different types of core research studies to be conducted is rigorously standardized in terms of both extent and frequency (Citation24,Citation25). Modification of statistical methodology has enabled interpretation of data that may need to take into account a plethora of information (a Bayesian approach) (Citation26,Citation27).
This rigorous pattern of testing required in regulatory toxicology is not typically followed in hypothesis-driven work, where studies addressing similar problems may vary widely in their characteristics. In recognizing issues that can confound study findings, a recent editorial in Nature, reporting the outcome of a meeting of editors of 30 major scientific journals under the aegis of the American Association for the Advancement of Science, pointed out that the source, species, strain and characteristics (including inbred), sex, age, and husbandry for animals should all be reported to achieve reproducible results in animal studies. For cell lines used in in vitro studies, the source, authentication, and mycoplasma contamination status should all be known (Citation25,Citation28). Regulatory guidance for conducting research programs to evaluate the safety of a new food ingredient embodies these types of recommendations, as can be seen in the Organisation for Economic Co-operation and Development (OECD) research study protocols (internationally agreed-upon protocols for regulatory safety investigations published by the OECD) Citation(25).
In contrast to certain hypothesis-driven studies, where variability may be desirable in the hypothesis-testing context, protocols recommended for inclusion in holistic safety testing programs are intentionally designed to minimize variability and maximize the possibility of producing an unspecified result (e.g., tumor production), sometimes at the expense of realistic exposures. Variability in animal strains, conditions, husbandry, housing, etc. in such studies can confound data interpretation. As such, food safety and health regulatory agencies typically require study designs that minimize background “noise.” This is addressed by rigorous standardization. It is difficult to demonstrate a null effect with confidence unless the methods are highly standardized. It is also important to evaluate “noise” by taking into account the full information available (e.g., is the finding reproducible; do other studies support the finding; is the finding consistent with known chemical structure and/or reactivity of the substance being tested; etc.).
Notably, recent advances in techniques, in knowledge, and databases created from analyses of accumulated data are available to investigate causality and predisposing factors related to carcinogenesis. The quality of data can vary across studies and not all findings may be confirmed in independent repeat studies. Regulators worldwide must consider the strength of the data in order to promulgate use regulations, which are intended to limit the risk for health effects on the public Citation(8). Similarly, it is also critical to assess new research in the context of the whole of the data available, particularly when studies have small sample sizes and/or were conducted utilizing unconventional methodology. Results of observational studies, which do not, by their nature, assess cause and effect, must be especially interpreted in the context of all available data. It is critical that correlation data not be misinterpreted as evidence of cause and effect.
Methods of data acquisition
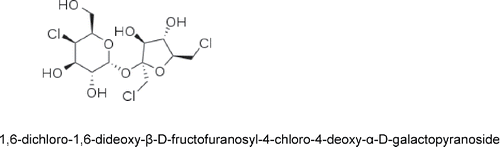
Literature searches were conducted in MEDLINE®, TOXFILE, BIOSIS Toxline®, FOODLINE®, CAB Abstracts, Food Science and Technology Abstracts, NTIS, and EMBASE using variations of the following key words, sucralose, Splenda, 6-Dichloro-1,6-dideoxy-β-D-fructofuranosyl-4-chloro-4-deoxy-α-D-galactopyranoside, CAS 56038-13-2, mutagen*, genotox*, genetic tox*, safety, metabolism, carcinogen*, cancer, tumour, tumor, etc. Additionally, studies submitted as part of the Sucralose Food Additive Petition, FAP 7A9387, are considered in this review.
Findings
Scientific evidence supporting regulatory decisions
International and country- or region-specific guidelines identify the types of evidence required to support the risk assessment of a substance intended for human consumption. The types of evidence required can be classified into three broad categories: 1) physicochemical characterization; 2) in vitro and short-term in vivo toxicological testing, including toxicokinetic studies [e.g., absorption, distribution, metabolism, and elimination or ADME studies]; and 3) subchronic to chronic biochemical profiling and tests for toxicity and carcinogenicity, using in vivo, in vitro, and ex vivo models; and specific additional studies, as needed, such as specialized testing (e.g., allergy, cognitive function, behavioral studies, and human tolerance studies) (). If the physicochemical and toxicological profiling of a substance does not identify carcinogenic risk, a need for additional profiling would not be anticipated. If carcinogenic potential is suggested or identified in these screening assays, health and food safety regulatory agencies may move to prohibit the use of the substance in the food supply or may require additional safety studies to evaluate dose–response and mode of action in the process of evaluating the safety of the studied ingredient (Citation6,Citation7,Citation9,Citation13,Citation25,Citation28,Citation29) ().
Figure 1. Scientific evidence required for assessing the carcinogenic potential of a new food ingredient.
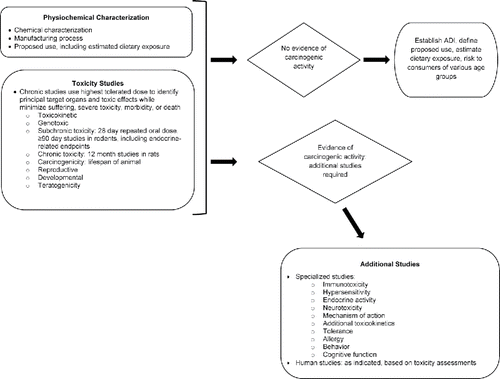
This tiered approach to the safety assessment of new food ingredients, used by regulatory agencies, limits the amount of data initially generated for a risk assessment, establishes the types of evidence to be considered, and potentially reduces the extent of animal research conducted by applying the 3-R approach: replace, refine, and reduce (Citation26,Citation27). The initial tier includes requirements for minimal testing and data, including determination of absorption by the gastrointestinal tract of the compound and the metabolic or manufacturing degradation products of a compound via in vitro, in vivo, and ex vivo models of absorption and bioavailability. If absorption by the gastrointestinal tract is identified, or if the compound exhibits toxicity, a second level of testing is required. Similarly, results of the second tier of evidence establish whether a third tier of testing is required. For example, if data generated from second-tier studies did not satisfy concerns of regulatory reviewers, additional second tier (e.g., additional animal studies) or third tier (e.g., studies in man) studies would be required.
Review of the data assembled for the safety assessment of a food additive by experienced pathologists is necessary for proper grouping of lesions for analysis of increased tumor incidence related to a compound. Several factors need to be considered, including the number of species or strains with an increased tumor incidence, number of positive studies, degree of tumor response, evidence of SAR, evidence of dose–response relationship, results of tests for genotoxicity, presence of preneoplastic lesions, and reduced latency for tumor development or increase in severity (malignancy) of the neoplasm Citation(16).
Furthermore, expert panels of toxicologists, pathologists, and statisticians review the body of evidence to enable regulatory agency review to estimate the acceptable daily intake (ADI) of a food additive. ADIs are set on the basis of the highest no-observed-effect level (NOEL) and expert judgment about the potential significance of the target effect, with a safety factor to allow for differences in human variability and interspecies differences Citation(14). The ADI represents the average intake over a lifetime of exposure that is expected to be safe Citation(15). ADIs are highly conservative, with safety factors of 100 or 1,000 often used Citation(9). Daily intake excursions that are sometimes higher than the ADI may have no associated safety concern, when this intake is considered in the scope of the average daily intake and given the safety factors employed at deriving an ADI. For new food ingredients, including sucralose, safety is finally considered by comparison of the estimated daily intake (EDI), to the ADI. When the EDI does not exceed the ADI (an acceptable/safe level), this provides an additional margin of safety. It is worth noting that the EDI for sucralose is very conservative. The EDI is calculated based on the assumption that sucralose would replace virtually all added sugar in all aspects of the diet. Thus, the EDI for sucralose is likely to significantly overestimate actual intakes experienced by consumers.
Scientific evidence assessing the carcinogenic potential of substances
Observations reported in scientific and clinical literature and reported to regulatory agencies are components of a knowledge database (e.g., SAR) that identifies chemical structures with likely genotoxic activity—and therefore possible carcinogenic potential. These “structural alerts” are available in several computerized databases and publications (Citation30,Citation31). Beyond structure, the manner in which molecules are metabolized under physiological conditions, in concert with their inherent reactivity, is central to an evaluation of possible chemical carcinogenicity ().
Chemical carcinogens can be broadly classified into two categories; those chemicals and their metabolites that react with DNA causing genetic damage or mutations and those which do not directly interact with DNA but which perturb other cellular processes that lead indirectly to DNA damage Citation(32). These two classes of carcinogens are referred to as genotoxic and nongenotoxic carcinogens, respectively (Citation33,Citation34). Studies that investigate the potential for a substance to cause genetic damage and/or mutations are therefore some of the earliest studies conducted in evaluating possible carcinogenic risk.
Nongenotoxic carcinogens are believed to produce cancer by binding to and altering cellular receptors responsible for effects such as gene expression, cellular metabolism, enhanced cell proliferation, inhibition of cell–cell communication, and DNA methylation.
As a result, bioassays are the types of studies given the greatest weight in considering the potential for a substance to be carcinogenic. Nevertheless, “positive” findings in bioassays, particularly shorter-term bioassays, may also require further investigation. Positive findings in bioassays can sometimes result from confounding factors, including issues with technical performance, animal housing and maintenance issues (e.g., problems with diet quality and cage conditions); unexpected viral infections (e.g., hepatitis virus); or concurrent infectious diseases (e.g., mycoplasma pneumonia) Citation(25). Species-specific responses can also occur that may have no relevance to humans (e.g., bladder cancer in long-term, extremely high-dose studies of saccharin in rats) Citation(35). As such, positive findings must be critically considered in the context of the whole of the experimental evidence.
Beyond risk assessments from chemical characterization and mutagenicity/genotoxicity studies, toxicity studies (bioassays), which assess the short- and long-term effect of exposure to a substance, can help to characterize carcinogenic risk. Subchronic toxicity studies (e.g., 30- to 90-day studies) are conducted to estimate the appropriate doses to be used in chronic toxicity studies, to determine NOELs for some toxicology endpoints, and to support the appropriate design of long-term toxicity studies (i.e., identify target organs). In general, subchronic toxicity studies do not determine the carcinogenic activity of a substance; however, subchronic studies can provide important supportive data Citation(36). Advances in research have found that carcinogenic substances begin to exert observable effects earlier than had been supposed (i.e., within ≤90 days) (Citation37,Citation38). As a result, histopathologic and biochemical evaluations from subchronic studies should be considered when evaluating the carcinogenic potential of a compound categorized as preneoplastic. Preneoplastic changes are not always, however, a harbinger for the development of cancer. They can instead be spontaneous changes or precursor changes to the development of benign tumors (Citation37–39).
Both chronic toxicity and carcinogenicity studies are designed to help characterize the dose–response, define toxicity and/or safety thresholds, provide data on health effects at human exposure levels in appropriate animal models, and provide data to assess mechanism of action Citation(40). Chronic toxicity and carcinogenicity research is frequently carried out in tandem, to maximize the ability to interpret findings, by conducting research under similar circumstances and in same-sourced animals. Studies classically defined as carcinogenicity studies (whether performed in tandem with toxicity studies or not) are still considered the gold standard for assessing potential carcinogenicity, i.e., they are the key studies relied upon by regulatory agencies around the world for evaluating carcinogenicity potential.
Other studies can also provide important information when assessing carcinogenic potential. Toxicokinetic studies define the relationship between systemic exposure to a substance, dose, and toxicity. Reproductive studies identify the effect a substance has on the fertility and gestation of the test animal. Developmental studies characterize the physical, cognitive, and behavioral effects a substance has on the test animal. Unusual findings in such studies can be further evaluated and add to the total comprehension of the substance's safety, including carcinogenic risk profile. It is important to note that adverse effects can often be exhibited in bioassays when doses are taken to extreme levels. Using very high dose levels is a planned design element in many studies conducted in a program to assess the overall safety of a new food ingredient. Assessment of carcinogenicity potential hinges on careful review of available SAR/chemical characteristics data, mutagenicity data, measures of health and development from all types of bioassays, comprehension of the substance's metabolic fate and toxicokinetics, and the appropriate evaluation of preneoplastic and neoplastic findings.
Scientific evidence assessing carcinogenic potential of sucralose
Chemical structure and stability of sucralose
Sucralose (1,6-dichloro-1,6-dideoxy-β-D-fructofuranosyl-4-chloro-4-deoxy-α-D-galactopyranoside) is a substituted disaccharide Citation(41). It is synthesized by selective chlorination of sucrose (common table sugar) at three of the primary hydroxyl groups, involving inversion of configuration at carbon-4, from the gluco- to the galacto-analogue. Based on its chemical structure, the reactivity of sucralose would be expected to be similar to that of polyhydric alcohols, such as sucrose, with modification by the pyranose and furanose ring systems (). The hydroxyl groups of sucralose are more acidic (i.e., have a lower pKa) than that of water. As such, any reaction of the multiple hydroxyl groups on the sucralose molecule could only occur in the absence of water and in the presence of highly reactive substances, and then only under strongly alkaline conditions—conditions that are not consistent with normal human physiologic conditions. The expected reactivity of sucralose with use in foods or in physiologic systems, based on chemical structure, has been borne out in early chemical research studies (Citation42,Citation48). Under conditions representative of normal human physiological conditions, sucralose had no opportunity to produce chemical interactions Citation(42). The results are consistent with the fact that the chlorine moieties in sucralose are not in the form of alkyl halides, being bonded to the stable ring structures, and their presence creates steric hindrance on the rings, also limiting the interactions. Hence, there is very limited opportunity for biotransformation. Chemistry studies also confirm that the sucralose molecule is not electrophilic, nor does it contain molecular structures capable of being bioactivated to electrophilic reactive metabolites. In all, sucralose contains no identified “structural alerts” (Citation30,Citation43) for either genotoxic or carcinogenic activity. Further, sucralose is highly water soluble and has a comparatively low fat solubility (very low octanol–water partition coefficient: logKow = −0.51 ± 0.05), so that accumulation in fat stores would not be predicted and indeed have not been found, based on studies using radiolabeled sucralose Citation(44).
From a chemical structure perspective, the sucralose molecule is expected to be exceptionally stable. No evidence of hydrolysis of the sucralose molecule has been found in vivo (Citation44–48). This is partly explained by the fact that the glycosidic linkage of sucralose is significantly more resistant to hydrolysis than is the glycosidic linkage of sucrose Citation(49)—the parent disaccharide used in sucralose synthesis. This increased resistance to hydrolysis (or enzymatic cleavage) is related to the increased steric hindrance created by the replacement of the three sucrose hydroxyl groups with three atoms of chlorine. In an acidic solution, sucralose can slowly hydrolyze to its two constituent substituted monosaccharides Citation(44); these hydrolysis products are resistant to further degradation Citation(50). After storage for 1 yr, detectable sucralose loss in model (1% sucralose) aqueous systems is less than 1% at 25°C Citation(44). No loss of sucralose is detected at pH 4 or 6 after 1 yr. While data suggest the possibility of hydrolysis product formation, exposures are not definitive, and the estimated possible exposure is in the ng/kg quantities (Citation51,Citation52). Nonetheless, the safety of the sucralose hydrolysis products was fully investigated prior to health and safety rulings permitting the use of sucralose. FDA and other health regulatory agencies have found the sucralose hydrolysis products to represent no carcinogenic or toxicologic risk (Citation53–55). Several studies under conditions involving extremes of pH and/or temperature (as might occur in baking) and ingredients found in various food matrices demonstrated the chemical stability and lack of reactivity of sucralose (Citation56–58). Certain studies have shown the production of different breakdown products under highly artifactual conditions or conditions irrelevant to food usage (Citation58–61). These studies are not investigations of the fate of sucralose in actual usages for production of foods/beverages and some also introduce variables into the testing that would alter normal reaction kinetics for the tested ingredients. For example, pyrolysis studies using a 1:2 molar ratio mixture of sucralose and glycerol can yield reaction products, which are not found in studies designed and recommended for the assessment of food ingredient stability Citation(62). Overall, the findings suggest no potential for reaction of sucralose with biological molecules under physiological conditions to result in either adduct products or dechlorination.
Bioassays assessing genotoxic potential of sucralose
Results in a variety of in vitro (e.g., Ames, and DNA damage/clastogenicity) and in vivo (e.g., mouse lymphoma, rodent micronucleus, and human lymphocyte) assays demonstrate that sucralose does not cause gene mutations or chromosomal damage (Citation63–80) ().
Table 1. Sucralose and derivatives genotoxicity assays.
Expert reviewers at regulatory agencies evaluated these data and concurred that there is no concern for genotoxic or mutagenic activity related to sucralose Citation(50).
A single mouse lymphoma study, conducted very early in the sucralose safety testing program, gave an equivocal result with sucralose concentration of 10 mg/ml. This is a very high concentration, which can result in conditions (e.g., osmolality changes) that can affect the assay and lead to effects on DNA. An updated analysis of the study (i.e., per updated international criteria) also determined that the equivocal result would be viewed as a negative result, for other technical reasons, under updated criteria Citation(63). Two Comet assays reported a positive finding with sucralose. In one Citation(74), review of these data identified a high incidence of false positive findings in the study and lack of corroborating studies with positive results Citation(63). In the other Citation(73), positive findings were reported for a range of popular NNSs based on assays using two human colon cancer cell lines (Caco-2 and HT-29) and one human embryonic kidney cell line (HEK-293). The cells were exposed to extremely high concentrations of sweetener solutions (up to 50 mM or approximately 20 g/l sucralose) for up 72 h. The cell lines used are not representative of normal human tissue. Together, the relevance of the results is questionable.
Absorption, distribution, metabolism, and excretion of sucralose
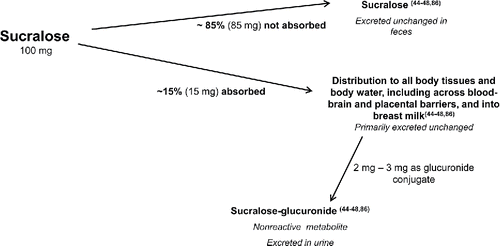
Studies show that sucralose is not catabolized, and therefore is not a source of energy, in either humans or animals. Following oral intakes, it is not dechlorinated or degraded into smaller chlorinated compounds (Citation44–48). Following ingestion, most sucralose (approximately 85%) is not absorbed and so is eliminated unchanged in the feces (Citation44–48). A small percent of an oral intake undergoes common phase II metabolism, specifically glucuronidation, about 2–3% in humans Citation(46). Glutathione conjugation is a phase II detoxification reaction (Citation81–84) that does not normally generate reactive intermediates, and instead neutralizes electrophiles Citation(82). There are no unique types of, or greater exposure to, glucuronide conjugates formed in man, compared to surrogate species. Both unchanged sucralose and its glucuronide conjugates are excreted in urine, and are readily eliminated with no bioaccumulation (Citation44–48,Citation85,Citation86).
In humans, peak sucralose plasma concentrations of 262 ng equivalents/ml were achieved between 1.5 and 3.0 h after a single oral dose of 1 mg/kg body weight Citation(46). The half-life of sucralose is approximately 13 h Citation(86).
The metabolic profile and pharmacokinetics are similar among rodents, dogs, and man (Citation44–48,Citation86). No adaptive change in sucralose metabolism was seen in rats after 18 mo of daily sucralose intakes in amounts thousands of times greater than estimated average daily intakes Citation(47).
Consistent with this, there is no evidence of sucralose being a substrate for gut microflora, with only intact sucralose found in feces. As such, exposure to gut microflora is not a pathway for exposure to metabolites that could be of concern Citation(85).
Studies of radiolabeled compound confirm that absorbed sucralose is distributed to essentially all tissues, indicating incorporation into total body water, consistent with its highly water-soluble nature. While sucralose moves readily with body water, there is no active transport into milk, transplacentally, or across the blood–brain barrier into the central nervous system, consequently any distribution to those sites is limited (Citation44,Citation47) (). Due to its high sweetness potency (about 600 times sweeter than sucrose on a weight-for-weight basis), total intakes are also remarkably low (on average, <3 mg/kg/day for an adult), and, combined, with a low level of absorption, any sucralose available to the systemic circulation is exceedingly low, reflected by the very low peak plasma levels following an oral dose.
Figure 3. Absorption, disposition, metabolism, and excretion of sucralose (Citation44–48,Citation86).
Separately, toxicokinetic and metabolic data established that rats, mice, and dogs are the most appropriate surrogate species for humans for toxicity ass-essments.
Toxicity studies with sucralose
In short-term and long-term subchronic toxicity studies in rats and dogs, sucralose was included in the diet continuously at up to 5.0% (50,000 ppm, approximately equivalent to 5,000 mg/kg body weight/day) concentrations. In the 4- and 8-wk dietary studies and in the 26-wk gavage study in rats, no histopathological changes were observed that could indicate any concern for carcinogenic activity of sucralose Citation(87). The only histological findings noted were decreased splenic mass and reduction in the lymphocyte mass of the thymus in the high-dose females in the 8-wk study. These data were ascribed to insufficient caloric intake—a common finding in high-dose dietary studies of high-potency sweeteners (Citation44,Citation88), which affect both the overall dietary caloric value and palatability and slight premature involution of the thymus, respectively, and were not replicated in a longer-term 26-wk gavage study Citation(87). To investigate these histologic findings, special studies were designed to specifically investigate the effect of oral gavage of sucralose to rats at doses of 4000, 3000, and 2000 mg/kg body weight/day for periods of, respectively, approximately 4, 8, and 13 wk. Thymic weight effects or pathological findings were not observed Citation(89) (Table S1).
Premature thymic involution (thymic involution occurring developmentally early) is a known potential outcome when caloric intake is restricted sufficiently (Citation90–92). In the 8-wk sucralose dietary study, the very high concentration of sucralose in the diet (5%, ∼5,000 mg/kg body weight/day) decreased the overall nutrient density. In addition, diet palatability was suboptimal (based on results of separate exploratory palatability studies) and lower food consumption was observed. The absence of the finding of premature thymic involution when sucralose was administered by gavage supports the interpretation that the dietary study findings were secondary effects resulting from reduced food intake and not directly related to the systemic presence of sucralose. In the 26-wk oral gavage study, sucralose intakes were up to 3,000 mg/kg body weight/day—comparable to the achieved average daily intake in the 8-wk dietary study (1–5% of diet, approximately equivalent to 1000–5000 mg/kg body weight/day). In this study and dietary and gavage studies with lower daily sucralose exposures, no effects on spleen or thymus weight or pathology were observed (Citation87,Citation89).
As discussed above, dietary and gavage studies expose animals to high concentrations and total daily amounts of the agent being tested in an effort to elicit a response. Overall, the minimal observations reported in sucralose dietary and gavage studies are significant, since substances that show carcinogenic activity in standard 104-wk studies usually display some evidence of tissue perturbation in subchronic toxicity testing. In contrast, the sucralose studies revealed no evidence of changes that might be considered preneoplastic.
In the 4- and 8-wk sucralose dietary studies, but not the oral gavage studies, rats in the highest diet group (5%, ∼5,000 mg/kg body weight/day) gained statistically significantly less weight and consumed less food than control animals likely owing to the decreased palatability of the food Citation(87).
Chronic toxicity studies
In a two-phase chronic toxicity–carcinogenicity study in rats Citation(93), sucralose was provided in the diet at 0, 3000, 10000, and 30000 ppm, equivalent to an average lifetime daily sucralose intake of 0, 150, 1000, and 1500 mg/kg body weight, respectively. In the chronic toxicity phase, rats received the diet for 52 wk. In the carcinogenicity phase, rats received the diet for 104 wk, considered a lifetime exposure. In both phases of the study, sucralose had no effect on the survival, clinical symptoms, or behavior of the rats, or in the carcinogenic potential of sucralose. Non-neoplastic findings consistent with aging of the rats were observed and were not increased in incidence or severity with sucralose dosing. There was no evidence of any neoplasia or type, distribution, or multiplicity of tumors associated with the sucralose treatment. Rats receiving sucralose in the diet did have a lower body weight gain and lower food consumption than control animals—consistent with palatability effects and with 4- and 8-wk dietary studies ().
Table 2. Abbreviated animal model carcinogenicity studies—sucralose and sucralose hydrolysis products.
In a 104-wk carcinogenicity study in mice Citation(94), sucralose was provided in the diet at 0, 3000, 10000, and 30000 ppm, equivalent to an average lifetime daily sucralose intake of 0, 450, 1500, and 4500 mg/kg body weight. Sucralose had no effect on survival, clinical condition, or behavior of the mice nor were there any gross physical abnormalities. Likewise, there was no evidence of benign or malignant neoplasms, or increases in total number of tumor bearing mice associated with sucralose dosing. Like rats in the 104-wk study, mice had non-neoplastic findings consistent with aging mice, but mostly similar in incidence and severity as controls. Female mice receiving the highest dose had peripheral blood erythrocyte counts 7–9% lower than controls. This change was not observed in males and considered unlikely to be a direct effect of sucralose Citation(94) ().
In the 52-wk chronic toxicity study in dogs, there were no sucralose-related histological changes despite the use of high (up to 3%) dietary concentrations or intakes up to approximately 900/mg/kg body weight/day. The lack of histological changes after 52 wk of high-dose administration is not consistent with substances that are carcinogenic Citation(95).
The sum of these chronic toxicity studies, even in the absence of carcinogenicity studies on sucralose, provides a strong basis from which to conclude, a priori, that sucralose is without carcinogenic potential.
In summary, regulatory compliant investigations in animal models revealed no evidence suggesting that even extremely high doses of sucralose will produce carcinogenicity. There were also no signs of toxicity that might support a concern for potential carcinogenic or other safety risk.
Other studies
Studies in rats and rabbits showed an absence of effect on fetal growth or development at doses up to 2,000 mg/kg body weight/day and 1,000 mg/kg body weight/day, respectively. In the rabbit study, maternal gastrointestinal effects were observed; however, these were considered to be a recognized species-specific response to poorly absorbed substances Citation(50). Sucralose was found to be nonteratogenic—with no effect on skeletal formation or organogenesis.
In a randomized, double-blind, placebo-controlled, 13-wk study, healthy human adults ingested increasing amounts of sucralose: 125 mg daily for 3 wk, followed by 250 mg daily for 4 wk and then 500 mg daily for 5 wk Citation(86). Tolerance was assessed through hematology, urinalysis, and biochemical analyses and ECGs, conducted prior to and at the end of the study, including a detailed ophthalmological examination in a subset of the subjects. Three subjects withdrew from the study for personal reasons, but not due to adverse reactions. Hematological, biochemical, and urinanalysis values remained stable; ECG readings were not affected; and ophthalmoscopic tests identified no abnormalities attributable to sucralose. None of the biochemical data indicated an issue of concern relative to carcinogenicity.
Human epidemiology studies are not typically conducted in the absence of data suggesting a possible effect of a substance on human health. There have been, however, two human epidemiology studies on cancer incidence in persons exposed to NNS since the first regulatory approval permitting sucralose use in foods (1991) (). While these studies were focused on aspartame, the study findings are related to NNSs, and, thus, could be considered, without due review, applicable to sucralose. Review of both of these studies, however, reveals that subjects in the studies would have had little to no exposure to sucralose, due to the time frame during which the studies were conducted. Notably, these studies included evaluation for the loosely defined category of non-Hodgkins lymphoma (NHL) rather than for a specific, molecular/cytogenetic characterized form of lymphoma, which limits their interpretation with regard to carcinogenic risk determination.
Table 3. Epidemiology studies evaluating the effect of non-nutritive sweeteners on risk of cancers.
In the Cancer Prevention Study II Nutrition Cohort study, a prospective study of the incidence of cancer and mortality in the US begun in 1999, subject recall was used to monitor the daily consumption of sugar-sweetened and artificially sweetened beverages Citation(96). Subjects provided follow-up information every 2 yr over the course of 10 yr. Neither sugar-sweetened nor artificially sweetened carbonated beverages were associated with an increased risk of NHL, or subtypes of NHL during the 10-yr follow-up.
In a prospective study analyzing initial and follow-up data collected in the Nurses' Health Study and Health Professionals Follow-up Study, subject recall was used to monitor daily consumption of sodas Citation(97). Subjects provided follow-up information every 2 yr over the course of 22 yr. In this cohort, a higher risk of NHL and multiple myeloma was observed in men who consumed sugar-sweetened sodas; no association was observed in women for NHL.
These studies, although not designed to test for a potential relationship of sucralose, specifically, to cancer, do not add any evidence that sucralose might be carcinogenic.
An older study of sucralose conducted by the Ramazzini Institute (RI) was published after this review was completed. It reported an increased incidence of certain tumors, notably hematopoietic tumors, in male Swiss mice Citation(98). The design of the study was similar to other carcinogenicity studies conducted by the RI (Citation99,Citation100) and has previously been considered to be the one that can lead to erroneous conclusions Citation(101). Historically, RI studies have posed other difficulties: the accuracy of histopathologic diagnosis of hematopoietic neoplasms and the uncertain relevance to humans of such findings in mice (Citation101,Citation102). The appropriateness of the statistical analyses used by RI has also been questioned (Citation101,Citation103).
Conclusions
International regulatory authorities consider physicochemical characteristics; toxicological and biochemical profiling, and carcinogenicity testing results; and specific additional testing (e.g., allergy, cognitive functioning) as needed in their risk assessment of a substance intended for human consumption and their decision to approve or ban the substance. This type of rigorous risk assessment resulted in sucralose being permitted for use by the US FDA and numerous other prominent regulatory authorities/agencies from other countries—including the Joint Food and Agricultural Organization/World Health Organization (FAO/WHO) Expert Committee on Food Additives, Health Canada, the Japanese Ministry of Health and Welfare, the Scientific Committee on Food, the European Union, and the Food Standards of Australia and New Zealand (Citation53–55,Citation104–108).
The chemical structure of sucralose predicts a low order of reactivity, no biotransformation potential, and no identified structural alerts for genotoxic or carcinogenic activity. Stability testing shows sucralose is remarkably resistant to both chemical and enzymatic degradation.
The results of in vitro and in vivo assays of sucralose revealed no confirmed genotoxic activity, consistent with the chemical structure and metabolism of sucralose.
Following ingestion, sucralose is not metabolized in the gut and approximately 85% of sucralose is excreted intact. The small amount absorbed is not metabolized to reactive intermediates and neither the parent molecule nor metabolites react with biological macromolecules (e.g., DNA). The small percentage of sucralose that undergoes metabolism is not catabolized (broken down), but is biotransformed to glucuronide conjugates that are toxicologically and biologically insignificant.
Sucralose was tested at doses up to 50,000 ppm, the maximum dose the FDA recommends for any compound due to the potential for nutrition impact at higher exposure levels. The FDA established an NOEL of 1.0% sucralose in the diet, equivalent to about 500 mg/kg body weight/day and the Joint FAO/WHO Expert Committee on Food Additives established an “Acceptable Daily Intake” (ADI) of 0–15 mg/kg body weight/day.
In some animal toxicity studies, certain effects on the thymus and spleen were observed at high doses. However, these findings appear to be an indirect effect related to diet palatability and/or potential nutritional impact. It is also important to keep in mind that, in these studies, sucralose daily intakes were equivalent in sweetness to 74–495 pounds of sugar per day for an average weight (e.g., 75 kg) adult—and hence clearly not representative of any anticipated human intake. Subsequent studies specifically assessed immune system cells, tissues, and function and supported an NOEL of 3,000 mg/kg body weight/day. Likewise, teratogenic and developmental studies in animals showed no effects related to sucralose. Randomized, double-blind clinical trials conducted over a period of approximately 3 mo in which sucralose was consumed daily in amounts greater than the maximum EDI showed that sucralose was well-tolerated and without evidence of toxicity or other changes that might suggest a potential for carcinogenic effects (Citation86,Citation109).
In summary, review of the evidence retrieved, including key studies recommended by international regulatory bodies and toxicology experts, confirm that sucralose is noncarcinogenic and safe to ingest. Sucralose does not demonstrate carcinogenic activity even when exposure levels are several orders of magnitude greater than the range of anticipated daily ingestion levels.
Declaration of interest
All authors contributed to the preparation and interpretation of the data and the writing, review, and approval of the manuscript. All authors were employees of, or consultants to, McNeil Nutritionals at the time the manuscript was prepared.
Suppl._Table.docx
Download MS Word (12.7 KB)Acknowledgments
The authors acknowledge Intertek Scientific and Regulatory Consultancy (Mississauga, Ontario, Canada), who conducted the literature review and prepared a summary report. They also acknowledge Kathleen Boyle, PhD, from KE Boyle Consultants, LLC (Exton, PA) who, under the direction of the authors, supported manuscript preparation from the summary report and managed the review process.
Funding
Funding for both the independent literature review (prepared by Intertek Scientific & Regulatory Consultancy, Missisauga, Ontario, Canada), which formed the basis for the current article, and the preparation of the manuscript, was provided by McNeil Nutritionals, Fort Washington, PA.
References
- National Institutes of Health; National Cancer Institute: Artificial Sweeteners and Cancer, 2009. Available at http://www.cancer.gov/about-cancer/causes-prevention/risk/diet/artificial-sweeteners-fact-sheet
- Mayo Clinic: Artificial Sweeteners and Other Sugar Substitutes, 2015. Available at http://www.mayoclinic.org/healthy-lifestyle/nutrition-and-healthy-eating/in-depth/artificial-sweeteners/art-20046936
- Medicine Net: Artificial Sweeteners, 2005. Available at http://www.medicinenet.com/script/main/art.asp?articlekey=47816
- de Vries RBM, Wever KE, Avey MT, Stephens ML, Sena ES, et al.: The usefulness of systematic reviews of animal experiments for the design of preclinical and clinical studies. ILAR J 55(3), 427–437, 2014.
- Williams GM, Iatropoulos MJ, and Enzmann HG: Principles of Testing for Carcinogenic Activity. Principles and Methods of Toxicology. 5th ed. Boca Raton, FL: CRC Press, Taylor & Francis Group, 2007.
- Environment Directorate: Joint meeting of the chemicals committee and the working party on chemicals, pesticides and biotechnology. Guidance Document on the Validation of (Quantitative)Structure-Activity Relationships [(Q)SAR] Models. OECD Environment Health Safey Publications Series on Testing and Assessment. No. 69. Organisation for Economic Co-operation and Development, Paris, France, 2007.
- EFSA Panel on Food Additives and Nutrient Sources Added to Food: Guidance for submission for food additive evaluations. EFSA J 10, 2760–2819, 2012.
- Berry CL: Relativism, regulation and the dangers of indifferent science. The Sir Roy Cameron lecture of the Royal College of Pathologists. Toxicology 267, 7–13, 2010.
- US Department of Health and Human Services; Food and Drug Administration; Office of Food Additive Safety; Center for Food Safety and Applied Nutrition: Chapter II. Agency review of toxicology information in petitions for direct food additives and color additives used in food. Redbook 2000. (Updated July 2007) Guidance for Industry and Other Stakeholders. Toxicological Principles for the Safety Assessment of Food Ingredients. Silver Spring, MD: US Department of Health and Human Services. Food and Drug Administration, 2000, pp. 9–18.
- Organisation for Economic Co-operation and Development: OECD Quantitative Structure-Activity Relationships Project [(Q)SARs]. Organisation for Economic Co-operation and Development. Paris, France, 2015. Available at http://www.oecd.org/chemicalsafety/risk-assessment/oecdquantitativestructure-activityrelationshipsprojectqsars.htm.
- Organisation for Economic Co-operation and Development: OECD Chemical Safety and Biosafety. OECD Guidelines for the Testing of Chemicals and Related Documents. Organisation for Economic Co-operation and Development. Paris, France, 2015. Available at http://www.oecd.org/chemicalsafety/testing/oecdguidelinesforthetestingofchemicalsandrelateddocuments.htm.
- Joint Food and Agriculture Organization of the United Nations and the World Health Organization Expert Committee on Food Additives: Seventy-Ninth Meeting. June 2014. JECFA/79/SC. World Health Organization, Geneva, Switzerland, 2014. Available at www.fao.org/fileadmin/templattes/agns/pdf/jecfa/JECFA_79_Summary_Version_Final.pdf.
- Joint Food and Agriculture Organization of the United Nations and the World Health Organization: EHC 240: Food Safety. Project to Update the Principles and Methods for the Assessment of Chemicals in Food. Principles and Methods for the Risk Assessment of Chemicals in Food. World Health Organization, Monograph EHC 240. Geneva, Switzerland: WHO Press, 2009.
- Dorne JLCM and AG Renwick: The refinement of uncertainty/safety factors in risk assessment by the incorporation of data on toxicokinetic variability in humans. Toxicol Sci 86 (1), 20–26, 2005.
- Herrman JL and Younes M: Background to the ADI/TDI/PTWI. Regul Toxicol Pharmacol 30(2 Pt 2), S109–S113, 1999.
- US Department of Health and Human Services; Food and Drug Administration; Office of Food Additive Safety; Center for Food Safety and Applied Nutrition: Chapter IV.C.4.a. Subchronic toxicity studies with rodents. Toxicological Principles for the Safety Assessment of Food Ingredients. Redbook 2000 (November 2003), Silver Spring, MD: US Department of Health and Human Services. Food and Drug Administration, 2003. Available at http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/IngredientsAdditivesGRASPackaging/ucm078345.htm.
- Redbook 2000: Chapter IV.B.1. General guidelines for designing and conducting toxicity studies. Toxicological Principles for the Safety Assessment of Food Ingredients. U.S. Department of Health and Human Services, Food and Drug Administration, Silver Spring, MD. Available at http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/IngredientsAdditivesGRASPackaging/ucm078315.htm
- OECD: Test No. 421: Reproduction/Developmental Toxicity Screening Test, OECD Guidelines for the Testing of Chemicals, Section 4, Paris: OECD Publishing, 2015. DOI: http://dx.doi.org/10.1787/9789264242692-en. Available at http://www.oecd-ilibrary.org/docserver/download/9715241e.pdf?expires=1453499610&id=id&accname=guest&checksum=F7F8F5FDBD5E9F59C0C74FEEC314714D
- Edler L, Hart A, Greaves P, Carthew P, Coulet M, et al.: Selection of appropriate tumour data sets for Benchmark Dose Modeling (BMD) and derivation of a Margin of Exposure (MoE) for substances that are genotoxic and carcinogenic considerations of biological relevance of tumour type, data quality and uncertainty. Food Chem Toxicol 70, 264–89, 2014.
- US Department of Health and Human Services; Food and Drug Administration; Office of Regulatory Affairs: Guidance for Industry. Good Laboratory Practices Questions and Answers. Rockville, MD: US Department of Health and Human Services. Food and Drug Administration, 2007. Available at www.fda.gov.downloads/ICECI/EnforcementActions/BioresearchMonitoring/UCM133748.pdf.
- US Department of Health and Human Services; Food and Drug Administration: Code of Federal Regulations Title 21 Part 58. Good Laboratory Practice for Nonclinical Laboratory Studies. Rockville, MD: US Department of Health and Human Services. Food and Drug Administration, 2015. Available at www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRsearch/cfm?CRFPart=58.
- Organisation for Economic Co-operation and Development: Good Laboratory Practice (GLP). Chemical safety and biosafety. Testing of chemicals. Paris, France: Organisation for Economic Co-operation and Development, 2015. Available at www.oecd.org/chemicalsafety/testing/goodlaboratorypracticeglp.htm.
- Medicines and Healthcare Products Regulatory Agency: Good Laboratory Practice (GLP) for Safety Tests on Chemicals. Medicines, Medical Devices and Blood Regulation and Safety—Guidance. London UK: Medicines and Healthcare products Regulatory Agency, 2014. Available at www.gov.uk/good-laboratory-practice-glp-for-safety-tests-on-chemicals.
- Keenan C, Elmore S, Francke-Carroll S, Kemp R, Kerlin R, et al.: Best practices for use of historical control data of proliferative rodent lesions. Toxicol Pathol 37(5), 679–93, 2009
- Hayes AW, Dayan AD, Hall WC, Kodell RL, Williams GM, et al.: A review of mammalian carcinogenicity study design and potential effects of alternate test procedures on the safety evaluation of food ingredients. Regul Toxicol Pharmacol 60(1Suppl), S1–S34, 2011.
- Holmes AM, Creton S, and Chapman K: Working in partnership to advance the 3Rs in toxicity testing. Toxicology 267, 14–19, 2010.
- EFSA Scientific Committee (Scientific Committee). Existing approaches incorporating replacement, reduction and refinement of animal testing: applicability in food and feed risk assessment. EFSA J, 7, 1–77, 2009. Available at http://www.efsa.europa.eu/en/efsajournal/pub/1052.htm. doi:10.2903/j.efsa.2009.1052
- Nature: Consensus on reporting principles aims to improve quality control in biomedical research and encourage public trust in science. Nature 515, 7, 2014.
- Williams GM, Iatropoulos MJ, and Enzmann HG: Principles of Testing for Carcinogenic Activity. Principles and Methods of Toxicology. 5th ed. Boca Raton, FL: CRC Press, 2008.
- International Programme on Chemical Safety: Environmental Health Criteria 70. Principles for the Safety Assessment of Food Additives and Contaminants in Food. Geneva, Switzerland: World Health Orgnization, 1987. Available at www.inchem.org/documents/ehc/ehc/ejc70.htm.
- Ashby J and Paton D: The influence of chemical structure on the extent and sites of carcinogenesis for 522 rodent carcinogens and 55 difference human carcinogen exposures. Mutat Res 286, 3–74, 1993.
- Benigni R and Bossa C: Structure alerts for carcinogenicity and the Salmonella assay system: a novel insight through the chemical relational databases technology. Mutat Res 659, 248–261, 2008.
- Broustas CG and Lieberman HB: DNA damage response genes and the development of cancer metastasis. Radiation Res 181, 111–130, 2014
- Weisburger JH and Williams GM: Profiles in toxicology. the distinction between genotoxic and epigenetic carcinogens and implication for cancer risk. Toxicol Sci 57, 4–5, 2004.
- Williams GM and Whysner J: Epigenetic carcinogens: evaluation and risk assessment. Exp Toxicol Pathol 48, 189–195, 1996.
- IARC: Saccharin and its salts. IARC Monographs on the Evaluation of Carcinogenesis Risks to Humans. Lyon, France: IARC, 1999; vol. 73, pp. 517–624.
- US Department of Health and Human Services; Food and Drug Administration; Center for Food Safety and Applied Nutrition: Chapter IV. Guidelines for toxicity studies. Redbook 2000 (Updated July 2007). Guidance for Industry and Other Stakeholders. Toxicological Principles for the Safety Assessment of Food Ingredients. Silver Spring, MD: US Department of Health and Human Services. Food and Drug Administration, 2000. Available at http://www.fda.gov/downloads/Food/GuidanceRegulation/UCM222779.pdf.
- Cohen SM: Evaluation of possible carcinogenic risk to humans based on liver tumors in rodent assays: The two-year bioassay is no longer necessary. Toxicol Pathol 38, 487, 2010.
- Cohen SM: Human carcinogenic risk evaluation: an alternative approach to the two-year rodent bioassay. Toxicol Sci 80, 225–229, 2004.
- Wood CE, Hukkanen RR, Sura R, Jacobson-Kram D, Nolte T, et al.: Scientific and regulatory policy committee (SRPC) review: interpretation and use of cell proliferation data in cancer risk assessment. Toxicol Pathol 43, 760–775, 2015.
- Organisation for Economic Co-operation and Development: OECD Guidance Document for the Performance of Chronic Toxicity and Carcinogenicity Studies, Supporting TG 451, 452, and 453. Paris, France: Organisation for Economic Co-operation and Development, 2008. Available at http://www.oecd.org/chemicalsafety/testing/41829966.pdf.
- National Center for Biotechnology Information: PubChem Compound Database; CID = 71485. Sucralose. Bethesda, MD: U.S. National Library of Medicine. Available at http://pubchem.ncbi.nlm.nih.gov/compound/Sucralose#section=Top. Accessed June 19, 2015.
- Parker KJ: The Potential Interaction of Sucralose with Food Components. Report prepared for Dr WM Nicol, Divisional Director, Speciality Sweeteners. Reading, UK: Tate & Lyle Plc, 1986.
- Benigni R, Bossa C, Battistelli CL, and Tcheremenskaia O: IARC Classes 1 and 2 carcinogens are successfully identified by an alternative strategy that detects DNA-reactivity and cell transformation ability of chemicals. Mutat Res 758, 58–61, 2013.
- Grice HC and Goldsmith LA: Sucralose—an overview of the toxicity data. Food Chem Toxicol 38(Suppl 2), S1–S6, 2000.
- John BA, Wood G, and Hawkins DR: The pharmacokinetics and metabolism of sucralose in the mouse. Food Chem Toxicol 38 (Suppl 2), S107–S110, 2000.
- Roberts A, Renwick AG, Sims J, and Snodin DJ: Sucralose metabolism and pharmacokinetics in man. Food Chem Toxicol 38 (Suppl 2), S31–S41, 2000.
- Sims J, Roberts A, Daniel JW, and Renwick AG: The metabolic fate of sucralose in rats. Food Chem Toxicol 38(Suppl 2), S115–S121, 2006.
- Wood SG, John BA, and Hawkins DR: The pharmacokinetics and metabolism of sucralose in the dog. Food Chem Toxicol 38(Suppl 2), S99–S106, 2000.
- Nabors LO (ed.): Chapter 11. Sucralose. Alternative Sweeteners, 3rd ed. New York, NY: Marcel Dekker, 2001.
- European Commission; Health & Consumer Protection Directorate-General: Opinion of the Scientific Committee on Food on sucralose. Sucralose—SCF/CS/EDUL/190Final, 19/9/2000. Brussels, Belgium: European Commission; Health & Consumer Protection Directorate-General, 2000. Available at http://ec.europa.eu/food/fs/sc/scf/out68_en.pdf.
- US Department of Health and Human Services; Food and Drug Administration: Food Additives Permitted for Direct Addition to Food for Human Consumption; Sucralose. Final rule. Docket No 87F-0086. Federal Register, 1998; vol. 63 (64), pp. 16417–16433.
- US Department of Health and Human Services; Food and Drug Administration: Food Additives Permitted for Direct Addition to Food for Human Consumption; Sucralose. Final Rule. Federal Register, 1999; vol. 64 (155), pp. 43908–43909.
- Food and Agriculture Organization of the United Nations and the World Health Organization; Expert Committee on Food Additives: Evaluation of Certain Food Additives and Contaminants. Thirty-fourth Report of the Joint FAO/WHO Expert Committee on Food Additives. World Health Organization Technical Report Series, No 776. Geneva, Switzerland : WHO Press, 1989.
- Food and Agriculture Organization of the United Nations and the World Health Organization; Expert Committee on Food Additives: Evaluation of Certain Food Additives and Contaminants. Forty-ninth Report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, No 884. Geneva, Switzerland: WHO Press, 1999.
- Food and Agriculture Organization of the United Nations and the World Health Organization; Expert Committee on Food Additives: Evaluation of Certain Food Additives and Contaminants. Thirty-seventh Report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, No 806. Geneva, Switzerland: WHO Press, 1991.
- Beyts P. Interaction of TGS with Food Components—Model System Study. Reading, UK: Tate & Lyle, 1986.
- National Food Laboratory: Stability of Sucralose in Heat Processed Foods (a feasibility study). Dublin, CA: National Food Laboratory, Inc, 1986.
- Barndt RL and Jackson G: Stability of sucralose in baked goods. Food Technol 44(1), 62–66, 1990.
- Hutchinson SA, Ho GS, and Ho C-T: Stability and degredation of the high intensity sweeteners: Aspartame, alitame, and sucralose. Food Rev Int 15, 249–261, 1999.
- Bannach G, Almeida RR, Lacerda L, Schnitzler E, and Ionashiro M: Thermal stabiliyt and thermal decomposition of sucralose. Edetica Qumia 34, 21–26, 2009.
- Rahn A and Yaylayan VA: Thermal degredation of sucralose and its potential in generating chloropropanols in the presence of glycerol. Food Chem 118, 56–61, 2010.
- Grotz VL and Brusick D: Response to the Letter to the Editor by S Schiffman and M Abou-Donia: Additional evidence to clarify the evidence of sucralose safety. Regul Toxicol Pharmacol 63, 509–513, 2012.
- Brusick D, Grotz VL, Slesinski R, Kruger CL, and Hayes AW: The absence of genotoxocity of sucralose. Food Chem Toxicol 48, 3067–3072, 2010.
- Bootman J and Lodge DC: 1,6-Dichloro-1,6-Dideoxyfructose: Assessment of Its Mutagenic Potential in Histidine Auxotrophs of Salmonella typhimurium. Essex, UK: Life Science Research, Ltd. to the World Health Organization by Tate & Lyle, 1980.
- Haworth SR, Lawlor TE, Smith TK, Williams NA, Simmons RT, et al.: Salmonella/Mammalian-Microsome Plate Incorporation Mutagenesis Assay (1,6-dichlorofructose). New Brunswick, NJ: EG & G Mason Research Institute to the World Health Organization by Tate & Lyle, 1981.
- Bootman J and Lodge DC: 4-Chloro-4-Deoxy-D-Galactose: Assessment of Its Mutagenic Potential in Histidine Auxotrophs of Salmonella typhimurium. Essex, UK: Life Science Research, Ltd. to the World Health Organization by Tate & Lyle, 1980.
- Bootman J and May K: The Detection of Mutagenic Activity by Increased Reversion of Histidine Auxotrophs of Salmonella typhimurium. Essex, UK: Life Science Research, Ltd. to the World Health Organization by Tate & Lyle, 1981.
- Kirby PE, Pizzarello RF, Brauninger RM, Vega RA, and Reichard GL: Evaluation of Test Article 1,6-Dichlorofructose (MRI No. 536) for Mutagenic Potential Employing the L5178Y TK+/− Mutagenesis Assay. Rockville, MD: EG & G Mason Research Institute. Submitted to the World Health Organization by Tate & Lyle, 1981.
- Kirby PE, Pizzarello RF, Cohen A, Williams PE, Reichard GL, et al.: Evaluation of Test Article 1,6-Dichlorofructose (MR1 No. 629) for Mutagenic Potential Employing the L5178Y TK+/− Mutagenesis Assay. Rockville, MD: EG & G Mason Research Institute. Submitted to the World Health Organization by Tate & Lyle, 1981.
- Jeffrey AM and Williams GM: Lack of DNA-damaging activity of five non-nutritive sweeteners in the rat hepatocyte/DNA repair assay. Food Chem Toxicol 38(4), 335–338, 2000.
- Molecular Toxicology (Microtest): Study to Evaluate the Potential of 1,6-Dichlorofructose to Induce Unscheduled DNA Synthesis in Isolated Rat Hepatocytes In Vitro. Hazleton Europe Study Number 178/2. Reading, UK: Tate & Lyle Speciality Sweeteners, 1994.
- Kirby PE, Pizzarello RF, Williams PE, Reichard GL, Wattam RE, et al.: Evaluation of Test Article 4-Chlorogalactose (MRI No. 628) for Mutagenic Potential Employing the L5178Y TK+/− Mutagenesis Assay. Rockville, MD: EG & G Mason Research Institute. Submitted to the World Health Organization by Tate & Lyle, 1981.
- van Eyk AD: The effect of five artificial sweeteners on Caco-2, HT-29, and HEK-293 cells. Drug Chem Toxicol 38(3), 318–327. doi: 10.3109/01480545.2014.966381.
- Sasaki YF, Kawaguchi S, Kamaya A, Ohshita M, Kabasawa K, et al.: The comet assay with 8 mouse organs: results with 39 currently used food additives. Mutat Res 519, 103–119, 2002.
- Bootman J and Whalley HE: Dominant Lethal Study in the Mouse After Subacute Treatment With an Equimolar Mixture of the Hydrolysis Products Derived From 1,6-Dichloro-1,6-Dideoxy-β-D-Fructofuranosyl-4-Chloro-4-Deoxy-Alpha-D-Galactopyranoside (TGS). Essex, UK: Life Science Research, Ltd. to the World Health Organization by Tate & Lyle, 1983.
- Ivett JL: 6-Dichloro-1,6-Dideoxyfructose: In Vivo Micronucleus Assay in the Mouse. Rockville, MD: Hazleton Laboratories America, Inc. to the World Health Organisation by Tate & Lyle, 1988.
- Ivett JL: 6-Dichloro-1,6-Dideoxyfructose: Single Exposure Dose Selection Study for the In Vivo Micronucleus Assay in the Mouse. Rockville, MD: Hazleton Laboratories America, Inc. to the World Health Organisation by Tate & Lyle, 1988.
- Bootman J and Rees R: In Vitro Assessment of the Clastogenic Activity of 1,6-DCF, 4-CG, and TGS in Cultured Human Peripheral Lymphocytes. Essex, UK: Life Science Research, Ltd. to the World Health Organization by Tate & Lyle, 1981.
- Cimino MC and Lebowitz H: Mutagenicity Evaluation of 4-Chloro-Galactose (4-CG), Batch P3/102/15, in the Rat Bone Marrow Cytogenetic Assay. Kensington, MD: Litton Bionetics Inc. to the World Health Organization by Tate & Lyle, 1981.
- Ivett JL: 6-Dichloro-1,6-Dideoxyfructose: In Vivo Sister Chromatid Exchange Micronucleus Assay in the Mouse. Rockville, MD: Hazleton Laboratories America, Inc. to the World Health Organisation by Tate & Lyle, 1981.
- Dauterman WC: Chapter 5: Metabolism of toxicants: Phase II Reactions. Introduction to Biochemical Toxicology, 2nd ed. Levi PE and Hodgson E (eds.). Norwalk, CT: Appleton & Lange, 1994.
- Timbrell JA: Chapter 5. Biotransformation of xenobiotics. General and Applied Toxicology, 2nd ed., Marrs TC, Syversen T, and Ballantyne B (eds.). New York: Grove's Dictionaries Inc, 1999; vol. 1.
- Parkinson A: Chapter 6: Biotransformation of xenobiotics. Unit Two. Disposition of toxicants. Casarett and Doull's Toxicology: The Basic Science of Poisons. New York, NY: McGraw Hill Companies, 2001.
- Kemper RA, Hayes JR, and Bogdanffy MS: Chapter 3. A determinant of toxicity. Principles and Methods of Toxicology, 5th ed., Hayes AW (ed.). Boca Raton, FL: CRC Press Taylor & Francis Group, 2007.
- Grotz VL and Munro IC: An overview of the safety of sucralose. Regul Toxicol Pharmacol 55, 1–5, 2009.
- McLean Baird I, Shephard NW, Merritt RJ, and Hildick-Smith G: Repeated dose study of sucralose tolerance in human subjects. Food Chem Toxicol 38(Suppl 2), S123–S129, 2000.
- Goldsmith LA: Acute and subchronic toxicity of sucralose. Food Chem Toxicol 38(Suppl2), S53–S69, 2000.
- Flamm WG, Blackburn GL, Comer CP, Mayhew DA, and Stargel WW: Long-term food consumption and body weight changes in neotame safety studies are consistent with the allometric relationship observed for other sweeteners and during dietary restrictions. Regul Toxicol Pharmacol 38, 144–156, 2003.
- Perry CJ, Hudson P, and Finn JP: Sucralose: Special Investigation in the Rat of the Effect of Administration by Oral Gavage on Body Weight and Selected Organs. Musselburgh, Scotland: Inveresk Research International to the World Health Organization by Tate & Lyle, 1988.
- Konno A, Utsuyama M, Kurashima C, Kasai M, Kimura S, et al.: Effects of a protein-free diet or food restriction on the immune system of Wistar and Buffalo rats at different ages. Mech Ageing Dev 72(3), 183–197, 1993.
- Savino W1, Dardenne M, Velloso LA, and Dayse Silva-Barbosa S: The thymus is a common target in malnutrition and infection. Br J Nutr 98 (Suppl 1), S11–S16, 2007.
- Duggan C, Watkins JB, and Walker WA: Nutrition in Pediatrics: Basic Science, Clinical Applications. Hamilton, ON: BC Decker, 2008. 4th ed. Available at https://books.google.com/books?id=wSTISCdSIosC&pg=PA264&lpg=PA264&dq=premature+thymic+involution+nutrition&source=bl&ots=K_9wIZBkcy&sig=4cS1iCCl2I5v9mWGfO5_qPyF8SM&hl=en&sa=X&ved=0ahUKEwjf_a_4hrfKAhWKbD4KHWafCpwQ6AEIQjAE#v=onepage&q=premature%20thymic%20involution%20nutrition&f=false (page 264)
- Mann SW, Yuschak MM, Amyes SJG, Aughton P, and Finn JP: A combined chronic toxicity/carcinogenicity study of sucralose in Sprague Dawley Rats. Food Chem Toxicol 38 (Suppl 2), S71–S89, 2000.
- Mann SW, Yuschak MM, Amyes SJG, Aughton P, and Finn JP: A carcinogenicity study of sucralose in the CD-1 mouse. Food Chem Toxicol 38(Suppl 2), S91–S98, 2000.
- Sistare FD, Morton D, Alden C, Christensen J, Keller D, et al.: An analysis of pharmaceutical experience with decades of rat carcinogenicity testing: support for a proposal to modify current regulatory guidelines. Toxicol Pathol 39(4), 716–744, 2011. doi: 10.1177/0192623311406935.
- McCollough ML, Teras LR, Shah R, Diver WR, Gaudet MM, et al.: Artificially and sugar-sweetened carbonated beverage consumption is not associated with risk of lymphoid neoplasms in older men and women. J Nutr 144, 2014–2019, 2014.
- Schernhammer ES, Bertrand KA, Birmann BM, Sampson L, Willett WC, et al.: Consumption of artificial sweetener- and sugar-containing soda and risk of lymphoma and leukemia in men and women. Am J Clin Nutr 96, 1419–1428, 2012.
- Soffritti M, Padovani M, Tibaldi E, Falcioni L, Manservisi F, et al.: Sucralose administered in feed, beginning prenatally through lifespan, incudes hematopoietic neoplasias in male swiss mice. Int J Occup Env Health, 22, 7–17, 2016. Available at: http://www.tandfonline.com/doi/full/10.1080/10773525.2015.1106075)
- Soffritti M, Belpoggi F, Manservigi M, Tibaldi E, Lauriola M, et al.: Aspartame administered in feed, beginning prenatally through life span, induces cancers of the liver and lung in male Swiss mice. Am J Ind Med 53(12), 1197–1206, 2010.
- Soffritti M, Belpoggi F, Degli Esposti D, Lambertini L, Tibaldi E, et al.: First experimental demonstration of the multipotential carcinogenic effects of aspartame administered in the feed to Sprague-Dawley rats. Environ Health Perspect 114(3), 379–385, 2006.
- European Food Safety Authority (EFSA): Statement of EFSA on the scientific evaluation of two studies related to the safety of artificial sweeteners. EFSA J 9(2), 2089, 2011.
- National Toxicology Program (NTP): Summary Report of the National Toxicology Program and Environmental Protection Agency-Sponsored Review of Pathology Materials from Selected Ramazzini Institute Rodent Cancer Bioassays. Research Triangle Park, NC: NTP. 2011. Available at: https://ntp.niehs.nih.gov/ntp/about_ntp/partnerships/international/summarypwg_report_ri_bioassays.pdf
- European Food Safety Authority (EFSA): Scientific opinion on the re-evaluation of aspartame (E 951) as a food additive. EFSA J 11(12), 3496, 2013.
- Canada Gazette: Food and drug regulations, amendment [sucralose] (SOR/91/527). Can Gaz Part II 125 (20), 3125–3130, 1991.
- JMHW (Japanese Ministry of and Welfare), Approval of New High-Intensity Sweetener: Sucralose: Revision of the Enforcement Regulations under the Food Sanitation Law and of the Standards and specifications for Food and Food Additives, etc. (published in Official Gazette, No 2678, July 30, 1999). Japanese Ministry of Health and Welfare Ordinance No 75 (Ministerial Ordinance to Revise Part of the Enforcement Regulations Under the Food Sanitation Law) and Ministry of Health and Welfare Announcement No 167 [Translation]. 1999. Available at http://www.ffcr.or.jp/saidan/FFCRHOME.nsf/pages/e-kousei-sucra2.
- SCF (Scientific Committee on Food): Opinion of the Scientific Committee on Food on Sucralose (adopted by the SCF on 7 September 2000). European commission health & consumer protection directorate-general directorate C-scientific opinions C3—management of scientific committees II; scientific cooperation and networks. Scientific Committee on Food (SCF/CS/ADDS/EDUL/190 Final 12/9/2000]. Available at http://ec.europa.eu/food/fs/sHealthc/scf/out68_en.pdf.
- EU (European Union): Directive 2003/115/EC of the European Parliament and of the Council of 22 December 2003 amending Directive 94/35/EC on sweeteners for use in foodstuffs. Off J Eur Union 47 (L24), 65–71, 2004.
- FSANZ (Food Standards of Australia and New Zealand): Chapter 1: General food standards. Standard 1.3.1: Food additives. Schedule 1, in: Food Standards Code, October 16, 2008. Food Standards Australia New Zealand, Kingston ACT, Australia. Available at http://www.foodstandards.gov.au/_srcfiles/Standard_1_3_1_Additives_Part_2_v98.pdf.
- Grotz VL, Henry RR, McGill JB, Prince MJ, Shamoon H, et al.: Lack of effect of sucralose on glucose homeostasis in subjects with type 2 diabetes. J Am Diet Assoc 103, 1607–1612, 2003.