ABSTRACT
OSCC is the most common malignant cancer of the head and neck. EMT is an essential cellular process critical to the morphogenesis and homeostasis of solid tissues. It is also involved in the initial stage of cancer metastasis and invasion in which cells lose epithelial characteristics. While cancer therapy protocols such as surgery, radiation, and chemotherapy are effective and useful, the drug tolerance and toxicity of OSCC patients remain a problem. Resveratrol is mainly produced in red grape skin and exhibits anti-oxidative, anti-inflammatory, anti-proliferative, and anti-cancer properties.
This study was undertaken to investigate the underlying mechanisms giving rise to the induction of apoptosis by resveratrol in the human tongue squamous cell carcinoma cell line. Resveratrol treatment resulted in a time- and dose-dependent decrease in cell viability and increased the apoptotic cell ratio in CAL-27, SCC15, and SCC25 cells. Resveratrol treatment of CAL-27 cells showed that several lines of apoptotic manifestation and decreased cell migration, invasion, and EMT-inducing transcription factor.
Taken together, our findings demonstrate the inhibitory effect of resveratrol in human OSCC cells via the mitochondrial pathway and that resveratrol is able to inhibit cell invasion and migration by inhibiting the EMT-inducing transcription factors.
Introduction
Oral squamous cell carcinoma (OSCC) is the sixth most common malignant cancer of the head and neck Citation(1), and despite recent advancements in cancer treatment it has been reported that the 5-year survival rate is still very low Citation(2). Although cancer therapy protocols, such as surgery, radiation, and chemotherapy, are effective and useful, drug tolerance and toxicity remains an issue for oral cancer patients Citation(1,2). Many dietary substances are becoming known in the treatment of cancer, and their molecular events are attracting increasing attention Citation(3). Therefore, a better understanding of the molecular mechanisms driving oral carcinogenesis may lead to new diagnostic and therapeutic approaches to this disease and help to improve the prognosis of OSCC patients.
Many natural compounds found in herbal medicines have potent biological effects, including anticancer activity Citation(4,5). Resveratrol is a natural polyphenolic compound (trans-3, 4, 5-trihydroxystilbene) () that is found in red grapes, berries, and peanuts and in food products derived from them, such as wine Citation(6,7), and has strong anti-inflammatory, anti-oxidant, cardioprotective, and anti-tumor properties Citation(8–10) Recent studies have shown that resveratrol inhibits the growth of a wide variety of tumor cells and modulates multiple pathways involved in cell growth inhibition, including cell-cycle arrest, apoptosis, the suppression of transcription factors, and inflammations Citation(3,11).
Apoptosis plays a critical role in embryonic development, tissue homeostasis, and the elimination of damaged cells Citation(12). Apoptosis can be initiated through two signaling pathways, the intrinsic (mitochondrial) pathway and the extrinsic (death receptor) pathway Citation(13). The mitochondria-dependent mechanism is regulated by pro-apoptotic Bax, which belongs to the Bcl-2 family of proteins, and releases cytochrome c Citation(14–16). These mitochondrial alterations lead the activation of a caspase cascade that induces the last stage of cell apoptosis Citation(17). The induction of apoptosis has been proposed as a potential mechanism to stimulate the elimination of cancer cells Citation(18). Therefore, in order to develop anti-cancer drugs with fewer side effects than are associated with current cancer treatment options, it is important to study the anti-cancer capacities of natural compounds Citation(19).
Epithelial-to-mesenchymal transition (EMT) is an essential cellular process critical to the morphogenesis and homeostasis of solid tissues. It is also involved in the initial stage of cancer metastasis and invasion in which cells lose epithelial characteristics Citation(20–22). EMT has been known to render cancer cells refractory to various types of therapy, including several types of chemotherapy and targeted agents, often by reducing apoptotic sensitivity Citation(23) In addition, a number of studies have demonstrated that the presence of EMT could be a predictor of OSCC progression and prognosis Citation(11).
However, the exact mechanism of the apoptosis-inducing effect and EMT of resveratrol on human OSCC is still not known. In the present study, we demonstrate that resveratrol has an anti-cancer and anti-metastasis effects are deriving from the signaling of mitochondria mediated apoptosis and the inhibitory process of EMT in OSCC cell lines.
Materials and Methods
Materials
Resveratrol (≥98% (HPLC) was purchased from Enzo Life Sciences (Farmingdale, NY, USA). 3-[4,5-dimethylthiazol-2-yl]2,5-diphenyl tetrazolium bromide (MTT), Hoechst33342, JC-1, protease inhibitor and RNase A were obtained from Sigma (St. Louis, MO, USA). Bcl-2 (Cat No. SC-7382), bcl-xl (Cat No. SC-8392), bax (Cat No. SC-23959), bak (Cat No. SC-832), cytochrome c (Cat No. SC-13560), caspase-activated DNase (CAD) (Cat No. SC-8342), inhibitor of caspase-activated DNase (ICAD) (Cat No. SC-9066), and β-actin (Cat No. SC-47778) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Apaf-1 (Cat No. #8969), procaspase-3 (Cat No. #9662), cleaved caspase-3 (Cat No. #9661), procasapse-9 (Cat No. #9508), PARP (Cat No. #9542), E-cadherin (Cat No. #3195), N-cadherin (Cat No. #13116), smad2/3 (Cat No. #8685), snail (Cat No. #3879) and slug (Cat No. #9585) were obtained from Cell Signaling Technology (Beverly, MA, USA). Goat anti-mouse IgG, (HRP conjugate) (Cat No. BML-SA204-0100) and Goat anti-rabbit IgG, polyclonal antibody (HRP conjugate) (Cat No. ADI-SAB-300-J). antibodies were purchased from Enzo Life Sciences.
Cell Culture and Resveratrol Treatment
Three human OSCC cell lines, CAL-27, SCC15, and SCC25, were purchased from American Tissue Culture Collection (Rockville, MD, USA). CAL-27 cells were cultured in Dulbecco's Modified Eagle Medium (DMEM; Hyclone, Logan, UT, USA) with 10% fetal bovine serum (FBS; Hyclone) and 1% penicillin-streptomycin antibiotics (Hyclone). SCC15 and SCC25 cells were cultured in DMEM and Ham F12 medium (DMEM/F12) 1:1 (Hyclone) with the same concentrations of FBS and penicillin-streptomycin antibiotics. All cells were incubated in a humidified atmosphere of 5% CO2 at 37°C. Resveratrol (100 mM) was dissolved in dimethyl sulfoxide (DMSO) and stored at −20°C until use. Before the resveratrol treatment, the cells were grown to 80–90% confluence and then exposed to resveratrol at different concentrations (10–500 μM) for at least 24 h and up to 72 h.
Cell Cytotoxicity Assay
The cytotoxicity of resveratrol was assessed using an MTT assay according to the manufacturer's instructions. Briefly, the cells (1 × 104) were seeded in a 96-well plate. After 24 h, CAL27, SCC15, and SCC25 cells were treated with resveratrol at various concentrations (10–500 μM) for 24–72 h. After the treatment, supernatants were removed and 0.5 mg/ml MTT solution was added to 96 -well and incubated for 4 h at 37°C in the dark. The MTT solution was aspirated, and the formazan crystals that formed were dissolved in DMSO. Cell viability was measured using an enzyme-linked immunosorbent assay (ELISA) reader (Sunrise Remote Control, Tecan, Austria) at an excitation emission wavelength of 570 nm.
Fluorescence Microscopy
CAL27, SCC15, and SCC25 (2 × 104) cells were cultured on Lab-Tek chambered coverglass and allowed to attach overnight. Cells were treated with 100 μM resveratrol for 24 h. After the treatment, the cells were rinsed with PBS, fixed with 4% PFA for 15 min, stained with Hoechst 33342, labelled with rhodamine-tagged phalloidin, and left in the dark for 15 min at 37°C. The samples were observed and photographed under an epifluorescence microscope (Carl Zeiss, Göettingen, Germany). The number of cells that showed condensed or fragmented nuclei was determined by a blinded observer from a random sampling of 3 × 102 cells per experiment.
Annexin V- FITC/PI Staining
Apoptotic cells were detected using an Annexin V- FITC/PI apoptosis detection kit (Enzo Life Sciences) according to the manufacturer's protocol, as described previously Citation(24). Apoptosis was detected using a CYTOMICS FC500 instrument (Beckman Coulter, Porterville, CA, USA) with excitation and emission settings of 488 nm (FL1 filter) and 530 nm (FL3 filter), respectively.
JC-1 Staining for Mitochondrial Membrane Potential (MMP)
Mitochondrial membrane potential (MMP) changes were monitored using JC-1 dye. After treatment, cells were washed twice with PBS, and 1 μg/ml of JC-1 was added directly to the cell culture medium and incubated at 37°C with 5% CO2 for 30 min. The analysis of MMP was performed using a CYTOMICS FC500 flow cytometry system (Beckman Coulter). Data were acquired and analyzed using CXP software, version 2.2.
Immunofluorescent Staining
Cytochrome c (1:200 dilution) and CAD (1:200 dilution) were detected in CAL27 cells plated on Lab-Tek chambered coverglass and allowed to attach for 1 d. After treatment with 100 μM resveratrol for 24 h, the cells were stained with MitoTracker Deep Red (Molecular Probes), labelled with rhodamine-tagged phalloidin (Invitrogen), and left for 30 min in the dark at 37°C. An immunostaining protocol was used as described previously Citation(25). Fluorescent images were observed and analyzed using a Zeiss LSM 750 laser-scanning confocal microscope (Göettingen, Germany).
Western Blot Assay
CAL27 (2 × 106) cells were plated in 100 mm culture dishes and were treated the next day with various concentrations of resveratrol for 24 h. The cells were harvested and washed twice with ice-cold PBS. The total cell proteins were lysed with a radio immunoprecipitation assay (RIPA) buffer (Invitrogen) at 4°C for 1 h. The protein lysis and immunoblotting protocols were employed using the previous method Citation(26). Immunostaining with antibodies was performed with a SuperSignal West Femto enhanced chemiluminescence substrate and was detected using an Alpha Imager HP (Alpha Innotech, Santa Clara, CA, USA). Equivalent protein loading was confirmed by Ponceau S staining. All of primary antibodies used in this study were diluted 1:1000 and secondary antibodies were diluted 1:5000.
Wound Healing Assay
We used a common wound healing assay to study cell migration. CAL27 (1 × 106) cells were cultured in 6-well culture plates and incubated at 37°C with 5% CO2 for 1 d. When the cells were grown to 90–100% confluency, a single wound was scratched in the center of the well using a sterile 1000 µl pipette tip and was then treated immediately with 10 μM resveratrol. After 24 h and 48 h of culturing, the cells that migrated into the wounded area were visualized and photographed under an inverted microscope. Each experiment was performed at least three times independently.
Invasion Assay
A transwell with an 8.0 μm pore polycarbonate membrane (Corning Costar, Cambridge, MA, USA) was coated with 40 μl Matrigel at 200 μg/ml and incubated for 2 h. CAL 27 cells were treated with 50 μM resveratrol for 24 h. The next day, the cells were harvested and seeded (1 × 105 cells in 200 μl) into the upper chamber of the transwell with serum-free medium. The lower chamber was filled with 800 μl medium containing 10% FBS. Following 72 h of incubation at 37°C in a humidifed 5% CO2 atmosphere, the cells were fixed in methanol and stained with hematoxylin for 30 min, and the cells that invaded through the pores to the lower surface of the filter were counted under an inverted microscope (Olympus, Tokyo, Japan).
Statistical Analysis
All the experiments were performed in triplicate, and the results were expressed as the mean ± SD. A Student's t-test or a one-way ANOVA was used for multiple comparisons in the statistical analysis. Statistical analyses were performed using GraphPad Prism Version 5.0 (GraphPad Prism, San Diego, CA, USA).
Results
Resveratrol Reduces Viability on OSCC Cell Lines
To examine the cytotoxicity of resveratrol on OSCC cells, we used the MTT assay for the CAL27, SCC15, and SCC25 cells. These three cell lines were treated with 10 to 500 μM for 24 to 72 h. The viability of CAL27 cells were reduced by 92.5% with 50 μM) ranging to 33.9% with 500 μM) at 24 h. The cell viability of SCC15 and SCC25 cells was slightly decreased at 24 h. The cell viability of all three cell lines was reduced by resveratrol treatment in a time-dependent manner (). The half maximal inhibitory concentrations (IC50) of resveratrol were 100 μM at 24 h in CAL27, 200 μM at 72 h in SCC15, 500 μM at 48 h, and 300 μM at 72 h in SCC25. The resveratrol concentration of 100 μM was used for further assessment of the apoptosis experiment.
Figure 1. Chemical structure of resveratrol. A natural polyphenol, resveratrol is the benefical compound found in various plant species such as berries, peanuts, and particularly grape.
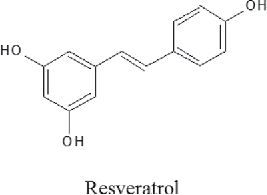
Figure 2. Cytotoxic effect of resveratrol on OSCC cells. OSCC (CAL27, SCC15, and SCC25) cells were treated with various concentrations of resveratrol (10–500 μM) for 24 to 72 h, and cell viability was determined with an MTT assay. Data were expressed as the mean ± SD (n = 6) and analyzed by one-way ANOVA using Dunnett's multiple-comparison test. (*p < 0.05, **p < 0.01, ***p < 0.001 at 24 h; #p < 0.05, ##p < 0.01, ###p < 0.001 at 48 h; †p < 0.05, ††p < 0.01, †††p < 0.001 at 72 h for the difference between the non-treatment and treatment groups).
Resveratrol Increases the Nuclear Condensation and Apoptosis Ratio of OSCC Cells
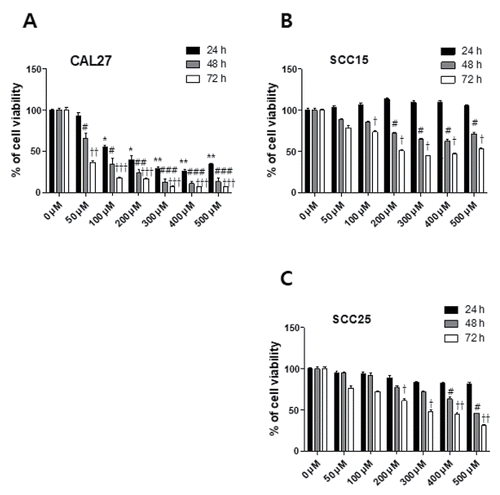
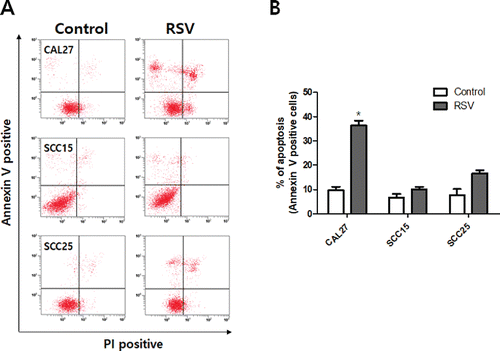
Resveratrol treatment resulted in morphological changes associated with the apoptosis of OSCC cells. Hoechst staining demonstrated that resveratrol induced a change in nuclear morphology; rhodamine phalloidin dye was used to stain actin fiber and to counter stain CAL27, SCC15, and SCC25 cells. The control cells had typical round nuclei, whereas CAL27 cells treated with 100 μM resveratrol showed many condensed and fragmented nuclei, and SCC15 and SCC25 cells treated with the same concentrations of resveratrol had very few condensed nuclei (). We quantified the extent of apoptosis by the fluorescence-activated cell sorting (FACS) of cells stained with Annexin V-PI. The three cell lines were treated with 100 μM resveratrol for 24 h, with the result that the apoptotic cell population (Annexin V positive population; early and late stage apoptotic cells) was higher than in the untreated control group. The ratio of apoptosis in resveratrol-treated CAL27 cells was 36.3%, which was markedly higher than that in SCC15 and SCC25 cells ().
Figure 3. Morphological changes in resveratrol-treated OSCC. Fluorescent images of CAL27, SCC15, and SCC25 cells. These cells were stained with rhodamine-phalloidin to visualize actin filament and Hoechst staining to highlight nuclei. A, B, and C: Cells not treated with resveratrol had round nuclei and actin filament (0 μM). Cells treated with resveratrol (100 μM) for 24 h showed the production of nuclear condensation. (D, E, and F) The values below the micrographs are the mean ± SD (n = 3) of the mean of apoptotic cells as determined by Hoechst staining (*p < 0.05).
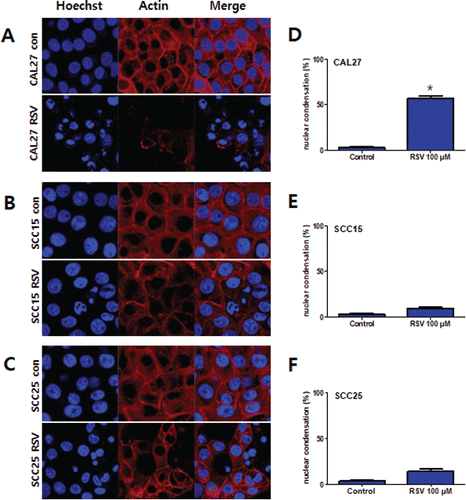
Figure 4. Annexin V-FITC/PI staining of CAL27, SCC15, and SCC25 cells treated with resveratrol. Cells were treated with 100 μM resveratrol for 24 h. A: Cells were collected and subjected to Annexin V-FITC/PI staining and analyzed using flow cytometry. B: The graph visualizes early and late stage apoptotic cells. Results were expressed as the mean ± SD (n = 3) (*p < 0.05).
Resveratrol Induces the Mitochondrial Intrinsic Pathway and Caspase Cascade
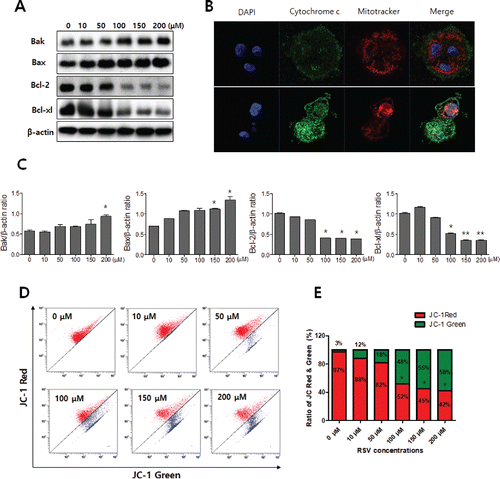
The Bcl-2 protein family plays a crucial role in the induction of apoptosis Citation(27). Bcl-2 family proteins have a function as inhibit and promote apoptosis; bcl-2 and bcl-xl have an anti-apoptotic function; and Bax and Bak have a pro-apoptotic function Citation(28). The role of Bcl-2 family proteins in resveratrol-induced apoptosis was examined using Western blot assays. Bak and bax were up-regulated, and Bcl-2 and Bcl-xl were down-regulated by resveratrol in a dose-dependent manner in CAL27 cells ( and ). Pro-apoptotic bax and bak protein induces the loss of MMP and releases cytochrome c from mitochondrial membranes Citation(29) The mitochondria were stained with JC-1, and the MMP (△Ψm) was measured using flow cytometry. CAL27 cells treated with various concentrations of resveratrol showed a loss of MMP (△Ψm) in a dose-dependent manner ( and ). The release of cytochrome c from the mitochondria was examined using confocal microscopy. Cytochrome c was released from mitochondria into the cytosol in CAL27 cells treated with 100 μM resveratrol ().
Figure 5. Resveratrol-induced loss of MMP (△Ψm) and the release of cytochrome c. A: Bak, Bax, Bcl-2, and Bcl-xl expression levels in resveratrol-treated CAL27 cells were detected using Western blot analysis. Pro-apoptotic factor Bax was significantly up-regulated in a dose-dependent manner, whereas anti-apoptotic factor Bcl-2 was down-regulated. The levels of β-actin were used as an internal standard for quantifying Bak, Bax, Bcl-2, and Bcl-xl expression. B: Confocal microscopy showed that cytochrome c was released from mitochondria into the cytosol in resveratrol-treated CAL27 cells. C: Densitometry analysis of Western blots. Data were expressed as the mean ± SD (n = 3) and analyzed by one-way ANOVA using Dunnett's multiple-comparison test (*p < 0.05, **p < 0.01). D and E: MMP (△Ψm) in resveratrol-treated CAL27 cells was evaluated using JC-1 staining and flow cytometry analysis. Three independent assays were performed.
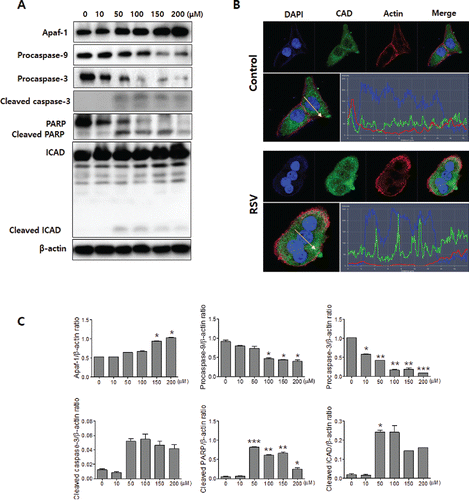
When the cells were undergoing apoptosis, the release of cytochrome c activated the caspase cascade Citation(29). Resveratrol treatment induced caspase activation of CAL27 cells, assessed using Western blot assays. Apoptotic protease activating factor 1 (Apaf-1) was increased and procaspase9, procasapase3, cleaved caspase3, PARP, and ICAD were showed activation form and cleaved products ( and ). Confocal microscopy showed that resveratrol treatment promoted the translocation of CAD from the cytosol into the nuclei ().
Figure 6. Resveratrol activates caspases and CAD. A: Western blot analyses of Apaf-1, pro-caspase-9, pro-caspase-3, cleaved-caspase-3, PARP, and ICAD. Resveratrol treatment induced pro-caspase-9, pro-caspase-3, and PARP degradation and produced the cleaved caspase-3, PARP, and ICAD cleaved products. The levels of β-actin were used as an internal standard for quantifying Apaf-1, procaspase-9, procaspase-3, cleaved caspase-3, PARP, and ICAD expression. B: CAD translocated from the cytosol into the nuclei as a result of the resveratrol treatment. The profile of CAD and nuclei fluorescence intensity is depicted. The intensity of CAD is shown in green, nuclei is blue, and actin in red. The green, blue, and red intensity overlap in the resveratrol-treated group. C: Densitometry analysis of Western blots. Data were expressed as the mean ± SD (n = 3) and analyzed by one-way ANOVA using Dunnett's multiple-comparison test (*p < 0.05, **p < 0.01, ***p < 0.001).
Resveratrol Inhibits Cell Migration and Invasion of CAL27 Cells
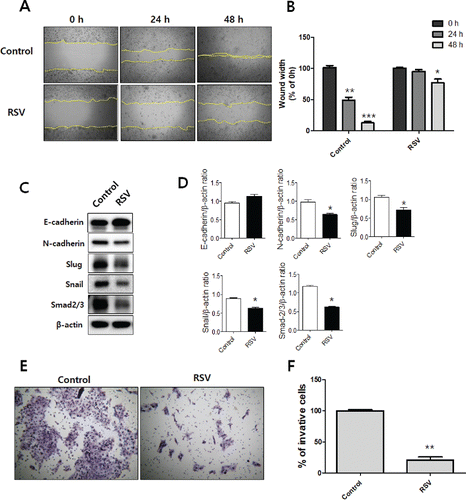
To investigate the cell migration of resveratrol-treated CAL27 cells, a wound healing assay was performed. The CAL27 cells were imaged following treatment with 50 μM resveratrol for 0 h and 48 h at the same marked site. The difference in wound width was measured at three time points (0 h, 24 h, and 48 h). The wound width ratio of the group treated with 50 μM resveratrol was 95.0% at 24 h and 76.6% at 48 h, and for the control group it was 49.0% at 24 h and 12% at 48 h. This result suggests that resveratrol significantly inhibits the cell migration of tumor cells ( and ). The effect of resveratrol on the invasion of CAL27 cells was measured using a Boyden chamber invasion assay. The ratio of the number of invasive cells in the treatment group to that in the corresponding control group was calculated and is presented in a bar graph; the results indicate that resveratrol markedly inhibited the cell invasion ( and ). To investigate whether resveratrol altered the EMT process in the metastatic progression of OSCC, we examined the expression level of E-cadherin and N-cadherin, the cell markers of EMT, in CAL27 cells. As shown in and , the resveratrol treatment group showed up-regulation of E-cadherin and down-regulation of \N-cadherin compared with the control group. The transcription factors snail and slug negatively regulate E-cadherin expression Citation(30). The snail and slug expression levels were dramatically down-regulated in the resveratrol treatment group. These results indicate that resveratrol suppress the malignant progression of OSCC by inhibiting the EMT process.
Figure 7. Resveratrol inhibits OSCC cell migration and invasion. A and B: A wound healing assay was used to detect the migration ability of the resveratrol-treated CAL27 cells. The demarcated yellow lines were cleared area C: The expression of EMT makers. D: Densitometry analysis of Western blots. Data were expressed as the mean ± SD (n = 3) and analyzed by t-test (*p < 0.05 vs Control). E and F: A Boyden chamber assay with matrigel-coated membrane was employed to examine the invasion ability of the resveratrol-treated CAL27 cells.
Discussion
Over the past several decades, many studies have reported that natural phenolic compounds derived from medicinal herbs and dietary plants have biological advantages against various types of cancer Citation(31–33). Resveratrol, a polyphenolic phytoalexin, is mainly produced in red grape skin and exhibits anti-oxidative, anti-inflammatory, anti-proliferative, and anti-cancer properties Citation(3,34). Malignant OSCC has been reported that 5-year survival rate is lower and incidence rate is to be younger than other tumors (approximately 30–50 years of age) worldwide. Citation(35,36). The biological mechanism behind resveratrol-induced cell death in OSCC cell lines has not been well studied. However, our preliminary experiments revealed that resveratrol inhibits cell viability in OSCC cell lines (). Many other studies have demonstrated that resveratrol at ranging from 50 to 200 μM has the ability to lead cell death in pancreatic, prostate, hepatic, and breast cancer cells Citation(37–41). Therefore, we first tested cell viability of resveratrol concentrations ranging from 10 to 500 μM. The effects of resveratrol on the viability of CAL27, SCC15, and SCC25 cells, revealing that resveratrol produces a dose- and time-dependent reduction, particularly in the viability of CAL27 cells. These data indicate that resveratrol exerts a specific cytotoxic effect on CAL27 cells.
When cells undergo apoptosis, the cells show specific morphological changes, such as chromatin condensation, a reduction in cell volume, and endonuclease cleavage of DNA into oligonucleosome length fragments Citation(42). In the present study, morphological changes in OSCC cells, including chromatin condensation and nuclear fragmentation, were observed using Hoechst 33258 staining. CAL27, SCC15, and SCC25 cells were treated with 100 μM resveratrol for 24 h, and it was shown that nuclei were condensed and fragmented morphology, especially in CAL27 cells. The analysis was carried out using a fluorescence microscope (). Apoptosis is accompanied by specific alterations to the plasma membrane that promote the recognition and engulfment of these cells by phagocytes Citation(43). The annexin V-binding assay provides a very specific and reliable technique to detect apoptosis Citation(44,45). We also assessed the annexin V positive apoptotic cells using flow cytometry and annexin V-PI staining of the CAL27, SCC15, and SCC25 cells. As shown in , the apoptotic ratio was much higher in CAL27 cells than in other cells due to the resveratrol treatment (). These results suggest that resveratrol should be recognized as a potential agent in the prevention and treatment of OSCC.
The mitochondrial signaling pathway of apoptosis plays a key role in regulating cell death in response to various stimuli Citation(46). During apoptosis, mitochondrial outer membrane permeability transition is induced by certain Bcl-2 family members as activation of pro-apoptotic (e.g., Bax and Bak) and inhibition of anti-apoptotic proteins (Bcl-2, Bcl-XL), and it triggers cytochrome c release into the cytosol Citation(47). Released cytochrome c binds to Apaf–1, cytochrome c/Apaf-1 complex and oligomerizes with caspase-9 to produce the apoptosome, which subsequently activates caspase-3 and caspase-7. This results in the cleavage of critical cellular proteins and apoptosis Citation(48).Caspase-3 is a main executioner that cleaves cellular proteins, including poly(ADP-ribose) polymerase (PARP) Citation(49). In addition, CAD and its inhibitor (ICAD) are responsible for DNA fragmentation. During apoptotic stimuli, ICAD is cleaved by active caspase-3 in response to apoptotic stimuli, and CAD is activated Citation(49–51). In the present study, we investigated the resveratrol-induced apoptosis occurring via the mitochondrial signaling pathway. We found that resveratrol down-regulates MMP, activates Bax and Bak, inhibits Bcl-2 and Bcl-XL, and releases cytochrome c from the mitochondria. In addition, we clarified the activation of caspases ICAD and PARP, which are associated with apoptosis by resveratrol. The expression levels of Apaf-1 caspase-9, caspase-3, ICAD, and PARP proteins activated and cleaved caspase-3, causing significant increases in CAL27 cells. These findings clearly suggest that resveratrol induces apoptosis via activation of the mitochondrial pathway and caspase cascades in OSCC cells.
EMT is critical to tumor progression which follows significant changes occur, including the down-regulation of E-cadherin and the translocation of β-catenin Citation(52,53). The transcription factors snail, slug, N-cadherin, and vimentin are up-regulated in this process Citation(52,54). Resveratrol inhibits EMT by down-regulating the expression of Zeb-1, slug, and snail, which are EMT-inducting transcription factors that slow migration and invasion in pancreatic cancer cells Citation(55). In this study, we showed that resveratrol significantly decreased cell migration, invasion, and inhibited EMT-inducing transcription factors in CAL27 cells at a concentration of 50 μM (). The results in show that the expression of slug, snail smad2/3, and N-cadherin was reduced by resveratrol. However, the expression of E-cadherin was increased. These results may be explained by the EMT-inducing transcription factor. Snail plays a central role in EMT, as it can bind to the promoter E-box that represses E-cadherin transcription Citation(56). Therefore, the expression of E-cadherin increases when snail, an EMT-inducing transcription factor, is inhibited. In conclusion, the present study demonstrated the inhibitory effect of resveratrol in human OSCC cells via the mitochondrial pathway and that resveratrol is able to inhibit cell invasion and migration by inhibiting the EMT-inducing transcription factors. However, confirmation of this result in a larger number of OSCC cell lines is necessary and further in vivo studies are required.
Conflicts of Interest
There is no conflict of interest.
Compliance with Ethical Standards
This article does not contain studies on animal models or human subjects.
Authors' Contributions
In-Ryoung Kim, Seong-Eon Kim, Sang-Hun Shin, In-Kyo Chung, Hae-Ryoun Park, and Bong-Soo Park conceived and designed the study. In-Ryoung Kim, Hae-Ryoun Park, Hae-Mi Kang, Jae-Yeol Lee and Chul-Hoon Kim performed the experiments. In-Ryoung Kim, Seong-Eon Kim and Sang-Hun Shin wrote the manuscript. All authors read and approved the final version of the manuscript.
Funding
This study was supported by Pusan National University Research Grant, 2017.
References
- Shiga K, Tateda M, Katagiri K, Nakanome A, Ogawa T, et al.: Distinct features of second primary malignancies in head and neck cancer patients in Japan. The Tohoku J Exp Med 225, 5–12, 2011.
- Sudbø J, and Reith A: Retracted: The evolution of predictive oncology and molecular-based therapy for oral cancer prevention. Int J Cancer 115, 339–345, 2005.
- Athar M, Back JH, Tang X, Kim KH, Kopelovich L, et al.: Resveratrol: A review of preclinical studies for human cancer prevention. Toxicol Appl Pharmacol 224, 274–283, 2007.
- Huang Y-L, Kou J-P, Ma L, Song J-X, and Yu B-Y: Possible Mechanism of the Anti-inflammatory Activity of Ruscogenin: Role of Intercellular Adhesion Molecule-1 and Nuclear Factor-. KAPPA. B. J Pharmacol Sci 108, 198–205, 2008.
- Tan W, Lu J, Huang M, Li Y, Chen M, et al.: Anti-cancer natural products isolated from chinese medicinal herbs. Chin Med 6, 1, 2011.
- Wang Y, Catana F, Yang Y, Roderick R, and Van Breemen RB: An LC-MS method for analyzing total resveratrol in grape juice, cranberry juice, and in wine. J Agric Food Chem 50, 431–435, 2002.
- Burns J, Yokota T, Ashihara H, Lean ME, and Crozier A: Plant foods and herbal sources of resveratrol. J Agr food Chem 50, 3337–3340, 2002.
- Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, et al.: Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Sci 275, 218–220, 1997.
- Qadri SM, Föller M, and Lang F: Inhibition of suicidal erythrocyte death by resveratrol. Life Sci 85, 33–38, 2009.
- Baur JA, and Sinclair DA: Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discovery 5, 493–506, 2006.
- Bhardwaj A, Sethi G, Vadhan-Raj S, Bueso-Ramos C, Takada Y, et al.: Resveratrol inhibits proliferation, induces apoptosis, and overcomes chemoresistance through down-regulation of STAT3 and nuclear factor-κB–regulated antiapoptotic and cell survival gene products in human multiple myeloma cells. Blood 109, 2293–2302, 2007.
- Tuzlak S, Kaufmann T, and Villunger A: Interrogating the relevance of mitochondrial apoptosis for vertebrate development and postnatal tissue homeostasis. Genes Dev 30, 2133–2151, 2016.
- Rosato RR, Almenara JA, Dai Y, and Grant S: Simultaneous activation of the intrinsic and extrinsic pathways by histone deacetylase (HDAC) inhibitors and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) synergistically induces mitochondrial damage and apoptosis in human leukemia cells. Mol Cancer Ther 2, 1273–1284, 2003.
- NunÄez G, Benedict MA, Hu Y, and Inohara N: Caspases: the proteases of the apoptotic pathway. Oncogene 17, 3245, 1998.
- Ott M, Gogvadze V, Orrenius S, and Zhivotovsky B: Mitochondria, oxidative stress and cell death. Apoptosis 12, 913–922, 2007.
- Iverson SL, and Orrenius S: The cardiolipin–cytochrome c interaction and the mitochondrial regulation of apoptosis. Arch Biochem Biophys 423, 37–46, 2004.
- Hsin Y-H, Chen C-F, Huang S, Shih T-S, Lai P-S, et al.: The apoptotic effect of nanosilver is mediated by a ROS- and JNK-dependent mechanism involving the mitochondrial pathway in NIH3T3 cells. Toxicol Lett 179, 130–139, 2008.
- Pozo-Guisado E, Alvarez-Barrientos A, Mulero-Navarro S, Santiago-Josefat B, and Fernandez-Salguero PM: The antiproliferative activity of resveratrol results in apoptosis in MCF-7 but not in MDA-MB-231 human breast cancer cells: cell-specific alteration of the cell cycle. Biochem Pharmacol 64, 1375–1386, 2002.
- Ahn G, Lee W, Kim KN, Lee JH, Heo SJ, et al.: A sulfated polysaccharide of Ecklonia cava inhibits the growth of colon cancer cells by inducing apoptosis. EXCLI J 14, 294–306, 2015.
- Thiery JP, Acloque H, Huang RY, and Nieto MA: Epithelial-mesenchymal transitions in development and disease. Cell 139, 871–890, 2009.
- Li J, Yang B, Zhou Q, Wu Y, Shang D, et al.: Autophagy promotes hepatocellular carcinoma cell invasion through activation of epithelial-mesenchymal transition. Carcinogenesis 34, 1343–1351, 2013.
- Ledford H: Cancer theory faces doubts. Nat 472, 273–273, 2011.
- Singh A, and Settleman J: EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 29, 4741–4751, 2010.
- Kwak H-H, Kim I-R, Kim H-J, Park B-S, and Yu S-B: α-Mangostin Induces Apoptosis and Cell Cycle Arrest in Oral Squamous Cell Carcinoma Cell. Evid-Based Complement Altern Med 2016, 1–10, 2016.
- Sang-Hun O, Sung-Jin P, Su-Bin Y, Yong-Ho K, In-Ryoung K, et al.: Mechanism underlying shikonin-induced apoptosis and cell cycle arrest on SCC25 human tongue squamous cell carcinoma cell line. Int J Oral Biology 40, 51–61, 2015.
- Son JH, Cho YC, Sung IY, Kim IR, Park BS, et al.: Melatonin promotes osteoblast differentiation and mineralization of MC3T3‐E1 cells under hypoxic conditions through activation of PKD/p38 pathways. J Pineal Res 57, 385–392, 2014.
- Zhou H-B, Yan Y, Sun Y-N, and Zhu J-R: Resveratrol induces apoptosis in human esophageal carcinoma cells. World J Gastroenterol 9, 408–411, 2003.
- Youle RJ, and Strasser A: The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 9, 47–59, 2008.
- Newmeyer DD: Ferguson-Miller S: Mitochondria: releasing power for life and unleashing the machineries of death. Cell 112, 481–490, 2003.
- Batlle E, Sancho E, Francí C, Domínguez D, Monfar M, et al.: The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol 2, 84–89, 2000.
- Huang W-Y, Cai Y-Z, and Zhang Y: Natural phenolic compounds from medicinal herbs and dietary plants: potential use for cancer prevention. Nutrition Cancer 62, 1–20, 2009.
- Treasure J: Herbal medicine and cancer: An introductory overview. Semin Oncol Nurs 21, 177–183, 2005.
- Birt DF, Hendrich S, and Wang W: Dietary agents in cancer prevention: flavonoids and isoflavonoids. Pharmacol Ther 90, 157–177, 2001.
- Seve M, Chimienti F, Devergnas S, Aouffen M, Douki T, et al.: Resveratrol enhances UVA-induced DNA damage in HaCaT human keratinocytes. Med Chem 1, 629–633, 2005.
- Wu J-Y, Yi C, Chung H-R, Wang D-J, Chang W-C, et al.: Potential biomarkers in saliva for oral squamous cell carcinoma. Oral Oncol 46, 226–231, 2010.
- Marocchio LS, Lima J, Sperandio FF, Corrêa L, and Sousa SOd: Oral squamous cell carcinoma: an analysis of 1,564 cases showing advances in early detection. J Oral Sci 52, 267–273, 2010.
- Jiang Z, Chen X, Chen K, Sun L, Gao L, et al.: YAP Inhibition by Resveratrol via Activation of AMPK Enhances the Sensitivity of Pancreatic Cancer Cells to Gemcitabine. Nutrients 8, 546, 2016.
- Li G, Rivas P, Bedolla R, Thapa D, Reddick RL, et al.: Dietary resveratrol prevents development of high-grade prostatic intraepithelial neoplastic lesions: involvement of SIRT1/S6K axis. Cancer Prev Res 6, 27–39, 2013.
- Sheth S, Jajoo S, Kaur T, Mukherjea D, Sheehan K, et al.: Resveratrol reduces prostate cancer growth and metastasis by inhibiting the Akt/MicroRNA-21 pathway. PloS one 7, e51655, 2012.
- Venkatadri R, Muni T, Iyer A, Yakisich J, and Azad N: Role of apoptosis-related miRNAs in resveratrol-induced breast cancer cell death. Cell Death & Dis 7, e2104, 2016.
- Ou X, Chen Y, Cheng X, Zhang X, and He Q: Potentiation of resveratrol-induced apoptosis by matrine in human hepatoma HepG2 cells. Oncology Rep 32, 2803–2809, 2014.
- Cohen G, Sun X, Snowden R, Dinsdale D, and Skilleter D: Key morphological features of apoptosis may occur in the absence of internucleosomal DNA fragmentation. Biochem J 286, 331–334, 1992.
- Brumatti G, Sheridan C, and Martin SJ: Expression and purification of recombinant annexin V for the detection of membrane alterations on apoptotic cells. Methods 44, 235–240, 2008.
- Niu G, and Chen X: Apoptosis imaging: beyond annexin V. J Nucl Med 51, 1659–1662, 2010.
- Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, et al.: Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. The J Exp Med 182, 1545–1556, 1995.
- Tait SW, and Green DR: Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biology 11, 621–632, 2010.
- Kang MH, and Reynolds CP: Bcl-2 Inhibitors: Targeting Mitochondrial Apoptotic Pathways in Cancer Therapy. Clin Cancer Res 15, 1126–1132, 2009.
- Kim H-E, Jiang X, Du F, and Wang X: PHAPI, CAS, and Hsp70 Promote Apoptosome Formation by Preventing Apaf-1 Aggregation and Enhancing Nucleotide Exchange on Apaf-1. Mol Cell 30, 239–247, 2008.
- Kim CH, Ko AR, Lee SY, Jeon HM, Kim SM, et al.: Hypoxia switches glucose depletion-induced necrosis to phosphoinositide 3-kinase/Akt-dependent apoptosis in A549 lung adenocarcinoma cells. Int J Oncol 36, 117, 2010.
- Elmore S: Apoptosis: A review of programmed cell death. Toxicol Pathol 35, 495–516, 2007.
- Nagata S, Nagase H, Kawane K, Mukae N, and Fukuyama H: Degradation of chromosomal DNA during apoptosis. Cell Death & Differ 10, 108–116, 2003.
- Wells A, Yates C, and Shepard CR: E-cadherin as an indicator of mesenchymal to epithelial reverting transitions during the metastatic seeding of disseminated carcinomas. Clin Exp Metastasis 25, 621–628, 2008.
- Shin NR, Jeong EH, Choi CI, Moon HJ, Kwon CH, et al.: Overexpression of Snail is associated with lymph node metastasis and poor prognosis in patients with gastric cancer. BMC Cancer 12, 1, 2012.
- Zeisberg M, and Neilson EG: Biomarkers for epithelial-mesenchymal transitions. The J Clin Invest 119, 1429–1437, 2009.
- Shankar S, Nall D, Tang S-N, Meeker D, Passarini J, et al.: Resveratrol inhibits pancreatic cancer stem cell characteristics in human and Kras G12D transgenic mice by inhibiting pluripotency maintaining factors and epithelial-mesenchymal transition. PLoS One 6, e16530, 2011.
- Gao Q, Yuan Y, Gan HZ, and Peng Q: Resveratrol inhibits the hedgehog signaling pathway and epithelial-mesenchymal transition and suppresses gastric cancer invasion and metastasis. Oncol Lett 9, 2381–2387, 2015.
