ABSTRACT
Background: Serum vitamin B12 levels have been proposed as one of the survival prediction factors, although no survival analysis in metastatic cancer patients has been conducted yet. This study examined whether serum vitamin B12 levels could be a prognostic factor in metastatic cancer patients. Methods: Data from a retrospective chart review were used to perform Kaplan-Meier and multivariate analyses of the Cox proportional hazards. Subgroup analysis was performed on patients without a liver lesion (hepatocellular carcinoma or liver metastasis). Results: A total of 523 patients were included. The median survival time was 1.8 months (mo) in the high B12 group (>911 pg/mL) and 5.1 mo in the normal B12 group (211–911 pg/mL) (p < 0.001). In patients without a liver lesion, the median survival times were 2.1 and 6.1 mo in the high and normal B12 groups, respectively (p < 0.001). Multivariate analysis revealed that serum vitamin B12 level was an independent prognostic factor for overall survival (hazard ratio [HR]: 1.62; 95% confidence interval [CI]: 1.34–1.96, p < 0.001). Conclusion: Serum vitamin B12 level can be used to predict survival time in metastatic cancer patients. Further large-scale cohort studies are required to confirm these findings.
Introduction
Metastatic cancer is incurable and its prognosis is poor. Medical teams have to make a choice between anticancer therapies to prolong the survival time and supportive care to manage patient symptoms according to individual circumstances. Although anticancer therapies could improve overall survival time in metastatic cancer patients Citation(1), the toxic effects of aggressive treatment can be detrimental Citation(2). Therefore, the risks and benefits should be considered for patients with a short life expectancy Citation(3). According to a study on terminal cancer patients, medical teams tend to optimistically predict survival time Citation(4), which can lead to the implementation of aggressive treatment rather than supportive care Citation(3). However, serious adverse events have been reported due to the application of aggressive treatment regimens on vulnerable patients Citation(5). Therefore, the accurate prediction of survival time is important for the welfare of terminal cancer patients Citation(6).
Several prognostic tools have been developed to more accurately predict survival time in cancer patients. As their performance status worsen, poor prognosis is observed Citation(7), and specific symptoms such as cancer-anorexia-cachexia syndrome (CACS), dyspnea, delirium, and cognitive failures have been suggested as prognostic factors of survival time Citation(8–10). Biological factors such as leukocytosis, lymphocytopenia, and elevated C-reactive protein (CRP) levels have also been used as survival time prediction markers Citation(11–13). Additionally, assessment tools such as palliative prognostic score (PaP) and palliative prognostic index (PPI), in which the symptoms listed above and biological factors are combined, have been developed (Citation14,Citation15).
Although studies on the significance of elevated vitamin B12 levels in cancer patients are limited, an association has recently been reported Citation(16). Furthermore, the diagnostic applicability of vitamin B12 levels as a nonspecific cancer marker has been under discussion Citation(17) and higher mortality has been observed in hepatocellular carcinoma patients with elevated serum vitamin B12 levels compared to those with normal levels Citation(18). In addition, there has been an effort to develop an index which reflects vitamin B12 levels as an indicator of survival time Citation(19), but survival time analysis research using serum vitamin B12 levels in metastatic cancer patients is limited despite the fact that survival time prediction is most needed for this population.
This study investigated the characteristics of patients with elevated serum vitamin B12 levels and the relationship between survival time and serum vitamin B12 levels among metastatic cancer patients with various solid tumors. A retrospective chart review was conducted to identify factors that could be used as indicators for poor survival time in patients with metastatic cancer.
Materials and Methods
Subjects
Data were collected from cancer patients who visited the Kyung Hee University Hospital at Gangdong, Seoul between March 2006 and June 2015. Those above 18 years of age whose serum vitamin B12 levels were measured were included. Patients who were not in the metastatic stage were excluded. Of the remaining included patients, except for those with leukemia or lymphoma, only patients with solid tumors were included. Patients who had undergone a procedure that could affect the absorption of vitamin B12, had received vitamin B12 therapy within the past two years, had gastric cancer, or had received a gastrectomy were excluded from the study. Patients with a low vitamin B12 levels (<211 pg/mL) were also excluded.
The protocol was approved by the Kyung Hee University Hospital at Gangdong Institutional Review Board (IRB approval number KHNMCOH 2015-08-004).
Clinical Data
Patient information including gender, age, cancer origin, liver metastasis, performance status, use of parenteral nutrition, infection status, vitamin B12 level, and creatinine level was collected during the chart review. If vitamin B12 measurements had been performed multiple times, the last measurement was used. High-risk groups (absolute neutrophil count <100 cells/μL, for >7 days) with chemotherapy-induced neutropenia that required the prophylactic use of antibiotics Citation(20) were not included in this study; so, it was assumed that individuals receiving antibiotic treatment had an infection. Therefore, infection status was defined as a patient who received antibiotic treatment. Patient survival was evaluated through December 31, 2015. Patients were divided into two groups according to serum vitamin B12 levels: normal (211–911 pg/mL) and high (>911 pg/mL). The clinical characteristics and survival times were compared between the two groups.
Tumor origin was defined as follows: non-small cell lung cancer and small cell lung cancer were included in the lung cancer group; the hepatobiliary cancer group included hepatocellular carcinoma, gallbladder cancer, and cholangiocarcinoma; the gynaecological cancer group included endometrial carcinoma, cervical cancer, and ovarian cancer; the urological cancer group included bladder cancer and renal cell carcinoma; and the other tumor group included esophageal cancer, ampulla of vater cancer, pancreatic cancer, prostate cancer, thyroid cancer, and unknown primary cancer.
Statistical Analysis
The results are presented as n (%) or mean ± standard deviation (SD). Continuous and categorical variables were compared between the two groups using Student's t-test and chi-squared test, respectively. The overall survival time analysis was performed using the Kaplan-Meier method, and the differences between the two groups were compared using log-rank test. We analyzed the overall survival time for all patients together and for each tumor group. Subgroup analysis was performed on data from patients without infection or liver lesion (hepatocellular carcinoma or liver metastasis). We investigated 30-day and 90-day mortality rates with corresponding 95% confidence intervals (CIs) according to vitamin B12 levels. To identify the predictors of survival, univariate and multivariate analyses were performed using the Cox proportional hazards model. Data were analyzed using PASW Statistics for Windows, version 18.0, and statistical significance was defined as p-values less than 0.05.
Results
Patient Characteristics
This study included 523 of 1,093 patients except for those who did not meet the inclusion criteria (four patients with low vitamin B12 levels were excluded); of the included patients, 269 (51.4%) were men and 254 (48.6%) were women. As shown in , 302 patients (57.7%) were in the normal B12 group and 221 patients (42.3%) were in the high B12 group. The mean vitamin B12 levels were 1360.8 pg/mL (range: 215–18,540) in total patients, 567.8 pg/mL (range: 215–906) in the normal B12 group, and 2,444.5 pg/mL (range: 923–18,540) in the high B12 group.
Table 1. Patient characteristics according to vitamin B12 levels.
There were no differences between the two groups in gender and age distribution, the proportion of patients who were currently receiving chemotherapy and radiotherapy, and creatinine levels, but, tumor origin differed significantly (p = 0.003); in the normal B12 group, the number of lung cancer patients was the highest, while in the high B12 group, the number of hepatobiliary cancer patients was the highest. There were also differences in liver lesion, administration of parenteral nutrition, and infection status between the two groups: the percentages of patients with a liver lesion were 33.1% and 55.2% in the normal and high B12 groups, respectively (p < 0.001); the percentages of patients who received parenteral nutrition were 19.9% and 33.5% in the normal and high B12 groups, respectively (p < 0.001); and the percentages of patients with infection were 28.8% and 47.1% in the normal and high B12 groups, respectively (p < 0.001). In addition, there were significant differences in Eastern Cooperative Oncology Group (ECOG) performance status scores between the two groups (p < 0.001); in the normal B12 group, 37.1% of patients had good performance status (ECOG <2), compared with 19.9% of patients in the high B12 group.
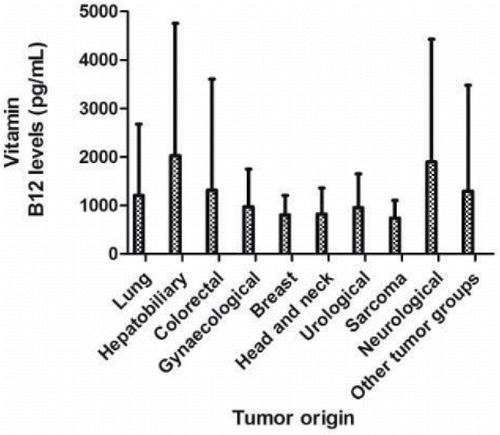
The mean vitamin B12 level of the patients differed according to the cancer origin. Hepatobiliary cancer patients had the highest mean vitamin B12 level (2,025.8 ± 2724.7 pg/mL), followed by neurological cancer (1,896 ± 2,527.8 pg/mL), colorectal cancer (1,314.3 ± 2,287.5 pg/mL), and lung cancer (1,205.9 ± 1,470.2 pg/mL). However, other breast cancer (803.6 ± 401.7 pg/mL), head and neck cancer (823.1 ± 531.9 pg/mL), and sarcoma (739.1 ± 361.8 pg/mL) had mean vitamin B12 levels within the normal range ().
Overall Survival Time
The median follow-up period, defined as the time from the test date to the survival time analysis period, was 57.9 ± 31.0 months (mo).
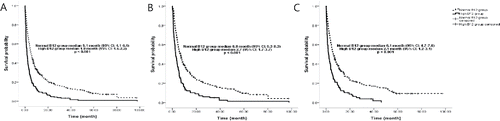
The 30-day mortality differed significantly between the two groups: 10.6% (95% CI: 7.3–14.6) in the normal B12 group and 38.9% (95% CI: 32.6–44.8) in the high B12 group (p < 0.001). The 90-day mortality also differed significantly between the two groups: 34.1% (95% CI: 29.5–40.1) in the normal B12 group and 63.8% (95% CI: 57.5–70.6) in the high B12 group (p < 0.001), as shown in .
Factors Influencing Overall Survival Times
In univariate analysis, the factors that influenced on overall survival time included age, liver lesion, performance status (ECOG ≥2), presently undergoing chemotherapy, administration of parenteral nutrition, infection status, serum creatinine level >1.2 mg/dL, and serum vitamin B12 levels > 911 pg/mL (see ). In multivariate analysis, performance status (HR: 1.81; 95% CI: 1.46–2.25, p < 0.001), administration of parenteral nutrition (HR: 1.69; 95% CI: 1.34–2.14, p < 0.001), serum vitamin B12 level (HR: 1.62; 95% CI: 1.34–1.96, p < 0.001), infection status (HR: 1.45; 95% CI: 1.17–1.80, p = 0.001), liver lesion (HR: 1.45; 95% CI: 1.20–1.75, p < 0.001), and serum creatinine level (HR: 1.40; 95% CI: 1.08–1.83, p = 0.012) were identified as factors affecting the overall survival time.
Discussion
We compared the characteristics and survival times of metastatic cancer patients with normal and high B12 levels and found that those with elevated serum vitamin B12 levels had a shorter survival time than those with normal serum vitamin B12 levels. Therefore, serum vitamin B12 level may be a useful prognostic factor in metastatic cancer patients.
In this study, among metastatic cancer patients with high serum vitamin B12 levels, infections, liver lesions, and parenteral nutrition support were more common, and their performance status tended to be lower than those with normal vitamin B12 levels. In particular, comparison of vitamin B12 levels for each tumor origin revealed the highest level among hepatobiliary cancer patients. The median survival time of the high B12 group was significantly lower than that of the normal B12 group and there was a significant difference in the 30-day and 90-day mortality rates between the groups. Subgroup analysis performed according to the cancer origin revealed significant differences in survival time according to serum vitamin B12 levels among patients with lung, hepatobiliary, colorectal, breast, or head and neck cancers. In patients with gynaecological, urological, and neurological cancers and sarcoma, statistically significant differences in survival times according to vitamin B12 levels were not observed, which might be due to the limited number of patients. Infection status and the presence of a liver lesion might affect serum vitamin B12 levels and may also be associated with elevated levels Citation(17). Therefore, subgroup analysis was performed after excluding patients with either infection or liver lesion in order to confirm whether vitamin B12 level could be used as an independent factor for cancer survival. In each analysis, patients with elevated vitamin B12 levels also had shorter survival times than those with normal vitamin B12 levels, which suggest that elevated levels are independently related to poor cancer survival. According to the results of the Cox proportional hazard analysis, patient performance status, administration of parenteral nutrition, vitamin B12 level, infection status, liver lesion, and creatinine level were independent prognostic factors of overall survival. In the institution in which this study was performed, the administration of parenteral nutrition is generally initiated when the patient's total intake is less than 30% of the daily nutritional requirements due to poor general condition and impaired oral intake. Therefore, the administration of parenteral nutrition could be considered an indicator of malnourishment instead of a prognostic factor. Furthermore, parenteral nutrition formulas contain a maximum of 5 μg vitamin B12 per day, which suggests a negligible contribution to serum vitamin B12 levels.
Generally, vitamin B12 measurements are performed only when a deficiency is suspected Citation(16). According to Stabler Citation(21), vitamin B12 deficiency is associated with megaloblastic anemia and neuropsychiatric disorders. However, elevated serum vitamin B12 levels were more prevalent than vitamin B12 deficiency among patients who underwent serum vitamin B12 measurement and only 11% of patients had vitamin B12 deficiency compared to 15% with high vitamin B12 levels Citation(16). Although elevated vitamin B12 levels are not unusual among patients, clinical information on elevated vitamin B12 is limited. Recent studies have shown that high serum vitamin B12 levels are observed with diseases such as solid tumor, hematologic malignancy, autoimmune disease, liver disease, infectious disease, and kidney disease, which suggests the importance of the clinical interpretation of elevated vitamin B12 levels (Citation22,Citation23). Moreover, in this study, the prevalence of vitamin B12 deficiency among metastatic solid tumor cancer (except gastric) patients was 0.8% (4/527 patients), while 41.9% (221/527 patients) of the patients had high vitamin B12 levels, which shows that, in particular, elevation of vitamin B12 is frequently observed in metastatic cancer patients.
Progress has been made regarding the significance of elevated vitamin B12 levels in cancer patients, with a reported 73% of patients diagnosed with cancer having elevated serum vitamin B12 levels Citation(22). Cancer risk increases with elevated vitamin B12 level, mostly within the first year of the follow-up period, suggesting that vitamin B12 level could be used as a cancer diagnostic marker Citation(24). In addition, the relationship between elevated vitamin B12 level and poor cancer survival time has been reported. Hepatocellular carcinoma patients with elevated vitamin B12 levels have a shorter median survival time in a dose-dependent manner Citation(18). According to a recent cohort study, cancer patients with an elevated vitamin B12 level prior to cancer diagnosis tended to have poorer one-year survival and higher 30-day mortality Citation(25). In addition, the vitamin B12/CRP index (BCI), an index generated from vitamin B12 and CRP levels, has been developed as a survival time predictor for cancer patients. In advanced cancer patients, the median survival time of patients with a BCI level over 40,000 is around 29 days (Citation19,Citation26,Citation27); unfortunately, 29 days is too short for the implementation of an appropriate intervention. However, in addition to being a prognostic factor, elevated CRP can also reflect transient, curable infection Citation(28). Therefore, measurement of vitamin B12 levels for survival time analysis of cancer patients instead of BCI has the advantage of not being influenced by confounding factors such as infection. Other survival time prognostic factors such as PaP and PPI have a survival prediction time of 30 days, which is fairly short (Citation14,Citation15), while that using vitamin B12 is around two months. In addition, vitamin B12 level is an objective value and not a symptom with subjective characteristics; thus, it is expected to have a wider range of application.
Vitamin B12 is metabolized and stored in the liver and a specific binding protein, transcobalamin (TC), is involved in the blood circulation and cellular uptake of vitamin B12 Citation(29). It is unclear why serum vitamin B12 levels are elevated, although the following mechanisms have been suggested. First, increased serum vitamin B12 levels may occur due to the direct disintegration or release of vitamin B12 from the liver due to liver disease. Second, increased TC levels may result in increased serum vitamin B12 levels because vitamin B12 only circulates in the blood when it is bound to TC; free vitamin B12 is excreted in the urine. TC levels may increase from their decreased clearance from circulation due to impaired liver or kidney function or by excessive TC synthesis induced by cancer or inflammation Citation(30). Third, serum vitamin B12 levels might increase because of decreased cellular uptake of vitamin B12 from the blood. In this case, excess vitamin B12 in the blood cannot be fully utilized by the cells, which leads to a vitamin B12 deficiency-like state, including problems in methionine synthesis, as indicated by elevated levels of homocysteine, an intermediate in methionine metabolism (Citation17,Citation23,Citation31). Of the three mechanisms mentioned above, the mechanism associated with elevated levels of vitamin B12 in cancer patients may be related to the excessive synthesis of TC by the cancer cells; in the case of cancer patients with a liver lesion, it is also related to direct dissolution of vitamin B12 from the liver.
This study has several limitations. It is a retrospective study based on chart reviews and includes various types of cancers; however, cancer-specific survival time analyses were performed to overcome this limitation. For several cancer origins, significant differences were found in survival times according to serum vitamin B12 levels. However, no significant differences in survival times were observed in others, which might be due to the limited number of patients in these groups. Additional large-scale cohort or experimental studies are required to elucidate the exact mechanism and to confirm the significance of vitamin B12 as a predictor of cancer patient survival time.
Conclusion
In conclusion, the results of this study suggest that vitamin B12 level is a useful independent prognostic factor in metastatic cancer patients. Therefore, serum vitamin B12 levels may be useful for the prediction of life expectancy in metastatic solid cancer patients. Prediction based on vitamin B12 levels could help clinician make better decisions regarding the appropriateness of aggressive anticancer therapy.
Funding
This study was supported by the Traditional Korean Medicine R&D program funded by the Ministry of Health & Welfare through the Korea Health Industry Development Institute (KHIDI) (grant no. HB16C0067) and Research Institute of Human Ecology at Seoul National University.
References
- Folprecht G, Seymour MT, Saltz L, Douillard JY, Hecker H, et al.: Irinotecan/fluorouracil combination in first-line therapy of older and younger patients with metastatic colorectal cancer: combined analysis of 2,691 patients in randomized controlled trials. J ClinOncol 20, 1443–1451, 2008.
- Jatoi A, Foster NR, Egner JR, Burch PA, Stella PJ, et al.: Older versus younger patients with metastatic adenocarcinoma of the esophagus, gastroesophagealjunction, and stomach: a pooled analysis of eight consecutive North Central Cancer Treatment Group (NCCTG) trials. Int J Oncol 36, 601–606, 2010.
- Weeks JC, Cook EF, O'Day SJ, Peterson LM, Wenger N, et al.: Relationship between cancer patients'predictions of prognosis and their treatment preferences. JAMA 279, 1709–1714, 1998.
- Glare P, Virik K, Jones M, Hudson M, Eychmuller S, et al.: A systematic review of physicians' survival predictions in terminally ill cancer patients. BMJ 327, 195–198, 2003.
- Hamaker ME, Seynaeve C, Wymenga AN, van Tinteren H, Nortier JW, et al.: Baseline comprehensive geriatric assessment is associated with toxicity and survival in elderly metastatic breast cancer patients receiving single-agent chemotherapy: Results from the OMEGA study of the Dutch breast cancer trialists' group. Breast 23, 81–87, 2014.
- den Daas N: Estimating length of survival in end-stage cancer: a review of the literature. J Pain Symptom Manage 10, 548–555, 1995.
- Kim YJ, Kim SJ, Lee JK, Choi WS, Park JH, et al.: Prediction of survival in terminally ill cancer patients at the time of terminal cancer diagnosis. J Cancer Res ClinOncol 140, 1567–1574, 2014.
- Reuben DB, Mor V, and Hiris J: Clinical symptoms and length of survival in patients with terminal cancer. Arch Intern Med 148, 1586–1591, 1988.
- Caraceni A, Nanni O, Maltoni M, Piva L, Indelli M, et al.: Impact of delirium on the short term prognosis of advanced cancer patients: Italian multicenter and study group on palliative care. Cancer 89, 1145–1149, 2000.
- Bruera E, Miller MJ, Kuehn N, MacEachern T, and Hanson J: Estimate of survival of patients admitted to a palliative care unit: a prospective study. J Pain Symptom Manage 7, 82–86, 1992.
- Maltoni M, Pirovano M, Nanni O, Marinari M, Indelli M, et al.: Biological indices predictive of survival in 519 Italian terminally ill cancerpatients. Italian multicenter study group on palliative care. J Pain Symptom Manage 13, 1–9, 1997.
- Frydenberg H, Thune I, Lofterød T, Mortensen ES, Eggen AE, et al.: Pre-diagnostic high-sensitive C-reactive protein and breast cancer risk, recurrence, and survival. Breast Cancer Res Treat 155, 345–354, 2016.
- Maltoni M, Caraceni A, Brunelli C, Broeckaert B, Christakis N, et al.: Prognostic factors in advanced cancer patients: evidence-based clinical recommendations—a study by the steering committee of the european association for palliative care. J ClinOncol 23, 6240–6248, 2005.
- Mendis R, Soo WK, Zannino D, Michael N, and Spruyt O: Multidisciplinary prognostication using the palliative prognostic score in an Australian cancer center. Palliat Care 9, 7–14, 2015.
- Subramaniam S, Thorns A, Ridout M, Thirukkumaran T, and Osborne TR: Accuracy of prognosis prediction by PPI in hospice inpatients with cancer: a multi-centre prospective study. BMJ Support PalliatCare 5, 399–404, 2015.
- Arendt JF, and Nexo E: Cobalamin related parameters and disease patterns in patients with increased serum cobalamin levels. PLoSOne 7, e45979, 2012. doi: 10.1371/journal.pone.0045979.
- Arendt JF, and Nexo E: Unexpected high plasma cobalamin: proposal for a diagnostic strategy. ClinChem Lab Med 51, 489–496, 2013.
- Lin CY, Kuo CS, Lu CL, Wu MY, and Huang RF: Elevated serum vitamin B12 levels in association with tumor marker as the prognostic factors predictive for poor survival in patients with hepatocellular carcinoma. NutrCancer 62, 190–197, 2010.
- Kelly L, White S, and Stone PC: The B12/CRP index as a simple prognostic indicator in patients with advanced cancer: a confirmatory study. Ann Oncol 18, 1395–1399, 2007.
- Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, et al.: Infectious diseases society of America: clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis 52, e56–93, 2011. doi: 10.1093/cid/cir073.
- Stabler SP: Clinical practice. Vitamin B12 deficiency. N Engl J Med 368, 149–160, 2013.
- Chiche L, Jean R, Romain F, Roux F, Thomas G, et al.: Clinical implications of high cobalamin blood levels for internal medicine. Rev Med Interne 29, 187–194, 2008.
- Andrès E, Serraj K, Zhu J, and Vermorken AJ: The pathophysiology of elevated vitamin B12 in clinical practice. QJM 106, 505–515, 2013.
- Arendt JF, Pedersen L, Nexo E, and Sørensen HT: Elevated plasma vitamin B12 levels as a marker for cancer: a population-based cohort study. J Natl Cancer Inst 105, 1799–1805, 2013.
- Arendt JF, Farkas DK, Pedersen L, Nexo E, and Sørensen HT: Elevated plasma vitamin B12 levels and cancer prognosis: a population-based cohort study. Cancer Epidemiol 40, 158–165, 2016.
- Geissbühler P, Mermillod B, and Rapin CH: Elevated serum vitamin B12 levels associated with CRP as a predictive factor of mortality in palliative care cancer patients: a prospective study over five years. J Pain Symptom Manage 20, 93–103, 2000.
- Tavares F: Is the B12/CRP Index more accurate than you at prediction life expectancy in advanced cancer patients? J Pain Symptom Manage 40, e12–13, 2010. doi: 10.1016/j.jpainsymman.2010.03.007.
- Smith RP, Lipworth BJ, Cree IA, Spiers EM, and Winter JH: C-reactive protein. a clinical marker in community-acquired pneumonia. Chest 108, 1288–1291, 1995.
- Solomon LR: Disorders of cobalamin (vitamin B12) metabolism: emerging concepts in pathophysiology, diagnosis and treatment. Blood Rev 21, 113–130, 2007.
- Ermens AA, Vlasveld LT, and Lindemans J: Significance of elevated cobalamin (vitamin B12) levels in blood. Clin Biochem 36, 585–590, 2003.
- Carmel R, and Eisenberg L: Serum vitamin B12 and transcobalamin abnormalities in patients with cancer. Cancer 40, 1348–1353, 1977.