ABSTRACT
The review discusses the effects of Epigallocatechin-3-gallate Gallate (EGCG) on glioma as a basis for future research on clinical application of EGCG. Epidemiological studies on the effects of green tea or EGCG on the risk of glioma is inconclusive due to the limited number of studies, the inclusion of all tea types in these studies, and the focus on caffeine rather than EGCG. In vivo experiments using EGCG monotherapy are inconclusive. Nevertheless, EGCG induces cell death, prevents cellular proliferation, and limits invasion in multiple glioma cell lines. Furthermore, EGCG enhances the efficacy of anti-glioma therapies, including irradiation, temozolomide, carmustine, cisplatin, tamoxifen, and TNF-related apoptosis-inducing ligand, but reduces the effect of bortezomib. Pro-drugs, co-treatment, and encapsulation are being investigated to enhance clinical applicability of EGCG. Mechanisms of actions of EGCG have been partly elucidated. EGCG has both anti-oxidant and oxidant properties. EGCG inhibits pro-survival proteins, such as telomerase, survivin, GRP78, PEA15, and P-gp. EGCG inhibits signaling of PDGFR, IGF-1R, and 67LR. EGCG reduces invasiveness of cancer cells by inhibiting the activities of various metalloproteinases, cytokines, and chemokines. Last, EGCG inhibits some NADPH-producing enzymes, thus disturbing redox status and metabolism of glioma cells. In conclusion, EGCG may be a suitable adjuvant to potentiate anti-glioma therapies.
Introduction
Glioma is a heterogeneous group of tumors originating from the support cells of the brain and accounts for approximately 30% of all central nervous system tumors and 80% of malignant brain tumors (Citation1). In the United States, there are more than 18,500 newly diagnosed cases and 13,000 deaths due to malignant glioma every year (Citation2). In the Netherlands, the incidence of glioma in adults has increased over time (from 4.9 per 100,000 person years in 1989 to 5.9 in 2010), but this may in part result from improved diagnosis (Citation3). Glioma has higher numbers of lost life years than any other type of cancer (Citation4). The World Health Organisation (WHO) categorizes glioma into ependymoma, pilocytic astrocytoma, oligodendroglioma, and astrocytoma (Citation5). Additional classification of tumor grade (I–IV) is defined by histopathological features, specifically by the levels of nuclear polymorphism, increased mitotic activity and cellularity, and the presence of neovascularization and necrosis (Citation6). Recent advances in next-generation sequencing allowed the identification of genetic abnormalities in glioma that added another layer to the diagnosis of glioma. A recent WHO re-classification also considers molecular characteristics such as the mutational status of genes encoding for isocitrate dehydrogenase 1 and 2 (IDH1/2) and loss of heterozygosity of chromosomes 1p and 19q (Citation6). The stage of glioma has implications for the prognosis of patients. Ependymoma and pilocytic astrocytoma (grade 1) are typically benign and slowly growing tumors; however, when becoming malignant, patients have a poor prognosis (Citation1). Life expectancy for patients with grade II astrocytomas ranges from 5 to over 15 yr (Citation1). Grade III astrocytoma often progresses to grade IV astrocytoma, i.e., secondary glioblastoma, and has a 5-yr survival rate of only 30% (Citation1). Oligodendroglioma has a better outcome with a 5-yr survival rate of 80% (Citation1). Grade IV glioma (glioblastoma) is the most aggressive entity of glioma with a 5-yr survival rate of merely 5%, and represents approximately 15% of all primary brain and central nervous system tumors and 55% of all glioma (Citation1). A characteristic of all grades II–IV gliomas is that they grow diffusely in the brain parenchyma, often behind the blood–brain–barrier (BBB), shielding cells from most systemically administered drugs (Citation5).
Within these pathological classes of glioma, several prognostic and predictive distinctions can be made with implementation of molecular markers such as mutational status of IDH1/2 and TP53, hypermethylation of the MGMT promoter and loss of (parts of) chromosome arms 1p and 19q (Citation7). Hypermethylation of the promoter of the DNA repair protein MGMT is a predictive marker of response to temozolomide (TMZ) treatment in glioblastoma patients (Citation8). In fact, the combination of IDH1 mutation and MGMT promoter hypermethylation predicts survival of glioblastoma patients better than either IDH1 mutation or MGMT promoter hypermethylation alone (Citation9). On the basis of mRNA expression profiling, Verhaak et al. suggested classification of glioblastoma into subtypes: classical, mesenchymal, proneural, and neural, which are defined by amplifications of or mutations in genes encoding for epidermal growth factor receptor, neurofibromatose type 1, platelet-derived growth factor receptor α (PDGFRα), and IDH1 (Citation10). There is no single molecular alteration that defines the neural subtype (Citation10). These molecular subclasses of glioblastoma have an implication for the responses of glioblastoma to current therapies, for instance TMZ and irradiation (IR) (Citation10).
The current treatment of newly diagnosed glioblastoma includes maximum safe tumor resection, 6 wk of IR with concomitant TMZ chemotherapy, and six cycles of adjuvant TMZ (Citation2). The median progression-free survival interval is 7–10 mo after initial surgery (Citation11). Post-operation radiotherapy extends survival to approximately 12 mo (Citation11). The use of TMZ in combination with surgical resection and radiotherapy extends the median survival period up to 14.6 mo and increases the 2-yr survival rate from 10% to 27% (Citation11). Overall, the current treatment scheme for malignant glioma has increased the life expectancy by approximately only 1 yr, when compared to the natural course of the disease (Citation11). Despite all research, the standard treatment scheme that is currently offered for malignant gliomas has limited efficacy. Virtually all glioblastomas eventually relapse due to drug-resistant glioma stem-like cells (GSLCs) (Citation12).
Besides the poor outcome of these aggressive treatments, the associated adverse events and high costs pose problems. Direct costs per patient for surgery and radiotherapy alone are in the range of €50,000–92,000 per patient (Citation2). Estimated indirect costs of the disease, based on sick leave, early retirement and mortality, mounted up to almost 200 million US dollars for the Swedish population in 1996 and accounted for 74% of the total cost of illness per case of glioma (Citation13). Additionally, the Food and Drug Administration granted approval to bevacizumab in 2009 for treatment of recurrent glioblastoma in the United States, which means an additional economic burden (Citation2). One year of bi-weekly treatment with 10 mg/kg dose of bevacizumab costs approximately $120,000–240,000 per patient (Citation2). Because of the poor outcome of the present treatment options and the high costs of treatment, there is an urgent need to improve the treatment outcome and reduce economic burden. The use of dietary nutrients or phytochemicals to improve prognosis for glioma patients is a poorly explored, yet highly interesting option by virtue of their putative favorable safety profile and low costs.
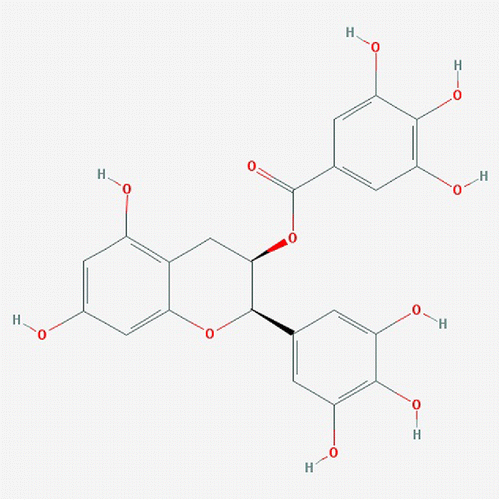
Tea is the second most consumed beverage in the world after water, and has been studied extensively for its health benefits (Citation14). The three main types produced from the leaves of the Camellia sinensis plant are green tea, oolong tea, and black tea. These types of tea differ in their after-harvest treatment process, which strongly affects the chemical composition of each tea type (Citation15). Green tea is produced by steaming freshly harvested leaves to prevent fermentation of polyphenols. The production of black tea involves withering, rolling, and crushing of fresh tea leaves, facilitating the fermentation process. Oolong tea is an intermediate product of the production process for green and black tea, with partial oxidation of the leaves. Among all tea types consumed over the world, green tea is best known for its health benefits. The polyphenol flavonoids, which are preserved in green tea, are the major chemical compounds contributing to its anti-microbial, immune-regulating and anti-inflammatory effects (Citation16). Claims are that polyphenols have beneficial effects in prevention and treatment of various diseases, such as cardiovascular diseases, arthritis, and diabetes (Citation16). There are four major polyphenol flavonoids in green tea: epigallocatechin-3-gallate (EGCG), epicatechin-3-gallate (ECG), epigallocatechin (EGC), and epicatechin (EC) (Citation15). Among these, EGCG () is the most abundant and most active catechin with an anti-oxidant activity that is 25–100 times more potent than that of vitamins C and E (Citation15). As a result, EGCG has long been considered to be a dietary compound with therapeutic potential.
In the last decades, EGCG has been frequently examined for its preventive and curative effects on various types of cancer. Phase II clinical trials have demonstrated an inhibitory effect of green tea extract on the progression of prostate pre-malignant lesions, even though observational studies do not support a beneficial role of tea intake on prostate cancer risk (Citation17). Other studies have also shown protective effects of green tea on cervical cancer, and on breast cancer in premenopausal women (Citation17,Citation18). In vivo and in vitro studies have demonstrated various molecular mechanisms of the anticancer activities of EGCG. EGCG has both anti-oxidant and pro-oxidant properties, inhibits the activities of multiple cellular compounds by direct binding, and regulates various pathways involved in cellular proliferation, apoptosis, and migration (Citation15).
Recently, a number of studies, both observational and experimental, has shown effects of green tea and EGCG intake on glioma. However, a critical review of these studies is not available to evaluate the effects of green tea and EGCG on glioma, and to determine whether green tea or EGCG can be added to the treatment schemes for glioma. Hence, this review aims to evaluate the current state of understanding of the effects of EGCG on glioma, as a basis for future research on the application of EGCG in glioma prevention and treatment. A systematic search using PubMed and ScienceDirect was conducted to sample all relevant peer-reviewed studies. Relevant publications that were referred to in these studies were also included.
Epidemiological Evidence
A large number of studies investigated the associations between tea consumption and the risk of developing different types of cancer (Citation17). However, only one case–control study, three prospective cohort studies and one meta-analysis were performed to determine the effects of tea on the risk of developing glioma (). Besides these five studies, there are three other studies examining dietary categories and glioma risk (Citation19–21). However, these three studies do not allow reliable conclusions on EGCG, as tea was lumped with other caffeinated beverages in these studies.
Table 1. Tea intake and its effects on the risk of glioma development.
The only case–control study to examine the association between the risk to develop glioma and tea intake was conducted by Burch et al. almost 30 yr ago and no association (risk ratio (RR) = 1.26; 95% confidence interval (CI): 0.70–2.25) was found for high intake compared with “never used regularly” (Citation22). In 2010, Holick et al. investigated the effects of tea on glioma development in a prospective cohort study (Citation23). The results of this study were drawn from a pooled population of participants in three US cohorts (Nurses’ Health Study I and II, and the Health Professionals Follow-up Study) and included 335 glioma cases (Citation23). The effects of tea and/or coffee were studied with 0–1 cup per day as a reference (Citation23). Intake of eight or more cups of tea per week did not provide any advantage (RR = 0.71, 95% CI: 0.45–1.12), whereas daily consumption of five or more cups of coffee and tea as compared to no consumption was associated with a decreased risk of glioma development (RR = 0.60; 95% CI: 0.41–0.87) (Citation23).
Michaud et al. reported in 2010 on the effects of coffee and tea on glioma development in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort (Citation24). The analysis was performed on 410,309 male and female subjects with a total of 343 glioma cases from ten countries in Europe, including Denmark, France, Germany, Greece, Italy, the Netherlands, Norway, Spain, Sweden, and the United Kingdom (Citation24). The study population was divided into four quantiles depending on the amount of daily tea intake, and the association between daily tea intake and the risk to develop glioma was analyzed by comparing the lowest tea intake quintile with the other quintiles (Citation24). There was no effect of tea consumption alone on the risk of developing glioma (Citation24). The authors also compared the risk of the population that had a combined coffee and tea intake of >100 ml/day with the risk of the population that consumed <100 ml/day (Citation24). The RR of each individual country showed no significant association (except for Spain with RR = 1.03; 95% CI: 0.54–0.95), whereas the pooled results showed that coffee and tea consumption of >100 ml/day provided a reduced risk of glioma occurrence (RR = 0.66; 95% CI: 0.45–0.96) (Citation24).
The most recent prospective cohort study was performed by Dubrow et al. in the United States with a population of 545,771 participants (Citation25). This population showed a high incidence of glioma (904 cases) (Citation25). The base-multivariate model showed a significant difference in the risk to develop glioma between the highest level of tea intake and no tea intake (three or more cups per day vs. none: RR = 0.75; 95% CI: 0.57–0.99) (Citation25). In addition, consumption of five cups or more per day of “coffee and tea” reduced the risk to develop glioma (RR = 0.65; 95% CI: 0.43–0.97) (Citation25).
In the meta-analysis of Marleba et al. (Citation26), the results of the studies of Dubrow et al. (Citation25), Holick et al. (Citation23), and Burch et al. (Citation22) were pooled to investigate the association between tea intake and the risk to develop glioma by comparing drinkers vs. non/occasional drinkers. The study of Michaud et al. (Citation24) was excluded from the analysis, because the lowest quintile of tea intake was used in their study as a reference instead of no intake or occasional intake (<1 cup per day) as was done in the other three studies (Citation22,Citation23,Citation25). The analysis showed a health benefit of tea consumption to prevent the development of glioma (RR = 0.86; 95% CI: 0.78–0.94). However, no difference in glioma risk was found between the highest tea intake group and the lowest tea intake group (RR = 0.88; 95% CI: 0.69–1.12).
A critical interpretation of the outcomes of the five studies leads to the conclusion that there is no consensus with respect to the preventive effect of tea intake on glioma development because of confounding effects, differences in study design and/or limited evidence. The lack of classification of tea types and inclusion of coffee [a result of the focus in these studies on the effects of caffeine, which is known to affect cell-cycle checkpoints, DNA repair, and apoptosis (Citation23)] may have created a major confounding effect on the results of these studies. The three tea types have different chemical compositions that significantly affect their health effects. For instance, Cabrera et al. examined the levels of multiple tea components in 45 samples of commercially available tea in Spain, and reported vast differences in concentrations of catechins and other health-benefit-providing compounds, such as gallic acid and caffeine (Citation27). Lin et al. confirmed these differences in extract samples of six commercially available tea types and showed that green tea had the highest EGCG level as compared to oolong tea, black tea, or red tea (Citation28). The authors also demonstrated that the apoptosis-inducing capacity in HL-60 cells was highest in green tea, then in oolong, followed by black tea, and lowest in red tea (Citation28). The different chemical compounds in black tea and green tea are considered to provide different health benefits (Citation29). In the western world, the most commonly consumed type of tea is black tea, whereas green tea is the main tea type in Japan and parts of China. Oolong tea only accounts for 2% of tea consumed worldwide and is used mainly in southern China and Taiwan (Citation30). It has been shown that there is around a fourfold difference in the incidence of primary malignant brain tumors between countries of high incidence (US, Canada, New Zealand, Australia, and Finland), and countries of low incidence (Philippines and India) (Citation4). In addition, the malignant brain tumor rate in Northern European countries is double that in Japan (Citation4). Thus, differences in environmental factors, including the consumption of different tea types, may account for some of the observed geographic differences (Citation4). Hence, future research should include the tea type as an important variable in questionnaires and control for this variable when analyzing the data.
The small number of epidemiological studies investigating the health benefit of tea on glioma reduces the reliability of the conclusions. Unlike malignancies of the prostate, breast, esophagus, stomach, colon, lung, liver or pancreas, glioma have not been intensively investigated in relation to tea intake (Citation17). Thus, more epidemiological studies, especially case-control studies, should be performed to establish the effects of tea on the risk of glioma development. Furthermore, such studies should examine specifically the effects of EGCG as a pure compound to allow reliable conclusions on its effects.
In conclusion, it is not yet possible to draw conclusions on the preventive effects of green tea or EGCG on the development of glioma. Hence, it is necessary to perform more studies on the association between tea, particularly green tea, and the risk to develop glioma with these recommendations in mind. As green tea is more frequently consumed in Eastern Asian countries and the average daily intake of green tea is high, future research should be performed in those countries.
The Effects of EGCG Monotherapy on Glioma in Animal Models
The anticancer effects of EGCG have been investigated in multiple animal models of cancer in over 100 publications (Citation31). EGCG in doses of 30–45 mg/kg has demonstrated significant anti-tumor effects in mice carrying xenografts of melanoma, lung carcinoma, colon carcinoma, pancreatic cancer, and prostate carcinoma (Citation31–33), but not breast carcinoma (Citation34), suggesting that identification of predictive markers is important. Recently, two studies examined the effects of EGCG on glioma in animal models (). The results of these studies are discussed in this section.
Table 2. The effects of EGCG monotherapy in animal models of glioma.
In 2011, Chen et al. examined the effects of EGCG monotherapy on survival of nude mice with intracranial U251 or U87 glioma xenografts (Citation35). U251 (mutated TP53) or U87 (wild-type TP53) cells were injected into the right frontal lobe of the mouse brain. Seven days after tumor cell delivery, EGCG (50 mg/kg) was administered daily by oral gavage in a 7-day-on/7-day-off cycle. This 14-day cycle was repeated until the animals became moribund and were sacrificed. EGCG treatment did not prolong survival in both animal models. The authors reasoned that the BBB may have limited EGCG distribution to the cancer cells. Indeed, pharmacodynamics studies in rat confirmed low brain:plasma ratios of EGCG, which suggested poor penetration of EGCG over the BBB due to the bipolar functional groups in EGCG (Citation36). However, the BBB in orthotopic U87 xenografts is characteristically leaky (Citation37), so the results of Chen et al. suggested that EGCG did not have direct anti-glioma effects in this model (Citation35).
On the other hand, EGCG has been shown to potentiate the anti-glioma effect of TMZ in orthotopically grown U87 and U251 glioma xenografts in nude mice (Citation35). Moreover, EGCG monotherapy inhibited growth of subcutaneous U251 xenografts in a study of Zhang et al. (Citation38). Mice received daily 50 mg/kg EGCG via intraperitoneal injection for 35 days, starting at one day after xenografting (Citation38). Tumor growth was significantly inhibited in the treatment group (P = 0.0052). Hence, EGCG can potentially inhibit the growth of glioma (Citation38).
There is a number of explanations for the differences in EGCG effects in both studies. First, the site of implantation is important, since it directly impacts on cell metabolism and behavior (Citation37). In the avascular subcutaneous space, tumors will rapidly experience hypoxia and are forced to induce a neovascular bed, contrasting the situation in brain in which cancer cells may use pre-existent vessels (Citation39). Furthermore, glioma cells may be protected to some extent by the BBB, although this issue seems less relevant in case of U87 (Citation37). Another explanation for the difference in efficacy may be in the dosing and route of administration. Chen et al. administered EGCG orally and with longer intervals between doses than Zhang et al. (Citation35,Citation38). Furthermore, the primary endpoint of the study of Chen et al. was overall mouse survival, whereas Zhang et al. directly measured tumor volumes (Citation35,Citation38). Therefore, future studies should test the effects of different dosages of EGCG, preferably administered via direct intravenous or intraperitoneal injection on orthotopic models of glioma. Moreover, longitudinal evaluation of EGCG effects via non-invasive imaging (e.g., MRI, luminescence imaging) provides better read-outs of anti-tumor effects (Citation37). Another area of research that also requires much attention is the transportation of EGCG over the BBB (Citation36).
EGCG Inhibits Glioma Cell Growth in Vitro
EGCG inhibits the growth of various types of human cancer cells in vitro, including glioma cells (Citation16,Citation31). Cell proliferation, apoptosis, and invasive behavior of glioma cells are affected by EGCG (), whereas the growth inhibitory effect of EGCG is not observed in control human astrocytes, which increases its potential for clinical applications (Citation40–42).
Table 3. The effects of EGCG on glioma cells in vitro.
Cell Proliferation
The cytotoxic effects of a drug are usually determined by its effects on cell viability. Viability assays measure the activities of metabolically active proteins. Tetrazolium compounds, such as MTT, MTS, and WST-8, are frequently used in these assays as they are reduced by viable cells to their purple formazans (Citation43). In addition, viability can be measured in ATP-quantification assays. Multiple studies have demonstrated that EGCG inhibits viability of human glioma cell lines (U251, MO59J, U373, U87, and U87 GSLC, 1321N1, SW1783, LN18) and the rat glioma cell line C6 (Citation40,Citation42,Citation44–47). However, since EGCG has important effects on metabolism, as explained in later sections, outcome of all these assays needs to be interpreted with care since quiescence may be confused with cell death.
Colony-formation assays have been performed to measure long-term effects of EGCG treatment. At 10–20 μM, EGCG did not inhibit colony formation of LN229 and U251 glioma cells in the study of Golen et al. (Citation48), but a dose-dependent inhibition of colony formation by EGCG was reported by Chen et al. (0–100 μM of EGCG applied to U87 and U251 cells) (Citation35) and Zhang et al. (0–200 μM of EGCG applied to U87 and U87 GSLC (Citation47). U87 GSLCs express stem cell markers such as CD133 and ALDH1 and are considered to make up the tumorigenic cell population in glioblastoma with an unlimited proliferation potential to support tumor development and maintenance (Citation12). Furthermore, two independent studies reported that 20–50 μM EGCG prevented spheroid formation of A172 glioma cells in semi-solid agar (Citation49,Citation50). These results further confirm the growth inhibitory effect of EGCG on glioma cells.
However, there are contradictory results showing no significant effects of EGCG, which may have resulted from different durations of treatment and, possibly, oxidation during storage (Citation34). Interestingly, one study showed that low concentrations of EGCG (<5 μM) stimulate colony formations of U87 and U251 cells (Citation35). This phenomenon can be explained by the multiple modes of action of EGCG, which are discussed in sections below.
Cell Death
A drug can exert its cytotoxic effects by induction of cell death. Dead cells can be detected by using various assays based on, for example, terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL), annexin-V, trypan blue, propidium iodide (PI), or Wright staining. Multiple studies reported tumoricidal effects of EGCG on human glioma cells (U87, T98G, U373, U251, A172, MO59J) and the rat glioma cell line C6 (Citation41,Citation42,Citation44,Citation46,Citation51–53), whereas normal human astrocytes and rat pituitary tumor cells (MtT/E) were resistant to the effects of EGCG (Citation41,Citation42). Another way to detect cell death is to measure the mitochondrial membrane potential, the activity of apoptosis-associated proteins, and the activity of anti-apoptotic proteins. Indeed, disrupted mitochondrial potential, decreased levels of BCL-2 and phosphorylated AKT, and increased levels of BAX, activated caspases, free calcium, cleaved BID, and cleaved PARP have been found after EGCG treatment (Citation41,Citation47,Citation51).
Controversies exist in the literature with respect to the apoptosis-inducing effects of EGCG. Chen et al. did not observe increased death of U87 cells after 48 h of treatment with 20 μM EGCG using PI and TUNEL assays (Citation35), while McLaughlin et al. reported cell death-induction effects of EGCG on U76 cells at lower EGCG concentrations and shorter incubation periods (6 h) (Citation52). Therefore, it is important that these studies are repeated with proper preparations of (non-oxidized) EGCG under different conditions with respect to concentration and period of incubation. Given the large number of studies that do report cell-killing effects of EGCG, it seems appropriate to conclude that EGCG can induce cell death in glioma cells in vitro.
Invasion
Glioma tumors are highly invasive that limits the effectiveness of surgery and IR. Therefore, inhibition of invasiveness of glioma cells is of utmost importance for the outcome of glioma patients, because it allows more radical local therapy (further explained in section V.1). Investigation of this aspect of EGCG in clinically relevant animal models of diffuse glioma, representing angiogenic and diffuse growth patterns, have unfortunately not been performed so far. An alternative way to test invasiveness of cells is the matrigel assay and multiple studies reported inhibitory effects of EGCG on invasiveness of multiple glioma cell lines (U87, U87 GSLC, and U251) (Citation46,Citation47,Citation51,Citation54). Validation of these results in vivo settings would be of great importance, because glioblastoma characteristically contains angiogenic areas with leaky vessels and disrupted BBB, which may be amenable for EGCG targeting.
The Effects of EGCG in Combination with other Therapies
EGCG has been shown to enhance efficacy of chemotherapeutic agents in in vitro and in vivo settings for various types of cancer. For example, EGCG can boost the effects of paclitaxel therapy in breast cancer, doxorubicin therapy in liver cancer, and interferon therapy in melanoma (Citation27,Citation29,Citation55). Since the standard care for malignant glioma has limited efficacy, adjuvant treatments with EGCG may be a highly interesting option ().
Table 4. The effects of the combination of EGCG with other anti-glioma therapies in vitro and in vivo.
Radiotherapy
McLaughlin et al. examined whether EGCG treatment potentiated anti-glioma effects of IR using U87 cells (Citation52). First, the radio-resistant properties of U87 cells were demonstrated using high doses of IR (10–30 Gy), and were compared to other cell lines such as DAOY, U118, and U138. U87 cells showed a 40% decrease in proliferation rate after exposure to 10 Gy IR and 50% decrease after exposure to 30 Gy IR. The combined treatment of EGCG and 10 Gy IR reduced the proliferation rate of U87 cells by 60%, whereas cells treated with EGCG alone or IR alone only showed a proliferation rate reduction of 35% and 40%, respectively. Therefore, EGCG can potentially be used to enhance anti-glioma IR. Strikingly, EGCG has been shown to protect DU145 cells against IR (Citation56). Thus, it is important to identify the underlying mechanisms explaining the different effects of EGCG on different cell lines to avoid radioprotection of glioma by EGCG instead of radiosensitization.
Chemotherapeutic Agents
TMZ is an alkylating chemotherapeutic agent that is used for newly diagnosed glioblastoma but, as discussed, its effects are limited to a few months of prolonged overall survival (Citation57), emphasizing that more efficient treatment options are urgently needed. Apart from synergizing with TMZ (Citation35), EGCG also potentiated the effects of carmustine (BCNU), cisplatin, and tamoxifen in various glioma cell lines (Citation38,Citation47). Carmustine is an alkylating agent, cisplatin causes DNA damage in cells, and tamoxifen inhibits proliferation of cancer cells by blocking estrogen receptors (Citation47,Citation58,Citation59). The effects were also observed in U87 cells when grown as neurospheres (Citation45). Although U87 is now recognized as being a less relevant model of glioma (Citation37), these results warrant intense investigations on the potential of EGCG to reverse or prevent chemoresistance in glioblastoma patients.
TNF-related apoptosis-inducing ligand (TRAIL) is a relatively safe and promising chemotherapeutic agent to trigger apoptosis specifically in cancer cells via death receptors (Citation53). However, cancer cells often develop resistance against TRAIL over time (Citation53). Seigelin et al. provided evidence that EGCG enhanced TRAIL-mediated apoptosis in three human glioblastoma cell lines, independently of TP53 status (Citation53).
On the other hand, EGCG reduces the therapeutic effects of bortezomib (BZM) on glioma cells. BZM is a selective inhibitor of the proteasome 26S which is responsible for the degradation of ubiquitinated proteins (Citation48). Inhibition of the proteasome 26S subunit has several consequences, including excessive accumulation of misfolded proteins, which ultimately generates endoplasmic reticulum (ER) stress and cell death. Golden et al. tested the effects of EGCG in combination with BZM on U251 and LN229 cells using two assays: the MTT assay to determine immediate effects on cell viability and the colony-formation assay to reveal consequences for long-term survival (Citation48). In both experimental settings, EGCG effectively blocked proteasome inhibition by BZM, thereby reducing the efficacy of BZM to kill glioma cells. Antagonism of EGCG and BZM was confirmed in other cell lines in independent studies (Citation44). Possibly, EGCG forms a stable cyclic boronate adduct with the boronic acid moiety of BZM, thus preventing BZM from acting as a proteasome inhibitor (Citation48). Via similar mechanisms, EGCG inhibits the effects of fenretinide, an agent that induces ER stress (Citation44). Consequently, these studies show that patients undergoing BZM or fenretinide therapy should avoid EGCG intake.
In summary, EGCG can enhance the effects of various chemotherapeutic agents, but potential antagonistic interactions between EGCG and drugs need to be considered.
Stability and Bioavailability of EGCG
Although EGCG has been demonstrated to possess anticancer properties in vitro and in several animal models, its stability and bioavailability have been a point of discussion for its clinical applicability. The health-promoting effects of EGCG can be hampered due to degradation of EGCG in biological fluids, or even during tea processing and storage (Citation60). This degradation takes place in two major reactions: epimerization and auto-oxidation (Citation60). The rates of these reactions are dependent on several factors, such as EGCG concentration, pH, and temperature (Citation60). For example, the half-life of EGCG in solution increases from 30 min at 20 μM to 150 min at 96 μM (Citation61). EGCG is stable at pH 2.0–5.5 and the exposure to various pH levels after oral administration has been reported as one of the reasons for its poor bioavailability (Citation62,Citation63). Low temperature helps stabilize EGCG as the half-life of a 109 mM EGCG solution decreases from 7 days at 4°C to 2 days at room temperature.
Furthermore, the oral bioavailability of EGCG has been documented to be low in animal models and in men. For instance, when measured in conscious and freely moving rats, Lin et al. observed an oral bioavailability of 4.95% for EGCG (Citation36). Chen et al. stated a lower percentage of 1.6% in a different rat model and Lambert et al. reported a percentage of 25% in mice (Citation64,Citation65). EGCG has been reported to be weakly absorbed by the intestines (Citation64). In addition, whereas Lin et al. measured an average concentration of 5 ng/ml in various brain regions in rat at 15 min after intravenous administration of EGCG (5 ng/kg), Nakagawa and Miyazawa detected a level of 0.5 nmol/g in rat brain tissue at 60 min after oral administration (500 mg/kg) (Citation36,Citation66). It is argued that low levels of EGCG in brain may be due to its bipolar functional group (), causing difficulty in penetrating the BBB (Citation36). Additionally, 92.4% EGCG in rat plasma is shown to be protein-bound and such large complexes may not be easily transported transcellularly over the endothelial cells that are part of the BBB, resulting in limited EGCG levels in brain tissue (Citation36). In men, EGCG levels after oral administration of green tea (3 tea bags containing 213 mg of EGCG) and green tea supplements (3 capsules containing 193 mg of EGCG) is 80 nmol/l and 150 nm/l, respectively (Citation67). The bioavailability of EGCG is calculated to be 0.1–1.1% (Citation67). Besides, a plasma polyphenol concentration of 7.5 μmol/l was required for increased anti-oxidant activity; however, the maximal plasma concentration of total flavanols, including EC, EGC, ECG, and EGCG after oral administration of green tea was only 1.2 μmol/l (Citation67). Thus, innovative methods to increase bioavailability of EGCG are of significance to increase its clinical applicability.
Current research is exploring different options, including pro-drugs, co-treatment, and encapsulation, to enhance bioavailability of EGCG. Multiple pro-forms of EGCG have been investigated and have shown increased stability, bioavailability, and biological activities in vivo and in vitro (Citation68–72). Co-treatment of EGCG with piperine (from black pepper) enhances the bioavailability of EGCG by 1.3-fold in mice (Citation73). Several studies have demonstrated that encapsulation of EGCG inside a polymer matrix, such as chitosan-based nanocarriers, improves its pharmacokinetics and pharmacodynamics (Citation74). Hence, the potential of EGCG in clinical applications is boosted by these technologies.
Mechanism of Actions of EGCG
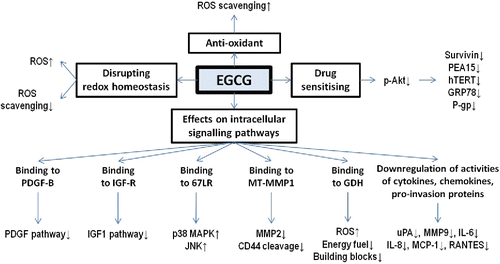
EGCG has a wide range of biological activities that may be involved in its effects on cancer cells (Citation15). Since different types of cancer harbor different protein expression profiles and cancer cells are genetically unstable, growth inhibitory effects of EGCG may be different in various types of cancer and also in individual patients. This review focuses primarily on the effects of EGCG that have been found in glioma cell lines which are summarized in .
Figure 2. Mechanisms of anti-glioma effects of EGCG. EGCG reduces the risk to develop glioma with its anti-oxidant property. At the same time, EGCG can be beneficial to treat glioma patients because it can disrupt redox homeostasis, enhance the efficacy of anti-glioma therapies, and regulate multiple pathways involved in survival and progression of glioma cells.
Anti-Oxidant and Oxidative Properties
In order to explain the cancer-preventive effects of tea that have been observed in epidemiological studies, it has been hypothesized that EGCG acts as a scavenger of ROS and consequently prevents tumor development (Citation31). Furthermore, these anti-oxidative effects of EGCG are more prominent in old than in young rats (Citation75). At low concentrations (<5 μM), EGCG has been reported to provide a growth advantage for U251 and U87 glioma cells, which may be attributed to the ROS-scavenging capacity of EGCG (Citation35). However, the ROS-scavenging properties of EGCG have not yet been extensively investigated in glioma cells.
On the other hand, at higher concentrations EGCG reduces the activity of NADPH-generating enzymes (Citation38) and consequently decreases the ROS-scavenging potential, which contributes to the apoptosis-inducing effects of EGCG. Agarwal et al. found that treatment of U87 cells with 25 μM EGCG did not induce ROS production, but a significant 1.7- and 2-fold increase in ROS production was detected in cells treated with 50 μM and 100 μM EGCG, respectively (Citation51). To examine the relevance of increased oxidative stress induced by EGCG, U87 cells were treated with EGCG, 1 mM N-acetyl cysteine (a ROS scavenger), or a combination of both for 48 h. The 22-fold increase in cell death after treating U87 cells with 100 μM EGCG was annihilated when EGCG treatment was combined with N-acetyl cysteine. This suggests that the anticancer effects of EGCG are mediated by induction of ROS. Furthermore, EGCG suppressed expression of the molecules associated with the maintenance of redox homeostasis (Citation51). Specifically, EGCG drastically decreased expression of thioredoxin-1 (TRX-1) and ceruloplasmin (CP), both of which play an important roles in the cellular defense against ROS (Citation51).
Das et al. confirmed the results of Agarwal by showing that EGCG (50 μM) treatment for 24 h increased oxidative stress in T98G and U87 cells (Citation41). Taking it a step further, Das et al. also found that EGCG-induced ROS accumulation triggered cell death via phosphorylation of p38 and JNK1 (Citation41), ultimately leading to the expression and activation of pro-apoptotic proteins and suppression of pro-survival proteins (Citation41). Furthermore, EGCG treatment downregulated NF-кB, a survival factor for glioma (Citation41).
Therapy Sensitization
| 1) | Telomerase | ||||
Treatment resistance is a major problem in glioma. A central dogma in cancer biology is that elevated telomerase levels ensure that telomere length is maintained. Moreover, telomerase levels are often correlated with tumor grade and progression in glioma (Citation76). Shervington et al. showed that addition of EGCG significantly reduced expression of telomerase mRNA in glioma cells (Citation40), an effect also observed in lung cancer, cancer of oral cavity, thyroid cancer, and liver cancer (Citation77,Citation78). Inhibition of telomerase may lead to shortening of telomeres to a critical length, resulting in genome instability and ultimately activation of the apoptotic pathway. This phenomenon may explain the therapy-sensitizing effects of EGCG in cancer, including glioma (Citation40,Citation77).
| 2) | P-glycoprotein | ||||
P-glycoprotein (P-gp) is a transmembrane multidrug-resistance protein that pumps many toxic compounds, including anticancer drugs, out of cells (Citation47). Zhang et al. presented evidence that EGCG suppressed P-gp activity (Citation47). Both mRNA levels and protein levels of P-gp were reduced in a dose-dependent manner after EGCG treatment (Citation47). This may provide an additional explanation of the synergy between EGCG and chemotherapies, including TMZ and BCNU (Citation47).
| 3) | GRP78 | ||||
GRP78 is a chaperone protein with several anti-apoptotic functions, including binding to and preventing the activation of caspase-7 and may be involved in chemoresistance of cancer cells (Citation48). The high expression levels of GRP78 are associated with poor outcome in glioblastoma patients (Citation79). Inhibition of GRP78 is considered as an attractive mean to induce chemosensitization (Citation48,Citation80). EGCG has been shown to interact with GRP78 at the ATP-binding domain of the protein, reducing its activity (Citation81,Citation82).
| 4) | Survivin | ||||
McLaughlin et al. demonstrated that EGCG enhanced the therapeutic efficacy of anti-glioma therapies, specifically IR, by downregulating expression of the pro-survival protein survivin (Citation52). Survivin has been found to enhance radio-resistance in human glioblastoma cells (Citation83). Survivin expression is induced by IR and suppresses caspase-mediated apoptosis by binding and inhibiting caspase-9 or smac-Diablo (Citation83). The TRAIL-sensitizing effects of EGCG to glioma cells via suppression of survivin expression has been confirmed in other studies (Citation52,Citation53).
| 5) | PEA15 | ||||
Seigelin et al. found that 24 h treatment of U87 and A172 cells with 20 μM EGCG significantly downregulated the levels of both survivin and PEA15 (Citation53), consequently sensitizing TRAIL-resistant glioma cells to TRAIL treatment (Citation53). PEA15 is an inhibitor of caspase-8 and its expression has been found to be upregulated in different glioma cell lines and correlates with resistance of human glioma cells to anticancer drugs, such as TRAIL (Citation53).
Downregulation of PEA15 and survivin by EGCG has been shown to occur via reduced phosphorylation of Akt in U87 cells (Citation53). In addition, downregulation of telomerase expression was also found to occur by suppression of phosphorylation of AKT (Citation84). Moreover, GRP78 has been reported to be responsible for tamoxifen resistance in breast cancer which is regulated by phosphorylated AKT (Citation85). P-gp is also under control of phosphorylated AKT in other cancer types (Citation86,Citation87). Therefore, it seems that EGCG sensitizes glioma cells to anticancer therapies by decreasing AKT phosphorylation in glioma cells.
Modulation of Intracellular Signaling
| 1) | Proliferation and survival | ||||
One of the mechanisms of inhibition of cell growth by EGCG is its effect on the PDGF pathway (Citation50). PDGF receptor β (PDGF-Rβ) conveys mitogenic signals by autophosphorylation of tyrosine residues after binding to ligand PDGF-BB (Citation50). The roles of PDGF-BB and its receptor in cell proliferation have been investigated in a range of cancer types, such as glioma, sarcoma, breast cancer, colon cancer, and melanoma (Citation50,Citation88,Citation89). Specifically, it has been proposed that autocrine activation of PDGF-Rβ is involved in the development and progression of glioma (Citation88,Citation90). Treatment of A172 glioblastoma cells with EGCG (50 μM) led to a significant inhibition of the PDGF-BB-induced tyrosine phosphorylation of PDGF-Rβ (Citation50). Inhibition of PDGF-Rβ corresponded with inhibition of spheroid formation of A172 cells (Citation50). EGCG has also been shown to inhibit PDGF-induced proliferation of other cancer types by inhibiting phosphorylation of PDGF-Rβ (Citation91,Citation92). Furthermore, EGCG has been shown to directly interact with PDFG-BB via its galloyl group and thus prevents PDFG-BB from binding to PDGF-Rβ (Citation93). Therefore, it is likely that EGCG inhibits the phosphorylation of PDGF-Rβ in glioma cells via blocking PDFG-BB from binding to its receptor.
IGF-1 promotes tumor growth via inhibition of apoptosis by interacting with IGF-1 receptors (Citation94). Yokoyama et al. observed that anti-IGF-1 antibodies further reduced the viability of EGCG-pretreated U87 and MtT/E cells (Citation42). This effect was more prominent in the EGCG-resistant rat non-functioning pituitary adenoma MtT/E cells with a higher IGF-1 expression level as compared to U87 cells (Citation42). These results suggest that high IGF-1 expression restricts the inhibitory effects of EGCG on cell proliferation. Binding of EGCG to IGF-1 receptors at the ATP-binding site has been demonstrated by Li et al. (Citation95). Binding of EGCG to IGF-1 receptors inhibits phosphorylation of the receptors and activation of the downstream signaling pathway, resulting in reduced tumor growth (Citation96,Citation97). Hence, expression and release of IGF-1 in cell types, such as MtT/E, may reduce inhibitory effects of EGCG. In summary, EGCG can inhibit the pro-apoptotic activity of IGF-1 by competitively binding to IGF-1R.
67LR is frequently overexpressed in glioma cells and is associated with tumor progression and poor prognosis (Citation98,Citation99). This receptor functions via MAPK pathways and regulates multiple physiological processes, such as cell proliferation, differentiation and invasion (Citation99). Cytotoxic effects of EGCG may occur via 67LR (Citation46). Transfection of cells with siRNA silencing 67LR (shRNA-67LR) before EGCG treatment reduces the apoptosis-inducing effects of EGCG. Hence, EGCG may induce glioma cell death via 67LR as 67LR is a natural binding target of EGCG (Citation100).
| 2) | Cell migration | ||||
EGCG inhibits invasion of glioma cells by inhibition of MT1-MMP (Citation54,Citation101,Citation102). EGCG reduces the expression of MT1-MMP via gene silencing (Citation54). MT1-MMP promotes invasion of glioma cells by activation of pro-MMP2 into MMP2 (Citation101). Strong dose-dependent inhibition of EGCG on the activity of MMP2 in U87 has been detected by gelatin zymography (Citation46,Citation103). Even when U87 cells are exposed to concanavalin A, a strong inducer of MT1-MMP expression and activator of pro-MMP2, EGCG still inhibits the activation of pro-MMP2 into MMP2 by MT1-MMP (Citation103). Since MMP2 is a major protease involved in invasive behavior of cells (Citation104), inhibition of MMP2 activation via MT1-MMP by EGCG leads to reduced invasiveness of glioma cells.
Besides, MT1-MMP regulates invasion of glioma cells via CD44 expression and the actin cytoskeleton (Citation102). CD44 is a transmembrane receptor for extracellular matrix components, such as osteopontin and hyaluronan (HA) (Citation102,Citation105). Both CD44 and its ligand HA have been demonstrated to promote tumor migration (Citation106,Citation107). By binding to CD44, HA induces cleavage of CD44 from the cell surface, which is critical for cellular detachment and cellular invasive behavior (Citation106). Through its cytoplasmic domain, MT1-MMP regulates the MAPK signaling pathway, leading to profound cytoskeletal restructuring and increased expression of RhoA (Citation101,Citation102). Increased RhoA expression results in activation of ROK which phosphorylates the cytoplasmic domain of CD44 and facilitates CD44/HA binding (Citation101). Hence, by inhibition of the expression and activity of MT1-MMP, EGCG reduces the HA-induced cleavage of CD44. Additionally, suppression of invasion of U87 cells by EGCG has been shown to occur by reduction of F-actin levels, which disrupts cytoskeletal architecture (Citation51). Since MT1-MMP affects the actin cytoskeleton to facilitate cell migration via the MAPK pathway, inhibition of MT1-MMP by EGCG may lead to a disrupted actin network (Citation51,Citation101). In summary, EGCG limits the invasion of glioma cells by disturbing the cleavage of CD44 and the restructuring of the cytoskeleton via inhibition of MT1-MMP (Citation101).
Furthermore, EGCG inhibits the activity of MMP9 in U87 and U251 cells (Citation46,Citation103). Increased expression of MMP9 has been shown to be associated with increased invasive behavior of U251 cells (Citation108). Agarwal et al. reported a significant twofold decrease in levels of interleukin 6 (IL-6), IL-8, monocyte chemoattractant protein-1 (MCP-1), and chemokine ligand 5 (CCL5 or RANTES) levels after treating U87 cells with 100 μM of EGCG (Citation51). These cytokines and chemokines induce invasion of glioma cells (Citation109). Similar effects have been found in human prostate carcinoma cells (Citation94). Pro-inflammatory cytokine IL-6 plays a role in the progression of astrocytoma to higher grade glioma by inducing expression of VEGF (Citation111). Moreover, IL-6 can increase invasiveness of U87 via upregulation of MMP-2 (Citation112). Additionally, EGCG inhibits urokinase-type plasminogen activator (uPa) activity in U87 cells, a protease that can activate MMPs and thus enhance degradation of the extracellular matrix for invasion purposes (Citation51,Citation113). Similar effects of EGCG on uPa has been reported for human fibrosarcoma (Citation110).
| 3) | Metabolism | ||||
One of the hallmarks of cancer is metabolic remodeling, as proliferating cancer cells need large amounts of nutrients, including glucose and glutamine, and shunt metabolites into anabolic pathways (Citation114,Citation115). Glutaminolysis and glutamatolysis occur in mitochondria, involving sequential conversion of glutamine to glutamate, and then to alpha-ketoglutarate (α-KG). α-KG is a co-factor for many enzymes and an intermediate of the tricarboxylic acid cycle that produces ATP and supplies anabolic carbons for generating amino acid, lipid, and nucleotides (Citation38). The conversion of glutamate to α-KG is catalyzed by glutamate dehydrogenase (GDH), glutamate pyruvate transaminase 2 (GPT2), or glutamate oxaloacetate transaminase 2 (GOT2) (Citation38). Besides, IDH1/2 are responsible for catalyzing the conversion of isocitrate to α-KG. Mutations in IDH1/2 are oncogenic drivers of low-grade glioma and secondary glioblastoma (Citation115). IDH1/2 mutations result in production of the oncometabolite D-2-hydroxyglutarate (D-2HG), depletion of α-KG, reduced NADPH production capacity, increased NADPH consumption, and inhibition of α-KG-dependent enzymes (Citation116,Citation117). It has been hypothesized that IDH1/2 mutations render glioma cells dependent on production of α-KG by importing the neurotransmitter glutamate from the brain microenvironment and its subsequent conversion to α-KG (Citation116). EGCG binds directly to GDH and thereby suppresses its activity, reducing intracellular α-KG levels, and NAD (P)H production during the GDH reaction (Citation118,Citation119). Activators of GDH are ADP and leucine, whereas important inhibitors are GTP, palmitoyl CoA, and ATP (Citation119). Li et al. demonstrated that EGCG specifically and allosterically inhibited GDH by binding to the ADP-activating site (Citation118,Citation119). Consequently, it reduces cell viability and colony formation in GDH-overexpressing U251 cells both in vivo and in vitro (Citation38). As a result, interaction between EGCG and GDH in glioma cells can block an important metabolic pathway that supplies building blocks for tumor growth, facilitate ROS accumulation in mitochondria, and induce apoptosis.
Conclusion
Although limited, there is evidence of the inhibitory effects of EGCG on glioma from different types of studies and experimental settings. Epidemiological studies so far have only focused on tea as a caffeinated drink and hence it is not possible to draw a conclusion on the effects of EGCG or green tea on reducing the risk of glioma development. Further epidemiological studies are needed with careful control for confounders, especially tea types. In vivo studies are also limited and report conflicting findings. These studies have to be repeated to demonstrate effects of EGCG on both survival benefit and tumor growth in animal models. Most in vitro studies have demonstrated cytotoxic effects of EGCG on various glioma cell lines in different assays, such as proliferation assays, apoptosis assays, and migration assays. The most promising potential of EGCG is as an adjuvant to conventional anti-glioma therapies, such as IR and TMZ, but caution is needed when patients are treated with drugs with a boronic acid moiety, such as BMZ. Although the instability and low bioavailability of EGCG create concerns for its clinical applicability, multiple scientific solutions such as pro-drugs, co-treatment, and encapsulation are being investigated to exploit EGCG's anticancer effects in clinics. Finally, mechanisms of action of EGCG have been partly elucidated with the involvement of multiple pathways and proteins. However, more research is certainly required to complete the conundrum of all the molecular mechanisms that govern the cytotoxic effects of EGCG on the survival, proliferation, migration, and metabolism of glioma.
Additional information
Funding
References
- Goodenberger ML and Jenkins RB: Genetics of adult glioma. Cancer Genet 205(12), 613–621, 2012. https://doi.org/10.1016/j.cancergen.2012.10.009.
- Raizer JJ, Fitzner KA, Jacobs DI, Bennett CL, Liebling DB, et al.: Economics of malignant gliomas: a critical review. J Oncol Practice/Am Soc Clin Oncol 11(1), e59–65, 2014. https://doi.org/10.1200/JOP.2012.000560.
- Ho VKY, Reijneveld JC, Enting RH, Bienfait HP, Robe P, Baumert BG, et al.: Changing incidence and improved survival of gliomas. Eur J Cancer 50(13), 2309–2318, 2014. https://doi.org/10.1016/j.ejca.2014.05.019.
- Schwartzbaum JA, Fisher JL, Aldape KD, and Wrensch M: Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol 2(9):494–503, 2006. quiz 1 p following 516. https://doi.org/10.1038/ncpneuro0289.
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, et al.: The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131(6):803–820, 2016. https://doi.org/10.1007/s00401-016-1545-1.
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, et al.: The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114(2):97–109, 2007. https://doi.org/10.1007/s00401-007-0243-4.
- The Cancer Genome Atlas Research Network: Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med 372(26), 2481–2498, 2015. https://doi.org/10.1056/NEJMoa1402121.
- Kim YS, Kim SH, Cho J, Kim JW, Chang JH, et al.: MGMT gene promoter methylation as a potent prognostic factor in glioblastoma treated with temozolomide-based chemoradiotherapy: a single-institution study. Int J Radiat Oncol Biol Phys 84(3), 661–667, 2012. https://doi.org/10.1016/j.ijrobp.2011.12.086.
- Molenaar RJ, Verbaan D, Lamba S, Zanon C, Jeuken JWM, et al.: The combination of IDH1 mutations and MGMT methylation status predicts survival in glioblastoma better than either IDH1 or MGMT alone. Neuro-Oncol 16(9), 1263–1273, 2014. https://doi.org/10.1093/neuonc/nou005.
- Verhaak, RGW , Hoadley, KA , Purdom, E, Wang, V, Qi, Y, et al.: Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer 19(1), 38–46, 2010.
- Richard Lee P, and Chiocca EA: Evolution of malignant glioma treatment: from chemotherapy to vacines to viruses. Neurosurgery 61(0 1), 74–83, 2014.
- Hira VV V., Ploegmakers KJ, Verbovšek U, Roing CS, Aronica EMA, et al.: CD133+ and nestin+ glioma stem-like cells reside around CD31+ arterioles in niches that express SDF-1α, CXCR4, osteopontin and cathepsin K. J Histochem Cytochem 63(7), 481–493, 2015. https://doi.org/10.1369/0022155415581689.
- Blomqvist P, Lycke J, Strang P, Törnqvist H, and Ekbom A: Brain tumours in Sweden 1996: care and costs. J Neurol Neurosurg Psychiatry 69(6), 792–798, 2000. https://doi.org/10.1136/jnnp.69.6.792.
- Graham HN: Green tea composition, consumption, and polyphenol chemistry. Prev Med 21(3), 334–350, 1992. https://doi.org/10.1016/0091-7435(92)90041-F.
- Khan N, and Mukhtar H: Multitargeted therapy of cancer by green tea polyphenols. Cancer Lett 269(2), 269–280, 2008. https://doi.org/10.1016/j.canlet.2008.04.014.
- Chakrawarti L, Agrawal R, Dang S, Gupta S, and Gabrani R: Therapeutic effects of EGCG: a patent review. Expert Opin Ther Patents 3776(August):1–10, 2016.
- Yuan JM, Sun C, and Butler LM: Tea and cancer prevention: epidemiological studies. Pharmacol Res 64(2), 123–135, 2011. https://doi.org/10.1016/j.phrs.2011.03.002.
- Ahn W-S, Yoo J, Huh S-W, Kim C-K, Lee J-M, et al.: Protective effects of green tea extracts (polyphenon E and EGCG) on human cervical lesions. Eur J Cancer Prev : Off J Eur Cancer Prev Organ 12(5), 383–390, 2003. https://doi.org/10.1097/00008469-200310000-00007.
- Lee M, Wrensch M, and Miike R: Dietary and tobacco risk factors for adult onset glioma in the San Francisco Bay area (California, USA). Cancer Causes Control 8(1), 13–24, 1996. https://doi.org/10.1023/A:1018470802969.
- Blowers L, Preston-martin S, and Mack WJ: Dietary and other lifestyle factors of women with brain gliomas in Los Angeles county (California, USA). Cancer Causes Control 8, 5–12, 1997.
- Giles GG, MacNeil JJ, Donnan G, Webley C, Staples MP, et al.: Dietary factors and the risk of glioma in adult Melbourne: results of a case-control study in Melbourne, Australia. Int J Cancer 59, 357–362, 1994. https://doi.org/10.1002/ijc.2910590311.
- Burch J, Craib KJ., Miller A, and Howe G: An exploratory case-control study of brain tumors in adults. J Nat Cancer Inst 31(4):601–609, 1987.
- Holick CN, Smith SG, Giovannucci E, and Michaud DS: Coffee, tea, caffeine intake, and risk of adult glioma in three prospective cohort studies. Cancer Epidemiol Biomark Prev 19(1), 39–47, 2010. https://doi.org/10.1158/1055-9965.EPI-09-0732.
- Michaud DS, Gallo V, Schlehofer B, Tjønneland A, Olsen A, et al.: Coffee and tea intake and risk of brain tumors in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort study 1–3. Education 92(5), 1145–1150, 2010.
- Dubrow R, Darefsky AS, Freedman ND, Hollenbeck AR, and Sinha R: Coffee, tea, soda, and caffeine intake in relation to risk of adult glioma in the NIH-AARP diet and health study. Cancer Causes Control 23(5), 757–768, 2012. https://doi.org/10.1007/s10552-012-9945-6.
- Malerba S, Galeone C, Pelucchi C, Turati F, Hashibe M, et al.: A meta-analysis of coffee and tea consumption and the risk of glioma in adults. Cancer Causes Control 24(2), 267–276, 2013. https://doi.org/10.1007/s10552-012-0126-4.
- Cabrera C, Giménez R, and López MC: Determination of tea components with antioxidant activity. J Agric Food Chem 51(15), 4427–4435, 2003. https://doi.org/10.1021/jf0300801.
- Lin YS, Tsai Y, Tsay JS, and Lin JK: Factors affecting the levels of teapolyphenols and caffeine in tea leaves. J Agric Food Chem 31, 1864–1873, 2003. https://doi.org/10.1021/jf021066b.
- Lorenz M, Urban J, Engelhardt U, Baumann G, Stangl K, and Stangl V: Green and black tea are equally potent stimuli of NO production and vasodilation: new insights into tea ingredients involved. Basic Res Cardiol 104(1), 100–110, 2009. https://doi.org/10.1007/s00395-008-0759-3.
- Zhang M, Holman CDJ, Huang J, and Xie X: Green tea and the prevention of breast cancer: a case-control study in Southeast China. Carcinogenesis 28(5), 1074–1078, 2007. https://doi.org/10.1093/carcin/bgl252.
- Yang CS, Wang X: Green tea and cancer prevention. Nutr Cancer 62(7), 931–937, 2010. https://doi.org/10.1080/01635581.2010.509536.
- Nihal M, Ahsan H, Siddiqui IA, Mukhtar H, Admad N, et al.: (−)-Epigallocatechin-3-gallate (EGCG) sensitizes melanoma cells to interferon induced growth inhibition in a mouse model of human melanoma. Cell Cycle 8(13), 2057–2063, 2009. https://doi.org/10.4161/cc.8.13.8862.
- Shankar S, Marsh L, and Srivastava RK: EGCG inhibits growth of human pancreatic tumors orthotopically implanted in Balb C nude mice through modulation of FKHRL1/FOXO3a and neuropilin. Mol Cell Biochem 372(1–2), 83–94, 2013. https://doi.org/10.1007/s11010-012-1448-y.
- Luo T, Wang J, Yin Y, Hua H, Jing J, et al.: (−)-Epigallocatechin gallate sensitizes breast cancer cells to paclitaxel in a murine model of breast carcinoma. Breast Cancer Res 12(1), R8, 2010. https://doi.org/10.1186/bcr2473.
- Chen TC, Wang W, Golden EB, Thomas S, Sivakumar W, et al.: Green tea epigallocatechin gallate enhances therapeutic efficacy of temozolomide in orthotopic mouse glioblastoma models. Cancer Lett 302(2), 100–108, 2011. https://doi.org/10.1016/j.canlet.2010.11.008.
- Lin LC, Wang MN, Tseng TY, Sung JS, and Tsai TH: Pharmacokinetics of (−)-epigallocatechin-3-gallate in conscious and freely moving rats and its brain regional distribution. J Agric Food Chem 55(4), 1517–1524, 2007. https://doi.org/10.1021/jf062816a.
- Lenting K, Verhaak R, Ter Laan M, Wesseling P, and Leenders W: Glioma: experimental models and reality. Acta Neuropathol 133(2), 1–20, 2017. https://doi.org/10.1007/s00401-017-1671-4.
- Zhang J, Wang G, Mao Q, Li S, Xiong W, Lin Y, et al.: Glutamate dehydrogenase (GDH) regulates bioenergetics and redox homeostasis in human glioma. Oncotarget 31, 1–12, 2016.
- Leenders WP, Kusters B, and de Waal RM: Vessel co-option: how tumors obtain blood supply in the absence of sprouting angiogenesis. Endothelium 9(2), 83–87, 2002. https://doi.org/10.1080/10623320212006.
- Shervington A, Pawar V, Menon S, Thakkar D, and Patel R: The sensitization of glioma cells to cisplatin and tamoxifen by the use of catechin. Mol Biol Rep 36(5), 1181–1186, 2009. https://doi.org/10.1007/s11033-008-9295-3.
- Arabinda Das, Naren B, and Swapan R: Flavonoids activated caspases for apoptosis in human glioblastoma T98G and U87MG cells but not in human normal astrocytes. Cancer 48(Suppl 2), 1–6, 2010.
- Yokoyama S, Hirano H, Wakimaru N, and Sarker KP: Inhibitory effect of epigallocatechin-gallate on brain tumor cell lines in vitro. Neuro-Oncol 31(1), 22–28, 1999.
- Riss TL, Moravec RA, Niles AL, Benink HA, Worzella TJ, et al.: Cell viability assays. Assay Guid Manual, 2013.
- Martin S, Lamb HK, Brady C, Lefkove B, Bonner MY, et al.: Inducing apoptosis of cancer cells using small-molecule plant compounds that bind to GRP78. Br J Cancer 109(2), 433–43, 2013. https://doi.org/10.1038/bjc.2013.325.
- Rahman AA, Makpol S, Jamal R, Harun R, Mokhtar N, et al.: Tocotrienol-rich fraction, [6]-gingerol and epigallocatechin gallate inhibit proliferation and induce apoptosis of glioma cancer cells. Molecules 19(9), 14528–14541, 2014. https://doi.org/10.3390/molecules190914528.
- Li H, Li Z, Xu YM, Wu Y, Yu KK, et al.: Epigallocatechin-3-gallate induces apoptosis, inhibits proliferation and decreases invasion of glioma cell. Neurosci Bull 30(1), 67–73, 2014. https://doi.org/10.1007/s12264-013-1394-z.
- Zhang Y, Wang SX, Ma JW, Li HY, Ye JC, et al.: EGCG inhibits properties of glioma stem-like cells and synergizes with temozolomide through downregulation of P-glycoprotein inhibition. J Neuro-Oncol 121(1), 41–52, 2015. https://doi.org/10.1007/s11060-014-1604-1.
- Golden EB, Lam PY, Kardosh A, Gaffney KJ, Cadenas E, et al.: Green tea polyphenols block the anticancer effects of bortezomib and other boronic acid-based proteasome inhibitors. Therapy 113(23), 5927–5937, 2009.
- Ahn HY, Hadizadeh KR, Seul C, Yun YP, Vetter H, et al.: Epigallocathechin-3 gallate selectively inhibits the PDGF-BB-induced intracellular signaling transduction pathway in vascular smooth muscle cells and inhibits transformation of sis-transfected NIH 3T3 fibroblasts and human glioblastoma cells (A172). Mol Biol Cell 10(4), 1093–1094, 1999. https://doi.org/10.1091/mbc.10.4.1093.
- Sachinidis A, Seul C, Seewald S, Ahn HY, Ko Y, et al.: Green tea compounds inhibit tyrosine phosphorylation of PDGF β-receptor and transformation of A172 human glioblastoma. FEBS Lett 471(1), 51–55, 2000. https://doi.org/10.1016/S0014-5793(00)01360-0.
- Agarwal A, Sharma V, Tewari R, Koul N, Joseph C, et al.: Epigallocatechin-3-gallate exhibits anti-tumor effect by perturbing redox homeostasis, modulating the release of pro-inflammatory mediators and decreasing the invasiveness of glioblastoma cells. Mol Med Rep. 1(4), 511–515, 2008.
- McLaughlin N, Annabi B, Bouzeghrane M, Temme A, Bahary JP, et al.: The Survivin-mediated radioresistant phenotype of glioblastomas is regulated by RhoA and inhibited by the green tea polyphenol (−)-epigallocatechin-3-gallate. Brain Res 1071(1), 1–9, 2006. https://doi.org/10.1016/j.brainres.2005.10.009.
- Siegelin MD, Habel A, and Gaiser T: Epigalocatechin-3-gallate (EGCG) downregulates PEA15 and thereby augments TRAIL-mediated apoptosis in malignant glioma. Neurosci Lett 448(1), 161–165, 2008. https://doi.org/10.1016/j.neulet.2008.10.036.
- Annabi B, Lachambre MP, Bousquet-Gagnon N, Pagé M, Gingras D, et al.: Green tea polyphenol (−)-epigallocatechin-3-gallate inhibits MMP-2 secretion and MT1-MMP-driven migration in glioblastoma cells. Biochim Biophys Acta – Mol Cell Res 1542(1–3), 209–220, 2002. https://doi.org/10.1016/S0167-4889(01)00187-2.
- Gang L, Tang A, Lin X, Li L, Zhang S, et al.: Green tea catechins augment the antitumor activity of doxorubicin in an in vivo mouse model for chemoresistant liver cancer. Int J Oncol 37, 111–123, 2010.
- Thomas F, Holly JMP, Persad R, Bahl A, and Perks CM: Green tea extract (epigallocatechin-3-gallate) reduces efficacy of radiotherapy on prostate cancer cells. Urology 78(2), 475.e15–475.e21, 2011. https://doi.org/10.1016/j.urology.2011.03.031.
- Stupp, R, Mason, WP , Van Den Bent, MJ , Weller, M, et al.: Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10), 987–996, 2005. https://doi.org/10.1056/NEJMoa043330.
- Shaloam D, and Tchounwou PB: Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol 740, 364–378, 2014. https://doi.org/10.1016/j.ejphar.2014.07.025.
- Gupta V, Su YS, Wang W, Kardosh A, Liebes LF, et al.: Enhancement of glioblastoma cell killing by combination treatment with temozolomide and tamoxifen or hypericin. Neurosurg Focus 20(4), E20, 2006. https://doi.org/10.3171/foc.2006.20.4.13.
- Krupkova O, Ferguson SJ, and Wuertz-kozak K: Stability of (−)-epigallocatechin gallate and its activity in liquid formulations and delivery systems. J Nutr Biochem 37, 1–12, 2016. https://doi.org/10.1016/j.jnutbio.2016.01.002.
- Sang S, Lee M-J, Hou Z, Ho C-T, and Yang CS: Stability of tea polyphenol (−)-epigallocatechin-3-gallate and formation of dimers and epimers under common experimental conditions. J Agric Food Chem 53, 9478–9484, 2005. https://doi.org/10.1021/jf0519055.
- Komatsu Y, Suematsu S, Hisanobu Y, Saigo H, Matsuda R, et al.: Effects of pH and temperature on reaction kinetics of catechins in green tea infusion effects of pH and temperature on reaction kinetics of catechins in green tea infusion. Biosci Biotechnol Biochem 57(6), 907–910, 1993. https://doi.org/10.1271/bbb.57.907.
- Zimeri J, and Tong CH: Degradation kinetics of (−)-epigallocatechin gallate as a function of pH and dissolved oxygen in a liquid model system. J Food Sci 64(5), 753–758, 1999. https://doi.org/10.1111/j.1365-2621.1999.tb15905.x.
- Chen L, Lee M, Li HE, Yang CS, and Al CET: Absportion, distribution, and elimination of tea polyphenols in rats. Drug Metab Dispos 25(9), 0–5, 1997.
- Lambert JD, Lee M, Lu H, Meng X, Ju J, et al.: Epigallocatechin-3-gallate is absorbed but extensively glucuronidated following oral administration to mice 1, 2. J Nutr 133(12), 4172–4177, 2003. https://doi.org/10.1093/jn/133.12.4172.
- Nakagawa K, and Miyazawa T: Absorption and distribution of tea catechin, (−)-epigallocatechin-3-gallate, in the rat. J Nutr Sci Vitaminol 43(6), 679–684, 1997. https://doi.org/10.3177/jnsv.43.679.
- Henning SM, Niu Y, Lee NH, Thames GD, Minutti RR, et al.: Bioavailability and antioxidant activity of tea flavanols after consumption of green tea, black tea, or a green tea extract. Am J Clin Nutr 80, 1558–1564, 2004. https://doi.org/10.1093/ajcn/80.6.1558.
- Saber R, Ahmed I, Liu G, Renzetti A, Farshi P, et al.: Biological and mechanistic characterization of noval prodrugs of green tea polyphenol epigallocatechin gallate analogs in human leiomyoma cell lines. J Cell Biochem 117, 2357–2369, 2016. https://doi.org/10.1002/jcb.25533.
- Lam WH, Kazi A, Kuhn DJ, Chow LMC, Chan ASC, et al.: A potential prodrug for a green tea polyphenol proteasome inhibitor: evaluation of the peracetate ester of (−)-epigallocatechin gallate [(−)-EGCG]. Bioorg Med Chem 12, 5587–5593, 2004. https://doi.org/10.1016/j.bmc.2004.08.002.
- Landis-piwowar KR, Huo C, Chen D, Milacic V, Shi G, et al.: A novel prodrug of the green tea polyphenol (À)-epigallocatechin-3-gallate as a potential anticancer agent. Cancer Res 2(11), 4303–4311, 2007. https://doi.org/10.1158/0008-5472.CAN-06-4699.
- Lee S, Chan W, Lee T, Lam W, Chan T, et al.: Effect of a prodrug of the green tea polyphenol (−)-epigallocatechin-3-gallate on the growth of androgen-independent prostate cancer in vivo. Nutr Cancer 60(4), 483–491, 2008. https://doi.org/10.1080/01635580801947674.
- Chiu C, Hui W, Gene X, Wai C, Tao M, et al.: Prodrug of green tea epigallocatechin-3-gallate (Pro-EGCG) as a potent anti-angiogenesis agent for endometriosis in mice. Angiogenesis 31, 59–69, 2013. https://doi.org/10.1007/s10456-012-9299-4.
- Lambert JD, Hong J, Kim DH, Mishin VM, and Yang CS: Piperine enhances the bioavailability of the tea polyphenol (−)-Epigallocatechin-3-gallate in mice. J Nutr 134(8), 1948–1952, 2004. https://doi.org/10.1093/jn/134.8.1948.
- Tyagi N, De R, and Popat A: Cancer therapeutics with epigallocatechin-3-gallate encapsulated in biopolymeric nanoparticles. Int J Pharm 518(1–2), 220–227, 2017. https://doi.org/10.1016/j.ijpharm.2016.12.030.
- Srividhya R, Jyothilakshmi V, Arulmathi K, Senthilkumaran V, and Kalaiselvi P: Attenuation of senescence-induced oxidative exacerbations in aged rat brain by (−)-epigallocatechin-3-gallate. Int J Dev Neurosci 26(2), 217–223, 2008. https://doi.org/10.1016/j.ijdevneu.2007.12.003.
- Boldrini L, Pistolesi S, Gisfredi S, Ursino S, Alì G, et al.: Telomerase activity and hTERT mRNA expression in glial tumors. Int J Oncol 28(6), 1555–1560, 2006.
- Sadava D, Whitlock E, and Kane SE: The green tea polyphenol, epigallocatechin-3-gallate inhibits telomerase and induces apoptosis in drug-resistant lung cancer cells. Biochem Biophys Res Commun 360(1), 233–237, 2007. https://doi.org/10.1016/j.bbrc.2007.06.030.
- Lin SC, Li WC, Shih JW, Hong KF, Pan YR, and Lin JJ: The tea polyphenols EGCG and EGC repress mRNA expression of human telomerase reverse transcriptase (hTERT) in carcinoma cells. Cancer Lett 236(1), 80–88, 2006. https://doi.org/10.1016/j.canlet.2005.05.003.
- Lee AS: GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res 67(8), 3496–3499, 2007. https://doi.org/10.1158/0008-5472.CAN-07-0325.
- Pyrko P, Schöntha AH, Hofman FM, Chen TC, and Lee AS: The unfolded protein response regulator GRP78/BiP as a novel target for increasing chemosensitivity in malignant gliomas. Cancer Res 67(20), 9809–9816, 2007. https://doi.org/10.1158/0008-5472.CAN-07-0625.
- Bhattacharjee R, Devi A, and Mishra S: Molecular docking and molecular dynamics studies reveal structural basis of inhibition and selectivity of inhibitors EGCG and OSU-03012 toward glucose regulated protein-78 (GRP78) overexpressed in glioblastoma. J Mol Model 21(10), 2015. https://doi.org/10.1007/s00894-015-2801-3.
- Ermakova SP, Kang BS, Choi BY, Choi HS, Schuster TF, et al.: (−)-Epigallocatechin gallate overcomes resistance to etoposide-induced cell death by targeting the molecular chaperone glucose-regulated protein 78. Cancer Res 66(18), 9260–9269, 2006. https://doi.org/10.1158/0008-5472.CAN-06-1586.
- Chakravarti A, Zhai GG, Zhang M, Malhotra R, Latham DE, et al.: Survivin enhances radiation resistance in primary human glioblastoma cells via caspase-independent mechanisms. Oncogene 23(45), 7494–7506, 2004. https://doi.org/10.1038/sj.onc.1208049.
- Peek GW, and Tollefsbol TO: Down-regulation of hTERT and cyclin D1 transcription via PI3K/Akt and TGF-β pathways in MCF-7 cancer cells with PX-866 and raloxifene. Exp Cell Res 344(1), 1–8, 2016. https://doi.org/10.1016/j.yexcr.2016.03.022.
- Pujari R, Jose J, Bhavnani V, Kumar N, Shastry P, et al.: Tamoxifen-induced cytotoxicity in breast cancer cells is mediated by glucose-regulated protein 78 (GRP78) via AKT (Thr308) regulation. Int J Biochem Cell Biol 77, 57–67, 2016. https://doi.org/10.1016/j.biocel.2016.05.021.
- Wang L, Wang C, Jia Y, Liu Z, Shu X, et al.: Resveratrol increases anti-proliferative activity of bestatin through downregulating P-glycoprotein expression via inhibiting PI3K/Akt/mTOR pathway in K562/ADR cells. J Cell Biochem 117(5), 1233–1239, 2016. https://doi.org/10.1002/jcb.25407.
- Xia Y, Yang L, Xue G, Zhang C, and Guo C: Combining GRP78 suppression and MK2206-induced Akt inhibition decreases doxorubicin-induced P-glycoprotein expression and mitigates chemoresistance in human osteosarcoma. Oncotarget 7(35), 56371–56382, 2016.
- Strawns LM, Mann E, Elligerm SS, Chus LM, Germains LL, et al.: Inhibition of glioma cell growth by a truncated platelet-derived growth factor beta receptor. J Biol Chem 269(33), 21215–21222, 1994.
- Zhang J, Chen T, Mao Q, Lin J, Jia J, et al.: PDGFR-β-activated ACK1-AKT signaling promotes glioma tumorigenesis. Int J Cancer 136(8), 1769–1780, 2015. https://doi.org/10.1002/ijc.29234.
- Westermark B, Heldin CH, and Nister M: Platelet-derived growth factor in human glioma. Glia 15(3), 257–263, 1995. https://doi.org/10.1002/glia.440150307.
- Masamune A, Kikuta K, Satoh M, Suzuki N, and Shimosegawa T: Green tea polyphenol epigallocatechin-3-gallate blocks PDGF-induced proliferation and migration of rat pancreatic stellate cells. World J Gastroenterol 11(22), 3368–33674, 2005. https://doi.org/10.3748/wjg.v11.i22.3368.
- Sakata R, Ueno T, Nakamura T, Sakamoto M, Torimura T, et al.: Green tea polyphenol epigallocatechin-3-gallate inhibits platelet-derived growth factor-induced proliferation of human hepatic stellate cell line LI90. J Hepatol 40(1), 52–59, 2004. https://doi.org/10.1016/S0168-8278(03)00477-X.
- Weber AA, Neuhaus T, Skach RA, Hescheler J, Ahn HY, et al.: Mechanisms of the inhibitory effects of epigallocatechin-3 gallate on platelet-derived growth factor-BB-induced cell signaling and mitogenesis. FASEB J: Off Publ Fed Am Soc Exp Biol 18(1), 128–130, 2004. https://doi.org/10.1096/fj.03-0007fje.
- Lamm GM, and Christofori G: Impairment of survival factor function potentiates chemotherapy-induced apoptosis in tumor cells. Cancer Res 58(4), 801–807, 1998.
- Li M, He Z, Ermakova S, Zheng D, Tang F, et al.: Direct inhibition of Insulin-like growth Factor-I receptor kinase activity by (À)ÀEpigallocatechin-3-gallate regulates cell transformation. Cell Prolif 16(March), 598–605, 2007.
- Kang H-G, Jenabi JM, Liu XF, Reynolds CP, Triche TJ, et al.: Inhibition of the insulin-like growth factor I receptor by epigallocatechin gallate blocks proliferation and induces the death of Ewing tumor cells. Mol Cancer Ther 9(5), 1396–1407, 2010. https://doi.org/10.1158/1535-7163.MCT-09-0604.
- Vu HA, Beppu Y, Chi HT, Sasaki K, Yamamoto H, et al.: Green tea epigallocatechin gallate exhibits anticancer effect in human pancreatic carcinoma cells via the inhibition of both focal adhesion kinase and insulin-like growth factor-I receptor. J Biomed Biotechnol 31, 290516, 2010. https://doi.org/10.1155/2010/290516.
- Chen FX, Qian YR, Duan YH, Ren WW, Yang Y, et al.: Down-regulation of 67LR reduces the migratory activity of human glioma cells in vitro. Brain Res Bull 79(6), 402–408, 2009. https://doi.org/10.1016/j.brainresbull.2009.04.019.
- Menard S, Tagliabue E, and Colnaghi MI: The 67 kDa laminin receptor as a prognostic factor in human cancer. Breast Cancer Res Treat 137–145, 1998. https://doi.org/10.1023/A:1006171403765.
- Tachibana H, Koga K, Fujimura Y, and Yamada K: A receptor for green tea polyphenol EGCG. Nat Struct Mol Biol 11(4), 380–381, 2004. https://doi.org/10.1038/nsmb743.
- Annabi B, Bouzeghrane M, Moumdjian R, Moghrabi A, and Béliveau R: Probing the infiltrating character of brain tumors: Inhibition of RhoA/ROK-mediated CD44 cell surface shedding from glioma cells by the green tea catechin EGCg. J Neurochem 94(4), 906–916, 2005. https://doi.org/10.1111/j.1471-4159.2005.03256.x.
- Annabi B, Thibeault S, Moumdjian R, and Béliveau R: Hyaluronan cell surface binding is induced by type I collagen and regulated by caveolae in glioma cells. J Biol Chem 279(21), 21888–21896, 2004. https://doi.org/10.1074/jbc.M313694200.
- Demeule M, Brossard M, Pagé M, Gingras D, and Béliveau R: Matrix metalloproteinase inhibition by green tea catechins. Biochim Biophys Acta 1478(1), 51–60, 2000. https://doi.org/10.1016/S0167-4838(00)00009-1.
- Mook ORF, Frederiks WM, and Van Noorden CJ: The role of gelatinases in colorectal cancer progression and metastasis. Biochim Biophys Acta 1705, 69–89, 2004.
- Boyerinas B, Zafrir M, Yesilkanal AE, Price TT, Hyjek EM, et al.: Adhesion to osteopontin in the bone marrow niche regulates lymphoblastic leukemia cell dormancy. Blood 121(24), 4821–4832, 2013. https://doi.org/10.1182/blood-2012-12-475483.
- Sugahara KN, Murai T, Nishinakamura H, Kawashima H, Saya H, et al.: Hyaluronan oligosaccharides induce CD44 cleavage and promote cell migration in CD44-expressing tumor cells. J Biol Chem 278(34), 32259–32265, 2003. https://doi.org/10.1074/jbc.M300347200.
- Okada H, Yoshida J, Sokabe M, Wakabayashi T, and Hagiwara M: Suppression of CD44 expression decreases migration and invasion of human glioma cells. Int J Cancer 66(2), 255–260, 1996. https://doi.org/10.1002/(SICI)1097-0215(19960410)66:2%3c255::AID-IJC20%3e3.0.CO;2-A.
- Pan H, Wang H, Zhu L, Mao L, Qiao L, et al.: The role of Nrf2 in migration and invasion of human glioma cell U251. World Neurosurg 80(3–4), 363–370, 2013. https://doi.org/10.1016/j.wneu.2011.06.063.
- Dinarello CA: The paradox of pro-inflammatory cytokines in cancer. Cancer Metastasis Rev 25(3), 307–313, 2006. https://doi.org/10.1007/s10555-006-9000-8.
- Kim MH, Jung MA, Hwang YS, Jeong M, Kim SM, et al.: Regulation of urokinase plasminogen activator by epigallocatechin-3-gallate in human fibrosarcoma cells. Eur J Pharmacol 487(1–3), 1–6, 2004. https://doi.org/10.1016/j.ejphar.2003.12.031.
- Loeffler S, Fayard B, Weis J, and Weissenberger J: Interleukin-6 induces transcriptional activation of vascular endothelial growth factor (VEGF) in astrocytes in vivo and regulates VEGF promoter activity in glioblastoma cells via direct interaction between STAT3 and Sp1. Int J Cancer 115(2), 202–213, 2005. https://doi.org/10.1002/ijc.20871.
- Park S-S, Park S-K, Lim J-H, Choi YH, Kim W-J, et al.: Esculetin inhibits cell proliferation through the Ras/ERK1/2 pathway in human colon cancer cells. Oncol Rep 25, 223–230, 2011.
- Lakka SS, Gondi CS, Yanamandra N, Invasion GC, Growth T, et al.: Synergistic down-regulation of urokinase plasminogen activator receptor and matrix metalloproteinase-9 in SNB19 glioblastoma cells efficiently inhibits glioma cell invasion, angiogenesis, and tumor growth synergistic down-regulation of urokinase plasmin. Cancer Res (20), 2454–2461, 2003.
- Hanahan D, and Weinberg RA: Hallmarks of cancer: the next generation. Cell 144(5), 646–674, 2011. https://doi.org/10.1016/j.cell.2011.02.013.
- Wolf A, Agnihotri S, and Guha A: Targeting metabolic remodeling in glioblastoma multiforme. Oncotarget 1(7), 552–62, 2010.
- Navis AC, Verrijp K, Niclou SP, Bjerkvig R, Wesseling P et al.: Glutamate as chemotactic fuel for diffuse glioma cells: are they glutamate suckers? Biochim Biophys Acta – Rev Cancer 1846(1), 66–74, 2014. https://doi.org/10.1016/j.bbcan.2014.04.004.
- Molenaar RJ, Radivoyevitch T, Maciejewski JP, van Noorden CJF, and Bleeker FE: The driver and passenger effects of isocitrate dehydrogenase 1 and 2 mutations in oncogenesis and survival prolongation. Biochim Biophys Acta – Rev Cancer 1846(2), 326–341, 2014. https://doi.org/10.1016/j.bbcan.2014.05.004.
- Li C, Li M, Chen P, Narayan S, Matschinsky FM, et al.: Green tea polyphenols control dysregulated glutamate dehydrogenase in transgenic mice by hijacking the ADP activation site. J Biol Chem 286(39), 34164–34174, 2011. https://doi.org/10.1074/jbc.M111.268599.
- Li C, Allen A, Kwagh J, Doliba NM, Qin W, et al.: Green tea polyphenols modulate insulin secretion by inhibiting glutamate dehydrogenase. J Biol Chem 281(15), 10214–10221, 2006. https://doi.org/10.1074/jbc.M512792200.