Abstract
Lignan intake, and its richest food source, flaxseed, have been associated with reduced breast cancer risk. Endogenous sex hormones, such as estrogens, play a role in breast cancer development, and lignans may alter these sex hormone levels. To assess the effect of flaxseed on circulating sex hormones, a randomized controlled trial was conducted among 99 postmenopausal women in Toronto, Canada. The intervention arm consumed 2 tablespoons (15 g) of ground flaxseed daily for 7 weeks; the control arm maintained usual diet. Baseline and week 7 concentrations of 14 serum sex hormones were measured using liquid chromatography–tandem mass spectrometry (LC-MS/MS) and immunoassay, and serum enterolignans (lignan biomarker) using LC-MS/MS. Intervention effects on sex hormone levels were assessed using analysis of covariance. Serum enterolignans increased among the flaxseed arm (+516%). Women consuming flaxseed (vs. controls) had increased serum 2-hydroxyestrone [treatment effect ratio (TER) = 1.54; 95% CI: 1.18–2.00] and 2:16α-hydroxyestrone ratio (TER =1.54; 95% CI: 1.15–2.06); effects on other hormones were not statistically significant. Within the flaxseed arm, change in enterolignan level was positively correlated with changes in 2-hydroxyestrone and 2:16α-hydroxyestrone ratio, and negatively with prolactin. Findings suggest flaxseed affects certain circulating sex hormone levels with possible implications for future breast cancer prevention research.
Introduction
Lignans are a major class of phytoestrogens, estrogen-like compounds with cancer-preventive properties (Citation1). While lignans are generally found at low concentrations in a wide range of plant foods, flaxseed is by far the richest dietary source, containing predominantly secoisolariciresinol diglucoside (SDG) (Citation2). Once ingested, SDG is metabolized by intestinal bacteria to the biologically active mammalian lignans (enterolignans), enterodiol and enterolactone (Citation3,Citation4), whose circulating and urinary levels are strongly correlated with dietary lignan intake (Citation5–8). Enterolignans are structurally similar to endogenous sex hormones and have been shown to exert weak hormonal effects and inhibit carcinogenesis in animal models (Citation9,Citation10). As such, lignans, as well as flaxseed, have been investigated for their possible role in the prevention of hormone-related cancers (Citation4,Citation9,Citation11), particularly breast cancer (Citation12–17).
Epidemiologic studies provide growing evidence of an inverse association between lignans and breast cancer risk, especially among postmenopausal women (Citation14–16). Two meta-analyses of prospective cohort and case-control studies reported that high dietary lignan intake was associated with a modest statistically significant reduction in postmenopausal breast cancer risk (Citation14,Citation15). Another meta-analysis evaluated the association between circulating enterolactone levels and postmenopausal breast cancer risk and reported a 34% risk reduction (Citation16). In addition, a Canadian population-based case-control study found that flaxseed consumption was significantly associated with reduced breast cancer risk (Citation17).
The possible protective effects of flaxseed and lignans on breast cancer risk are thought to be mediated through modulation of sex hormone synthesis, metabolism, and activity (Citation12). It is widely accepted that endogenous sex hormones play a role in breast cancer etiology. In particular, higher circulating levels of estrogens (e.g., estradiol, estrone), androgens (e.g., testosterone), and prolactin have been associated with increased postmenopausal breast cancer risk (Citation18–20). Metabolites formed from the hydroxylation of estrogens, as well as their relative abundance, have also been linked to breast cancer development (Citation21–23). Notably, a high ratio of 2-hydroxylation to 16-hydroxylation pathway estrogen metabolites has been associated with reduced breast cancer risk (Citation22,Citation23). In addition, 2-hydroxyestrone has been shown to exert antiestrogenic activity, which may counteract the estrogen-agonist and cell proliferation effects of 16α-hydroxyestrone (Citation24–27). Although exact mechanisms remain to be elucidated, lignans have been suggested to exert anticancer effects by competing with estrogens for binding to estrogen receptors, resulting in altered estrogen-sensitive gene expression, and subsequently, decreased cell proliferation and increased apoptosis (Citation12). Furthermore, lignans may interact with key enzymes involved in hormone synthesis and metabolism (e.g., aromatase) to modulate relative levels of circulating sex hormones and their metabolites (Citation28,Citation29).
Despite biological plausibility and evidence from observational studies, only a few small intervention trials have assessed the effect of flaxseed on endogenous sex hormone levels among postmenopausal women, with inconsistent findings (Citation30–37). Flaxseed intake has been shown to either increase (Citation30–32) or decrease (Citation33) the ratio of 2-hydroxyestrone to 16α-hydroxyestrone, while significant effects on other sex hormones were reported by some (Citation33,Citation34), but not all (Citation32,Citation35–37), studies. Most previous trials had very small sample sizes, lacked a control group, and only assessed a limited number of sex hormones. In addition to studies examining the 2:16α-hydroxyestrone ratio (Citation30–33), only one small pre-post study assessed other estrogen metabolites (e.g., estriol) considered relevant to breast cancer risk (Citation33).
Our larger, randomized controlled intervention study was conducted among postmenopausal women to investigate the effect of daily flaxseed intake on circulating serum levels of a wide spectrum of sex hormones and their metabolites. In addition, the effect of flaxseed on circulating enterolignan levels, as well as correlations between changes in enterolignan levels and changes in sex hormone levels, were assessed.
Methods
The Women’s Flaxseed and Health Study was a two-arm, parallel, randomized controlled trial (RCT) consisting of a flaxseed intervention and a control arm designed to assess the effect of flaxseed intake on serum sex hormone levels among postmenopausal women. The study was conducted in Toronto, Ontario, Canada during 2015–2016. The study protocol was approved by the University of Toronto Health Sciences Research Ethics Board (Toronto, ON) and registered at ClinicalTrials.gov (NCT02501031). All participants provided written informed consent.
Participants
Eligible participants were women residing in Toronto, Ontario who were 57–64 years of age and postmenopausal (no menstrual period within the past year). Exclusion criteria included the following: regular consumption (more than once per week) of flaxseed (whole/ground flaxseed, foods containing flaxseed, or flaxseed oil containing lignans) or other lignan- or phytoestrogen-rich foods [sesame seeds, tahini, sesame oil, or soy foods/supplements (e.g., soy milk, tofu, tempeh, soybeans, soy nuts, supplements containing soy, red clover, or isoflavones)] in the past 6 months; use of oral antibiotics in the past 6 months; use of hormone replacement therapy or corticosteroids in the past month; current chronic conditions such as bowel disease (e.g., irritable bowel syndrome, inflammatory bowel disease), diabetes, heart disease, or cancer; current use of blood thinners (e.g., warfarin); and non-English speaking.
Participants were recruited between November 2015 and February 2016 by the Institute for Social Research at York University (Toronto, ON) using random-digit dialing of telephone numbers to reach a random sample of households in Toronto. All sampled numbers were called up to 12 times to identify eligible women (up to 1 per household) interested in participation. Contact information for identified women was forwarded to the study staff at Cancer Care Ontario (Toronto, ON), who then mailed these women invitation letters and consent forms. Eligible (confirmed through telephone) and consenting women were randomly assigned to either the intervention or the control arm using a computer-generated random allocation sequence.
Intervention and Control Arms
Women in the intervention arm were provided a bag of ground flaxseed at baseline and instructed to add 2 tablespoons (15 g) of ground flaxseed to their diet daily for 7 weeks. They were also asked to avoid soy foods/supplements. To reduce variability in lignan levels, the same commercially available brand and batch of ground flaxseed (Gold Top Organics Cold Milled Brown Flax Seed, Stony Plain, AB) was provided to the women, along with a tablespoon, storage instructions, and suggestions for how to add flaxseed to their daily diet (e.g., sprinkle on cereal, toast, yogurt, or in smoothies). To verify the lignan content stated on the product label (75 mg lignans/15 g flaxseed, i.e., 5 mg/g), a sample was analyzed at the Fred Hutchinson Cancer Research Center (Seattle, WA) using gas chromatography–mass spectrometry (Agilent 6890N/5975 GC/MS System; Agilent Technologies, Santa Clara, CA). The sample was indeed found to contain 5.3 mg SDG (lignans)/g flaxseed. The product was expected to retain its potency over the study period, as previous studies have shown that SDG in flaxseed remained relatively stable over several weeks to months, even after heat treatment during baking (Citation38,Citation39).
Women in the control arm were asked to maintain their usual diet during the study and to avoid foods/supplements containing flaxseed or soy.
To assess adherence, women in the intervention arm recorded flaxseed eaten each day over the 7 weeks in a “flaxseed diary”. Flaxseed consumption was also assessed by measuring serum enterolignan levels among all women at baseline and week 7 (see below).
Baseline and Follow-up Measures
Information on demographics, medical and reproductive history, lifestyle factors (e.g., smoking, alcohol consumption, physical activity), body mass index [BMI; calculated as weight (kg)/height squared (m2)], and dietary intake (e.g., phytoestrogen-containing foods/supplements, dietary fat) was collected using self-administered questionnaires completed by all participants both at baseline and at the end of the study (week 7). Women in the intervention arm were also asked to report any adverse effects in the week 7 questionnaire.
Primary outcome measures were serum levels of sex hormones, including estrogens and estrogen metabolites (estradiol, free estradiol, estrone, estrone sulfate, estriol, 2-methoxyestrone, 2-hydroxyestrone, 16α-hydroxyestrone, and 2:16α-hydroxyestrone ratio), androgens [androstenedione, dehydroepiandrosterone sulfate (DHEAS), and testosterone], prolactin, and sex hormone-binding globulin (SHBG). Secondary outcome measures were serum levels of enterolignans (enterodiol and enterolactone). All sex hormone and enterolignan measures were assessed at baseline and week 7 by laboratory personnel blinded to randomization status.
Blood Collection and Laboratory Assays
Blood was drawn from participants at baseline and week 7. Samples were allowed to clot for 45–60 min at room temperature prior to centrifugation, and serum was aliquoted into cryovial tubes and stored at –80 °C until analysis or shipment. All blood samples were collected, processed, and stored at Mount Sinai Services, Mount Sinai Hospital (Toronto, ON).
Sex Hormone Assays
Serum sex hormone assays were performed by the Mount Sinai Services laboratory. Concentrations of total [conjugated (glucuronidated and sulfated) plus unconjugated] estradiol, estrone, estriol, and 2-methoxyestrone were measured by liquid chromatography–tandem mass spectrometry (LC-MS/MS) as previously described (Citation40,Citation41). Briefly, standards for each estrogen and estrogen metabolite (Steraloids Inc., Newport, RI) and deuterated internal standards (Cambridge Isotope Laboratories, Andover, MA) were added to serum samples, followed by enzymatic hydrolysis with β-glucuronidase/arylsulfatase from Helix pomatia (Sigma Aldrich Canada Co., Oakville, ON). After incubation at 37 °C for 18 h, samples were extracted with methyl tert-butyl ether, evaporated under nitrogen gas, reconstituted in 70:30 water:methanol, and analyzed using the AB SCIEX Triple Quad™ 6500 LC-MS/MS System (AB Sciex, Concord, ON). Levels of 2-hydroxyestrone and 16α-hydroxyestrone were measured using Estramet™ enzyme immunoassay (EIA) kits (Immuna Care Corporation, Tampa, FL) (Citation42) (because the laboratory was unable to detect levels of 2-hydroxyestrone using LC-MS/MS). The ratio of 2-hydroxyestrone to 16α-hydroxyestrone was also calculated. Estrone sulfate and androstenedione were analyzed using enzyme-linked immunosorbent assay kits (MyBioSource, Inc., San Diego, CA). DHEAS was measured by a competitive binding immunoenzymatic assay on the Unicel DxI 600 Access Immunoassay System (Beckman Coulter Canada, Mississauga, ON). Testosterone, prolactin, and SHBG were quantified using electrochemiluminescence immunoassays on the Roche Modular Analytics immunoassay analyzer (Roche Diagnostics Canada, Laval, QC). Free (non-albumin- and non-SHBG-bound) estradiol concentration was calculated from measured estradiol and SHBG concentrations and an assumed constant for albumin (4 g/dl), according to a validated algorithm based on the law of mass action (Citation43). Calculated free estradiol concentrations have been shown to correlate strongly with directly measured values (Citation43).
The lower limit of quantification (LLOQ) was 1.0 pg/ml for estradiol, estrone, estriol, and 2-methoxyestrone and 20 pg/ml for 2- and 16α-hydroxyestrone. LLOQs for estrone sulfate, androstenedione, DHEAS, testosterone, prolactin, and SHBG were 0.625 ng/ml, 0.021 ng/ml, 0.074 µg/ml, 0.025 ng/ml, 1.0 ng/ml, and 1.0 nmol/l, respectively. Concentrations below the LLOQ at baseline (estradiol, n = 19; 2-methoxyestrone, n = 37; 2-hydroxyestrone, n = 19; 16α-hydroxyestrone, n = 5; testosterone, n = 21) and week 7 (estradiol, n = 19; estriol, n = 2; 2-methoxyestrone, n = 50; 2-hydroxyestrone, n = 6; 16α-hydroxyestrone, n = 2; testosterone, n = 23) were assigned a value of LLOQ/2 (e.g., 0.5 pg/ml for estradiol). Intra-assay coefficients of variation (CV) were <10% for all sex hormone assays, except for estrone sulfate (<15%), whereas inter-assay CVs were <15% for all assays.
Enterolignan Assays
Serum enterolignan (enterodiol and enterolactone) assays were performed by the Bioanalytics, Metabolomics and Pharmacokinetics Shared Resource at Roswell Park Comprehensive Cancer Center (Buffalo, NY). Total (conjugated plus unconjugated) concentrations of enterodiol and enterolactone were determined using an LC-MS/MS method adapted from the literature (Citation44,Citation45). Briefly, serum samples were treated with β-glucuronidase and sulfatase, followed by solid phase extraction and evaporation under nitrogen gas. Dried residues were reconstituted and chromatographed using an XBridge™ C18 column (Waters Corporation, Milford, MA) with a water/acetonitrile/acetic acid mobile phase. The individual enterolignans were analyzed and quantitated using a TSQ Vantage Mass Spectrometer (Thermo Fisher Scientific, Waltham, MA), and the total enterolignan concentration was calculated as the sum of the enterodiol and enterolactone concentrations.
The LLOQ was 0.500 ng/ml for both enterodiol and enterolactone. Concentrations below the LLOQ at baseline [enterodiol, n = 41 (18 intervention; 23 control); enterolactone, n = 1 control] and week 7 [enterodiol, n = 32 (3 intervention; 29 control); enterolactone, n = 3 control] were replaced with LLOQ/2 (i.e., 0.25 ng/ml). The large proportion of participants with enterodiol values below the LLOQ at baseline (and week 7 for the control arm) was expected since participants were not regular consumers of flaxseed or other lignan-rich foods (e.g., sesame seed). Intra-day assay CVs of the validation quality control samples for enterodiol and enterolactone were 3.0% and 3.6%, respectively, while inter-day assay CVs were 2.8% and 2.6%, respectively.
Statistical Analyses
Baseline demographic and lifestyle characteristics were summarized in each study arm using means and standard deviations (SD) for continuous variables and counts and percentages for categorical variables. Baseline sex hormone and enterolignan concentrations were reported as medians and interquartile ranges (IQR), as these measurements did not follow a normal distribution. Between-group differences were assessed using Student’s t or Wilcoxon rank-sum tests for continuous variables and Pearson’s chi-square or Fisher’s exact tests for categorical variables, as appropriate. In subsequent analyses, unless otherwise indicated, all sex hormone and enterolignan measurements were natural log-transformed.
Within each study arm, the geometric mean and corresponding 95% confidence intervals (CI) were calculated for sex hormones and enterolignans at baseline and follow-up (week 7). Change from baseline was expressed both as an absolute change (week 7 geometric mean – baseline geometric mean) and as a percent change [100% × (week 7 geometric mean – baseline geometric mean)/baseline geometric mean]. Analysis of covariance (ANCOVA) was used to compare change in sex hormone or enterolignan level from baseline between study arms, while adjusting for baseline level (Citation46). The ANCOVA linear regression model included week 7 hormone or enterolignan level as the dependent variable and randomization arm (intervention vs. control) and baseline hormone or enterolignan level as independent variables. Furthermore, to be on the conservative side, potential confounding was assessed in expanded multivariable models adjusted for additional covariates, including age, age at menopause, BMI, smoking, alcohol consumption, and physical activity (selected as they may be associated with postmenopausal sex hormone levels; refs. Citation47–50); however, none of the covariates resulted in >10% change in the effect estimates for any of the hormones. Thus, final models were adjusted only for baseline hormone level.
Since analyses were performed on log-transformed data, the regression coefficient for randomization arm was transformed back (i.e., exponentiated) to the original scale and interpreted as the treatment effect ratio (TER) for our main analysis on sex hormones. The TER represented the effect of the flaxseed intervention on change in hormone level during the study period, adjusted for baseline hormone level (i.e., intervention:control ratio of geometric means at week 7 relative to baseline). A TER <1 indicated a reduction, whereas a TER >1 indicated an increase, in hormone level in the intervention relative to the control arm. In addition, correlations between changes in enterolignan levels (untransformed) and changes in sex hormone levels (untransformed) among the intervention arm were assessed using the Spearman rank-order correlation test.
All statistical tests were two-sided with a significance level of 0.05. Analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC).
Results
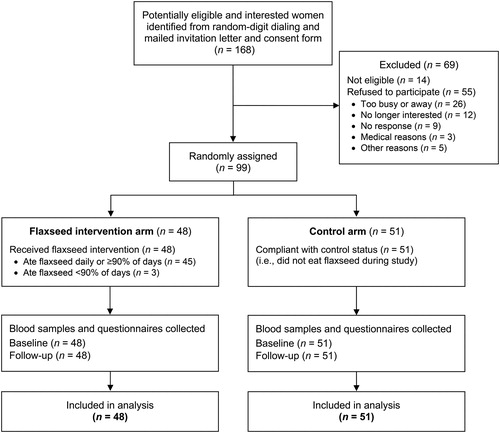
A total of 168 potentially eligible and interested women were identified from random-digit dialing of thousands of households in Toronto, and these women were then mailed invitation letters and consent forms, of which 14 were further deemed ineligible and 55 did not consent to participate (). Ninety-nine participating postmenopausal women were randomized (48 intervention; 51 control). All women (n = 99) from both study arms returned questionnaires and provided blood samples at both baseline and follow-up (100% retention) and were included in the analysis. In terms of compliance, 45 (94%) of the 48 women in the intervention arm reported consuming flaxseed daily or ≥90% of days, while all 51 (100%) women in the control arm reported no flaxseed intake. Minor side effects reported by women in the intervention arm included gas (33%), bloating (21%), constipation (15%), abdominal pain (13%), stomach ache (10%), diarrhea (10%), nausea (4%), and dry mouth/thirst (4%).
Figure 1. Flow diagram of the enrollment, randomization, and retention of participants in the Women’s Flaxseed and Health Study, Toronto, Canada, 2015–2016.
presents some baseline characteristics of participants stratified by study arm, as well as baseline serum enterolignan and sex hormone levels. On average, participants were 60 years of age, became postmenopausal at 52 years, were slightly overweight (mean BMI = 26 kg/m2), white (94%), nonsmokers (94%), and had at least a college/university degree (76%). There were no significant differences between the intervention and control arms at baseline (). No changes in body weight were observed in either study arm over the 7-week intervention period (data not shown).
Table 1. Baseline characteristics and serum enterolignan and sex hormone levels of postmenopausal women participants in the Women’s Flaxseed and Health Study, Toronto, Canada, 2015–2016.
presents baseline and week 7 geometric means and changes in serum enterolignan levels for each study arm. Consistent with the high compliance as assessed by self-report, serum concentrations of enterodiol, enterolactone, and total enterolignans were all significantly increased in the flaxseed intervention (+1,148%, +380%, and +516%, respectively) compared to the control (–21%, –34%, and –31%, respectively) arm (all P < 0.001).
Table 2. Changes in serum enterolignan concentrations from baseline to week 7, by study arm.
presents effects of the flaxseed intervention on serum sex hormone levels. Compared to the control arm, women in the intervention arm had significant increases in serum 2-hydroxyestrone concentration (TER = 1.54; 95% CI: 1.18–2.00; P = 0.002) and 2:16α-hydroxyestrone ratio (TER =1.54; 95% CI: 1.15–2.06; P = 0.004), while no effect was observed for 16α-hydroxyestrone (TER = 0.98; 95% CI: 0.83–1.15; P = 0.806). The intervention (vs. control) arm showed larger declines in serum levels of most other estrogens and estrogen metabolites, including estradiol (–12% vs. +20%), free estradiol (–6% vs. –2%), estriol (–7% vs. –4%), and 2-methoxyestrone (–25% vs. –9%), but not estrone (+5% vs. –7%) and estrone sulfate (+32% vs. +30%); however, baseline-adjusted TERs were not statistically significant for any of these hormones. Similarly, the intervention did not result in statistically significant changes in any of the androgens (androstenedione, DHEAS, and testosterone; TERs ranging from 0.98 to 1.01), prolactin (TER =0.91; 95% CI: 0.80–1.03), or SHBG (TER =0.93; 95% CI: 0.81–1.07).
Table 3. Effect of the dietary flaxseed intervention on changes in serum sex hormone concentrations from baseline to week 7.
shows correlations between changes in serum enterolignan levels and changes in serum sex hormones levels among women in the intervention arm. Significant positive correlations were found between change in total enterolignans and changes in both 2-hydroxyestrone (r = 0.33; P = 0.023) and 2:16α-hydroxyestrone ratio (r = 0.37; P = 0.009). Change in enterolactone, but not enterodiol, was also positively correlated with significant changes in 2-hydroxyestrone (r = 0.40; P = 0.005) and 2:16α-hydroxyestrone ratio (r = 0.41; P = 0.004). Additionally, changes in enterodiol and total enterolignans showed significant negative correlations with change in prolactin (r = –0.37; P = 0.009 and r = –0.32; P = 0.029, respectively). There were no statistically significant correlations between changes in enterolignans and changes in other sex hormones.
Table 4. Spearman correlation coefficients between changes in serum enterolignan concentrations and changes in sex hormone concentrations among women in the intervention arm (n = 48).
Discussion
This randomized intervention trial among postmenopausal women found that intake of ground flaxseed daily for 7 weeks significantly increased circulating 2-hydroxyestrone level and the 2:16α-hydroxyestrone ratio; however, no statistically significant effects were seen for other sex hormones assessed. In addition, flaxseed consumption substantially increased circulating enterolignans, which were in turn positively correlated with changes in 2-hydroxyestrone and 2:16α-hydroxyestrone ratio, supporting the hypothesis that effects of flaxseed on estrogen metabolism may be mediated through mechanisms involving lignans (Citation12). Our findings add to the literature suggesting flaxseed intake may alter estrogen profiles (Citation30–34). This is of particular importance for breast cancer prevention, as these estrogen profiles have been shown to be associated with breast cancer risk (Citation23,Citation24).
While previous trials measured urinary excretion of 2- and 16α-hydroxyestrone (Citation30–33), our study was the first to assess the effect of flaxseed on circulating blood levels of these estrogen metabolites, which may be a more representative measure of endogenous exposures (Citation51). Our findings that 2-hydroxyestrone level and 2:16α-hydroxyestrone ratio increased among postmenopausal women consuming flaxseed are consistent with three (of four) previous studies, all of which measured hormones using EIA (Citation30–32). Using a randomized crossover design, Haggans et al. found that consuming 10 g flaxseed/day for 7 weeks significantly increased urinary 2-hydroxyestrone (34%) and 2:16α-hydroxyestrone ratio (21%) (Citation30). The pre-post study by McCann et al. also reported significant increases in urinary 2-hydroxyestrone (73%) and 2:16α-hydroxyestrone ratio (77%) among women supplemented with 10 g flaxseed/day for 7 days (Citation31). In the RCT by Brooks et al., daily consumption of a flaxseed muffin (25 g flaxseed) for 16 weeks significantly increased urinary 2-hydroxyestrone (103%) and 2:16α-hydroxyestrone ratio (98%) (Citation32). Furthermore, consistent with our results, none of these studies reported significant changes in 16α-hydroxyestrone, suggesting that flaxseed improves the 2:16α-hydroxyestrone ratio mainly by increasing 2-hydroxyestrone, the presumably more optimal metabolite with antiestrogenic potential, without affecting the more estrogenic 16α-hydroxyestrone (Citation30–32). In contrast, a 12-week pre-post flaxseed intervention study (7.5 g/day for 6 weeks, followed by 15 g/day for 6 weeks) reported a significant increase in urinary 16α-hydroxyestrone and no change in 2-hydroxyestrone (i.e., decreased 2:16α-hydroxyestrone ratio); however, the lack of a control group makes it difficult to account for possible temporal changes unrelated to the intervention (Citation33).
As expected and reported by others (Citation52,Citation53), daily flaxseed intake significantly increased serum enterolignan levels. Our study further demonstrated that changes in enterolignans, especially enterolactone (primary SDG metabolite), are positively correlated with changes in 2-hydroxyestrone and 2:16α-hydroxyestrone ratio among women consuming flaxseed. This suggests that flaxseed intake led to changes in the estrogen profile by increasing enterolignans (Citation12). Brooks et al. also reported a positive correlation between change in total urinary lignan excretion and change in 2:16α-hydroxyestrone ratio (r = 0.58; P = 0.02) among women supplemented with flaxseed; however, specific enterolignans were not examined (Citation32). Our finding of a stronger correlation with enterolactone than enterodiol may be explained by the shorter half-life of enterodiol, which is further metabolized into enterolactone (Citation7,Citation8). Additionally, in vitro and in vivo studies have demonstrated differential effects of enterolactone and enterodiol on estrogen-related pathways in cancer development (Citation54–56).
Although exact mechanisms are unclear, it has been postulated that flaxseed/lignans may alter the profile of estrogen metabolites by modifying the expression or activity of cytochrome P450 enzymes responsible for estrogen hydroxylation to favor 2- over 16-hydroxylation (Citation24,Citation31,Citation57). For example, flaxseed supplementation in hens upregulated CYP1A1 expression, resulting in increased and decreased production of 2- and 16α-hydroxyestrone, respectively (Citation57). However, while the 2:16α-hydroxyestrone ratio has been inversely associated with breast cancer risk and suggested as a potential target for dietary interventions (Citation24), recent research indicated that overall and relative abundances of 2-, 4-, and 16-hydroxylation pathway metabolites may be more relevant with respect to breast cancer risk and prevention (Citation21–23,Citation58,Citation59). Our study additionally assessed 2-methoxyestrone and estriol (2- and 16-hydroxylated, respectively) and found no significant changes related to flaxseed intake. These results are in line with those of Sturgeon et al., who examined three other metabolites (2-hydroxyestradiol, 2-methoxyestradiol, and 4-hydroxyestradiol) in urine and reported no changes except for a statistically significant decline in 2-methoxyestradiol (Citation33).
Flaxseed intake did not significantly influence circulating levels of estradiol, free estradiol, estrone, or estrone sulfate in our study. This is consistent with other intervention studies assessing serum (Citation32,Citation35,Citation36), plasma (Citation37), or urinary (Citation33) estrogen levels in postmenopausal women, with the exception of a crossover trial reporting statistically significant reductions in serum estradiol and estrone sulfate with 10 g flaxseed/day for 7 weeks (Citation34). The most biologically active estrogens, estradiol, and estrone, have been associated with elevated postmenopausal breast cancer risk (Citation18,Citation19), thus factors decreasing their levels may be protective. In vitro studies have suggested that lignans may decrease estrogen synthesis by inhibiting the aromatase enzyme responsible for converting testosterone and androstenedione to estradiol and estrone, respectively (Citation28,Citation29,Citation60). However, evidence from intervention trials, including ours, provides stronger support for flaxseed’s role in modifying estrogen metabolism (e.g., 2-hydroxylation of estrone) rather than inhibiting estrogen synthesis.
Consistent with the other two intervention studies assessing androgens in postmenopausal women (Citation34,Citation35), flaxseed intake had no effect on serum levels of testosterone, androstenedione, and DHEAS. Conflicting results have been obtained from cross-sectional data, with studies reporting positive (Citation61), negative (Citation62), or no (Citation63,Citation64) correlation between enterolignans and androgens. In addition to inhibiting aromatase conversion of androgens to estrogens, lignans may affect androgen metabolism by interfering with 5α-reductase, which converts testosterone to the more potent dihydrotestosterone (Citation61,Citation65). Furthermore, lignans have been shown to stimulate SHBG synthesis in the liver (Citation66) and interact with SHBG (Citation67,Citation68) to alter biological activity and availability of circulating estrogens and androgens. Observational studies have also reported positive correlations between lignans and SHBG (Citation63,Citation64,Citation69). However, like other intervention studies (Citation34–36), our results do not support the role of flaxseed in modulating SHBG levels.
Although flaxseed intake only resulted in a small (and not statistically significant) reduction in prolactin level, a significant negative correlation between change in enterolignans and change in prolactin was observed among women consuming flaxseed. To our knowledge, only one previous study in humans has examined the effect of flaxseed on prolactin, reporting a statistically significant increase in serum prolactin among postmenopausal women consuming 10 g flaxseed/day for 7 weeks (Citation34). Additionally, animal models suggested that the estrogenic activity of lignans and other phytoestrogens may stimulate prolactin synthesis and secretion (Citation70–72); however, intervention studies generally reported no effect of soy (rich source of phytoestrogen) intake on prolactin (Citation73–76). Epidemiological evidence supports an association between increased circulating prolactin and increased breast cancer risk (Citation20), and our findings showed that flaxseed/lignans may decrease prolactin level, although additional studies are warranted.
Strengths of our study included the large sample size relative to other flaxseed intervention trials, complete follow-up of participants, and excellent adherence rates, as confirmed by both self-report and lignan biomarker measurements. In addition, compared to previous studies, a wider range of sex hormones (possibly relevant to breast cancer risk) were assessed, including estrogens and their metabolites, androgens, SHBG, and prolactin. Furthermore, the concurrent assessments of circulating sex hormones and enterolignans helped provide additional insights into potential underlying biological mechanisms. A possible limitation of our study is the potential for cross-reactivity in some of the hormone assays, which is an inherent problem for all immunoassay methods (Citation77). Moreover, a placebo was not used for the control arm. While some RCTs used pre-baked foods (e.g., muffins, bread) with or without flaxseed (Citation32,Citation37), the ability of participants to add flaxseed to any food, as demonstrated here, suggests that this intervention approach may have population-wide application.
In conclusion, our findings suggest that dietary flaxseed may modulate estrogen levels and metabolism among postmenopausal women, particularly by increasing circulating 2-hydroxyestrone levels, and consequently, improving the 2:16α-hydroxyestrone ratio. Our results add to the emerging evidence from previous intervention trials suggesting that flaxseed intake may have a role in altering sex hormone profiles which are thought to be involved in breast cancer risk (Citation30–32). In addition, despite minor gastrointestinal side effects reported by some women, a very high adherence to the daily flaxseed intervention was achieved, suggesting it may be a feasible approach at the population level if these findings are replicated in larger trials.
Author Contributions
VC contributed to the analysis and interpretation of data and wrote the first draft of the manuscript. MC contributed to the conception and design of the study, acquisition and interpretation of data, supervised the study, and critically revised the manuscript for its intellectual content. BB contributed to the conception and design of the study, acquisition and interpretation of data, and critically revised the manuscript for its intellectual content. DJ, LM, SM, and LT contributed to the conception and design of the study, interpretation of data, and critically revised the manuscript for its intellectual content. All authors have approved the final version of the manuscript submitted.
Acknowledgments
The authors thank Michelle Lee (Cancer Care Ontario, Toronto, ON) for her dedication as the Project Coordinator (data collection procedures); the Institute for Social Research staff at York University (Toronto, ON) for services provided in participant recruitment; Dr. Johanna Lampe (Fred Hutchinson Cancer Research Center, Seattle, WA) for analyzing the lignan content of flaxseed; Dr. Azar Azad, Dr. Hilde Vandenberghe, and Michelle Rodrigues (Mount Sinai Services, Toronto, ON) for services provided in overseeing the blood collection and completing the sex hormone assays; and Dr. John Wilton and Dr. Sarah Schihl (Roswell Park Comprehensive Cancer Center, Buffalo, NY) for services provided in completing the enterolignan assays. The enterolignan assays were completed with partial support from National Cancer Institute (NCI) grant P30CA016056 involving the use of Roswell Park’s Bioanalytics, Metabolomics and Pharmacokinetics (BMPK) Shared Resource. We also gratefully acknowledge all the participants of the Women’s Flaxseed and Health Study.
Disclosure Statement
No potential conflicts of interest were disclosed by any of the authors, except for D.J.A. Jenkins who declares the following potential conflicts of interest: he has received research grants from Saskatchewan Pulse Growers, the Agricultural Bioproducts Innovation Program through the Pulse Research Network, the Advanced Foods and Material Network, Loblaw Companies Ltd., Unilever, Barilla, the Almond Board of California, Agriculture and Agri-food Canada, Pulse Canada, Kellogg’s Company, Canada, Quaker Oats, Canada, Procter & Gamble Technical Centre Ltd., Bayer Consumer Care, Springfield, NJ, Pepsi/Quaker, International Nut & Dried Fruit (INC), Soy Foods Association of North America, the Coca-Cola Company (investigator initiated, unrestricted grant), Solae, Haine Celestial, the Sanitarium Company, Orafti, the International Tree Nut Council Nutrition Research and Education Foundation, the Peanut Institute, the Canola and Flax Councils of Canada, the Calorie Control Council (CCC), the CIHR, the Canada Foundation for Innovation and the Ontario Research Fund; he has received in-kind supplies for trial as a research support from the Almond board of California, Walnut Council of California, American Peanut Council, Barilla, Unilever, Unico, Primo, Loblaw Companies, Quaker (Pepsico), Pristine Gourmet, Bunge Limited, Kellogg Canada, WhiteWave Foods; he has been on the speaker’s panel, served on the scientific advisory board and/or received travel support and/or honoraria from the Almond Board of California, Canadian Agriculture Policy Institute, Loblaw Companies Ltd, the Griffin Hospital (for the development of the NuVal scoring system), the Coca-Cola Company, EPICURE, Danone, Diet Quality Photo Navigation (DQPN), FareWell, Verywell, True Health Initiative, Saskatchewan Pulse Growers, Sanitarium Company, Orafti, the Almond Board of California, the American Peanut Council, the International Tree Nut Council Nutrition Research and Education Foundation, the Peanut Institute, Herbalife International, Pacific Health Laboratories, Nutritional Fundamental for Health, Barilla, Metagenics, Bayer Consumer Care, Unilever Canada and Netherlands, Solae, Kellogg, Quaker Oats, Procter & Gamble, the Coca-Cola Company, the Griffin Hospital, Abbott Laboratories, the Canola Council of Canada, Dean Foods, the California Strawberry Commission, Haine Celestial, PepsiCo, the Alpro Foundation, Pioneer Hi-Bred International, DuPont Nutrition and Health, Spherix Consulting and WhiteWave Foods, the Advanced Foods and Material Network, the Canola and Flax Councils of Canada, the Nutritional Fundamentals for Health, Agri-Culture and Agri-Food Canada, the Canadian Agri-Food Policy Institute, Pulse Canada, the Saskatchewan Pulse Growers, the Soy Foods Association of North America, the Nutrition Foundation of Italy (NFI), Nutra-Source Diagnostics, the McDougall Program, the Toronto Knowledge Translation Group (St. Michael’s Hospital), the Canadian College of Naturopathic Medicine, The Hospital for Sick Children, the Canadian Nutrition Society (CNS), the American Society of Nutrition (ASN), Arizona State University, Paolo Sorbini Foundation and the Institute of Nutrition, Metabolism and Diabetes; he received an honorarium from the United States Department of Agriculture to present the 2013 W.O. Atwater Memorial Lecture; he received the 2013 Award for Excellence in Research from the International Nut and Dried Fruit Council; he received funding and travel support from the Canadian Society of Endocrinology and Metabolism to produce mini cases for the Canadian Diabetes Association (CDA); he is a member of the International Carbohydrate Quality Consortium (ICQC); his wife, A.L. Jenkins, is a director and partner of Glycemic Index Laboratories, Inc., and his sister received funding through a grant from the St. Michael’s Hospital Foundation to develop a cookbook for one of his studies.
Additional information
Funding
References
- Adlercreutz H: Phytoestrogens: epidemiology and a possible role in cancer protection. Environ Health Perspect 103, 103–112, 1995.
- Thompson LU, Boucher BA, Liu Z, Cotterchio M, and Kreiger N: Phytoestrogen content of foods consumed in Canada, including isoflavones, lignans, and coumestan. Nutr Cancer 54, 184–201, 2006.
- Heinonen S, Nurmi T, Liukkonen K, Poutanen K, Wähälä K, et al.: In vitro metabolism of plant lignans: new precursors of mammalian lignans enterolactone and enterodiol. J Agric Food Chem 49, 3178–3186, 2001.
- Touré A and Xueming X: Flaxseed lignans: source, biosynthesis, metabolism, antioxidant activity, bio-active components, and health benefits. Compr Rev Food Sci Food Saf 9, 261–269, 2010.
- Nesbitt PD, Lam Y, and Thompson LU: Human metabolism of mammalian lignan precursors in raw and processed flaxseed. Am J Clin Nutr 69, 549–555, 1999.
- Kilkkinen A, Valsta LM, Virtamo J, Stumpf K, Adlercreutz H, et al.: Intake of lignans is associated with serum enterolactone concentration in Finnish men and women. J Nutr 133, 1830–1833, 2003.
- Kuijsten A, Arts IC, Vree TB, and Hollman PC: Pharmacokinetics of enterolignans in healthy men and women consuming a single dose of secoisolariciresinol diglucoside. J Nutr 135, 795–801, 2005.
- Setchell KD, Brown NM, Zimmer-Nechemias L, Wolfe B, Jha P, et al.: Metabolism of secoisolariciresinol-diglycoside the dietary precursor to the intestinally derived lignan enterolactone in humans. Food Funct 5, 491–501, 2014.
- Adlercreutz H: Lignans and human health. Crit Rev Clin Lab Sci 44, 483–525, 2007.
- Thompson LU: Experimental studies on lignans and cancer. Baillieres Clin Endocrinol Metab 12, 691–705, 1998.
- Neill AS, Ibiebele TI, Lahmann PH, Hughes MC, Nagle CM, et al.: Dietary phyto-oestrogens and the risk of ovarian and endometrial cancers: findings from two Australian case-control studies. Br J Nutr 111, 1430–1440, 2014.
- Mason JK and Thompson LU: Flaxseed and its lignan and oil components: can they play a role in reducing the risk of and improving the treatment of breast cancer? Appl Physiol Nutr Metab 39, 663–678, 2014.
- Calado A, Neves PM, Santos T, and Ravasco P: The effect of flaxseed in breast cancer: a literature review. Front Nutr 5, 4, 2018.
- Velentzis LS, Cantwell MM, Cardwell C, Keshtgar MR, Leathem AJ, et al.: Lignans and breast cancer risk in pre- and post-menopausal women: meta-analyses of observational studies. Br J Cancer 100, 1492–1498, 2009.
- Buck K, Zaineddin AK, Vrieling A, Linseisen J, and Chang-Claude J: Meta-analyses of lignans and enterolignans in relation to breast cancer risk. Am J Clin Nutr 92, 141–153, 2010.
- Zaineddin AK, Vrieling A, Buck K, Becker S, Linseisen J, et al.: Serum enterolactone and postmenopausal breast cancer risk by estrogen, progesterone and herceptin 2 receptor status. Int J Cancer 130, 1401–1410, 2012.
- Lowcock EC, Cotterchio M, and Boucher BA: Consumption of flaxseed, a rich source of lignans, is associated with reduced breast cancer risk. Cancer Causes Control 24, 813–816, 2013.
- Key T, Appleby P, Barnes I, Reeves G, and Endogenous Hormones and Breast Cancer Collaborative Group. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst 94, 606–616, 2002.
- Zhang X, Tworoger SS, Eliassen AH, and Hankinson SE: Postmenopausal plasma sex hormone levels and breast cancer risk over 20 years of follow-up. Breast Cancer Res Treat 137, 883–892, 2013.
- Wang M, Wu X, Chai F, Zhang Y, and Jiang J: Plasma prolactin and breast cancer risk: a meta-analysis. Sci Rep 6, 25998, 2016.
- Fuhrman BJ, Schairer C, Gail MH, Boyd-Morin J, Xu X, et al.: Estrogen metabolism and risk of breast cancer in postmenopausal women. J Natl Cancer Inst 104, 326–339, 2012.
- Ziegler RG, Fuhrman BJ, Moore SC, and Matthews CE: Epidemiologic studies of estrogen metabolism and breast cancer. Steroids 99, 67–75, 2015.
- Sampson JN, Falk RT, Schairer C, Moore SC, Fuhrman BJ, et al.: Association of estrogen metabolism with breast cancer risk in different cohorts of postmenopausal women. Cancer Res 77, 918–925, 2017.
- Lord RS, Bongiovanni B, and Bralley JA: Estrogen metabolism and the diet-cancer connection: rationale for assessing the ratio of urinary hydroxylated estrogen metabolites. Altern Med Rev 7, 112–129, 2002.
- Schneider J, Huh MM, Bradlow HL, and Fishman J: Antiestrogen action of 2-hydroxyestrone on MCF-7 human breast cancer cells. J Biol Chem 259, 4840–4845, 1984.
- Bradlow HL, Telang NT, Sepkovic DW, and Osborne MP: 2-hydroxyestrone: the ‘good’ estrogen. J Endocrinol 150, S259–S265, 1996.
- Gupta M, McDougal A, and Safe S: Estrogenic and antiestrogenic activities of 16alpha- and 2-hydroxy metabolites of 17beta-estradiol in MCF-7 and T47D human breast cancer cells. J Steroid Biochem Mol Biol 67, 413–419, 1998.
- Brooks JD and Thompson LU. Mammalian lignans and genistein decrease the activities of aromatase and 17beta-hydroxysteroid dehydrogenase in MCF-7 cells. J Steroid Biochem Mol Biol 94, 461–467, 2005.
- Adlercreutz H, Bannwart C, Wähälä T, Mäkelä T, Brunow G, et al.: Inhibition of human aromatase by mammalian lignans and isoflavonoid phytoestrogens. J Steroid Biochem Mol Biol 44, 147–153, 1993.
- Haggans CJ, Hutchins AM, Olson BA, Thomas W, Martini MC, et al.: Effect of flaxseed consumption on urinary estrogen metabolites in postmenopausal women. Nutr Cancer 33, 188–195, 1999.
- McCann SE, Wactawski-Wende J, Kufel K, Olson J, Ovando B, et al.: Changes in 2-hydroxyestrone and 16α-hydroxyestrone metabolism with flaxseed consumption: modification by COMT and CYP1B1 genotype. Cancer Epidemiol Biomarkers Prev 16, 256–262, 2007.
- Brooks JD, Ward WE, Lewis JE, Hilditch J, Nickell L, et al.: Supplementation with flaxseed alters estrogen metabolism in postmenopausal women to a greater extent than does supplementation with an equal amount of soy. Am J Clin Nutr 79, 318–325, 2004.
- Sturgeon SR, Volpe SL, Puleo E, Bertone-Johnson ER, Heersink J, et al.: Effect of flaxseed consumption on urinary levels of estrogen metabolites in postmenopausal women. Nutr Cancer 62, 175–180, 2010.
- Hutchins AM, Martini MC, Olson BA, Thomas W, and Slavin JL: Flaxseed consumption influences endogenous hormone concentrations in postmenopausal women. Nutr Cancer 39, 58–65, 2001.
- Sturgeon SR, Heersink JL, Volpe SL, Bertone-Johnson ER, Puleo E, et al.: Effect of dietary flaxseed on serum levels of estrogens and androgens in postmenopausal women. Nutr Cancer 60, 612–618, 2008.
- Lucas EA, Wild RD, Hammond LJ, Khalil DA, Juma S, et al.: Flaxseed improves lipid profile without altering biomarkers of bone metabolism in postmenopausal women. J Clin Endocrinol Metab 87, 1527–1532, 2002.
- Simbalista RL, Sauerbronn AV, Aldrighi JM, and Arêas JA: Consumption of a flaxseed-rich food is not more effective than a placebo in alleviating the climacteric symptoms of postmenopausal women. J Nutr 140, 293–297, 2010.
- Hyvärinen HK, Pihlava JM, Hiidenhovi JA, Hietaniemi V, Korhonen HJ, et al.: Effect of processing and storage on the stability of flaxseed lignan added to bakery products. J Agric Food Chem 54, 48–53, 2006.
- Simbalista RL, Frota KM, Soares RA, and Arêas JA: Effect of storage and processing of Brazilian flaxseed on lipid and lignan contents. Ciênc Tecnol Aliment 32, 374–380, 2012.
- Xu X, Roman JM, Issaq HJ, Keefer LK, Veenstra TD, et al.: Quantitative measurement of endogenous estrogens and estrogen metabolites in human serum by liquid chromatography-tandem mass spectrometry. Anal Chem 79, 7813–7821, 2007.
- Vandenberghe H, Jarvis MJ, Quadri S, and Kafle A: Analysis of estrogens and their methoxy- and hydroxy- metabolites in serum using the SCIEX Triple Quad™ 6500+ LC-MS/MS System. AB Sciex Technical Note (Report No.: RUO-MKT-10-5223-A). AB Sciex, Framingham, MA, 2017.
- Klug TL, Bradlow HL, and Sepkovic DW: Monoclonal antibody-based enzyme immunoassay for simultaneous quantitation of 2- and 16 alpha-hydroxyestrone in urine. Steroids 59, 648–655, 1994.
- Endogenous Hormones and Breast Cancer Collaborative Group. Free estradiol and breast cancer risk in postmenopausal women: comparison of measured and calculated values. Cancer Epidemiol Biomarkers Prev 12, 1457–1461, 2003.
- Nørskov NP, Olsen A, Tjønneland A, Bolvig AK, Lærke HN, et al.: Targeted LC-MS/MS method for the quantitation of plant lignans and enterolignans in biofluids from humans and pigs. J Agric Food Chem 63, 6283–6292, 2015.
- Smeds A and Hakala K: Liquid chromatographic-tandem mass spectrometric method for the plant lignan 7-hydroxymatairesinol and its potential metabolites in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 793, 297–308, 2003.
- Vickers AJ and Altman DG: Statistics notes: analysing controlled trials with baseline and follow up measurements. BMJ 323, 1123–1124, 2001.
- Key TJ, Appleby PN, Reeves GK, Roddam AW, Helzlsouer KJ, et al.: Circulating sex hormones and breast cancer risk factors in postmenopausal women: reanalysis of 13 studies. Br J Cancer 105, 709–722, 2011.
- Verkasalo PK, Thomas HV, Appleby PN, Davey GK, and Key TJ: Circulating levels of sex hormones and their relation to risk factors for breast cancer: a cross-sectional study in 1092 pre- and postmenopausal women (United Kingdom). Cancer Causes Control 12, 47–59, 2001.
- Chan MF, Dowsett M, Folkerd E, Bingham S, Wareham N, et al.: Usual physical activity and endogenous sex hormones in postmenopausal women: the European prospective investigation into cancer-norfolk population study. Cancer Epidemiol Biomarkers Prev 16, 900–905, 2007.
- Chubak J, Tworoger SS, Yasui Y, Ulrich CM, Stanczyk FZ, et al.: Associations between reproductive and menstrual factors and postmenopausal sex hormone concentrations. Cancer Epidemiol Biomarkers Prev 13, 1296–1301, 2004.
- Chen WY: Exogenous and endogenous hormones and breast cancer. Best Pract Res Clin Endocrinol Metab 22, 573–585, 2008.
- Knust U, Spiegelhalder B, Strowitzki T, and Owen RW: Contribution of linseed intake to urine and serum enterolignan levels in German females: a randomised controlled intervention trial. Food Chem Toxicol 44, 1057–1064, 2006.
- Tarpila S, Aro A, Salminen I, Tarpila A, Kleemola P, et al.: The effect of flaxseed supplementation in processed foods on serum fatty acids and enterolactone. Eur J Clin Nutr 56, 157–165, 2002.
- Liu H, Liu J, Wang S, Zeng Z, Li T, et al.: Enterolactone has stronger effects than enterodiol on ovarian cancer. J Ovarian Res 10, 49, 2017.
- Zhu Y, Kawaguchi K, and Kiyama R: Differential and directional estrogenic signaling pathways induced by enterolignans and their precursors. PLoS One 12, e0171390, 2017.
- Carreau C, Flouriot G, Bennetau-Pelissero C, and Potier M: Enterodiol and enterolactone, two major diet-derived polyphenol metabolites have different impact on ERalpha transcriptional activation in human breast cancer cells. J Steroid Biochem Mol Biol 110, 176–185, 2008.
- Dikshit A, Filho MA, Eilati E, McGee S, Small C, et al.: Flaxseed reduces the pro-carcinogenic microenvironment in the ovaries of normal hens by altering the prostaglandin and estrogen pathways in a dose dependent manner. Br J Nutr 113, 1384–1395, 2015.
- Obi N, Vrieling A, Heinz J, and Chang-Claude J: Estrogen metabolite ratio: is the 2-hydroxyestrone to 16α-hydroxyestrone ratio predictive for breast cancer? Int J Womens Health 3, 37–51, 2011.
- Fuhrman BJ, Pfeiffer R, Xu X, Wu AH, Korde L, et al.: Soy intake is associated with increased 2-hydroxylation and decreased 16α-hydroxylation of estrogens in Asian-American women. Cancer Epidemiol Biomarkers Prev 18, 2751–2760, 2009.
- Wang C, Mäkelä T, Hase T, Adlercreutz H, and Kurzer MS: Lignans and flavonoids inhibit aromatase enzyme in human preadipocytes. J Steroid Biochem Mol Biol 50, 205–212, 1994.
- Granata OM, Traina A, Ramirez S, Campisi I, Zarcone M, et al.: Dietary enterolactone affects androgen and estrogen levels in healthy postmenopausal women. Ann N Y Acad Sci 1155, 232–236, 2009.
- Low YL, Dunning AM, Dowsett M, Folkerd E, Doody D, et al.: Phytoestrogen exposure is associated with circulating sex hormone levels in postmenopausal women and interact with ESR1 and NR1I2 gene variants. Cancer Epidemiol Biomarkers Prev 16, 1009–1016, 2007.
- Zeleniuch-Jacquotte A, Adlercreutz H, Shore RE, Koenig KL, Kato I, et al.: Circulating enterolactone and risk of breast cancer: a prospective study in New York. Br J Cancer 91, 99–105, 2004.
- Zeleniuch-Jacquotte A, Lundin E, Micheli A, Koenig KL, Lenner P, et al.: Circulating enterolactone and risk of endometrial cancer. Int J Cancer 119, 2376–2381, 2006.
- Evans BA, Griffiths K, and Morton MS: Inhibition of 5 alpha-reductase in genital skin fibroblasts and prostate tissue by dietary lignans and isoflavonoids. J Endocrinol 147, 295–302, 1995.
- Adlercreutz H, Mousavi Y, Clark J, Höckerstedt K, Hämäläinen E, et al.: Dietary phytoestrogens and cancer: in vitro and in vivo studies. J Steroid Biochem Mol Biol 41, 331–337, 1992.
- Martin ME, Haourigui M, Pelissero C, Benassayag C, and Nunez EA: Interactions between phytoestrogens and human sex steroid binding protein. Life Sci 58, 429–436, 1996.
- Schöttner M, Gansser D, and Spiteller G: Interaction of lignans with human sex hormone binding globulin (SHBG). Z Naturforsch C 52, 834–843, 1997.
- Adlercreutz H, Höckerstedt K, Bannwart C, Bloigu S, Hämäläinen E, et al.: Effect of dietary components, including lignans and phytoestrogens, on enterohepatic circulation and liver metabolism of estrogens and on sex hormone binding globulin (SHBG). J Steroid Biochem 27, 1135–1144, 1987.
- Stahl S, Chun TY, and Gray WG: Phytoestrogens act as estrogen agonists in an estrogen-responsive pituitary cell line. Toxicol Appl Pharmacol 152, 41–48, 1998.
- Troina AA, Figueiredo MS, Passos MC, Reis AM, Oliveira E, et al.: Flaxseed bioactive compounds change milk, hormonal and biochemical parameters of dams and offspring during lactation. Food Chem Toxicol 50, 2388–2396, 2012.
- Górski K, Gajewska A, Romanowicz K, and Misztal T: Genistein-induced pituitary prolactin gene expression and prolactin release in ovariectomized ewes following a series of intracerebroventricular infusions. Reprod Biol 7, 233–246, 2007.
- Foth D and Nawroth F: Effect of soy supplementation on endogenous hormones in postmenopausal women. Gynecol Obstet Invest 55, 135–138, 2003.
- Petrakis NL, Barnes S, King EB, Lowenstein J, Wiencke J, et al.: Stimulatory influence of soy protein isolate on breast secretion in pre- and postmenopausal women. Cancer Epidemiol Biomarkers Prev 5, 785–794, 1996.
- Martini MC, Dancisak BB, Haggans CJ, Thomas W, and Slavin JL: Effects of soy intake on sex hormone metabolism in premenopausal women. Nutr Cancer 34, 133–139, 1999.
- Duncan AM, Underhill KE, Xu X, Lavalleur J, Phipps WR, et al.: Modest hormonal effects of soy isoflavones in postmenopausal women. J Clin Endocrinol Metab 84, 3479–3484, 1999.
- Taylor AE, Keevil B, and Huhtaniemi IT: Mass spectrometry and immunoassay: how to measure steroid hormones today and tomorrow. Eur J Endocrinol 173, D1–D12, 2015.
