Abstract
Optimal nutrition formulas for colorectal cancer patients underwent surgery remains uncertainty. We constructed an indirect comparison study to assess comparative efficacy of different immunonutrition formulas and standard nutrition in colorectal cancer patients underwent surgery. PubMed, the Cochrane Library, EMBASE, ClinicalTrials.gov and Web of Science databases were searched to identify RCTs that compared immunonutrition with standard nutrition or different immunonutrition formulas. Data on length of hospital stays (LOS), infectious complications (IC), noninfectious complications (NIC) and anastomotic leakage (AL) were extracted from the included RCTs for Bayesian network analysis using a random-effect model. Twelve articles that included 1032 individuals were incorporated into this study. The indirect comparison confirmed the potential improvement of arginine-based immunonutrition on IC (odds ratios [OR] = 0.43, 95%confidence interval [CI]: 0.17 to 0.95), glutamine on NIC (OR = 0.07 CI: 0.00 to 0.78) and LOS (MD=-3.91 CI: −6.33 to -1.69) and omega-3 polyunsaturated fatty acids on LOS (OR=-3.49 CI: −5.46 to -1.00). Results indicated that glutamine had the highest probability of reducing complications and hospital stays. As for colorectal cancer patients underwent surgery, this indirect comparison suggested some superiority of glutamine. Future more RCTs with larger scale are required to provide evidence for the optimal immunonutrition formulas.
Introduction
According to global cancer statistics, it is currently estimated that there were more than 1.8 million new colorectal cancer diagnoses, in addition to more than half a million deaths, reported in 2018 (Citation1). As a common digestive tract tumor, patients with colorectal cancer are prone to malnutrition, which may be due to decreased intake, impaired digestion, malabsorption of nutrients and increased nutritional requirements (Citation2). Malnutrition is a key factor associated with the dysfunction of homeostasis and the immune system, which may lead to poor outcomes of surgery, such as postoperative complications, delay of wound healing and prolonged hospital stay (Citation3). Thus, proper nutrition support is necessary for perioperative colorectal cancer patients. Recently, many researchers have argued that immunonutrition was more effective than standard nutrition interventions in improving inflammation, promoting wound healing and shortening the length of hospital stay in surgical patients (Citation4).
Immunonutrition is defined as nutritional formulas with the aim of stimulating the host immune response with a combination of immunonutrients including arginine, omega-3 polyunsaturated fatty acids (n-3 PUFAs), glutamine, nucleotides, etc (Citation5).. Currently, the immunonutrition formulas used for colorectal cancer patients include n-3 PUFA supplements, glutamine supplements and arginine-based immunonutrition, which mainly comprise arginine and small amounts of n-3 PUFAs and nucleotides (Citation6). Arginine is a non-essential amino acid and an essential substrate for immune cells; arginine is especially important for lymphocyte function (Citation7, Citation8). N-3 PUFAs are bioactive lipids that can modulate metabolic and inflammatory responses in addition to providing energy. Glutamine is the major energy source for macrophages, lymphocytes, and enterocytes and could decrease inflammation (Citation5, Citation9).
Although the biological properties of immunonutrients have been well studied in experimental models, the role of immunonutrition in clinical settings was contrasting in relevant studies. Some studies (Citation10, Citation11) revealed a significant reduction in complications in individuals receiving immunonutrition, while other studies (Citation12, Citation13) revealed that immunonutrition had no advantage over standard nutrition in colorectal cancer patients undergoing surgery. We assumed that these different conclusions were caused by different immunonutrition formulas. Regarding these formulas, some studies (Citation14, Citation15) suggested that arginine-based immunonutrition and n-3 PUFAs can significantly reduce infectious complications while having no advantage for noninfectious complications. Glutamine had an advantage for anastomotic leakage over standard nutrition but had no advantage for infectious complications (Citation16).
Few trials have been planned to investigate the comparative efficacy of different immunonutrition formulas. As a result, it is unclear which formulas are the optimal nutrition support regimes for colorectal cancer patients undergoing surgery. Bayesian network meta-analysis, which is the expansion of pairwise meta-analysis, provides an option for investigators to indirectly compare the efficacies of multiple treatments (Citation17). Hence, in our study, a network meta-analysis was performed to assist in clinical decisions regarding the use of n-3 PUFAs, glutamine, arginine-based immunonutrition or standard nutrition for colorectal patients undergoing surgery.
Materials and Methods
The indirect comparisons we performed strictly adhered to the Preferred Reporting for Systematic reviews and Meta-Analysis (PRISMA) statement (Citation18).
Search Strategy
We searched electronic databases that included PubMed, the Cochrane Library, EMBASE, ClinicalTrials.gov and the Web of Science to identify all relevant randomized controlled trials (RCTs) that investigated the effects of immunonutrition on colorectal cancer patients who underwent surgery. The last retrieval was performed on October 15, 2019. The computerized search procedure was conducted using the following search terms based on the strategy of combining medical subject headings (MeSH) and key words: (“Colon” OR “Rectal” OR “Colorectal”) AND (“Neoplasms” OR “Cancer” OR “Adenomas” OR “Tumor”) AND (“Surgery” OR “Operation” OR “Resection” OR “Enterectomy” OR “Proctectomy” OR “Rectectomy” OR “Coloproctectomy”) AND (“Glutamine” OR “N-3 Fatty Acid” OR “Fish Oil” OR “Arginine” OR “Nucleotide” OR “RNA” OR “Omega-3 Fatty Acid” OR “Pharmaconutrition” OR “Immunonutrition”) AND (“Nutrition” OR “Supplement*”). We also performed manual supplemental searches of references cited by the retrieved articles.
Inclusion Criteria
In this systematic review and network meta-analysis, we included the RCTs meeting the following eligibility criteria: (1) Patients: all adults diagnosed with colorectal neoplasms who were scheduled for surgery, (2) Intervention: immunonutrition formulas including glutamine supplements, n-3 PUFA supplements and arginine-based immunonutrition, (3) Comparison: other active immunonutrition formulas or standard nutrition, and 4) Outcomes: length of hospital stays (LOSs), infectious complications (IC), noninfectious complications (NIC) and anastomotic leakage (AL). Nonoriginal research, including reviews, letters and specialist comments, and non-English publications were excluded.
Data Extraction
Data from the included articles were extracted by two investigators separately according to the predesigned data extraction forms. When differences existed, the senior investigator was consulted, and a consensus was reached by discussion. The following information was extracted from the studies: basic characteristics of each study (first author, publication year, country, sample size, age and BMI of the participants), study design (nutrients in the immunonutrition formula, duration of the nutrition support, type of nutrition supplement, treatment of control group), and outcomes of interest.
Quality Assessment
The methodological quality of the included studies was assessed with the Cochrane Collaboration tool, which was used to evaluate random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other biases related to RCTs (Citation19).
Statistical Analysis
The data used in this meta-analysis were clinical outcomes of different nutrition formulas. Indirect comparisons were conducted using WinBUGS version 1.4.3 (MRC Biostatistics Unit, Cambrigde, UK). We performed a random-effect model within a Bayesian framework for each endpoint (Citation17). Dichotomous data were analyzed to estimate the odds ratios (ORs) for binary variables and the mean difference (MD) for continuous outcomes. Models were computed with Markov chain Monte Carlo simulations, using three chains with over-dispersed initial values, with Gibbs sampling based on 20000 iterations after a burn-in phase of 50000 iterations. Each outcome was estimated from the combination of the direct evidence between the two treatments and the indirect evidence derived from the network meta-analysis. When a direct connection between two treatments was not available, the effect estimates were derived from only indirect evidence. The results were also presented using the surface under the cumulative ranking curve (SUCRA), and the higher SUCRA values corresponded to a higher possibility for the respective outcome.
We conducted a traditional pairwise meta-analysis by synthesizing studies that compared the same interventions with a random-effects model. We performed the analyses using Stata version 13.0.
Results
Study Selection
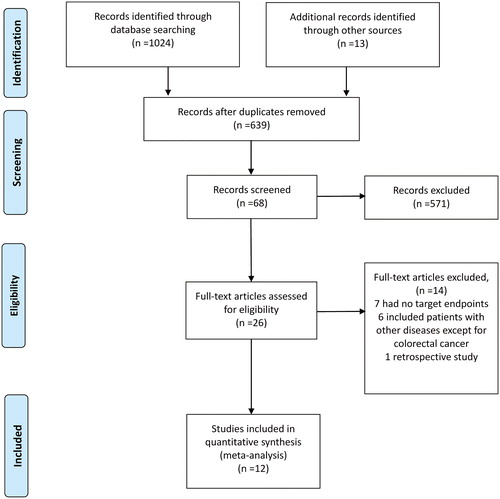
As shown in , a total of 1024 relevant articles were identified by the search strategy. After reading the titles and abstracts, we selected 26 articles to undergo full-text review. Of the 26 articles, 14 were excluded (most commonly because the target outcomes were not included or the participants were not only colorectal cancer patients), leaving twelve studies (Citation10–13, Citation20–27) eligible for the systematic review and network meta-analysis.
Study Characteristics
The key characteristics of the studies included in the network meta-analysis are summarized in . All twelve included studies were RCTs and involved a total of 1032 patients. Among the included trials, three were performed in Spain, 2 in Turkey, 2 in Italy, 2 in China, 1 in Japan, 1 in Germany and 1 in the UK. Study dates varied from December 1998 to August 2016, and the average age of the participants varied from 54.39 to 70.29 years. BMI ranged from 22.80 to 27.07. The sample sizes of the individual studies ranged from 26 to 200. Eleven studies assessed the length of hospital stays, ten studies assessed infectious complications, six studies assessed noninfectious complications, and eight studies assessed anastomotic leakage. The evidence network plot was shown in .
Figure 2. Network of indirect comparisons. The size of the nodes stands for the number of patients included and line width the number of articles comparing each pair of articles.
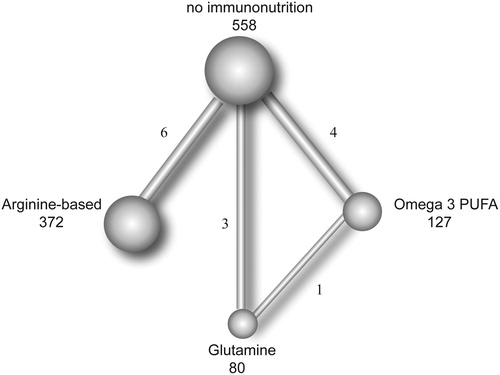
Table 1. Characteristics of the included studies.
Methodological Quality of the Included Studies
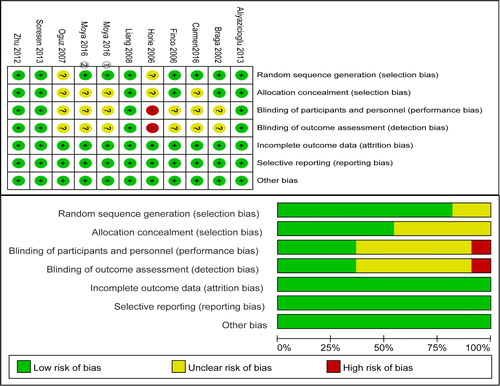
The quality assessment of the included papers is shown in . Most studies had a clear description of their random sequence generation. Half of the studies appropriately performed allocation concealment. Seven studies were open-label studies and were identified to have an unclear or high risk for blinding of participants and personnel and blinding of outcome assessment. In addition, almost all of the studies were identified to have a low risk of bias for incomplete outcome bias and selective reporting.
Length of Hospital Stays
Eleven articles, which included a total of 1006 participants, were synthesized for the network meta-analysis of length of hospital stays (). Our research showed that, compared to standard nutrition, glutamine appeared to have better effect of shortening hospitalization time (OR -3.91 95%CI: −6.33 to -1.69); this effect was also observed when compared to arginine-based immunonutrition (OR -3.28 95%CI: −6.31 to -0.45). n-3 PUFAs were superior to standard nutrition in reducing hospital stays (OR -3.49 95%CI: −5.96 to -1.00).
Table 2. Comparison of different immunonutrtion formulas in length of hospital stays between pairwise meta-analysis and network.
Infectious Complications
Ten articles, which included a total of 1100 participants, were synthesized for the network meta-analysis of infectious complications (). The results showed that, compared with standard nutrition, only arginine-based immunonutrition (OR = 0.43 95%CI [0.17, 0.95]) appeared to have an effect on reducing infectious complications, while n-3 PUFAs and glutamine did not show an effect. Three formulas did not show any differences either.
Table 3. Comparison of different immunonutrtion formulas in infectious complication between pairwise meta-analysis and network.
Noninfectious Complications
Six articles, which included a total of 890 participants, were synthesized for the network meta-analysis of noninfectious complications (). Our research suggested that, compared to standard nutrition, glutamine appeared to have better strength in reducing noninfectious complications (OR = 0.07 95%CI [0.00, 0.78]), and the same effect was observed when compared to n-3 PUFAs (OR = 0.05 95%CI [0.00, −0.83]) and arginine-based immunonutrition (OR = 0.08 95%CI [0.00, 0.99]).
Table 4. Comparison of different immunonutrtion formulas in noninfectious complication between pairwise meta-analysis and network.
Anastomotic Leakage
Eight articles, which included a total of 1002 participants, were synthesized for the network meta-analysis of anastomotic leakage (). Anastomotic leakage is an important noninfectious complication after colorectal resection. An unexpected result was found in which a natural number of one was included in all confidence intervals; therefore, no significant difference was found in these comparisons.
Table 5. Comparison of different immunonutrtion formulas in Anastomotic leakage between pairwise meta-analysis and network.
Ranking of the Different Formulas of Immunonutrition
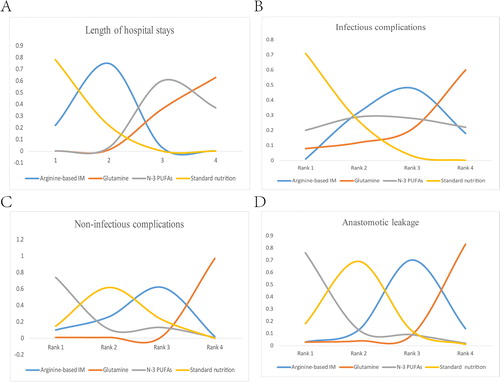
We used SUCRA to rank all nutrition support regimes for the corresponding outcomes. shows the distribution of the probabilities of each treatment being ranked in each of the possible four positions. Glutamine worked best for preventing infectious complications, followed by n-3 PUFAs, arginine-based formulas, and standard nutrition. The same results were found with respect to length of hospital stays, indicating a possible relationship between these outcomes. On the other hand, patients supplied with glutamine were less likely to have postoperative noninfectious complications and anastomotic leakage; arginine-based nutrition, standard nutrition and n-3 PUFAs followed in the ranking.
Figure 4. Ranking of outcomes for the included studies. Odds ratio (OR) and 95% confidence interval (CI) were used to expressed the difference between formulas. First rank indicates lowest probability to prevent occurrence of infectious, noninfectious and anastomotic leak complications. For length of hospitalization, first rank indicates longest stay in hospital.
Discussion
This network meta-analysis comparing different formulas of immunonutrition for colorectal cancer patients undergoing surgery from twelve RCTs (1032 individuals) had four main findings. First, glutamine had the highest probability of reducing postoperative complications and hospital stays. Second, arginine-based immunonutrition was significantly more effective than standard nutrition for infectious complication reduction. Third, n-3 PUFAs have shown a tendency to reduce hospital stays compared with the effects of standard nutrition. Fourth, regardless of the formulas, immunonutrition has demonstrated an advantage in terms of length of hospital stays, infectious complications and noninfectious complications compared with standard nutrition; an exception is the result obtained for anastomotic leakage.
Glutamine is the most abundant free amino acid in the body and plays an important role in nitrogen balance. Glutamine is a fuel for enterocytes, macrophages and lymphocytes, protects the gastrointestinal mucosa barrier and enhances immune function (Citation28). Previously, a direct meta-analysis examining the efficacy of glutamine in colorectal cancer patients undergoing surgery suggested that glutamine significantly reduced postoperative noninfectious complications and the length of hospital stays (Citation16). Our findings are consistent with the previous study; moreover, based on the SUCRA value of the current study, we discovered that glutamine had the highest probability of reducing postoperative complications and length of hospital stay compared with the effects of the other nutrition formulas.
Arginine-based immunonutrition is a specialized nutrition formula comprising arginine, omega-3 fatty acids and nucleotides (Citation14). Arginine-based immunonutrition is one of the most common immunonutrition formulas for gastrointestinal cancer patients because of its comprehensive immunonutrients. The results of the current study suggested that compared with standard nutrition, arginine-based immunonutrition resulted in a 57% reduction in the relative risk of infectious complications, which suggested that it is worthy of recommendation. In addition, arginine-based immunonutrition was initially expected by Waitzberg et al (Citation6) to reduce the hospital stays in surgical patients, but this finding was not significant in our indirect comparison. Despite this disparity, the result of a recent multicentre retrospective study performed in colorectal cancer patients was consistent with our results (Citation14). A possible explanation for this conflict was that the study of Waitzberg et al included gastrointestinal cancer patients with multiple diagnoses, which increased the heterogeneity.
N-3 PUFAs are the precursors of resolvins, which are shown to reduce cellular inflammation by inhibiting the transportation of inflammatory cells and mediators to the site of inflammation (Citation29). The potential effects of n-3 PUFAs have been demonstrated in a number of animal studies and model systems, while there is a lack of human studies to examine their clinical efficacy (Citation5). This meta-analysis synthesized relevant clinical trials and suggested that n-3 PUFAs could significantly reduce hospital stays by an average of 3.46 day. Previous studies were consistent with this result, while we also compared n-3 PUFAs with other immunonutrients (Citation15). From the SUCRA scores in this study, n-3 PUFAs showed a trend toward reducing infectious complications, but it was less effective in reducing noninfectious complications, including anastomotic leakage. A possible explanation is that n-3 PUFAs can significantly reduce the production of proinflammatory cytokines, including IL-6 and TNF-α, which may lead to improved immune function and reduced septic events in colorectal cancer patients undergoing surgery, while it may have little effect on noninfectious complications (Citation30).
A meta-analysis of immunonutrition has been previously performed in colorectal cancer patients to study its functional mechanism and clinical use. Xu et al performed a direct comparison of meta-analyses comparing immunonutrition with standard nutrition in colorectal cancer patients who underwent surgery and suggested that regardless of the enteral or parenteral route and different nutrition formulas, immunonutrition was effective in reducing infectious complications and length of hospital stays (Citation31). However, when comparing each immunonutrition formula alone with standard nutrition, the results suggested that different formulas had different advantages in clinical outcomes (Citation14–16). To the best of our knowledge, this is the first study to indirectly compare these nutrition formulas with each other; we eventually found the possible superiority of glutamine of all of the immunonutrition formulas for colorectal cancer patients.
There are still some limitations in this study. Above all, some estimates in our study were generated from individual RCTs with a small number of patients; however, most of our results were consistent with previous studies. Future well-designed RCTs with large sample sizes are needed. Additionally, we failed to identify the effects of different routes of nutrition supplementation (enteral or parenteral) and timing of nutrition supplementation (preoperative, postoperative or perioperative) on the outcomes as planned because of the limited number of studies in each group. Overall, our results should be interpreted with caution. Nonetheless, our research can provide clinicians with an optimal nutritional formula reference to effectively improve the nutritional status of patients and improve clinical outcomes.
Conclusion
Immunonutrition is an effective type of nutrition support for colorectal cancer patients undergoing surgery, while the ideal immunonutrition formulas may differ according to the parameters prioritized by surgeons and patients. Our indirect comparison suggested that arginine-based immunonutrition reduced the relative risk of infectious complications. N-3 PUFAs significantly reduced hospital stays. Overall, glutamine had the highest probability of reducing complications and hospital stays compared to the probabilities associated with other nutrition formulas. Future RCTs with larger sample sizes directly comparing these immunonutrition formulas with each other are warranted.
Author Contributions
Xiao-han Jiang and Xi-jie Chen have made substantial contribution to conception and design of the study. Xin-you Wang and Yun-zhi Chen searched literature, extracted data from the collected literature and analyzed the data. Qin-qin Xie assessed each study. Jun-sheng Peng was the guarantor of overall content. All authors revised and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
- Lopes JP, de Castro Cardoso Pereira PM, dos Reis Baltazar Vicente AF, Bernardo A, de Mesquita MF. Nutritional status assessment in colorectal cancer patients. Nutr Hosp. 2013;28(2):412–8. doi:10.3305/nh.2013.28.2.6173
- Seretis C, Kaisari P, Wanigasooriya K, Shariff U, Youssef H. Malnutrition is associated with adverse postoperative outcome in patients undergoing elective colorectal cancer resections. J BUON. 2018;23(1):36–41.
- Marik PE, Zaloga GP. Immunonutrition in high-risk surgical patients: a systematic review and analysis of the literature. J Parenter Enteral Nutr. 2010;34(4):378–86. doi:10.1177/0148607110362692
- Prieto I, Montemuino S, Luna J, de Torres MV, Amaya E. The role of immunonutritional support in cancer treatment: current evidence. Clin Nutr. 2017;36(6):1457–64. doi:10.1016/j.clnu.2016.11.015
- Waitzberg DL, Saito H, Plank LD, Jamieson GG, Jagannath P, Hwang T-L, Mijares JM, Bihari D. Postsurgical infections are reduced with specialized nutrition support. World J Surg. 2006;30(8):1592–604. doi:10.1007/s00268-005-0657-x
- Mizock BA. Immunonutrition and critical illness: an update. Nutrition. 2010;26(7-8):701–7. doi:10.1016/j.nut.2009.11.010
- Gerlach AT, Murphy C. An update on nutrition support in the critically ill. J Pharm Pract. 2011;24(1):70–7. doi:10.1177/0897190010388142
- Avenell A. Hot topics in parenteral nutrition. Current evidence and ongoing trials on the use of glutamine in critically-ill patients and patients undergoing surgery. Proc Nutr Soc. 2009;68(3):261–8. doi:10.1017/S0029665109001372
- Chen R, Cai JL, Zhou B, Jiang AF. Effect of immune-enhanced enteral diet on postoperative immunological function in patients with colorectal cancer. Zhonghua wei chang wai ke za zhi [Chin J Gastrointest Surg]. 2005;8:328–30. ‐
- Moya P, Soriano-Irigaray L, Ramirez JM, Garcea A, Blasco O, Blanco FJ, Brugiotti C, Miranda E, Arroyo A. Perioperative standard oral nutrition supplements versus immunonutrition in patients undergoing colorectal resection in an enhanced recovery (ERAS) protocol. Medicine (United States). 2016;95(21):e3704. doi:10.1097/MD.0000000000003704
- Liang B, Wang S, Ye Y-J, Yang X-D, Wang Y-L, Qu J, Xie Q-W, Yin M-J. Impact of postoperative omega-3 fatty acid-supplemented parenteral nutrition on clinical outcomes and immunomodulations in colorectal cancer patients. World J Gastroenterol. 2008;14(15):2434–9. doi:10.3748/wjg.14.2434
- Sorensen LS, Thorlacius-Ussing O, Schmidt EB, Rasmussen HH, Lundbye-Christensen S, Calder PC, Lindorff-Larsen K. Randomized clinical trial of perioperative omega-3 fatty acid supplements in elective colorectal cancer surgery. Br J Surg. 2014;101(2):33–42. doi:10.1002/bjs.9361
- Banerjee S, Garrison LP, Danel A, Ochoa Gautier JB, Flum DR. Effects of arginine-based immunonutrition on inpatient total costs and hospitalization outcomes for patients undergoing colorectal surgery. Nutrition. 2017; 42:106–13. doi:10.1016/j.nut.2017.06.002
- Xie H, Chang YN. Omega-3 polyunsaturated fatty acids in the prevention of postoperative complications in colorectal cancer: A meta-analysis. Onco Targets Ther. 2016; 9:7435–43. doi:10.2147/OTT.S113575
- Xin J, Xin-Tian X. Meta-analysis of the effects of glutamine on postoperative complications, immune function and nutritional status of colorectal cancer. Ann Nutrit Metabol. 2019; 75:79.
- Mills EJ, Ioannidis JPA, Thorlund K, Schünemann HJ, Puhan MA, Guyatt GH. How to use an article reporting a multiple treatment comparison meta-analysis. Jama. 2012;308(12):1246–53. doi:10.1001/2012.jama.11228
- Moher D, PRISMA-P Group, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015; 4:1doi:10.1186/2046-4053-4-1
- Munder T, Barth J. Cochrane's risk of bias tool in the context of psychotherapy outcome research. Psychother Res. 2018;28(3):347–55. doi:10.1080/10503307.2017.1411628
- Aliyazicioglu T, Canturk NZ, Simsek T, Kolayli F, Cekmen M. Effects of standard and/or glutamine dipeptide and/or omega-3 fatty ASCID-supplemented parenteral nutrition on neutrophil functions, interleukin-8 level and length of stay–a double blind, controlled, randomised study. East Afr Med J. 2013; 90:59–66.
- Braga M, Gianotti L, Vignali A, Carlo VD. Preoperative oral arginine and n-3 fatty acid supplementation improves the immunometabolic host response and outcome after colorectal resection for cancer. Surgery. 2002;132(5):805–14. ‐doi:10.1067/msy.2002.128350
- Finco C, Magnanini P, Sarzo G, Vecchiato M, Luongo B, Savastano S, Bortoliero M, Barison P, Merigliano S. Prospective randomized study on perioperative enteral immunonutrition in laparoscopic colorectal surgery. Surg Endosc. 2007;21(7):1175–9. doi:10.1007/s00464-007-9238-4
- Horie H, Okada M, Kojima M, Nagai H. Favorable effects of preoperative enteral immunonutrition on a surgical site infection in patients with colorectal cancer without malnutrition. Surg Today. 2006;36(12):1063–8. doi:10.1007/s00595-006-3320-8
- Moya P, Miranda E, Soriano-Irigaray L, Arroyo A, Aguilar M-D-M, Bellón M, Muñoz J-L, Candela F, Calpena R. Perioperative immunonutrition in normo-nourished patients undergoing laparoscopic colorectal resection. Surg Endosc. 2016;30(11):4946–53. doi:10.1007/s00464-016-4836-7
- Oguz M, Kerem M, Bedirli A, Mentes BB, Sakrak O, Salman B, Bostanci H. L-Alanin-L-glutamine supplementation improves the outcome after colorectal surgery for cancer. Colorect Dis. 2007;9(6):515–20. doi:10.1111/j.1463-1318.2006.01174.x
- Zhu M-W, Tang D-N, Hou J, Wei J-M, Hua B, Sun J-H, Cui H-Y. Impact of fish oil enriched total parenteral nutrition on elderly patients after colorectal cancer surgery. Chin Med J. 2012;125(2):178–81.
- Morlion BJ, Stehle P, Wachtler P, Siedhoff HP, Köller M, König W, Fürst P, Puchstein C. Total parenteral nutrition with glutamine dipeptide after major abdominal surgery: a randomized, double-blind, controlled study. Ann Surg. 1998;227(2):302–8. doi:10.1097/00000658-199802000-00022
- Kim H. Glutamine as an immunonutrient. Yonsei Med J. 2011;52(6):892–7. doi:10.3349/ymj.2011.52.6.892
- Alexander JW. Immunonutrition: the role of omega-3 fatty acids. Nutrition. 1998;14(7-8):627–33. doi:10.1016/s0899-9007(98)00004-5
- Mocellin MC, Camargo CQ, Nunes EA, Fiates GMR, Trindade E. A systematic review and meta-analysis of the n-3 polyunsaturated fatty acids effects on inflammatory markers in colorectal cancer. Clin Nutr. 2016;35(2):359–69. doi:10.1016/j.clnu.2015.04.013
- Xu J, Sun X, Xin Q, Cheng Y, Zhan Z, Zhang J, Wu J. Effect of immunonutrition on colorectal cancer patients undergoing surgery: a meta-analysis. Int J Colorectal Dis. 2018;33(3):273–83. doi:10.1007/s00384-017-2958-6