Abstract
Nutrition and metabolism are altered in patients with gastroenteropancreatic neuroendocrine tumors, which is related to excessive production of gastrointestinal hormones, peptides, and amines that can cause maldigestion, diarrhea, steatorrhea, and altered gastrointestinal motility. Patients with carcinoid syndrome are at risk of malnutrition due to tryptophan depletion, reduced intake of food, and loss of appetite because of diarrhea and/or flushing. To date, there is limited information on the nutritional issues faced by patients with neuroendocrine tumors, and on what specific recommendations should be made to patients concerning nutrition at various stages of the disease process. Dietary planning should therefore be an integral part of multidisciplinary management for patients with neuroendocrine tumors. Herein, we review current guidance for nutrition in patients with neuroendocrine tumors, focusing on intake of amines and foods to avoid, as well as concurrent medications. We also propose a new and practical food pyramid based on the principles of Mediterranean diet 4.0 that can be easily adapted according to the unmet needs of patients with neuroendocrine tumors at all stages of disease. The overarching goal of the present review is to create greater awareness of nutritional care and considerations that should be given to patients with neuroendocrine tumors.
Introduction
Gastroenteropancreatic neuroendocrine tumors (NETs) are a heterogeneous group of neoplasms arising from secretory cells of the neuroendocrine cell system (Citation1,Citation2). Gastroenteropancreatic NETs can be broadly considered as clinically functioning or nonfunctioning depending on whether the neoplasm produces peptide hormones such as insulin, gastrin, and glucagon which give rise to characteristic clinical syndromes (Citation1,Citation2). Since the secretion of hormones leads to symptoms that can facilitate diagnosis, nonfunctioning tumors are generally asymptomatic from a clinical standpoint (Citation3).
While gastroenteropancreatic NETs have been traditionally considered as fairly rare, their incidence has increased substantially in recent decades. In fact, the SEER database of the National Cancer Institute reported that the annual age-adjusted incidence rate increased from 1.09 per 100,000 in 1973 to 6.98 per 100,000 in 2012, which spanned all sites, stages, and grades (Citation4). It is widely held that this dramatic increase is due to improvements in imaging and detection (Citation1,Citation2). Considering all gastroenteropancreatic NETS, given the broad heterogeneity of these tumors it is not surprising that their clinical behavior also varies widely, and depends on histologic grade and tumor differentiation (Citation5).
Among all NETs, roughly 20% of patients will present with carcinoid syndrome (CS), which is characterized by flushing, abdominal cramps, and diarrhea, but also hypotension, tachycardia, and bronchoconstriction (Citation2,Citation6). CS has significant negative impact on all aspects of the quality of life, including diet, employment, physical functioning, and social activities, and is associated with increased costs of care compared to nonfunctioning NETs (Citation6). Therefore, minimizing the symptoms related to CS is an important goal in the management of these patients (Citation1). As a paraneoplastic syndrome, CS is related to secretion of humoral factors, comprising polypeptides, vasoactive amines, and prostaglandins (Citation6). In addition to the aforementioned peptide hormones, 5-hydroxytryptophan and its secondary breakdown product 5-hydroxyindoleacetic acid in urine are also key markers for CS (Citation3). The vast majority of tryptophan is used for synthesis of nicotinic acid, and only a small fraction is used for synthesis of 5-hydroxytryptophan (Citation7). However, it has been known for decades that in patients with CS the tumor can consume more than 50% of the tryptophan in the body (Citation8). Tryptophan is an essential amino acid that is a precursor of both nicotinic acid (vitamin B3) and 5-hydroxytryptophan. The unrestrained production of 5-hydroxytryptophan via tryptophan 5-hydroxylases and aromatic L-amino acid decarboxylase in carcinoid cells may thus deplete tryptophan from the pathway that produces nicotinic acid leading to its subsequent depletion (Citation8). For this reason, patients with uncontrolled CS are prone to develop nicotinic acid deficiency (Citation9). The ensuing and clinically-relevant alterations in tryptophan levels can thus lead to neurocognitive symptoms, pellagra, and possible carcinoid heart disease (Citation6).
For a variety of reasons as discussed below, nutrition and metabolism are altered in patients with NETs, and as such these patients have special nutritional requirements (Citation10). Despite this, there is limited information on what actions should be recommended to patients concerning nutrition at various stages of the disease (Citation10). Herein, we summarize current guidance for nutrition in patients with NETs, especially regarding intake of amines and food to avoid, as well as concurrent medications. We also propose a new and practical food pyramid with the overarching goal of creating greater awareness of nutritional care in patients with NETs.
Diagnosis and Clinical Management of NETs
Foods to Avoid during Diagnosis and Follow-up
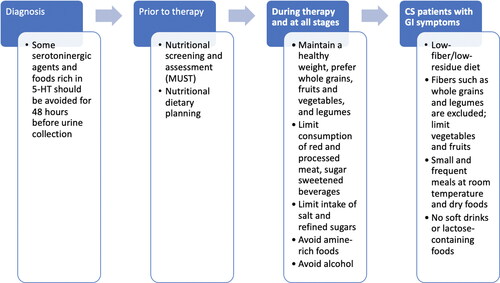
Diagnosis of CS depends on the presence of symptoms as well as the presence of elevated levels of urinary 5-hydroxyindoleacetic acid (Citation3). Clinicians should keep in mind that while sensitivity and specificity of 24-hour urinary 5-hydroxyindoleacetic acid is very high, some serotoninergic agents and foods rich in 5-hydroxytryptophan should be avoided for 48 h, before urine collection since they can give rise to a false positive result (Citation6) (). It is noted that assay of plasma 5-hydroxyindoleacetic acid may have similar accuracy to measurement of 24-hour urine 5-hydroxyindoleacetic acid (Citation11).
Table 1. Drugs and foods to avoid prior to testing for carcinoid syndrome by 24-hour urinary 5-hydroxyindoleacetic acid.
Chromogranin A is a sensitive, and effective marker for diagnosis and follow-up of NETs (Citation12). It has also been reported to have the highest accuracy and to be the most reliable marker that reflects the clinical status of NETs (Citation13). As such, its correct determination is essential for management of these tumors (Citation13). t has been reported that while intake of caffeine‐containing beverages prior to blood sampling appears to have no impact on measurement of chromogranin A in patients with gastroenteropancreatic NETs, intake of a 5‐item English breakfast (available items to choose from: one slice of bacon, one sausage, one fried egg or scrambled egg, fried bread, toast, baked beans, plum tomato, black pudding or one hash brown) moderately raised plasma chromogranin A levels in individuals who were not on treatment with a long‐acting somatostatin analog (Citation14). In addition, the same study found that proton pump inhibitors have the potential to interfere with sampling of chromogranin A (Citation14). Given these findings, fasting should be encouraged prior to measuring chromogranin A during screening and follow-up in patients with presumably resected disease (Citation14). Overnight fasting, or at least for 6 h, has also been recommended prior to fasting gut hormone profile (Citation15). Moreover, if the patient is on a proton pump inhibitor this should be temporarily discontinued (Citation15).
Treatment of CS
While surgery remains the mainstay of many gastroenteropancreatic NETs, somatostatin analogues, such as octreotide LAR and lanreotide, have revolutionized the management of these tumors (Citation16). Octreotide LAR and lanreotide bind to somatostatin receptors and inhibit the secretion of peptides causing CS (Citation17). These agents are associated with improvement in symptoms (diarrhea and flushing) in about 80% of patients (Citation18), and resolution or improvement of symptoms may occur as early as 3 mo, after initial therapy (Citation19). The median duration of symptomatic response is generally about 12 mo, because of the upregulation of other somatostatin receptor subtypes, leading to “escape from response” (Citation20). Today, somatostatin analogs are recommended as first-line treatment by European guidelines for patients with advanced functioning NETs, and especially for those with CS (Citation21).
Telotristat ethyl is a prodrug that is converted into its active metabolite telotristat etiprate after oral administration (Citation22). In CS patients, telotristat ethyl has been associated with significant improvement in the symptoms of CS, including diarrhea, over 1-3 mo, of therapy (Citation23). In addition, in the TELESTAR study in patients with CS, treatment with telotristat ethyl was associated with significant, dose-dependent weight gain and reduced severity of diarrhea in approximately one-third of cases (Citation24).
More recently, peptide receptor radionuclide therapy (PRRT) with 177Lu-Dotatate is a new therapeutic option in patients with metastatic gastroenteropancreatic NETs that express somatostatin receptors (Citation25). The β-emitting radionuclide lutetium-177, with a half-life of 6.7 days and a maximum β range of 2 mm in tissue, make it the ideal radionuclide for PRRT, and treatment with β-emitting radiolabeled somatostatin analogues results in impressive percentages of tumor regression (Citation25). Progression-free survival (PFS) was 29 mo, and overall survival for all NET patients was greater than 5 years (63 mo, 95% CI, 55–72 mo,) (Citation25). Based on these results, 177Lu-Dotatate is considered to be a valid therapeutic option in patients with gastroenteropancreatic NETs that express somatostatin receptors, and is associated with good response rates and few treatment-related adverse events (Citation26). All the available data indicate that many patients with NETs will achieve favorable long-term survival (Citation25).
Nutritional Concerns in Patients with NETs
The overall process of nutrition-metabolism is altered in gastroenteropancreatic NETs, which is related excessive production of gastrointestinal hormones, peptides, and amines that can lead to maldigestion, diarrhea, steatorrhea, and altered gastrointestinal motility that develops into various clinical syndromes (Citation27–29). Patients with NETs are at risk of protein-caloric malnutrition due to tryptophan depletion, reduced intake of food, and loss of appetite because of severe diarrhea and/or flushing (Citation27). Furthermore, it is important to consider that diarrhea can lead to changes in treatment, including dose reduction or discontinuation of therapy, which may have a negative effect on work productivity as well as increased direct and indirect costs (Citation30).
As noted by other authors, it cannot be overstated that there is limited information on the nutritional issues faced by patients with CS (Citation31). Moreover, malnutrition, vitamin deficiencies, and food intolerances appear to be prevalent, but unfortunately are not widely recognized (Citation31). If these complications are not adequately treated, they can have significant negative impact on quality of life and physical functioning (Citation31). Although not widely investigated, the association between nutrition-related complications and quality of life is being examined in a dedicated study in patients with gastroenteropancreatic NETs, and should help to provide a comprehensive description of the nutritional issues that these patients face (Citation32).
Not only are NETs a relatively rare clinical entity, but they are also very complex from both diagnostic and management perspectives, as are the associated nutritional issues (Citation10,Citation33). Both the tumor and therapies can have an influence on the nutritional concerns of the patient (Citation10). Food can interact with the metabolism of oral therapies and antineoplastic agents used to manage some of these tumors (Citation28). Malnutrition is a problem that is common to oncological pathologies, and which has been invariably associated with poorer response to treatment, increased rates of complications following surgical intervention, increased length of hospital stay, and poorer quality of life (Citation10,Citation29). Accordingly, cancer patients who are malnourished can be expected to have poorer outcomes from a variety of points of view (Citation34). Malnutrition has also been shown to have negative consequences on outcomes in patients with NETs considering the above outcomes (Citation10). Several studies have reported on the presence of malnutrition at first visit or at successive follow-up visits with percentages that vary widely, from 5% to up to 38% (Citation29,Citation35–39). In addition, body mass index (BMI) < 20 kg/m2 has been considered to be a negative prognostic factor in patients with NETs (Citation40), and is also a predictor of response to chemoembolization for liver metastases (Citation41). In a study on 203 patients with NETs, using malnutrition screening tools and anthropomorphic measurements including BMI, up to 25% of cases were considered to have malnutrition, which was associated with poorer outcomes; malnutrition was identified in multivariate analysis as an independent prognostic factor for NETs (Citation29).
Vitamin status has also been evaluated in NETs. Deficiency in vitamin D has been reported in at roughly half of patients with NETs (Citation42–44). Importantly, correction of vitamin D levels through supplementation has been shown to have a positive influence of progression-free survival (Citation43). There is also evidence that treatment with long-acting somatostatin analogues may impair exocrine pancreatic function, which may further lead to insufficiencies in fat soluble vitamins like vitamin D (Citation39). Nicotinic acid deficiency has also been documented in nearly 30% of patients with CS (Citation45).
Considering all of the above, nutritional screening and assessment should be an integral component of management of patients with NETS, and especially CS (Citation10). Nutritional dietary planning should therefore be an integral part of multidisciplinary management for patients with NETs (Citation36). For screening, the malnutrition universal screening tool (MUST) can be used both prior to and during therapy (Citation38), although it has not been validated to be diagnostic of malnutrition. Due to frequent bouts of diarrhea, patients can sometimes show severe malnutrition (MUST score > 2) that requires enteral and/or parenteral nutrition (Citation38). In addition to anthropometric measurements such as BMI, which is not diagnostic of malnutrition, nutritional status can be assessed with tools such as the Subjective Global Assessment (SGA) (Citation46) or Nutritional Risk Screening (Citation47), which are widely applied to evaluate nutritional status assessment in routine practice and also used in patients with NETs (Citation29).
Lastly, it is worthwhile mentioning the recently proposed Global Leadership Initiative in Malnutrition (GLIM) criteria (Citation48). This consensus effort proposed that diagnosis of malnutrition be guided through the use of five criteria: three phenotypic criteria (non-volitional weight loss, low body mass index, and reduced muscle mass) and two etiologic criteria (reduced food intake or assimilation and inflammation or disease burden) (Citation48). For a diagnosis of malnutrition, one phenotypic criterion and etiologic criterion are required (Citation48). Compared to the SGA, the GLIM criteria have been reported to have fair sensitivity and specificity (Citation49). In cancer patients, GLIM criteria using hand grip strength have been shown to correlate positivity with 6-month mortality (Citation50). To our knowledge, GLIM criteria have not been used to assess nutritional status in patients with NETs.
General Recommendations for Nutrition in Patients with NETs
The World Cancer Research Fund has provided a series of general recommendations for primary or secondary prevention of cancer (Citation51). These include maintaining a healthy weight, prefer whole grains, fruits and vegetables, legumes, and limiting red and processed meat as well as sugar sweetened beverages (Citation51). The American Cancer Society has recommended that all cancer survivors should eat at least five servings of fruits and vegetables per day (Citation52). The European Society for Clinical Nutrition and Metabolism has also issued nutritional guidance for cancer patients and for cancer-related nutrition (Citation53). In general, while not specific for NETs, many of the basic principles of these recommendations may be applied to NET patients, especially for those undergoing chemotherapy (Citation10,Citation54).
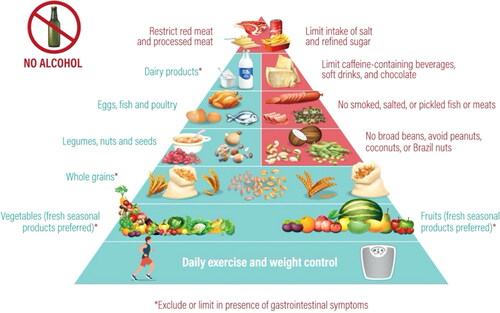
The Carcinoid Cancer Foundation has also issued a series of general recommendations (Citation55). These comprise increasing intake of protein, eating 5-10 portions of carbohydrates (fruits, vegetables, whole grains) daily, maintaining a low intake of fat, and eating foods that are rich in vitamins and minerals (Citation55). It is stressed that in patients with NETs, consumption of alcohol should be avoided (Citation55).
Considering that low levels of tryptophan are generally present in patients with CS, protein intake should be individualized based on referred eating patterns, also considering possible insufficiency in renal function (Citation36). Moreover, in consideration of the general recommendations from the societies cited above and recommendations for patients with NETs, a diet rich in fruits, vegetables, and whole grains than contain both macro- and micronutrients is likely to be of benefit (Citation36). Reducing the intake of fat may help to lower the incidence of steatorrhea and achieve better control of diarrhea, while eating foods that are rich in vitamins and minerals may help prevent vitamin deficiency (such as nicotinic acid deficiency, associated with pellagra) (Citation36).
In a review on nutrition in patients with NETs, Go et al. highlighted the recommendations of the 2005 Dietary Guidelines Advisory Committee from the US Department of Health and Human Services () (Citation28). Similar to the above recommendations, these provide general guidance for nutrition. It was considered that a healthy diet is primarily plant-based, with 5-10 portions of fruits and vegetables and limiting consumption of animal protein, replacing it with beans and other legumes (Citation28). It also consists of low-fat or fat-free dairy products; nuts and seeds, which are low in saturated fat and contain no trans fatty acids; and whole grains as a source of carbohydrates (Citation28). Limiting intake of salt and refined sugar is encouraged, and dietary supplements should be avoided unless specifically instructed by a physician (Citation28).
Table 2. Recommendations by the 2005 Dietary Guidelines Advisory Committee (Citation28).
Nutritional Concerns for Management of Diarrhea
The prevalence of CS-associated diarrhea in patients with NETs and elevated urinary 5-hydroxyindoleacetic acid has been reported to be as high as 80% (Citation6). Thus, some considerations on diarrhea and nutrition are warranted. Based on studies in patients with gastrointestinal conditions such as Crohn’s disease and ulcerative colitis, a low-fiber/low-residue diet is considered to be an integral part of management of a number of bowel diseases (Citation56). By applying these principles, patients presenting up to four bouts of diarrhea, a low-fiber diet ranging from 18 to 24 gm might be considered (Citation56). This suggestion is based on our unpublished experience in patients with colorectal cancer undergoing chemotherapy in which non-adherence to dietary recommendation is associated with a higher rate of cancer therapy-induced diarrhea. In this prospective interventional study on 107 consecutive patients, metastatic colorectal cancer who underwent fluoropyrimidine, oxaliplatin, irinotecan-based chemotherapy, a significant relation was found between cancer therapy-induced diarrhea and low adherence to a preemptive balanced fiber diet, according to World Cancer Research Fund recommendations (odds ratio 2.52; unpublished data).
In those with more than for 4–7 episodes, a maximum of 10 g/day might be considered (Citation56). Fibers such as whole grains and legumes are excluded, and vegetables and fruits are limited (Citation56). It should be noted that these are therapeutic considerations for diarrhea, and not general recommendations that are in contrast with what mentioned above. For patients with more than seven bouts of diarrhea, hospitalization may be necessary and should be treated with artificial nutrition (Citation56). In order to limit bouts of diarrhea, this nutritional treatment provides a low sugar level and appropriate distribution during the day (Citation56). Small and frequent meals at room temperature and dry foods are recommended (Citation56). Soft drinks are banned because they cause water osmotic pressure in the bowel, causing diarrhea (Citation57). Lactose-containing foods are not allowed in order to avoid abdominal pain and diarrhea for those who are intolerant and have congenital primitive lactase deficiency (68% of global population) (Citation58).
Intake of Amines in Patients with NETs
Regarding nutritional considerations for patients with symptomatic NETs, it is advisable to follow a healthy diet as recommended above (Citation28). However, all patients must weigh general advice against their own experience and individual symptoms. The most common symptoms include diarrhea, abdominal pain, gas and bloating, and flushing, and to a lesser extent fatigue, weakness, weight loss, and skin rash (Citation54). In addition, some key nutritional issues must be considered for patients with gastrointestinal symptoms, and it has been suggested that some amine-rich foods be avoided () (Citation28). Nonetheless, it is acknowledged that these recommendations are largely based on anecdotal reports and are not evidence-based (Citation31). Vasoactive amines are precursors for catecholamines like adrenaline, which possibly trigger carcinoid tumors to secrete vasoactive substances and aggravate symptoms or cause carcinoid crisis (Citation59). Tyramine is one of the most active of the vasoactive amines (Citation60). As a general consideration, tyramine and other pressor amines are usually present in high concentrations only in aged, fermented and spoiled protein products (Citation55). Thus, foods that have a high content in vasoamines should be avoided, such as aged cheeses, alcoholic beverages, and shrimp (Citation55). Other foods with a moderately high content, such as caffeine-containing beverages and chocolate, might be allowed depending on severity symptoms (Citation55). There is no need to avoid foods that are rich in 5-hydroxytryptophan (Citation55).
Table 3. Amine-rich foods and products (tyramine, dopamine). Adapted from (Citation28).
Concomitant Medications
As with any pharmacotherapy, food-drug interactions should be given due consideration in patients with NETs, which can alter the absorption rate or interfere with the metabolism of many drugs (Citation61). Some of the most relevant interactions relate to those involving CYPP450 3A4, which are especially relevant when on treatment with everolimus or sunitinib (Citation10). As one example, patients should be counseled to avoid drinking grapefruit juice and eating grapefruit when taking these medications as it interferes with the activity of CYPP450 3A4 (Citation10). Probiotics should be allowed if needed as they have been shown to prevent diarrhea in inflammatory bowel disease (Citation10). Pancreatic enzymes, such as pancrease, creon, and ultrase, are recommended for patients with steatorrhea, which may be related to somatostatin analog therapy (Citation10). Fat-soluble vitamins A, D, E, and K, and multiple vitamins are also advisable, especially if the patient has fat malabsorption (Citation10).
Summing up Dietary Recommendations
The American Institute of Cancer has issued a series of general recommendations for healthy eating that are in line with those discussed above (Citation62). This include eating more whole grains, vegetables, fruits and pulses (legumes), limiting consumption of fast foods and processed foods, red meat and processed meat, and sugar-sweetened soft drinks, with no need for dietary supplementation (Citation62). Nutritional assessment and screening, based on body composition, lifestyle, and specific tools to evaluate nutritional status should be an integral component of management of all patients with NETs, and particularly so in those with CS (Citation10,Citation54). While healthy eating should be a fundamental part of the daily routine of all patients, it is important in individuals with NETs since many tumors will have an indolent course, and patients are likely to undergo multiple lines of therapy during the long natural history of these tumors (Citation3,Citation33). The tumor itself may also have an impact on the patient’s overall nutritional status and needs, and poor nutritional status and low body weight can have a negative impact on outcomes (Citation10,Citation29,Citation40,Citation41). The main dietary recommendations in patients with NETs are summarized in .
In line with the above-described recommendations, we propose a new and practical food pyramid for use in patients with NETS, based on the principles of Mediterranean diet 4.0 (63). It is considered the most sustainable diet in terms of health benefits, environmental impact, economic returns and socio-cultural values. We believe that the Mediterranean diet 4.0 is a useful model to adopt since it can be easily adapted according to the unmet needs of patient with NETs () (Citation63). Our proposal is made with the realization that there are yet no studies supporting the adoption of this model in patients with NETs, although it is highly compatible with the principles and recommendations outlined herein. Nonetheless, a recent study has evaluated the Mediterranean diet in prevention of platinum-based gastrointestinal toxicity in women with gynecological malignancies, reporting that adherence to the diet was seen to reduce gastrointestinal toxicity and prevent impairment of nutritional status (Citation64). In particular, the study showed that adoption of a Mediterranean diet led to a reduction in the severity and frequency of nausea and gastrointestinal pain, abdominal bloating, and less interference with daily activities (Citation64).
A multidisciplinary team is increasingly used in management of NETS due to their complexity, ideally requiring multiple specialists (surgeons, radiologists, endocrinologists, nuclear medicine specialists) (Citation3,Citation65,Citation66). We believe that nutritionists with expertise in NETs should also be a fundamental component of the multidisciplinary management team as they can provide expert dietary approaches to improve the quality of life as well as nutritional status during the entire treatment pathway. Nutritionists can monitor the nutritional status of patients and suggest appropriate dietary changes to avoid the side effects of any therapy.
Lastly, solid data on nutrition in NETs is rather limited and large studies investigating specific aspects are lacking (Citation10). In the absence of such data, there are still several guiding principles that can direct the most appropriate nutritional therapy at any given stage, from diagnosis to long-term follow-up. Patients with CS merit additional consideration over those with nonfunctioning NETs due to their symptoms and the underlying pathophysiological causes. It is hoped that the present analysis can help to create greater awareness of nutritional care and considerations that should be given to patients with NETs.
Author Contributions
Conception/Design: Salvatore Artale. Collection and/or Assembly of Data: Nunziata Grillo, Claudia Maggi, Stefano Lepori, Chiara Butti, Antonella Bovio, Lucia Barbarini, Andrea Colombo and Laura Zanlorenzi. Data Analysis and Interpretatio: Salvatore Artale, Sabrina Barzaghi, Elena Castiglioni and Alessandra Trojani. Original Draft Preparation: Salvatore Artale, Sabrina Barzaghi, Claudia Maggi, Chiara Butti, Stefano Lepori and Laura Zanlorenzi. Review and Editing: Nunziata Grillo, Lucia Barbarini, Antonella Bovio, Andrea Colombo, Elena Castiglioni and Salvatore Artale. Supervision: Alessandra Trojani and Salvatore Artale. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
The daily support of physicians, nurses, and patients at the ASST Valle Olona, Department of Medical Oncology, is gratefully acknowledged. Special thanks to the nursing coordinators Enrica Aloisio, Giovanna Oliva, and Monica Muscarà.
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Cives M, Strosberg JR. Gastroenteropancreatic neuroendocrine tumors. CA Cancer J Clin. 2018;68(6):471–87. doi:https://doi.org/10.3322/caac.21493
- Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, Caplin M, Delle Fave G, Kaltsas GA, Krenning EP, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9(1):61–72.:doi:https://doi.org/10.1016/S1470-2045(07)70410-2
- Oberg K, Couvelard A, Delle Fave G, Gross D, Grossman A, Jensen RT, Pape U-F, Perren A, Rindi G, Ruszniewski P, Antibes Consensus Conference participants, et al. ENETS consensus guidelines for standard of care in neuroendocrine tumours: biochemical markers. Neuroendocrinology. 2017;105(3):201–11. doi:https://doi.org/10.1159/000472254
- Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3(10):1335–42. doi:https://doi.org/10.1001/jamaoncol.2017.0589
- Lloyd RV, Osamura RY, Kloppel G. WHO Classification of tumours of endocrine organs. 4th ed. Lyon, France: World Health Organization; 2017. p. 355.
- Rubin de Celis Ferrari AC, Glasberg J, Riechelmann RP. Carcinoid syndrome: update on the pathophysiology and treatment. Clinics (Sao Paulo). 2018;73(suppl 1):e490s. doi:https://doi.org/10.6061/clinics/2018/e490s
- Badawy AA. Modulation of tryptophan and serotonin metabolism as a biochemical basis of the behavioral effects of use and withdrawal of androgenic-anabolic steroids and other image- and performance-enhancing agents. Int J Tryptophan Res. 2018;11:1178646917753422. doi:https://doi.org/10.1177/1178646917753422
- Kvols LK. Metastatic carcinoid tumors and the malignant carcinoid syndrome. Ann N Y Acad Sci. 1994;733:464–70. doi:https://doi.org/10.1111/j.1749-6632.1994.tb17296.x
- Mota JM, Sousa LG, Riechelmann RP. Complications from carcinoid syndrome: review of the current evidence. Ecancermedicalscience. 2016;10:662. doi:https://doi.org/10.3332/ecancer.2016.662
- Altieri B, Barrea L, Modica R, Muscogiuri G, Savastano S, Colao A, Faggiano A. Nutrition and neuroendocrine tumors: An update of the literature. Rev Endocr Metab Disord. 2018;19(2):159–67. doi:https://doi.org/10.1007/s11154-018-9466-z
- Adaway JE, Dobson R, Walsh J, Cuthbertson DJ, Monaghan PJ, Trainer PJ, Valle JW, Keevil BG. Serum and plasma 5-hydroxyindoleacetic acid as an alternative to 24-h urine 5-hydroxyindoleacetic acid measurement. Ann Clin Biochem. 2016;53(Pt 5):554–60. doi:https://doi.org/10.1177/0004563215613109
- Al-Risi ES, Al-Essry FS, Mula-Abed WS. Chromogranin A as a biochemical marker for neuroendocrine tumors: a single center experience at Royal Hospital, Oman. Oman Med J. 2017;32(5):365–70. doi:https://doi.org/10.5001/omj.2017.71
- Bajetta E, Ferrari L, Martinetti A, Celio L, Procopio G, Artale S, Zilembo N, Di Bartolomeo M, Seregni E, Bombardieri E, et al. Chromogranin A, neuron specific enolase, carcinoembryonic antigen, and hydroxyindole acetic acid evaluation in patients with neuroendocrine tumors. Cancer. 1999;86(5):858–65. doi:https://doi.org/10.1002/(sici)1097-0142(19990901)86:5 < 858::aid-cncr23 > 3.0.co;2-8
- Robbins HL, Symington M, Mosterman B, Tranter F, Davies L, Randeva HS, Penedo A, Ferreira C, Darby C, Grammatopoulos D, et al. Effects of intake of breakfast or caffeine‐containing beverages on measurement of circulating chromogranin A in plasma. GastroHep. 2019;1(1):11–21. doi:https://doi.org/10.1002/ygh2.208
- NET Patient Foundation. https://www.netpatientfoundation.org/wp-content/uploads/npf_fastinggut_factsheet.pdf.
- Pusceddu S, Prinzi N, Raimondi A, Corti F, Buzzoni R, Di Bartolomeo M, Seregni E, Maccauro M, Coppa J, Milione M, et al. Entering the third decade of experience with octreotide LAR in neuroendocrine tumors: A review of current knowledge. Tumori. 2019;105(2):113–20. doi:https://doi.org/10.1177/0300891618765362
- Barbieri F, Bajetto A, Pattarozzi A, Gatti M, Würth R, Thellung S, Corsaro A, Villa V, Nizzari M, Florio T, et al. Peptide receptor targeting in cancer: the somatostatin paradigm. Int J Pept. 2013;2013:926295. doi:https://doi.org/10.1155/2013/926295
- Vinik AI, Wolin EM, Liyanage N, Gomez-Panzani E, Fisher GA, ELECT Study Group. Evaluation of lanreotide depot/autogel efficacy and safety as a carcinoid syndrome treatment (Elect): a randomized, double-blind, placebo-controlled trial. Endocr Pract. 2016;22(9):1068–80. doi:https://doi.org/10.4158/EP151172.OR
- Anthony L, Vinik AI. Evaluating the characteristics and the management of patients with neuroendocrine tumors receiving octreotide LAR during a 6-year period. Pancreas. 2011;40(7):987–94. doi:https://doi.org/10.1097/MPA.0b013e31821f66b4
- Hofland LJ, van der Hoek J, Feelders R, van der Lely AJ, de Herder W, Lamberts SWJ. Pre-clinical and clinical experiences with novel somatostatin ligands: advantages, disadvantages and new prospects. J Endocrinol Invest. 2005;28(11 Suppl International):36–42.
- Niederle B, Pape U-F, Costa F, Gross D, Kelestimur F, Knigge U, Öberg K, Pavel M, Perren A, Toumpanakis C, Vienna Consensus Conference participants, et al. ENETS consensus guidelines update for neuroendocrine neoplasms of the jejunum and ileum. Neuroendocrinology. 2016;103(2):125–38. doi:https://doi.org/10.1159/000443170
- Saavedra C, Barriuso J, McNamara MG, Valle JW, Lamarca A. Spotlight on telotristat ethyl for the treatment of carcinoid syndrome diarrhea: patient selection and reported outcomes. Cancer Manag Res. 2019;11:7537–56. doi:https://doi.org/10.2147/CMAR.S181439
- Strosberg J, Joish VN, Giacalone S, Perez‐Olle R, Fish‐Steagall A, Kapoor K, Dharba S, Lapuerta P, Benson AB. TELEPRO: patient-reported carcinoid syndrome symptom improvement following initiation of telotristat ethyl in the real world. The Oncol. 2019;24(11):1446–52. doi:https://doi.org/10.1634/theoncologist.2018-0921
- Weickert MO, Kaltsas G, Hörsch D, Lapuerta P, Pavel M, Valle JW, Caplin ME, Bergsland E, Kunz PL, Anthony LB, et al. Changes in weight associated with telotristat ethyl in the treatment of carcinoid syndrome. Clin Ther. 2018;40(6):952–62. doi:https://doi.org/10.1016/j.clinthera.2018.04.006
- Brabander T, van der Zwan WA, Teunissen JJM, Kam BLR, Feelders RA, de Herder WW, van Eijck CHJ, Franssen GJH, Krenning EP, Kwekkeboom DJ, et al. Long-term efficacy, survival, and safety of [177Lu-DOTA0,Tyr3]octreotate in patients with gastroenteropancreatic and bronchial neuroendocrine tumors. Clin Cancer Res. 2017;23(16):4617–24. doi:https://doi.org/10.1158/1078-0432.CCR-16-2743
- Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, Mittra E, Kunz PL, Kulke MH, Jacene H, et al. Phase 3 trial of (177)Lu-dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376(2):125–35. doi:https://doi.org/10.1056/NEJMoa1607427
- Clement DS, Tesselaar ME, van Leerdam ME, Srirajaskanthan R, Ramage JK. Nutritional and vitamin status in patients with neuroendocrine neoplasms. World J Gastroenterol. 2019;25(10):1171–84. doi:https://doi.org/10.3748/wjg.v25.i10.1171
- Go VL, Srihari P, Kamerman Burns LA. Nutrition and gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2010;39(4):827–37. doi:https://doi.org/10.1016/j.ecl.2010.08.003
- Maasberg S, Knappe-Drzikova B, Vonderbeck D, Jann H, Weylandt KH, Grieser C, Pascher A, Schefold JC, Pavel M, Wiedenmann B, et al. Malnutrition predicts clinical outcome in patients with neuroendocrine neoplasia. Neuroendocrinology. 2017;104(1):11–25. doi:https://doi.org/10.1159/000442983
- Dasari A, Joish VN, Perez-Olle R, Dharba S, Balaji K, et al. Work productivity burden and indirect costs associated with carcinoid syndrome diarrhea. Expert Rev Pharmacoecon Outcomes Res. 2019;20(5):1–5. doi:https://doi.org/10.1080/14737167.2019.1660646
- Laing E, Kiss N, Michael M, Krishnasamy M. Nutritional complications and the management of patients with gastroenteropancreatic neuroendocrine tumors. Neuroendocrinology. 2020;110(5):430–42. doi:https://doi.org/10.1159/000503634
- Laing E, Kiss N, Michael M, Gough K, Krishnasamy M. Investigating nutrition-related complications and quality of life in patients with gastroenteropancreatic neuroendocrine tumors: protocol for a mixed-methods prospective study. JMIR Res Protoc. 2018;7(12):e11228. doi:https://doi.org/10.2196/11228
- Oronsky B, Ma PC, Morgensztern D, Carter CA. Nothing But NET: a review of neuroendocrine tumors and carcinomas. Neoplasia. 2017;19(12):991–1002. doi:https://doi.org/10.1016/j.neo.2017.09.002
- Kim DH. Nutritional issues in patients with cancer. Intest Res. 2019;17(4):455–62. doi:https://doi.org/10.5217/ir.2019.00076
- Borre M, Dam GA, Knudsen AW, Gronbaek H. Nutritional status and nutritional risk in patients with neuroendocrine tumors. Scand J Gastroenterol. 2018;53(3):284–92. doi:https://doi.org/10.1080/00365521.2018.1430848
- Gallo M, Muscogiuri G, Pizza G, Ruggeri RM, Barrea L, Faggiano A, Colao A, NIKE Group The management of neuroendocrine tumours: a nutritional viewpoint. Crit Rev Food Sci Nutr. 2019;59(7):1046–57. doi:https://doi.org/10.1080/10408398.2017.1390729
- Glazer E, Stanko K, Ong E, Guerrero M. Decreased inpatient mortality in obese patients with abdominal nets. Endocr Pract. 2014;20(12):1–20. doi:https://doi.org/10.4158/EP14203.OR
- Qureshi SA, Burch N, Druce M, Hattersley JG, Khan S, Gopalakrishnan K, Darby C, Wong JLH, Davies L, Fletcher S, et al. Screening for malnutrition in patients with gastro-entero-pancreatic neuroendocrine tumours: a cross-sectional study. BMJ Open. 2016;6(5):e010765. doi:https://doi.org/10.1136/bmjopen-2015-010765
- Robbins HL, Symington M, Mosterman B, Goodby J, Davies L, Dimitriadis GK, Kaltsas G, Randeva HS, Weickert MO. Supplementation of vitamin D deficiency in patients with neuroendocrine tumors using over-the-counter vitamin D3 preparations. Nutr Cancer. 2018;70(5):748–54. doi:https://doi.org/10.1080/01635581.2018.1470650
- Ekeblad S, Skogseid B, Dunder K, Oberg K, Eriksson B. Prognostic factors and survival in 324 patients with pancreatic endocrine tumor treated at a single institution. Clin Cancer Res. 2008;14(23):7798–803. doi:https://doi.org/10.1158/1078-0432.CCR-08-0734
- Marrache F, Vullierme MP, Roy C, El Assoued Y, Couvelard A, O'Toole D, Mitry E, Hentic O, Hammel P, Lévy P, et al. Arterial phase enhancement and body mass index are predictors of response to chemoembolisation for liver metastases of endocrine tumours. Br J Cancer. 2007;96(1):49–55. doi:https://doi.org/10.1038/sj.bjc.6603526
- Lind A, Wangberg B, Ellegard L. Vitamin D and vitamin B12 deficiencies are common in patients with midgut carcinoid (SI-NET). Eur J Clin Nutr. 2016;70(9):990–4. doi:https://doi.org/10.1038/ejcn.2016.40
- Massironi S, Zilli A, Bernasconi S, Fanetti I, Cavalcoli F, Ciafardini C, Felicetta I, Conte D. Impact of vitamin D on the clinical outcome of gastro-entero-pancreatic neuroendocrine neoplasms: report on a series from a single institute. Neuroendocrinology. 2017;105(4):403–11. doi:https://doi.org/10.1159/000456619
- Motylewska E, Gawronska J, Niedziela A, Melen-Mucha G, Lawnicka H, Komorowski J, Swietoslawski J, Stepien H. Somatostatin analogs and tumor localization do not influence vitamin D concentration in patients with neuroendocrine tumors. Nutr Cancer. 2016;68(3):428–34. doi:https://doi.org/10.1080/01635581.2016.1152387
- Shah GM, Shah RG, Veillette H, Kirkland JB, Pasieka JL, Warner RRP. Biochemical assessment of niacin deficiency among carcinoid cancer patients. Am J Gastroenterol. 2005;100(10):2307–14. doi:https://doi.org/10.1111/j.1572-0241.2005.00268.x
- Detsky AS, Baker JP, Mendelson RA, Wolman SL, Wesson DE, Jeejeebhoy KN. Evaluating the accuracy of nutritional assessment techniques applied to hospitalized patients: methodology and comparisons. JPEN J Parenter Enteral Nutr. 1984;8(2):153–9. doi:https://doi.org/10.1177/0148607184008002153
- Kondrup J, Rasmussen HH, Hamberg O, Stanga Z. Ad Hoc EWG: Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22(3):321–36. doi:https://doi.org/10.1016/S0261-5614(02)00214-5
- Cederholm T, Jensen GL, Correia MITD, Gonzalez MC, Fukushima R, Higashiguchi T, Baptista G, Barazzoni R, Blaauw R, Coats A, GLIM Working Group, et al. GLIM criteria for the diagnosis of malnutrition - A consensus report from the global clinical nutrition community. Clin Nutr. 2019;38(1):1–9. doi:https://doi.org/10.1016/j.clnu.2018.08.002
- Allard JP, Keller H, Gramlich L, Jeejeebhoy KN, Laporte M, et al. GLIM criteria has fair sensitivity and specificity for diagnosing malnutrition when using SGA as comparator. Clin Nutr. 2019;39(9):2771–7. doi:https://doi.org/10.1016/j.clnu.2019.12.004
- Contreras-Bolivar V, Sanchez-Torralvo FJ, Ruiz-Vico M, Gonzalez-Almendros I, Barrios M, et al. GLIM criteria using hand grip strength adequately predict six-month mortality in cancer inpatients. Nutrients. 2019;11(9):2043. doi:https://doi.org/10.3390/nu11092043
- World Cancer Research Fund. [accessed 2020 Feb 16]. https://www.wcrf.org/dietandcancer/cancer-prevention-recommendations.
- Blanchard CM, Courneya KS, Stein K. American Cancer Society's SCS, II: Cancer survivors' adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society's SCS-II. JCO. 2008;26(13):2198–204. doi:https://doi.org/10.1200/JCO.2007.14.6217
- European Society for Clinical Nutrition and Metabolism. https://www.espen.org/guidelines-home/espen-guidelines.
- NHS. Food and neuroendocrine tumors. [accessed 2020 Sept 14]. https://tarawhyand.com/wp-content/uploads/2018/07/Food_and_NETs.pdf.
- The Carcinoid Cancer Foundation. [accessed 2020 Feb 16]. https://www.carcinoid.org/for-patients/general-information/nutrition/nutritional-concerns-for-the-carcinoid-patient-developing-nutrition-guidelines-for-persons-with-carcinoid-disease/.
- Vanhauwaert E, Matthys C, Verdonck L, De Preter V. Low-residue and low-fiber diets in gastrointestinal disease management. Adv Nutr. 2015;6(6):820–7. doi:https://doi.org/10.3945/an.115.009688
- Brown AC, Rampertab SD, Mullin GE. Existing dietary guidelines for Crohn's disease and ulcerative colitis. Expert Rev Gastroenterol Hepatol. 2011;5(3):411–25. doi:https://doi.org/10.1586/egh.11.29
- Storhaug CL, Fosse SK, Fadnes LT. Country, regional, and global estimates for lactose malabsorption in adults: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017;2(10):738–46. doi:https://doi.org/10.1016/S2468-1253(17)30154-1
- Lips CJ, Lentjes EG, Hoppener JW. The spectrum of carcinoid tumours and carcinoid syndromes. Ann Clin Biochem. 2003;40(Pt 6):612–27. doi:https://doi.org/10.1258/000456303770367207
- Skypala IJ, Williams M, Reeves L, Meyer R, Venter C. Sensitivity to food additives, vaso-active amines and salicylates: a review of the evidence. Clin Transl Allergy. 2015;5:34. doi:https://doi.org/10.1186/s13601-015-0078-3
- Bushra R, Aslam N, Khan AY. Food-drug interactions. Oman Med J. 2011;26(2):77–83. doi:https://doi.org/10.5001/omj.2011.21
- American Institute of Cancer. [accessed 2020 Feb 16]. https://www.aicr.org.
- Dernini S, Berry EM, Serra-Majem L, La Vecchia C, Capone R, Medina FX, Aranceta-Bartrina J, Belahsen R, Burlingame B, Calabrese G, et al. Med Diet 4.0: the Mediterranean diet with four sustainable benefits. Public Health Nutr. 2017;20(7):1322–30. doi:https://doi.org/10.1017/S1368980016003177
- Ghisoni E, Casalone V, Giannone G, Mittica G, Tuninetti V, Valabrega G. Role of Mediterranean diet in preventing platinum based gastrointestinal toxicity in gynecolocological malignancies: a single institution experience. WJCO. 2019;10(12):391–401. doi:https://doi.org/10.5306/wjco.v10.i12.391
- Granata V, Fusco R, Setola SV, Castelguidone E. d L d, Camera L, Tafuto S, Avallone A, Belli A, Incollingo P, Palaia R, et al. The multidisciplinary team for gastroenteropancreatic neuroendocrine tumours: the radiologist's challenge. Radiol Oncol. 2019;53(4):373–87. doi:https://doi.org/10.2478/raon-2019-0040
- Magi L, Mazzuca F, Rinzivillo M, Arrivi G, Pilozzi E, et al. Multidisciplinary management of neuroendocrine neoplasia: a real-world experience from a referral center. J Clin Med. 2019;8(6):910. doi:https://doi.org/10.3390/jcm8060910