ABSTRACT
Several studies have reported the preoperative control nutritional status (CONUT) score as an independent prognostic factor for the prognosis of lung cancer patients. Patients with severe chronic obstructive pulmonary disease usually have high cholesterol levels, cachexia, and muscle atrophy. Abnormal nutritional status and lymphopenia were also related to poor prognosis. We explored the relationship between the preoperative CONUT score and patient prognosis and predicted the efficacy of pembrolizumab in lung cancer treatment. A systematic literature search was performed to identify qualified articles reporting the prognostic prediction potential of CONUT scores in lung cancer patients. A meta-analysis was performed for the association between CONUT scores and survival outcomes and clinic-pathological parameters. Overall, eight articles and 1,220 cases were included. Abnormal preoperative CONUT scores were a poor prognostic factor for elderly lung cancer patients. Finally, higher CONUT scores of pembrolizumab were associated with poor survival. CONUT was an independent prognostic indicator of lung cancer, successfully predicting the efficacy and prognosis of pembrolizumab in lung cancer treatment.
Introduction
Lung cancer is one of the most common causes of cancer-related deaths worldwide, with more than one million deaths per year. Despite developments in new gene targeted therapeutic drugs and immunotherapy, the patient’s prognosis is still not optimistic (Citation1–3). Exploring clinical indexes is important in predicting patient prognosis and in guiding therapeutic selection and optimization, which improve patient prognosis.
Clinical indexes include patient health status, age, cancer stage, serum lactate dehydrogenase (LDH) level, and tumor classification, which are considered as prognostic indexes. Their application in the clinical setting is controversial. Malnutrition status was associated with a poor prognosis for lung cancer patients. Evaluation of malnutrition is important to predict prognosis and select a treatment strategy. Evidence shows that impaired immuno-nutritional status is closely related to patient prognosis. The prognostic nutritional index (PNI), calculated based on the serum albumin concentration and the total number of peripheral blood lymphocytes, related to the survival of lung cancer patients (Citation4).
Another nutritional status index is the controlling nutritional status (CONUT) score. It is a relatively new index and is considered as an immune nutritional status score. It has a significant relationship with prognosis in various cancers, improving both disease-free survival (DFS) and overall survival (OS). It is a potential predictor of cancer progression and postoperative complications (Citation5). The score is based on the serum albumin concentration, the total number of peripheral lymphocytes, and the index, calculated with the total cholesterol concentration. Compared with PNI, CONUT score focuses less on albumin concentration and more on the concentration of total cholesterol, which makes the assessment of nutritional status more accurate.
CONUT is considered to be the most important independent prognostic factor for lung cancer before surgery (Citation6–10). The albumin level is affected by patient nutrition and the level of inflammation. In addition, cancer cachexia can also affect albumin levels. Moreover, cancer patients with cachexia have a poor prognosis. Cancer can cause hypocholesterolemia, and low cholesterol is associated with poor prognosis of cancer. In addition, patients with severe chronic obstructive pulmonary disease present with high serum cholesterol levels. The cachexia and muscle atrophy in lung cancer patients with obstructive lung injury negatively affect the nutritional status (Citation4). Lymphopenia is a common clinical complication that results in poor prognosis and is seen on chest radiotherapy. Furthermore, lymphopenia can lead to anti-tumor immune suppression and poor OS (Citation11). CONUT is used as an immune nutritional status score, which can be used to enhance postoperative recovery (ERAS) or as a potential predictor of cancer progression and postoperative complications (Citation5). In recent years, CONUT scores have been extensively studied in colorectal cancer (Citation6), gastric cancer (Citation12), bladder cancer (Citation13), and breast cancer (Citation14). It has been related to a poor clinical prognosis and has been used in surgery. Moreover, it was related to the incidence of later complications. The CONUT score has been studied in the prognosis of lung cancer (Citation15). The use of CONUT score for lung cancer was first reported in 2017, and several articles have been published in the following years. Their result can be adopted. However, the results of these studies were controversial, owing to the use of different cutoff values of the CONUT score. The prognostic significance of the CONUT score has not been systematically studied in patients with lung cancer so far. The purpose of this study was to explore the association between the CONUT score and prognostic clinical parameters of patients following lung cancer resection. Moreover, it determined the potential application of CONUT scores for predicting the prognosis of patients receiving pembrolizumab treatment.
Materials and Methods
Search Method
The PRISMA guidelines were followed in this study (Citation16). A systematic literature search was conducted using five publication libraries: Embase, Medline Ovid, Web of Science, Cochrane CENTRAL, and Google scholar on March 9, 2020. Literature reporting the association between the CONUT score and outcomes in patients with lung cancer was identified. Eligible studies were independently screened by two investigators.
Preparation for Inclusion
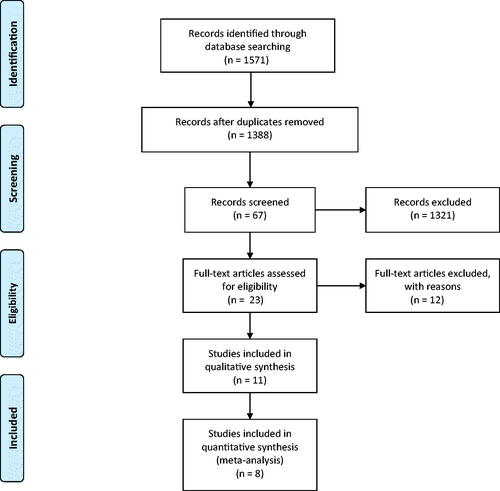
A total of 1,571 articles were searched using the database; 1,321 articles were excluded from the records, and 23 articles were evaluated for full-text articles. This included 11 articles of qualitative synthesis. Finally, eight meta-analysis articles were included.
Data Extraction and Quality Assessment
According to the guidelines, we independently screened the title, abstract, and full text. Articles that met the standards were included. Survival data were extracted from the author, year of publication, type of research, basic treatment method of the patient, follow-up time, number of patients, cutoff value of the CONUT score, and OS and DFS results in the included articles. The Newcastle–Ottawa Scale score was used to assess the quality of the meta-analysis study. The scoring criteria were: ≤5, low quality; 6 or 7, medium quality; ≥8, and high quality (Citation16).
Statistical Analysis
All calculations were performed using STATA 14.0 software. If I2 value <0.05 or P > 0.1, a fixed-effect model was used. If the conditions were not met, the random effects model was used to analyze the significant heterogeneity. The literature included in this study comprised retrospective studies, published by hospitals or research institutions in Japan. The literature sensitivity analysis was stable in all included patient data, and the funnel chart examination did not show asymmetry. P < 0.05 was considered significant.
Results
In the literature search step, the term “controlling nutritional status” was used to search the database, and 1,571 articles were retrieved. We further reviewed the title or abstract of the studies as well as removed duplicates and those with no available data, too little data, or inconsistencies. Finally, eight articles were considered qualified and included in the meta-analysis ().
The data included were from the literature published from 2017 to 2020, including a total of 1,220 patients. The cutoff values of the CONUT scores were different for all studies. This study conducted a systematic meta-analysis of the eight included studies and focused on OS or DFS. is the Newcastle–Ottawa Scale (NOS) scoring of these documents. All articles scored seven to eight points, and all were medium- or high-quality articles (Citation17). The fifth article concluded that among patients receiving pembrolizumab as first-line treatment, high CONUT scores were associated with poor DFS and OS. In addition, the third and eight articles concluded that abnormal preoperative CONUT score was a factor of poor prognosis in elderly patients with lung cancer complicated by other diseases. The fourth CONUT score was an independent prognostic factor for DFS and OS in lung cancer patients.
Table 1. Basic information.
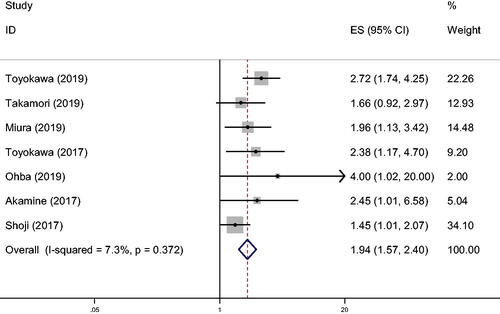
Seven of the articles studied the impact of CONUT score on OS (). The results, based on the fixed effects model, showed that the patients with high CONUT scores had a worse prognosis at 5 years than those with low CONUT scores (hazard ratio [HR] 1.94, 95% confidence interval [CI] 1.574–2.399, P < 0.001, I2 = 7.3%, Ph = 0.372).
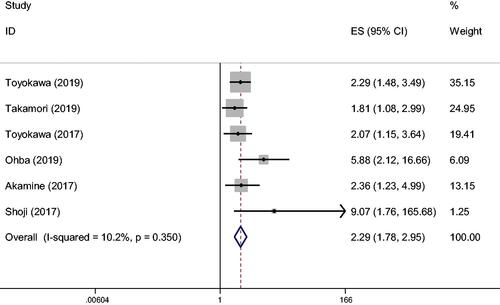
Six of the articles studied the impact of CONUT score on DFS (). The result, based on the fixed effects model, showed that lung cancer patients with high CONUT scores had a worse prognosis in 5-year DFS than those with low CONUT scores (HR 2.29, 95% CI 1.776–2.954, P < 0.001, I2 = 10.2%, Ph = 0.35). Correlation analysis of CONUT score OS and DFS ().
Table 2. CONUT score OS, DFS correlation analysis.
The number of study cases included, the number of patients, the model used, HR, P value, and heterogeneity were the research objects. The results showed that the surgical method (HR 1.915, 95% CI 1.548–2.369, P < 0.001, I2 = 9.9%, Ph = 0.352) used a fixed-effect model. The cutoff value of CONUT score was 1 or 2 (HR 1.651, 95% CI 1.238–2.201, P < 0.001, I2 = 0%, Ph = 0.46; HR 2.347, 95% CI 1.722–3.198, P = 0, I2 = 0%, Ph = 0.521, respectively). The number of cases studied was <100 or > 100 (HR 1.907, 95% 1.515–2.402, P = 0, I2 = 27.8%, Ph = 0.236; HR 2.138, 95% CI 1.272–3.592, P < 0.004, I2 = 0%, Ph = 0.379), and a fixed effects model was used. The results, based on the fixed effects model, showed that the surgical method, cutoff, and the stratified analysis of the number of cases studied were meaningful.
A correlation analysis was conducted for the CONUT score and clinical factors (). A stratified analysis based on smoking, BMI, tumor stage, and histology was performed. The results showed that smoking (HR 1.086, 95% CI 0.847–1.391, P < 0.516. I2 = 0%, Ph = 0.968), using fixed effects model, BMI (HR 0.63, 95% CI 0.222–1.781, P < 0.383, I2 = 83%, Ph = 0.003), so random effects model is used. Tumor stage (HR 1.133, 95% CI 0.704–1.824, P < 0.606, I2 = 79%, Ph = 0.008), so the random effects model is adopted. Histology (HR 1.355, 95% CI 0.494–3.715, P < 0.555, I2 = 93.3%, Ph = 0), so the random effects model is adopted. Except for smoking with a fixed-effect model, BMI, tumor staging, and tumor typing all used random effects models, and there was no statistical difference in P values.
Table 3. Correlation analysis of CONUT score on clinical factors.
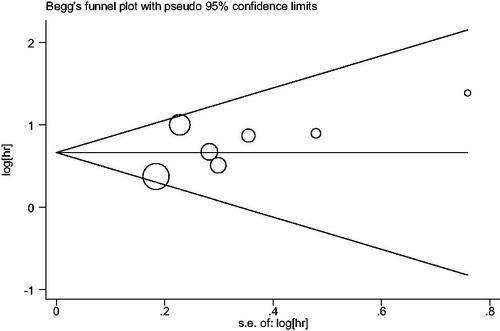
A funnel chart was made based on the data provided by the included articles. The test result indicated that there was no evidence of publication bias (Egger’s test P < 0.186). The sensitivity analysis chart showed that the current data research was relatively stable and reliable ( and ).
Discussion
This is the first meta-analysis investigating the use of the CONUT score as a prognostic indicator of lung cancer. Serum albumin is one of the most commonly used indicators to assess nutritional status. Previous studies have shown that low albumin levels were related to poor nutritional status and clinical outcomes in various cancers, including colorectal cancer, stomach cancer, breast cancer, and lung cancer. Patients with good nutrition and healthy livers, producing normal amounts of albumin, have good resistance to disease and tumor growth. Albumin is inhibited by inflammatory cytokines, such as tumor necrosis factor-α, IL-1, and IL-6, which promote tumor development (Citation18). Since cancer affects its metabolism, albumin can be used to reflect physical conditions. Different immune functions, especially cell-mediated immune functions, are affected by hypoproteinemia. Patients with postoperative hypoproteinemia have increased infections. It is not clear whether correcting hypoproteinemia can reduce postoperative complications or cancer recurrence. Further study is needed to investigate whether it can effectively improve the survival rate and reduce postoperative complications and tumor recurrence (Citation5).
Cholesterol metabolism is abnormal in malignant tumor cells, including prostate cancer, lung cancer, acute myeloid leukemia, breast cancer, and other tumors. Tumor cell proliferation may require new membrane synthesis. Cholesterol was higher in tumor tissues than in normal tissues. Cholesterol was related to cell proliferation, and cholesterol provides favorable conditions for cell growth. Cholesterol oxidase can increase the content of ROS by reducing cholesterol levels, which leads to cell apoptosis. Based on previous research, HDL-C played an active role in cancer development (Citation19). Low serum cholesterol levels were related to the poor prognosis of patients with malignant tumors, such as colorectal cancer, kidney cancer, and pancreatic cancer. In addition, hypocholesterolemia can cause systemic inflammation and cancer. CONUT score includes immune defense, calorie consumption, and protein savings. Therefore, the CONUT score can reflect the immune and nutritional status.
The high lymphatic count indicated that tumor proliferation, growth, and invasion were related (Citation20,Citation21). Low lymphatic count may lead to tumor progression and is related to a poor prognosis of gastrointestinal tumors. The decreased lymphocytes indicated the poor prognosis of lung cancer patients (Citation19). Numerous literature reports indicated that the number of lymphocytes was related to the survival rate of lung cancer patients, and the poor prognosis of advanced cancer was related to lymphopenia.
The CONUT score was significantly related to the long-term prognosis of lung cancer patients in this study due to the following reasons. First, patients with high CONUT scores had lower OS and DFS survival rates than those with low CONUT scores. This suggested the CONUT score can predict the prognosis of lung cancer (Citation4,Citation10,Citation22), and patients with high clinical CONUT scores should be followed up more carefully. Second, preoperative nutritional intervention may improve treatment results and hospital stay. In particular, it has a suggestive effect on the survival rate of radical surgery for pathological stage I non-small cell lung cancer (Citation4,Citation23). Third, normal preoperative and postoperative immune nutrition are important to reduce postoperative complications of lung cancer (Citation24).
This study has limitations. All studies were retrospective studies, and the number of cases was small. The articles based on this study is from Japan, so the application of its results to other countries and regions is limited. Further studies are needed in multi-center and with large-scale cases to further confirm the prognostic significance of the score.
This review emphasized that the CONUT score can be used as an indicator to predict the survival rate and complications of lung cancer patients. It was clinically simple, quick, and economic. However, prospective studies are needed for multi-center and large-scale cases, and further research is needed to determine the prognostic and therapeutic benefits of nutritional interventions as well as the underlying mechanism.
Disclosure Statement
The authors report no conflict of interest.
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi:https://doi.org/10.3322/caac.21254
- Cancer Genome Atlas Research N: Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519–525.
- Cancer Genome Atlas Research N: Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543–550.
- Akamine T, Toyokawa G, Matsubara T, Kozuma Y, Haratake N, Takamori S, Katsura M, Takada K, Shoji F, Okamoto T, et al. Significance of the preoperative CONUT score in predicting postoperative disease-free and overall survival in patients with lung adenocarcinoma with obstructive lung disease. Anticancer Res. 2017;37(5):2735–2742. doi:https://doi.org/10.21873/anticanres.11625
- Park S, Ahn HJ, Yang M, Kim JA, Kim JK, Park SJ. The prognostic nutritional index and postoperative complications after curative lung cancer resection: A retrospective cohort study. J Thorac Cardiovasc Surg. 2020;160(1):276–285.
- Iseki Y, Shibutani M, Maeda K, Nagahara H, Ohtani H, Sugano K, Ikeya T, Muguruma K, Tanaka H, Toyokawa T, et al. Impact of the preoperative controlling nutritional status (CONUT) score on the survival after curative surgery for colorectal cancer. PLoS One. 2015;10(7):e0132488. doi:https://doi.org/10.1371/journal.pone.0132488
- Yoshida N, Baba Y, Shigaki H, Harada K, Iwatsuki M, Kurashige J, Sakamoto Y, Miyamoto Y, Ishimoto T, Kosumi K, et al. Preoperative nutritional assessment by controlling nutritional status (CONUT) is useful to estimate postoperative morbidity after esophagectomy for esophageal cancer. World J Surg. 2016;40(8):1910–1917. doi:https://doi.org/10.1007/s00268-016-3549-3
- Toyokawa T, Kubo N, Tamura T, Sakurai K, Amano R, Tanaka H, Muguruma K, Yashiro M, Hirakawa K, Ohira M, et al. The pretreatment controlling nutritional status (CONUT) score is an independent prognostic factor in patients with resectable thoracic esophageal squamous cell carcinoma: results from a retrospective study. BMC Cancer. 2016;16:722. doi:https://doi.org/10.1186/s12885-016-2696-0
- Shoji F, Haratake N, Akamine T, Takamori S, Katsura M, Takada K, Toyokawa G, Okamoto T, Maehara Y. The preoperative controlling nutritional status score predicts survival after curative surgery in patients with pathological stage I non-small cell lung cancer. Anticancer Res. 2017;37(2):741–747.
- Takamori S, Toyokawa G, Shimokawa M, Kinoshita F, Kozuma Y, Matsubara T, Haratake N, Akamine T, Hirai F, Tagawa T, et al. A novel prognostic marker in patients with non-small cell lung cancer: musculo-immuno-nutritional score calculated by controlling nutritional status and creatine kinase. J Thorac Dis. 2019;11(3):927–935.
- Joseph N, McWilliam A, Kennedy J, Haslett K, Mahil J, Gavarraju A, Mistry H, Van Herk M, Faivre-Finn C, Choudhury A, et al. Post-treatment lymphocytopaenia, integral body dose and overall survival in lung cancer patients treated with radical radiotherapy. Radiother Oncol. 2019;135:115–119.
- Kuroda D, Sawayama H, Kurashige J, Iwatsuki M, Eto T, Tokunaga R, Kitano Y, Yamamura K, Ouchi M, Nakamura K, et al. Controlling Nutritional Status (CONUT) score is a prognostic marker for gastric cancer patients after curative resection. Gastric Cancer. 2018;21(2):204–212.
- Miyake M, Morizawa Y, Hori S, Marugami N, Shimada K, Gotoh D, Tatsumi Y, Nakai Y, Inoue T, Anai S, et al. Clinical impact of postoperative loss in psoas major muscle and nutrition index after radical cystectomy for patients with urothelial carcinoma of the bladder. BMC Cancer. 2017;17(1):237.
- Li W, Li M, Wang T, Ma G, Deng Y, Pu D, Liu Z, Wu Q, Liu X, Zhou Q, et al. Controlling nutritional status (CONUT) score is a prognostic factor in patients with resected breast cancer. Sci Rep. 2020;10(1):6633. doi:https://doi.org/10.1038/s41598-020-63610-7
- Zhang Y, Zhang X. Controlling nutritional status score, a promising prognostic marker in patients with gastrointestinal cancers after surgery: a systematic review and meta-analysis. Int J Surg. 2018;55:39–45.
- Takagi K, Domagala P, Polak WG, Buettner S, Ijzermans JNM. The controlling nutritional status score and postoperative complication risk in gastrointestinal and hepatopancreatobiliary surgical oncology: a systematic review and meta-analysis. Ann Nutr Metab. 2019;74(4):303–312.
- Takagi K, Domagala P, Polak WG, Buettner S, Ijzermans JNM. Prognostic significance of the controlling nutritional status (CONUT) score in patients undergoing hepatectomy for hepatocellular carcinoma: a systematic review and meta-analysis. BMC Gastroenterol. 2019;19(1):211.
- Guo X, Shao J, Zhai B, Zou Q, Yan J, et al. Relationship and prognostic significance between preoperative serum albumin to globulin ratio and CT features of non-small cell lung cancer. Eur J Radiol. 2020;128:10939.
- Ding N, Pang ZFei, Shen H, Ni Y, Du J, Liu Q. The prognostic value of PLR in lung cancer, a meta-analysis based on results from a large consecutive cohort. Sci Rep. 2016;6:34823. doi:https://doi.org/10.1038/srep34823
- Lianyuan T, Dianrong X, Chunhui Y, Zhaolai M, Bin J. The predictive value and role of stromal tumor-infiltrating lymphocytes in pancreatic ductal adenocarcinoma (PDAC). Cancer Biol Ther. 2018;19(4):296–305.
- Heinzel S, Marchingo JM, Horton MB, Hodgkin PD. The regulation of lymphocyte activation and proliferation. Curr Opin Immunol. 2018;51:32–38. doi:https://doi.org/10.1016/j.coi.2018.01.002
- Toyokawa G, Shoji F, Yamazaki K, Shimokawa M, Takeo S. Significance of the red blood cell distribution width in resected pathologic stage I nonsmall cell lung cancer. Semin Thorac Cardiovasc Surg. 2019;S1043-0679:30134.
- Toyokawa G, Kozuma Y, Matsubara T, Haratake N, Takamori S, Akamine T, Takada K, Katsura M, Shimokawa M, Shoji F, et al. Prognostic impact of controlling nutritional status score in resected lung squamous cell carcinoma. J Thorac Dis. 2017;9(9):2942–2951. doi:https://doi.org/10.21037/jtd.2017.07.108
- Shoji F, Miura N, Matsubara T, Akamine T, Kozuma Y, Haratake N, Takamori S, Katsura M, Takada K, Toyokawa G, et al. Prognostic significance of immune-nutritional parameters for surgically resected elderly lung cancer patients: a multicentre retrospective study. Interact Cardiovasc Thorac Surg. 2018;26(3):389–394. doi:https://doi.org/10.1093/icvts/ivx337
- Miura N, Shoji F, Kozuma Y, Toyokawa G, Yamazaki K, Takeo S. Preoperative immune-nutritional abnormality predicts poor outcome in elderly non-small-Cell lung cancer patients with comorbidities. Ann Thorac Cardiovasc Surg. 2020;26(5):240–247. doi:https://doi.org/10.5761/atcs.oa.19-00207
- Belanger MM, Gaudreau M, Roussel E, Couet J. Role of caveolin-1 in etoposide resistance development in A549 lung cancer cells. Cancer Biol Ther. 2004;3(10):954–959. doi:https://doi.org/10.4161/cbt.3.10.1112
- Krycer JR, Brown AJ. Cholesterol accumulation in prostate cancer: a classic observation from a modern perspective. Biochim Biophys Acta. 2013;1835(2):219–229. doi:https://doi.org/10.1016/j.bbcan.2013.01.002
- Todor IN, Lukyanova NY, Chekhun VF. The lipid content of cisplatin- and doxorubicin-resistant MCF-7 human breast cancer cells. Exp Oncol. 2012;34(2):97–100.
- Li HY, Appelbaum FR, Willman CL, Zager RA, Banker DE. Cholesterol-modulating agents kill acute myeloid leukemia cells and sensitize them to therapeutics by blocking adaptive cholesterol responses. Blood. 2003;101(9):3628–3634. doi:https://doi.org/10.1182/blood-2002-07-2283
- Jeon JH, Kim SK, Kim HJ, Chang J, Ahn CM, Chang YS. Lipid raft modulation inhibits NSCLC cell migration through delocalization of the focal adhesion complex. Lung Cancer. 2010;69(2):165–171. doi:https://doi.org/10.1016/j.lungcan.2009.10.014
- Liu J, Xian G, Li M, Zhang Y, Yang M, Yu Y, Lv H, Xuan S, Lin Y, Gao L, et al. Cholesterol oxidase from bordetella species promotes irreversible cell apoptosis in lung adenocarcinoma by cholesterol oxidation. Cell Death Dis. 2014;5:e1372. doi:https://doi.org/10.1038/cddis.2014.324
- Chi P-D, Liu W, Chen H, Zhang J-P, Lin Y, Zheng X, Liu W, Dai S. High-density lipoprotein cholesterol is a favorable prognostic factor and negatively correlated with C-reactive protein level in non-small cell lung carcinoma. PLoS One. 2014;9(3):e91080.