Abstract
Background
Pancreatic cancer is often associated with cachexia. It had been reported that eicosapentaenoic acid (EPA) improve cachexia. This study aimed to evaluate the efficacy and safety of gemcitabine with an EPA-enriched oral supplement in patients with advanced pancreatic cancer.
Methods
This open-label phase II study consisted of patients (pts) who were randomly categorized into the EPA group (1,000 mg/m2 gemcitabine was administered on day 1, 8, and 15, every 4 weeks while an EPA-enriched oral supplement (prosure®, EPA 1.056 mg per pack) was taken daily at the maximum of two packs or the gemcitabine monotherapy group with an allocation ratio of 2:1. The primary endpoint was the evaluation of the 1-year survival estimating 10% addition.
Results
Randomized 68 pts were examined (EPA: 45, gemcitabine: 23). The 1-year survival probability of the EPA group was 35% while the gemcitabine group was 19%. The median survival times were 8.2 and 9.7 mo, respectively. The hazard ratio for EPA group was 0.79 [95% CI 0.46–1.37]; (P = 0.40). The toxicities were mild and insignificant in both groups. More beneficial effects of EPA in survival were observed in men, pancreatic body-tail and low C-reactive protein patients.
Conclusion
An EPA-enriched oral supplement may be effective in advanced pancreatic cancer.
Introduction
Pancreatic cancer is eighth leading cause of cancer death with approximately 35,000 patients (Citation1). Gemcitabine has been the standard chemotherapy treatment in advanced pancreatic cancer (PC) since 1997 (Citation2). Recently, FOLFIRINOX and gemcitabine + nab-paclitaxel has become the standard chemotherapy treatment (Citation3, Citation4). However, the prognosis of PC is still dismal. PC often has a cachexia (Citation5). This is a metabolic alteration that depends on pro-inflammatory mediators, such as IL-6, TNF, and TWEAK, and tumor-derived catabolic factors, such as activins and myostatin, and serotonin, which causes appetite loss and decrease of body weight (Citation6–8). Such condition is one of the reasons of chemo-resistance in PC. Although the prevention of cachexia is important in performing chemotherapy, conventional nutritional support including oral supplementation was not enough to improve cachexia in the past (Citation9, Citation10).
Eicosapentaenoic acid (EPA), an n-3 fatty acid (omega-3), has shown an anticachectic effect in animal model (Citation11). These effects are related to the inhibition of PIF and downregulation of proinflammatory cytokine production. Moreover, additional protein and energy are required in body synthesis. Oral nutritional supplement including high protein and energy with EPA showed weight gain and improved quality of life (Citation6). On the other hand, the effect of chemotherapy and EPA is not widely known although it had been reported to improve response rates (RR) in non-small cell lung cancer (Citation12). This time we aimed to examine the effect of an EPA-enriched oral supplement with gemcitabine compared with gemcitabine monotherapy in advanced PC.
Methods
Study Design
This multicenter, open-labeled, randomized phase II study was conducted at two centers in compliance with the Declaration of Helsinki and Good Clinical Practice guidelines. The study protocol was approved by the ethics committee or institutional review board of each participating center. All patients provided written informed consent before study entry. This study is registered with the UMIN000003658. The department of Biostatistics and Epidemiology in Yokohama City University monitored patient safety, adverse events, and the progress of the trial.
Patients
The eligibility criteria for inclusion were as follows: clinical diagnosis of PC; unresectable or recurrent disease; over 20 years of age; histologically or cytologically confirmed diagnosis of adenocarcinoma; ability to maintain sufficient food intake with greater than two-thirds of the normal intake as judged by interview; an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0, 1/2, and adequate organ functions such as leucocyte count ≥ 3,000 ⁄mm3; neutrophil count ≥ 1,500⁄mm3; hemoglobin level ≥9.0 g⁄dL; platelet count ≥ 100,000⁄mm3; aspartate aminotransferase and alanine aminotransferase concentrations ≤100 IU⁄L (≤150 IU⁄L in patients with biliary drainage); total bilirubin level ≤ 2mg⁄dL (≤3mg⁄dL in patients with biliary drainage); creatinine concentration ≤ 1.5 mg⁄dL; Patients had no serious comorbidities. Patients with interstitial pneumonia, lung fibrosis, or watery diarrhea were excluded.
Randomization
After confirming eligibility, patients were randomly assigned in a 2:1 ratio to receive gemcitabine monotherapy (gemcitabine group) or gemcitabine with an EPA-enriched oral supplement (EPA group). Random assignment was carried out centrally at the data center, using the minimization method with institution, the UICC stage (III vs. IV, recurrence), and ECOG PS (0, 1, vs. 2) as allocation factors. The randomization sequence was generated by the department of Biostatistics and Epidemiology in Yokohama City University, using a validated computer system blinded from investigators. Both the investigators and patients were aware of the assigned treatment.
Treatment
Gemcitabine 1,000 mg/m2 was infused on day 1, 8, and 15 every 4 weeks. The EPA-enriched oral supplement (prosure®, EPA 1.056 g per pack, Abbott, IL, USA) was administered maximum two packs every day. In patients who had grade four leucopenia or neutropenia, grade four thrombocytopenia or grade three thrombocytopenia requiring transfusion and grade three nonhematologic toxicity, the dose of gemcitabine was reduced in steps of 200 mg/m2 until 600 mg/m2 from the next treatment cycle. If patients experienced grade three diarrhea or the grade three adverse events related to the EPA-enriched oral supplement, it was reduced to one pack. And patients were allowed to reduce the volume of supplements dependent on their appetite. Treatment was continued until disease progression, unacceptable toxic effects, or the withdrawal of consent.
Assessments
Physical examinations, complete blood counts, and biochemical tests were performed at least on day 1, 8, and 15 every 4 weeks. Thereafter all adverse events were evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0). CT or MRI was performed every 6 weeks until disease progression. Response and disease progression were assessed by the attending physicians and according to RECIST version 1.1. Data on patients in whom treatment had been discontinued before disease progression were censored on the last day of tumor assessment (loss of events).
Study Objectives and Endpoints
The primary endpoint was 1-year survival defined as the time from randomization. Secondary endpoints were progression-free survival (PFS), defined as the time from randomization to disease progression or death from any cause, RR, body weight change, performance status change, and adverse events.
Statistical Analysis
This study adopted selection design (Citation13), in that the regimen with a higher 1-year survival would be selected. The sample size was determined as follows using Simon’s selection design. We assumed that the 1-year survival for one regimen would be 25% and that it would be higher than 40% for the other regimen. Under these conditions, the sample size ensuring a probability of at least 78% for correct selection of the more effective regimen with one-sided α = 0.25 was 63 patients with an allocation ratio of 2:1. Because the anticipated drop-out rate of 5% required the addition of more patients, the total number of patients was set at 66. Overall survival and PFS were estimated by using the Kaplan–Meier method, and curves were compared using an unstratified log–rank test. Hazard ratios of treatment effects were estimated by using the unstratified Cox regression model. We carried out all the analyses based on full analysis set using SAS version 9.2 (SAS Institute, Cary, NC, USA). The full analysis set was defined as the population of patients who were given the study treatment at least once. Unless otherwise specified, two-sided P-values for superiority were used.
Results
Patients
Between May 2010 and October 2011, a total of 68 patients were enrolled and eligible. Two patients in the EPA group were discontinued before treatment due to cerebral infarction and worsening of performance status. Sixty-six patients were included in the analysis set as shown in .
Table 1. Patient characteristics.
Study Treatment
At the primary analysis, the protocol treatment continued in one patient (2.3%) in the EPA group. The main reason for treatment discontinuation was disease progression [39 patients (90.7%) in the EPA group, and 21 patients (91.3%) in the gemcitabine group]. Two patients [4.4%] in the EPA group, and two patients [8.7%] in the gemcitabine group discontinued the study treatment because of treatment-related adverse events, and one patient (2.2%) in the EPA group discontinued treatment because of non-related adverse events. Two patients (4.4%) in the EPA group could not be recognized with the reason and timing of treatment discontinuation. The mean volume of oral supplement was 1.13 packs/day.
Progression-Free and Overall Survival
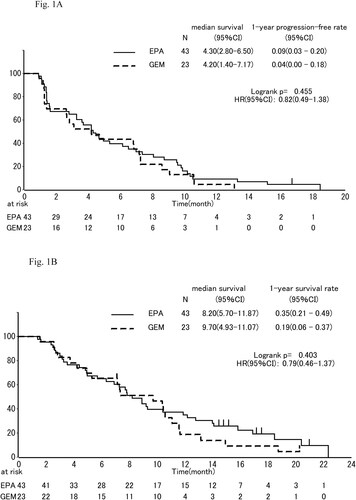
The median PFS was 4.30 mo, (95% confidence interval (CI), 2.8 to 6.5 mo,) in the EPA group and 4.20 mo, (95% CI, 1.4 to 7.2 mo,) in the gemcitabine group (). EPA group did not decrease the risk of disease progression (HR, 0.82; 95% CI, 0.49 to 1.38; P = 0.455).
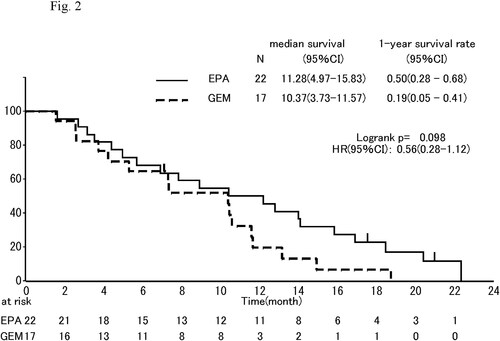
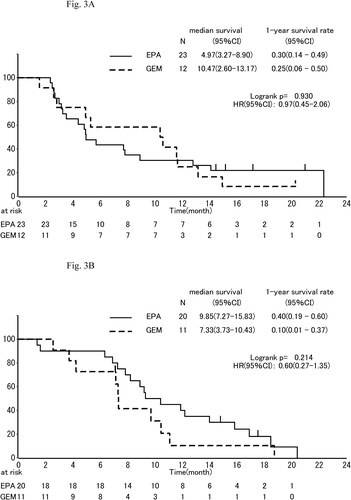
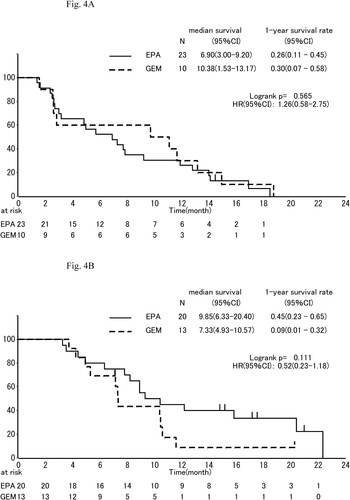
The median OS was 8.2 mo, (95% CI, 5.7 to 11.9 mo,) in the EPA group and 9.7 mo, (95% CI, 4.9 to 11.1 mo,) in the gemcitabine group (HR, 0.79; 95% CI, 0.460–1.3; P = 0.404; ). One-year survival which was primary endpoint was 35% (95% CI, 21% to 49%) in the EPA group and 19% (95% CI, 6% to 37%) in the gemcitabine group. In subgroup analyses of OS, the effect of EPA was better in males than females (), in pancreas body-tail than head (), and in patients of low C-reactive protein (CRP) than those of high CRP (divided into 0.61 mg/dl; ).
Response to Therapy
The RR was 4.7% (95% CI, 0.6% to 15.8%) in the EPA group and 8.7% (95% CI, 1.1% to 28.0%) in the gemcitabine group (P = 0.606; ). The DCR was 67.4% (95% CI, 51.5% to 80.9%) in the EPA group and 65.2% (95% CI, 42.7% to 83.6%) in the gemcitabine group (P = 1.000; ).
Table 2. Response rate.
Post-Protocol Treatment (Second-Line Chemotherapy)
Post-protocol treatment was given to 35 patients (81.4%) in the EPA group and 17 patients (73.9%) in the gemcitabine group. Most patients received S-1 monotherapy in both groups. Median CRP value was 0.73 mg/dl in the EPA group and 1.00 mg/dl in the gemcitabine group in the timing of treatment discontinuation. Median albumin value was 3.7 mg/dl in the EPA group and 3.5 mg/dl in the gemcitabine group in the timing of treatment discontinuation. Twelve patients (27.9%) in the EPA group and two patients (8.7%) in the gemcitabine group (P = 0.113) had low CRP value (≤1.0 mg/dl) and high albumin value (≥4.0 mg/dl) in the timing of treatment discontinuation.
Safety
It shows the adverse events recorded within 6 mo, after randomization. The major grade 3 or four adverse events had incidences of ≥ 5% were neutropenia (30.2% in the EPA group vs. 21.7% in the gemcitabine group), leukopenia (20.9% vs. 13.0%), anemia (14.0% vs. 8.7%), thrombocytopenia (9.3% vs. 8.7%), hyponatremia (9.3% vs. 4.3%), decreased appetite (7.0% vs. 0.0%), nausea (11.6% vs. 0.0%), and infection (mainly cholangitis; 7.0% vs. 13.0%). CRP elevation (11.6% vs. 39.1%) of all grades were significantly higher in the gemcitabine group (). Serious adverse events related to the protocol treatment did not occur in this study.
Table 3. Adverse events.
Discussion
PC is resistant to chemotherapy and poor prognosis. A standard chemotherapy treatment was gemcitabine monotherapy when this study started and the prognosis of advanced PC was about 7–8 mo. One of the reasons of chemotherapy resistance is a cachexia. PC patients often have symptoms such as appetite loss, body weight loss, and the decrease of performance status due to cachexia. As a result, the continuation of chemotherapy is often difficult (Citation14). The control of cachexia seems to be a key in the chemotherapy of advanced PC. EPA had been reported to inhibit cachexia and in addition nutritional support is also considered to be important in cachexia (Citation6). The oral supplement in this study is a promising treatment including EPA, high protein, and energy.
PFS is the same throughout and the early period of OS curves are not different; however, in the latter period of OS, the EPA group was better than the gemcitabine group and 1-year survival of the EPA group was 35% and that of the gemcitabine group was 19%. As a reason for that, there seemed to be CRP and albumin changes. CRP is a marker of cachexia (Citation15) and albumin is a marker of sarcopenia which is caused by cachexia (Citation16). In this study, there was low frequency of CRP elevation with the EPA group. Moreover, at the time of treatment discontinuation, the numbers of high albumin and low CRP patients were higher in the EPA group. This seemed to be linked to better second-line chemotherapy and better prognosis. We suppose that it caused the change in the latter half of OS period. The anti-inflammation effect of EPA seems to affect the CRP elevation. Recently, the importance of MENAC (Multimodal-Exercise, Nutrition and Anti-inflammatory medication for Cachexia) was reported (Citation17). Because EPA is one of the targeted cachexia treatments in the MENAC trial, our data support the importance of the MENAC trial.
In the subgroup analyses, male, patients with pancreatic body-tail cancer, and patients with low CRP had good prognosis. These parameters usually are not prognostic factors in PC. The averages of a total EPA-enriched oral supplement were 122.4 packs in male and 61.5 packs in female (P = 0.01). And these were 64.7 packs in pancreatic head cancer and 122.2 packs in pancreatic body-tail cancer (P = 0.01). The reason for gender differences is based on physiques and the food intake including oral supplements. And then the reason for low intake of oral supplements in the pancreatic head is duodenal stenosis or biliary tract obstruction since the food intake would decrease due to the difficulty of passage or the preparation of the endoscopic treatment. The effect of an EPA-enriched oral supplement may depend on the volume of EPA and food intake.
Although the patients with high CRP in this study expected better results, the patients with low CRP showed better results. It seemed that the effect of an EPA-enriched oral supplement was the prevention of the worsening in the nutritional status. The effect of an EPA-enriched oral supplement is sufficient for low level of cachexia, but insufficient for high level of cachexia. Fearon classified cachexia into three classes such as pre-cachexia, cachexia syndrome, and severe cachexia (Citation18). An EPA-enriched oral supplement may play a role in pre-cachexia.
Limitations of this study were as follows: First, due to this being a small randomized study, attention should first be paid to the interpretations of the small subgroup analyses, and future studies need to take them into account by stratification. Second, this was an open-label trial, and we cannot completely rule out the possibility of intake of an EPA-enriched oral supplement or EPA-containing food in the control arm that might have weakened the differences. However, the EPA-enriched oral supplements were obtained with research funding and distributed to the subjects and the effect of the intake of Japanese food containing EPA seemed to be small. Because the EPA content is reported to be 2–1,400 mg/100 g depending on the part or kind of fish and the EPA volume for most fish is under 500 mg/100 g (Citation19), it is quite difficult to achieve an intake of 1–2 g EPA every day. Third, in this study, it was difficult to achieve an intake of two packs of the total EPA-enriched oral supplement due to its taste (Citation20) and a feeling of fullness when combined with a normal meal. We may have to consider more palatable foods for this. Fourth, a recent pancreatic enzyme replacement therapy (PERT) has been reported to be an important factor in the pancreatic exocrine insufficiency in advanced PC (Citation21). We could not evaluate to what degree the patients received PERT. Nevertheless, the exclusion criteria excluded patients with severe diarrhea. Furthermore, only 17% of the patients in the control arm had Grade 1–2 diarrhea as an adverse event. As a result, the effect of PERT was small in this trial.
Currently, FOLFIRINOX and gemcitabine plus nab-paclitaxel are internationally recognized as standard regimens for the chemotherapy of metastatic PC. These chemotherapy treatments would result in increased numbers of adverse reactions compared with gemcitabine monotherapy. As regards the patients in this study, it might be difficult for some of them to receive the combined chemotherapy due to age and low performance status. Recently, adverse reactions to combined chemotherapy were reported to be increased in low albumin patients (Citation22). Thus, the addition of an EPA-enriched oral supplement would still be important in recent standard chemotherapy.
In conclusion, an EPA-enriched oral nutritional supplement with gemcitabine improved 1-year survival in randomized phase II study. The anti-inflammation effect of EPA seemed to prevent cachexia. Further investigation would be needed in combination with FOLFIRINOX and gemcitabine plus nab-paclitaxel.
Authors’ contributions
Makoto Ueno, Satoshi Kobayashi, Masataka Taguri and Takeharu Yamanaka conceived and designed the study. Makoto Ueno, Kazuya Sugimori, Shinichi Ohkawa, Satoshi Kobayashi, Haruo Miwa, Takashi Kaneko and Manabu Morimoto performed the experiments. All authors were involved in the writing of the manuscript. Makoto Ueno, Masataka Taguri and Takeharu Yamanaka analyzed the data. All authors read and approved the final version of the manuscript.
Disclosure statement
Makoto Ueno has received honoraria from Abbott Japan LLC and Eli Lilly Japan K.K. All remaining authors have declared no conflicts of interest.
Additional information
Funding
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi:https://doi.org/10.3322/caac.20107
- Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403–2413. doi:https://doi.org/10.1200/JCO.1997.15.6.2403
- Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul J-L, Gourgou-Bourgade S, de la Fouchardière C, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. doi:https://doi.org/10.1056/NEJMoa1011923
- Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703. doi:https://doi.org/10.1056/NEJMoa1304369
- Moses AW, Slater C, Preston T, Barber MD, Fearon KC. Reduced total energy expenditure and physical activity in cachectic patients with pancreatic cancer can be modulated by an energy and protein dense oral supplement enriched with n-3 fatty acids. Br J Cancer. 2004;90(5):996–1002. doi:https://doi.org/10.1038/sj.bjc.6601620
- Fearon KC, Von Meyenfeldt MF, Moses AG, Van Geenen R, Roy A, Gouma DJ, Giacosa A, Van Gossum A, Bauer J, Barber MD, et al. Effect of a protein and energy dense N-3 fatty acid enriched oral supplement on loss of weight and lean tissue in cancer cachexia: a randomised double blind trial. Gut. 2003;52(10):1479–1486. doi:https://doi.org/10.1136/gut.52.10.1479
- Fearon KC, Barber MD, Moses AG. The cancer cachexia syndrome. Surg Oncol Clin N Am. 2001;10(1):109–126.
- Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer-associated cachexia. Nat Rev Dis Primers. 2018;4:17105. doi:https://doi.org/10.1038/nrdp.2017.105
- Evans WK, Nixon DW, Daly JM, Ellenberg SS, Gardner L, Wolfe E, Shepherd FA, Feld R, Gralla R, Fine S, et al. A randomized study of oral nutritional support versus ad lib nutritional intake during chemotherapy for advanced colorectal and non-small-cell lung cancer. J Clin Oncol. 1987;5(1):113–124. doi:https://doi.org/10.1200/JCO.1987.5.1.113
- Ovesen L, Allingstrup L, Hannibal J, Mortensen EL, Hansen OP. Effect of dietary counseling on food intake, body weight, response rate, survival, and quality of life in cancer patients undergoing chemotherapy: a prospective, randomized study. J Clin Oncol. 1993;11(10):2043–2049. doi:https://doi.org/10.1200/JCO.1993.11.10.2043
- Beck SA, Smith KL, Tisdale MJ. Anticachectic and antitumor effect of eicosapentaenoic acid and its effect on protein turnover. Cancer Res. 1991;51(22):6089–6093.
- Murphy RA, Mourtzakis M, Chu QSC, Baracos VE, Reiman T, Mazurak VC. Supplementation with fish oil increases first-line chemotherapy efficacy in patients with advanced nonsmall cell lung cancer. Cancer. 2011;117(16):3774–3780. doi:https://doi.org/10.1002/cncr.25933
- Simon R, Wittes RE, Ellenberg SS. Randomized phase II clinical trials. Cancer Treat Rep. 1985;69(12):1375–1381.
- Caillet P, Liuu E, Raynaud Simon A, Bonnefoy M, Guerin O, Berrut G, Lesourd B, Jeandel C, Ferry M, Rolland Y, et al. Association between cachexia, chemotherapy and outcomes in older cancer patients: a systematic review. Clin Nutr. 2017;36(6):1473–1482. doi:https://doi.org/10.1016/j.clnu.2016.12.003
- Punzi T, Fabris A, Morucci G, Biagioni P, Gulisano M, Ruggiero M, Pacini S. C-reactive protein levels and vitamin d receptor polymorphisms as markers in predicting cachectic syndrome in cancer patients. Mol Diagn Ther. 2012;16(2):115–124. doi: https://doi.org/10.2165/11632380-000000000-00000.
- Baumgartner RN, Koehler KM, Romero L, Garry PJ. Serum albumin is associated with skeletal muscle in elderly men and women. Am J Clin Nutr. 1996;64(4):552–558. doi:https://doi.org/10.1093/ajcn/64.4.552
- Solheim TS, Laird BJA, Balstad TR, Bye A, Stene G, Baracos V, Strasser F, Griffiths G, Maddocks M, Fallon M, et al. Cancer cachexia: rationale for the MENAC (Multimodal-Exercise, Nutrition and Anti-inflammatory medication for Cachexia) trial. BMJ Support Palliat Care. 2018;8(3):258–265. doi:https://doi.org/10.1136/bmjspcare-2017-001440
- Fearon KC. Cancer cachexia: developing multimodal therapy for a multidimensional problem. Eur J Cancer. 2008;44(8):1124–1132. doi:https://doi.org/10.1016/j.ejca.2008.02.033
- Ministry of Education, Culture, Sports, Science and Technology (MEXT). Office for Resources, Policy Division Science and Technology Policy Bureau [Internet]. Standard Tables of Food Composition in Japan Seventh Revised Edition. MEXT; 2015. https://www.mext.go.jp/en/policy/science_technology/policy/title01/detail01/1374030.htm [last accessed 2015 Jan 27].
- Akita H, Takahashi H, Asukai K, Tomokuni A, Wada H, Marukawa S, Yamasaki T, Yanagimoto Y, Takahashi Y, Sugimura K, et al. The utility of nutritional supportive care with an eicosapentaenoic acid (EPA)-enriched nutrition agent during pre-operative chemoradiotherapy for pancreatic cancer: prospective randomized control study. Clin Nutr Espen. 2019;33:148–153. doi:https://doi.org/10.1016/j.clnesp.2019.06.003
- Pezzilli R, Caccialanza R, Capurso G, Brunetti O, Milella M, Falconi M. Pancreatic enzyme replacement therapy in pancreatic cancer. Cancers (Basel). 2020;12(2):275. doi:https://doi.org/10.3390/cancers12020275
- Ueno M, Okusaka T, Omuro Y, Isayama H, Fukutomi A, Ikeda M, Mizuno N, Fukuzawa K, Furukawa M, Iguchi H, et al. A randomized phase II study of S-1 plus oral leucovorin versus S-1 monotherapy in patients with gemcitabine-refractory advanced pancreatic cancerdagger. Ann Oncol. 2016;27(3):502–508. doi:https://doi.org/10.1093/annonc/mdv603