Abstract
Background
The preoperative prognostic nutritional index (PNI) is associated with postoperative complications and long-term survival of various cancers. However, its role in esophageal squamous cell carcinoma (ESCC) is inconclusive. The aim of this study was to identify the prognostic value of PNI in predicting survival in ESCC patients undergoing radical radiotherapy.
Methods
We retrospectively reviewed 354 ESCC patients undergoing radical radiotherapy. The time-dependent receiver operating characteristics was used to determine the optimal cutoff value. The association between PNI and survival was determined by Kaplan-Meier method and Cox regression model. Propensity score matching was applied to balance the baseline characteristics.
Results
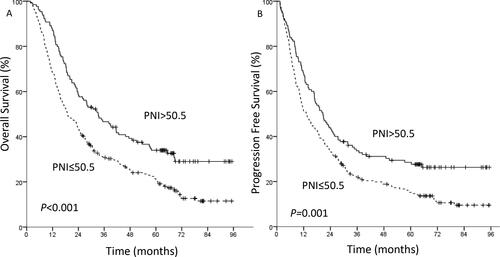
PNI was positively correlated with hemoglobin (P < 0.001) and prealbumin (P < 0.001). The optimal cutoff value of PNI was set at 50.5. The 5-year overall survival (OS) in low PNI group and high PNI group were 20.8% and 34.0%, respectively (P < 0.001). The 5-year progression free survival in patients with low PNI and high PNI were 15.2% and 28.5%, respectively (P = 0.001). Multivariate analysis showed that PNI was a significant predictor for OS (P = 0.038). In the PSM analysis, PNI still remained an independent predictor for OS (P = 0.015).
Conclusions
The PNI is a significant and independent predictor for OS of ESCC patients undergoing radical radiotherapy.
Esophageal cancer is the eighth most common cancer worldwide in incidence and the sixth most common cause in mortality (Citation1). Although multidisciplinary treatment is commonly applied, including surgery combined with radiotherapy and chemotherapy, the 5-year overall survival remains poor. Therefore, assessing the prognostic predictors in esophageal cancer remains very important.
During the past decade, there has been an increasing interest in the associations between nutrition and long-term outcomes of several malignancies for many clinical researchers (Citation2–4). The PNI, based on albumin and peripheral lymphocyte count, was originally developed by Buzby and optimized by Onodera et al. to predict the risk of postoperative morbidity and mortality after gastrointestinal surgery (Citation5, Citation6). In the recent researches, PNI has been demonstrated to be associated with postoperative complications, hospital mortality rate, length of hospital stay and prognosis prior to surgery in gastric, colorectal, lung and other cancers (Citation7–11). Some previous studies have showed that preoperative PNI is a prognostic marker for esophageal cancer undergoing esophagectomy. However, only a few retrospective studies have focused on PNI in esophageal cancer undergoing radical radiotherapy. Its role in ESCC receiving radical radiotherapy still remains inconclusive. Therefore, in this study, we evaluated the prognostic value of PNI in patients with ESCC who underwent radical radiotherapy. To reduce biases due to the different distributions of co-variables among the comparable groups and to increase statistical power, propensity score matching (PSM) was applied.
Materials and Methods
Patients
This retrospective analysis was conducted in 354 patients with ESCC who underwent radical radiotherapy from January 2011 to December 2017 at Fourth Hospital of Hebei Medical University in Shijiazhuang, China. All of the patients enrolled in the analysis met the following inclusion criteria: ESCC confirmed by histopathology; Karnofsky score ≥70 points; neither chemotherapy nor other anti-tumor therapy before radiotherapy; no history of malignant disease. All subjects gave their written informed consent to the study protocol, which was approved by the ethics committee of Fourth Hospital of Hebei Medical University, Shijiazhuang, China. According to TNM stage system issued by the American Joint Committee on Cancer (AJCC; 7th edition), 36 patients with Stage I, 134 patients with Stage II and 184 patients with Stage III were included in this study. The characteristics of the patients are summarized in .
Table 1. Relationships between PNI and clinicopathological features in ESCC patients before and after propensity score matching.
Treatment
In this study, all of patients received intensity-modulated radiotherapy (IMRT) or three-dimensional conformal radiotherapy (3 D-CRT). The gross tumor volume (GTV), clinical tumor volume (CTV), and planned tumor volume (PTV) were outlined in the RT treatment planning system, according to computed tomography simulation scan, barium esophagram, electronic gastroscopy and/or PET-CT. The prescribed dose was set at 54–68 Gy (in 27–34 fractions over a range of 5–7 weeks), a bi-lung V20 ≤ 28%–30%, a bi-lung V5 of <50–60%, a heart V30 of ≤30%, and a maximum dose to the spinal cord of <45 Gy. The patients receiving adjuvant chemotherapy mainly received cisplatin combined with paclitaxel or 5-fluorouracil for 1 to 6 cycles.
Calculation of PNI
The PNI was calculated by the serum albumin (g/L) + 5 × total lymphocyte count. The optimal cutoff value for PNI was calculated using the time-dependent receiver operating characteristics (ROC).
Follow-Up
At our institution, follow-up examinations were performed every 3-month intervals in the first 2 years, then 6-month intervals. The routine examination included physical examination, blood tests, biological investigations, tumor markers, thoracic CT scanning, and barium esophagram. The lowest peripheral blood counts (leukocytes, neutrophils, hemoglobin, and platelets) noted during radiotherapy were used to assess hematological toxicities. Overall survival (OS) was defined as the interval from the first day of radiotherapy to the date of death or last contact. Progression free survival (PFS) was defined as the interval from the first day of radiotherapy to the date of tumor progression or death or the last follow up.
Statistical Analysis
All recorded data were analyzed using SPSS (version 23.0, IBM Corporation, Armonk, NY, USA). Pearson chi-square test was used to assess the correlation between different categorical variables, and Spearman correlation analysis was used to evaluate the correlation between PNI and other clinicopathological factors. The OS rate was calculated using the Kaplan-Meier method, and a log-rank test was used to assess survival differences between groups. Cox proportional hazards regression analysis was performed to identify independent variables. Propensity score matching (PSM) was carried out to reduce biases due to the different distributions of co-variables among the comparable groups. P values <0.05 indicated statistical significance.
Results
Relationships between PNI and Clinicopathological Characteristics before and after Propensity Score Matching
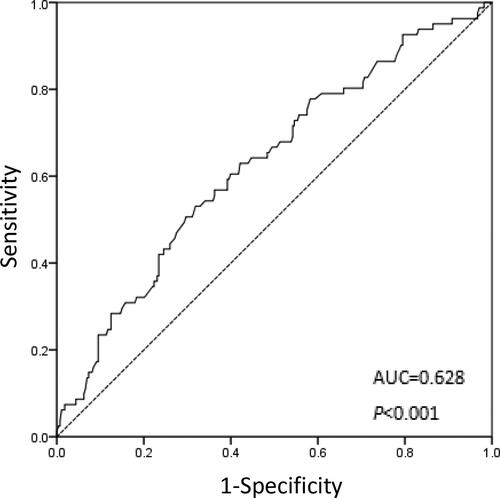
The mean value of the PNI was 48.37 ± 5.02, range from 32.75 to 63.45. We decided to set the optimal cutoff value for PNI levels at 50.5 in this study, based on the OS (sensitivity: 53.1%; specificity: 68.1%; AUC of the ROC curve: 0.628) (). Based on their PNI values, patients were categorized as having a low PNI (50.5 or less) or as having a high PNI (more than 50.5).
Figure 1. ROC curve for the PNI for predicting the overall survival of ESCC patients undergoing radiotherapy.
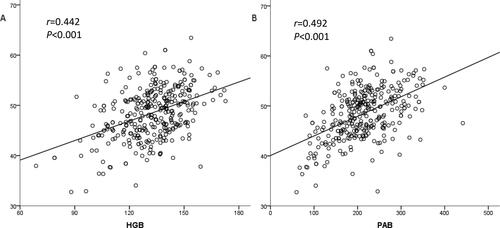
The characteristics of 354 ESCC patients undergoing radical radiotherapy are presented in . Among the 354 patients, 206 (58.2%) were male and 148 (41.8%) were female. The median age was 67 years (range, 41–90 years). The results of our analysis showed that the PNI value was significantly associated with age, T stage, TNM stage, body mass index (BMI), hemoglobin (HGB) and prealbumin (PAB). In addition, PNI was positively correlated with HGB (r = 0.442, P < 0.001) and PAB (r = 0.492, P < 0.001, ).
Survival Analysis Stratified by the PNI
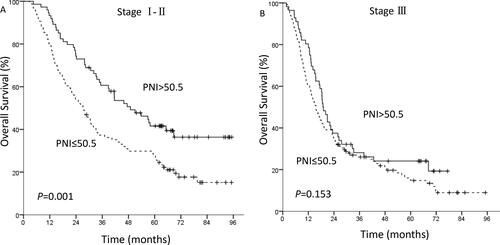
Overall, four patients were lost to follow-up. The 1-, 3- and 5-year OS rates were 75.1%, 36.6% and 25.7%, respectively. The 1-, 3- and 5-year PFS rates were 57.3%, 26.2% and 19.8%, respectively. For the patients with a PNI ≤50.5 (n = 224), the 1-, 3- and 5-year OS rates were 68.3%, 30.8% and 20.8%, respectively. The corresponding rates in patients with a PNI >50.5 (n = 130) were 86.9%, 46.7% and 34.0%, respectively. In the low PNI group and the high PNI group, the 1-, 3-, 5-year PFS rates were 51.3%, 21.9%, 15.2% and 67.7%, 33.6%, 28.5%, respectively. Kaplan-Meier analysis showed that overall the high PNI group had superior OS (P < 0.001, ) and PFS (P = 0.001, ) compared with the low PNI group. According to the results of Cox multivariate analysis, the survival analysis was stratified by TNM stage and chemotherapy. For TNM stage I-II patients, the high PNI group had superior OS (P = 0.001, ) than the low PNI group. However, there was no significant relationship between PNI and OS in stage III patients (P = 0.153, ). For the patients who received adjuvant chemotherapy (P = 0.021) and no chemotherapy (P = 0.006), Kaplan-Meier analysis and the log-rank test demonstrated that patients with a low PNI had a significantly worse prognosis in terms of OS than those with a high PNI.
Prognosis Factors of Survival in All Patients
In the univariate analysis, age, tumor location, TNM stage, irradiation method, radiotherapy dose, adjuvant chemotherapy and the PNI level were significantly associated with OS. The TNM stage (HR 1.649; 95%CI 1.282–2.121; P < 0.001), adjuvant chemotherapy (HR 0.651; 95%CI 0.494–0.857; P = 0.002) and PNI (HR 0.754; 95%CI 0.577–0.984; P = 0.038) were confirmed as independent prognostic factors for OS when multivariate analysis was applied ().
Table 2. Univariate and multivariate analyses of prognostic factors for OS in 354 ESCC patients.
Univariate analysis identified tumor location, TNM stage, irradiation method, radiotherapy dose, adjuvant chemotherapy and the PNI level as significant risk factors for PFS. In multivariate analysis, the TNM stage (HR 1.582; 95%CI 1.238–2.021; P < 0.001), irradiation method (HR 0.749; 95%CI 0.566–0.992; P = 0.044) and adjuvant chemotherapy (HR 0.696; 95%CI 0.545–0.889; P = 0.004) were identified as independent prognostic factors for PFS ().
Table 3. Univariate and multivariate analyses of prognostic factors for PFS in 354 ESCC patients.
Prognosis Factors of Survival in Propensity Score Matched Patients
According to the multivariate analysis results of all patients, considering the TNM stage, adjuvant chemotherapy and irradiation method were independent predictors, we applied a 1:1 ratio propensity score matching. After PSM, there were 224 patients in all, and the clinicopathological characteristics and treatment methods were balanced and evenly distributed between two groups. The analysis results showed that the PNI level was associated with HGB (P = 0.006) and PAB (P = 0.026, ). In the univariate analysis, tumor location, TNM stage, irradiation method, radiotherapy dose, adjuvant chemotherapy and the PNI level were significantly associated with OS and PFS. In multivariate analysis, the TNM stage (HR 1.570; 95%CI 1.139–2.165; P = 0.006) and PNI (HR 0.680; 95%CI 0.498–0.928; P = 0.015) were identified as independent prognostic factors for OS (), the TNM stage (HR 1.584; 95%CI 1.163–2.156; P = 0.003) was confirmed an independent predictor for PFS ().
Table 4. Univariate and multivariate analyses of prognostic factors for OS in propensity score matched 224 ESCC patients.
Table 5. Univariate and multivariate analyses of prognostic factors for PFS in propensity score matched 224 ESCC patients.
Relationships between PNI and Acute Adverse Reactions
The incidence rate of ≥ grade two acute hematological toxicity in the low PNI group was higher than that in the high PNI group. No significant differences in incidence rates of other acute adverse reactions were observed between low and high PNI groups ().
Table 6. Comparison between PNI and acute adverse reactions.
Discussion
Recently, many studies have described the role of pretreatment PNI in the prognosis of various malignancies. PNI has been demonstrated to be associated with postoperative prognosis in hepatocellular, gastric, lung and other cancers (Citation3, Citation7–10). Moreover, in esophageal carcinoma, the preoperative PNI was associated with long-term survival and postoperative complications, such as anastomotic fistula and pulmonary complications (Citation12–17). Matsumoto Y et al. (Citation18) analyzed 191 patients with esophageal cancer treated with at least one course of cisplatin and 5-fluorouracil and reported that patients with low PNI suffered more frequent severe adverse events than did those with high PNI, and the latter patients survived longer. However, few retrospective studies, are available regarding PNI in esophageal cancer undergoing radical radiotherapy. Its role in ESCC undergoing radical radiotherapy still remains inconclusive. Dai Y et al. (Citation19) retrospectively analyzed 106 CESCC patients who received radiotherapy with or without chemotherapy and reported that the OS was higher in the high PNI group than in the low PNI group. A retrospective study (Citation20), enrolled 97 patients with esophageal cancer treated with chemoradiotherapy, revealed that the baseline PNI or PNI at the end of CRT ≥45 was associated with improved 2-year OS than that <45, and the PNI at the third week of CRT was the independent risk factor for prognosis. In the present study, 354 ESCC patients received radical radiotherapy were analyzed retrospectively. The results showed that the OS and PFS were higher in the high PNI group than in the low PNI group. Cox regression analyses revealed that the TNM stage, adjuvant chemotherapy, and PNI level were independent risk factors for prognosis. To reduce biases due to the different distributions of co-variables among the comparable groups and to increase statistical power, propensity score matching (PSM) was applied. In 224 propensity matched ESCC patients, the PNI level was significantly associated with long-term survival. In the multivariate analysis, the TNM stage and PNI were identified as independent prognostic factors for OS.
Although there have been many studies that focused on PNI, the optimal cutoff value for predicting prognosis is still inconclusive. In Kanda M’s study, a PNI value of at least 50 was defined as normal, less than 50 was regarded as mild malnutrition, less than 45 as moderate to severe malnutrition, and less than 40 as serious malnutrition (Citation4). Therefore, the optimal cutoff value of the PNI was set at 45 or 50 in some studies (Citation21, Citation22). In other studies, the optimal cutoff value of PNI was determined by ROC curve (Citation19, Citation23). In this study, a receiver operating characteristic curve of the PNI was generated for the multiple logistic regression analysis of OS. The area under the curve estimation method was used to assess the ability of the PNI to predict OS. The optimal cutoff value of the PNI was set at 50.5, based on OS. Patients were stratified into a high or low PNI group based on the cutoff value. The Kaplan-Meier analysis and log-rank test demonstrated that patients with a low PNI value had a significantly poorer OS and PFS than those with a high PNI value.
The mechanism of the correlation between PNI and overall survival in patients with esophageal cancer remains unclear. Previous studies have suggested that albumin can help stabilize cell growth and DNA replication, buffer a variety of biochemical changes and maintain sex hormone homeostasis to protect against tumorigenesis. Albumin, reflective of the inflammation and immune status of cancer, therefore, plays an important role in the anti-tumor process (Citation24, Citation25). Lymphocytes are involved in the destruction and apoptosis of tumor cells (Citation26). Previous studies have shown that the level of inflammatory factors of patients with malnutrition is significantly higher than that of patients with normal nutrition. Low lymphocyte counts and the increased inflammatory factors, are associated with an immunosuppressed condition (Citation27). Meanwhile, elevated inflammatory factors may provide a favorable microenvironment for tumor recurrence and metastasis by up-regulating cytokines, producing inflammatory mediators, inhibiting apoptosis, promoting angiogenesis, and inducing DNA mutations (Citation28, Citation29). Furthermore, low nutritional and immunological condition may lead to delaying therapies. All of these factors may cause the poor prognosis. Therefore, the PNI, reflective of the nutritional and immune status of cancer, has certain significance in predicting the prognosis of malignant tumor patients.
As mentioned above, pretreatment PNI was associated with the prognosis of many cancers. Can nutrition support improve nutrition status and then improve survival? Qiu M et al. reported that for stage IV gastric cancer patients whose NRS ≥ 3, the nutrition support might be helpful to improve the prognosis (Citation30). In Ravasco P’s study, during radiotherapy, nutritional interventions positively influenced outcomes of patients with head and neck cancer (Citation31). Ruggeri E revealed that home artificial nutrition can be effective in avoiding death from malnutrition in 73% of cancer patients, and in maintaining or improving the KPS at one month in 90% of cancer patients (Citation32). However, there have been different conclusions in other studies. Van Bokhorst-De Van Der Schueren MA reported that nine days of preoperative tube feeding did not significantly improve nutritional status, reduce the surgery-induced immune suppression, or affect clinical outcome in severely malnourished head and neck cancer patients (Citation33). In Xu L’s study, short-term parenteral nutrition support did not significantly improve the short-term clinical outcomes of nutritionally at-risk gastric cancer patients (Citation34). From the above studies, short-term nutrition support may not always improve clinical outcome, long-term nutrition management perhaps has more prognostic significance. In all, the impact of nutrition support on survival of cancer patients is still undefined and will be one direction of our research in the future.
There are several potential limitations in this study. First, this is a retrospective, single-institute study, and there may be selection bias during patients collection, although the PSM has been applied to balance the baseline characteristics. Second, PNI data were collected only at a single time point prior to treatment, and fluctuations in PNI throughout the treatment and follow-up were not fully documented and analyzed. Therefore, larger prospective studies are required in the future to confirm these preliminary results.
In conclusion, the PNI is a significant and independent predictor for OS of ESCC patients undergoing radical radiotherapy. Based on simple and inexpensive standard laboratory measurements, PNI can be a potential marker for ESCC patients.
Authors’ Contributions
YZ and SCZ participated in the design. YZ, CYS, JWS, PWW, XW, KY and JRX participated in data collection. YZ and WBS participated in data analysis. All authors participated in data interpretation, drafting and finalizing the report.
Acknowledgments
We thank the patients for their time and participation in this study.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Funding
Additional information
Funding
References
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi:10.1002/ijc.29210
- Migita K, Takayama T, Saeki K, Matsumoto S, Wakatsuki K, Enomoto K, Tanaka T, Ito M, Kurumatani N, Nakajima Y, et al. The prognostic nutritional index predicts long term outcomes of gastric cancer patients independent of tumor stage. Ann Surg Oncol. 2013;20(8):2647–2654. doi:10.1245/s10434-013-2926-5
- Pinato DJ, North BV, Sharma R. A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: the prognostic nutritional index (PNI). Br J Cancer. 2012;106(8):1439–1445. doi:10.1038/bjc.2012.92
- Kanda M, Fujii T, Kodera Y, Nagai S, Takeda S, Nakao A. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg. 2011;98(2):268–274. doi:10.1002/bjs.7305
- Buzby GP, Mullen JL, Matthews DC, Hobbs CL, Rosato EF. Prognostic nutritional index in gastrointestinal surgery. Am J Surg. 1980;139(1):160–167. doi:10.1016/0002-9610(80)90246-9
- Onodera T, Goseki N, Kosaki G. [Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients]. Nihon Geka Gakkai Zasshi. 1984;85(9):1001–1005.
- Hirahara N, Tajima Y, Fujii Y, Kaji S, Yamamoto T, Hyakudomi R, Taniura T, Kawabata Y. Prognostic nutritional index as a predictor of survival in resectable gastric cancer patients with normal preoperative serum carcinoembryonic antigen levels: a propensity score matching analysis. BMC Cancer. 2018;18(1):285. doi:10.1186/s12885-018-4201-4
- Mohri Y, Inoue Y, Tanaka K, Hiro J, Uchida K, Kusunoki M. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg. 2013;37(11):2688–2692. doi:10.1007/s00268-013-2156-9
- Jin S, Cao S, Xu S, Wang C, Meng Q, Yu Y. Clinical impact of pretreatment prognostic nutritional index (PNI) in small cell lung cancer patients treated with platinum-based chemotherapy. Clin Respir J. 2018;12(9):2433–2440. doi:10.1111/crj.12925
- Okada S, Shimada J, Kato D, Tsunezuka H, Teramukai S, Inoue M. Clinical significance of prognostic nutritional index after surgical treatment in lung cancer. Ann Thorac Surg. 2017;104(1):296–302. doi:10.1016/j.athoracsur.2017.01.085
- Shoji F, Morodomi Y, Akamine T, Takamori S, Katsura M, Takada K, Suzuki Y, Fujishita T, Okamoto T, Maehara Y, et al. Predictive impact for postoperative recurrence using the preoperative prognostic nutritional index in pathological stage I non-small cell lung cancer. Lung Cancer. 2016;98(1):15–21. doi:10.1016/j.lungcan.2016.05.010
- Feng JF, Chen QX. Significance of the prognostic nutritional index in patients with esophageal squamous cell carcinoma. Ther Clin Risk Manag. 2014;10:1–7. doi:10.2147/TCRM.S56159
- Filip B, Scarpa M, Cavallin F, Cagol M, Alfieri R, Saadeh L, Ancona E, Castoro C. Postoperative outcome after oesophagectomy for cancer: nutritional status is the missing ring in the current prognostic scores. Eur J Surg Oncol. 2015;41(6):787–794. doi:10.1016/j.ejso.2015.02.014
- Matsumoto H, Okamoto Y, Kawai A, Ueno D, Kubota H, Murakami H, Higashida M, Hirai T. Prognosis prediction for postoperative esophageal cancer patients using Onodera’s Prognostic Nutritional Index. Nutr Cancer. 2017;69(6):849–854. doi:10.1080/01635581.2017.1339093
- Nakatani M, Migita K, Matsumoto S, Wakatsuki K, Ito M, Nakade H, Kunishige T, Kitano M, Kanehiro H. Prognostic significance of the prognostic nutritional index in esophageal cancer patients undergoing neoadjuvant chemotherapy. Dis Esophagus. 2017;30(8):1–7. doi:10.1093/dote/dox020
- Kubo N, Ohira M, Tamura T, Sakurai K, Toyokawa T, Tanaka H, Yashiro M, Yamashita Y, Hirakawa K. Prognostic significance of baseline nutritional index for patients with esophageal squamous cell carcinoma after radical esophagectomy. Esophagus. 2017;14(1):84–90. doi:10.1007/s10388-016-0548-2
- Zhang H, Shang X, Ren P, Gong L, Ahmed A, Ma Z, Ma R, Wu X, Xiao X, Jiang H, et al. The predictive value of a preoperative systemic immune-inflammation index and prognostic nutritional index in patients with esophageal squamous cell carcinoma. J Cell Physiol. 2019;234(2):1794–1802. doi:10.1002/jcp.27052
- Matsumoto Y, Zhou Q, Kamimura K, Moriyama M, Saijo Y. The prognostic nutrition index predicts the development of hematological toxicities in and the prognosis of esophageal cancer patients treated with cisplatin plus 5-fluorouracil chemotherapy. Nutr Cancer. 2018;70(3):447–452. doi:10.1080/01635581.2018.1445765
- Dai Y, Fu X, Li T, Yao Q, Su L, Su H, Li J. Long-term impact of prognostic nutritional index in cervical esophageal squamous cell carcinoma patients undergoing radical radiotherapy. Ann Transl Med. 2019;7(8):175. doi:10.21037/atm.2019.03.60
- Wang J, Yu B, Ye Y, Shen J, Ding N, Tang H, Xu Y, Song L, Zhu Z, Chen Y, et al. Predictive value of nutritional risk screening 2002 and prognostic nutritional index for esophageal cancer patients undergoing radical radiochemotherapy. Nutr Cancer. 2018;70(6):879–885. doi:10.1080/01635581.2018.1470656
- Nakatani M, Migita K, Matsumoto S, Wakatsuki K, Ito M, Nakade H, Kunishige T, Kitano M, Sho M. Prognostic significance of the prognostic nutritional index in patients with recurrent esophageal squamous cell carcinoma. Nutr Cancer. 2018;70(3):467–473. doi:10.1080/01635581.2018.1445771
- Xiao F, Wang L, Zhang W, Wang L, Zhao S Preoperative prognostic nutritional index is a significant predictor of survival in esophageal squamous cell carcinoma patients. Nutr Cancer. 2021;73(2):215–220. doi:10.1080/01635581.2020.1757129
- Hirahara N, Tajima Y, Fujii Y, Kaji S, Yamamoto T, Hyakudomi R, Taniura T, Miyazaki Y, Kishi T, Kawabata Y, et al. Preoperative prognostic nutritional index predicts long-term surgical outcomes in patients with esophageal squamous cell carcinoma. World J Surg. 2018;42(7):2199–2208. doi:10.1007/s00268-017-4437-1
- Seaton K. Albumin concentration controls cancer. J Natl Med Assoc. 2001;93(12):490–493.
- Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9:69. doi:10.1186/1475-2891-9-69
- O’Callaghan DS, O’Donnell D, O’Connell F, O’Byrne KJ. The role of inflammation in the pathogenesis of non-small cell lung cancer. J Thorac Oncol. 2010;5(12):2024–2036.
- Taniguchi K, Karin M. NF-κB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. 2018;18(5):309–324. doi:10.1038/nri.2017.142
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi:10.1016/j.cell.2010.01.025
- Koike Y, Miki C, Okugawa Y, Yokoe T, Toiyama Y, Tanaka K, Inoue Y, Kusunoki M. Preoperative C-reactive protein as a prognostic and therapeutic marker for colorectal cancer. J Surg Oncol. 2008;98(7):540–544. doi:10.1002/jso.21154
- Qiu M, Zhou Y-x, Jin Y, Wang Z-x, Wei X-l, Han H-y, Ye W-f, Zhou Z-w, Zhang D-s, Wang F-h, et al. Nutrition support can bring survival benefit to high nutrition risk gastric cancer patients who received chemotherapy. Support Care Cancer. 2015;23(7):1933–1939. doi:10.1007/s00520-014-2523-6
- Ravasco P, Monteiro-Grillo I, Marques Vidal P, Camilo ME. Impact of nutrition on outcome: a prospective randomized controlled trial in patients with head and neck cancer undergoing radiotherapy. Head Neck. 2005;27(8):659–668. doi:10.1002/hed.20221
- Ruggeri E, Giannantonio M, Agostini F, Ostan R, Pironi L, Pannuti R. Home artificial nutrition in palliative care cancer patients: impact on survival and performance status. Clin Nutr. 2020;39(11):3346–3353. doi:10.1016/j.clnu.2020.02.021
- van Bokhorst-De Van Der Schueren MA, Quak JJ, von Blomberg-van der Flier BM, Kuik DJ, Langendoen SI, Snow GB, Green CJ, van Leeuwen PA. Effect of perioperative nutrition, with and without arginine supplementation, on nutritional status, immune function, postoperative morbidity, and survival in severely malnourished head and neck cancer patients. Am J Clin Nutr. 2001;73(2):323–332. doi:10.1093/ajcn/73.2.323
- Xu L‐B, Huang Z‐X, Zhang H‐H, Chen X‐D, Zhang W‐T, Shi M‐M, Ma Y‐N, Shen X‐C, Lin J‐T, Cai Y‐Q, et al. Impact of preoperative short-term parenteral nutrition support on the clinical outcome of gastric cancer patients: a propensity score matching analysis. JPEN J Parenter Enteral Nutr. 2021;45(4):729–737. doi:10.1002/jpen.1944