Abstract
To evaluate the effectiveness of Helicobacter pylori (Hp) related gastric cancer screening (Hp-GCS) and cost-effectiveness of protocol candidates in a hospital-based cross-sectional study. A total of 163 gastric cancer patients in West China Hospital were retrospectively collected according to ICD-10 code and histologic proof between April 1, 2013 and March 31, 2014, and 15,599 cancer-free controls were simultaneously collected from the health checkup registry. Hp infection was examined by urea breath test (UBT). The prevalence of Hp infection was compared between patients and controls. The diagnostic performance of UBT-based predictive index was tested in both training and validation settings. Cost-effectiveness analysis was conducted to assess candidates of Hp-GCS protocols. The prevalence of Hp infection was 55.8% and 41.2% in gastric cancers and controls, respectively (p < 0.001). UBT-based model showed moderate diagnostic strength in Hp-GCS (AUC = 0.78, 95% CI 0.74–0.82), better than UBT alone (p < 0.001). The sensitivity and specificity of UBT-based index were 80.2% and 61.9% at optimal cutoff in training setting, comparable in validation setting, which sensitivity and specificity were 76.9% and 59.6%. Number needed to screen was decreased along with older age, as well as stronger positivity of UBT. The optimal cost-effective Hp-GCS protocol with detection rate (DR = 77.9%) was endoscopic screening in age 40–59 years and positive UBT, or age ≥60 years without UBT. Incremental analysis suggested a preferable protocol as endoscopic screening in age ≥40 years without UBT (DR = 93.3%). UBT had moderate diagnostic strength in massive gastric cancer screening, and might be cost-effective in middle-aged population (40–59 years). More robust Hp-GCS protocol needs further investigate in test methods and individual biologic features.
Introduction
Gastric cancer is a common malignant tumor with high morbidity and mortality in China (Citation1, Citation2). In recent years, gastric cancer has become the third largest burden of cancer in China and an important part of disease burden in China (Citation3). China is one of the countries with the most bloated burden of gastric cancer around the globe, with 421,540 people dying from the disease in 2019, contributes the biggest number of incident cases, deaths, and disability-adjusted life-years (DALYs) from gastric cancer on earth (Citation4, Citation5). Moreover, due to the aging of the population and other factors, the number of new cases and deaths of gastric cancer in China will further grow in the next 25 years, the disease burden of gastric cancer in China is still grievous (Citation4). Although the 5-year survival rate of early gastric cancer can reach more than 90% (Citation6), the rate for advanced gastric cancer is quite low, only 15.8% (Citation7). Therefore, early detection of tumor would be helpful to improve survival. Massive screening can increase the proportion of detected patients with early gastric cancer. Currently, Japan and South Korea have relatively complete screening strategies for gastric cancer (Citation8). Smoking and high-salt diet are momentous risk factors for gastric cancer (Citation9–11). China has formulated a series of tobacco-control strategies (Citation12, Citation13), and advocated citizens’ low-salt diet (Citation14). Since 2005, China has plotted and implemented a range of screening and early detection projects, which have been expanded from high-risk areas to the whole country in 2019 (Citation15). Presently, the target population of a screening program is people aged 40–69 (Citation16, Citation17). However, China is a country with a huge population, as the most effective measures of gastric cancer screening, the cost of gastroscopy is also high, it is generally assumed that the entire population endoscopic screening is impracticable (Citation18, Citation19). In addition, due to limited medical resources and lower population participation, there is no programmatic massive gastric cancer screening in China (Citation20). It is indispensable to develop an economical and effective screening program for gastric cancer to improve detection rate based on the high selected candidates for endoscopy (Citation21, Citation22).
Helicobacter pylori (Hp) is closely related to the development of gastric cancer, which was listed as a class I carcinogen by the World Health Organization (WHO) in 1994 (Citation23, Citation24). China was a country with a high infection rate of Helicobacter pylori, and the prevalence of Helicobacter pylori infection for the general population was 55.8% (Citation25). It was reported that the infection rate of Helicobacter pylori in Chengdu, Sichuan Province was about 53.1% (Citation26). Long-term infection of Hp can cause atrophic gastritis, a precancerous lesion, and further generate gastric cancer (Citation27, Citation28). The role of Hp-related gastric cancer screening (Hp-GCS) for endoscopy candidates has been reported in a large number of literature studies (Citation29, Citation30). Iris, et al. pointed out that due to the low cost of screening test, Hp-GCS had certain cost-effectiveness value (Citation31). For the diagnosis of Hp, urea breath test (UBT) has relatively high sensitivity and specificity, with low cost (Citation32). Therefore, this study was aimed to identify the diagnostic strength of UBT for gastric cancer and the cost-effectiveness of Hp-GCS.
Methods
Study Design
This was a retrospective, high-volume, hospital-based, cross-sectional study within the Sichuan Gastric Cancer Early Detection and Screening (SIGES) project. The SIGES research group was conducted in West China Hospital, Sichuan University, a central hospital in Sichuan province. The period of the study was between April 2013 and March 2014 (Citation33). The subjects covered healthy controls and gastric cancer patients, who were managed or treated in West China Hospital. The West China Hospital is a renowned national medical center in China, located in Chengdu, Sichuan Province of western China. Compared with other hospitals in Sichuan, even in China, it has the most advanced medical equipment all around the world and is one of the hospitals with the highest medical level in China. It is estimated that the West China Hospital receives about 1000 patients with gastric cancer for surgery annually. A total of 15,762 observations were included in this SIGES study.
Ethics
This study retrospectively collected the observations’ basic information and results of 14-C UBT. The SIGES study was approved by the Biomedical Ethical Committee of West China Hospital, Sichuan University (id: 2015-151-V2; 2018-215-V1). The informed consent was waived by the approval of the Biomedical Ethical Committee due to the retrospective nature, while the personal information was anonymized during the whole procedure of analyzing and reporting data.
Eligibility
The eligibility was assessed based on the electronic medical records reported by general practitioners, gastroenterologists, or gastrointestinal surgical oncologists. The healthy controls were collected from the health checkup in the Center of Health Checkup. The general practitioners recorded them as the asymptomatic status and cancer-free status. The gastric cancer patients were collected from the Department of Gastrointestinal Surgery. The diagnosis was proved by the ICD-10 code C16 and histology, regardless of TNM stage. Other kinds of malignancies were excluded, such as lymphoma and gastrointestinal stromal tumor. In addition, all the included observations should be tested by the 14-C UBT. For the gastric cancer patients, the UBT should be performed preoperatively, while the postoperative tests would not be considered.
General Information
The results of gender (male, female), age (<20, 20–39, 40–59, ≥60), ethnicity (Han, Tibetan, Yi, others), 14-C urea breath test (–, 1+, 2+, 3+, 4+) and direct medical expenses of patients with gastric cancer and healthy control group were collected. All the data were collected from the information management system of West China Hospital. When patients visit the hospital, their relevant information will be registered.
14-C Urea Breath Test
Fasting (at least for 3 h) tests took in a 14-C urea capsule (1μCi) with warm water, and then their breath was collected 15 mins, later by blowing to the breath card for 5 mins. The sample was tested by YH04 Helicobacter Pylori Analyzer (Anhui Young Hearty Medical Appliance & Equipment Co.; Ltd., China), and disintegrations per minute (dpm) was measured. The definitions of positivity and severity were according to manufacturer’s instructions. The outcomes were automatically classified as negative (−), mild positive (1+), moderate positive (2+), strong positive (3+), and very strong positive (4+). The cases were diagnosed with Hp infection for any 1+ to 4+ UBT results, and the cases with 3+ or 4+ UBT results were considered severe infections.
Statistics
Demographic characteristics and UBT results of the observations were compared by Chi-square test for categorical variables, and Wilcoxon rank-sum test for ranked variables or continuous variables without normality distribution. Shapiro-Wilk test for normal data was used to check the normality of variables. Multivariable analyses were performed by Logistic regression, and adjusted odds ratios (aORs) with 95% confidence intervals (CIs) were estimated. Non-parametric ROC analysis was used to assess the capability of predicting gastric cancer cases, while sensitivity (SEN) and specificity (SPE) were estimated. The area under the curve (AUC) with standard error (SE) was calculated. The diagnostic strength of UBT alone for gastric cancer was not high. Age, sex and ethnicity was related to gastric cancer in Logistic regression analysis. Therefore, UBT-based index was generated from the model of Logistic regression. The maximal Youden index (=SEN + SPE) was calculated to determine the optimal cutoff of UBT-based index. The difference of AUCs was compared by the Z test (Citation34, Citation35). Number needed to screen (NNS) was calculated in diverse subpopulation, stratified by sex, age group, and positivity strength of UBT, and relative ratio (RR) was estimated. The cost-effectiveness ratio (CER) was estimated throughout nine candidate protocols of Hp-GCS. The direct medical cost contained UBT and endoscopy according to different protocols. The effect was number of detected cases by endoscopy. The detection rate (DR) of each protocol of Hp-GCS was estimated. Additionally, incremental cost-effectiveness ratio (ICER) was estimated to identify the protocol of higher detection rate regarding willing-to-pay manner. The strength of AUC, SEN, SPE or DR was classified as weak (0.5–0.7), moderate (0.7–0.9), and strong (0.9–1). All the tests were two-sided and statistical significance was defined as p < 0.05. The STATA/SE 12.0 software was used for statistical analysis.
Results
In this study, a total of 15,599 healthy controls and 163 gastric cancer patients were analyzed. The basic characteristics of gastric cancer patients and cancer-free controls are shown in . There were statistically significant differences, older age and more males in gastric cancer patients, comparing to controls (p < 0.001), but not in terms of ethnicity (p = 0.119). The prevalence of Hp infection was 55.8% and 41.2% in gastric cancers and controls, respectively (aOR = 1.79, 95% CI 1.31–2.46, p < 0.001) (). Age ≥ 40 years and males were associated with higher prevalence of Hp infection.
Table 1. Baselines between cancer-free controls and gastric cancer cases.
Table 2. Hp prevalence between cancer-free controls and gastric cancer cases.
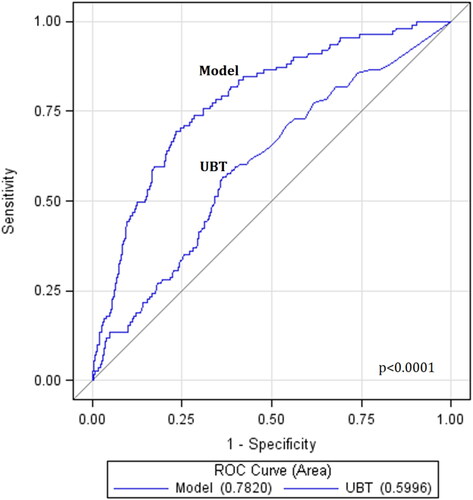
UBT-based multivariate model showed moderate diagnostic strength in predicting gastric cancer (AUC = 0.78, 95% CI 0.74–0.82), better than UBT alone (AUC = 0.60, 95% CI 0.55–0.65) (p < 0.001) (). The diagnostic strength of UBT-based model in each subset of age group or sex was not a significant difference (p > 0.05) (). The SEN and SPE of UBT-based index were moderate 80.2% (95% CI 71.5–87.1%) and weak 61.9% (61.1–62.8%) at optimal cutoff in training setting (), comparable in validation setting moderate 76.9% (63.2–87.5%) and weak 59.6% (57.9–61.3%), respectively. Providing SPE at 80%, SEN of UBT-based index was estimated as weak 59.5% (49.7–68.7%).
Table 3. AUC values of UBT predicting gastric cancer in training setting.
Table 4. Diagnostic performance of the UBT-based indexTable Footnote a generated from UBT for predicting gastric cancer.
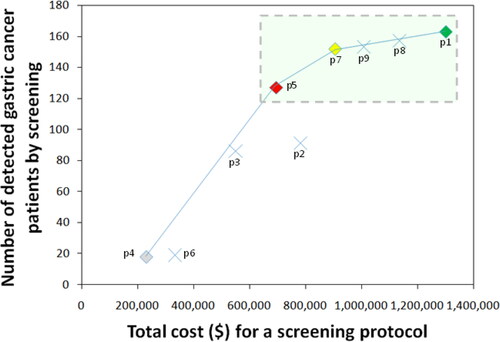
NNS was significantly lower along with older age, as well as stronger positivity of UBT (). NNSs were 109 males (RR = 145.23, 95% CI 17.43–1,228.73) and 268 females (RR = 58.73, 95% CI 7.87–534.55) in age group ≥60 years with UBT positivity 3+∼4+, compared to reference male <40 years with negative UBT (NNS = 15,762). The optimal CER of Hp-GCS protocol was endoscopic screening in age 40–59 years and positive UBT, or age ≥60 years without UBT (DR = 77.9%) (, ). ICER suggested a preferable protocol as endoscopic screening in age ≥40 years without UBT (DR = 93.3%). The protocols containing age group <40 years might not obviously improve DRs ().
Table 5. Subpopulation strategy for endoscopic screening.
Table 6. Cost-effectiveness analysis of protocol candidates for Hp-GCS.
Discussion
In this hospital-based cross-sectional study, the higher prevalence of Hp infection was observed in gastric cancer patients, especially in age group ≥40 years or males. UBT-based model containing demographic covariates showed just moderate diagnostic strength for predicting gastric cancer. Strong and very strong positivity of UBT might additionally decrease the NNSs in any age groups of either males or females. The cost-effectiveness analysis demonstrated that endoscopic screening might not bring obvious benefit of detection rate in age group <40 years, but the selection of positive UBT individuals for Hp-GCS obtained optimal cost-effectiveness with a moderate detection rate 77.9%.
Hp infection is a common cause of gastric cancer (Citation36). The proportion of Hp infection in Chinese residents was estimated to be 55.8% (Citation25). And a 14-year follow-up study in rural China found that the incidence of gastric cancer in patients with Hp infection was about 23.1‰, which was significantly higher than that of uninfected patients (5.93‰) (Citation37). The present study confirmed the association between gastric cancer and higher Hp prevalence. However, Hp-GCS has not been widely carried out around the world, with the exception of Japan and South Korea (Citation20). A western study in the United States showed that the screening and treatment of Hp for high-risk groups was also potentially cost-effective in preventing gastric cancer (Citation38). The Taiwan region also launched a population-based screening and eradication project of Hp in 2004 (Citation39). A prospective study in Japan found that Hp infection could predict gastric cancer well, and as the aggravation of infection, the incidence of gastric cancer increased (Citation40).
Regarding the Hp-GCS, either serologic antibody test (Citation41, Citation42) or UBT (Citation43–45) was a convenient and noninvasive approach. Besides UBT, serological screening of Hp might be helpful to identify high-risk individuals of gastric cancer development (Citation46). Although a Japanese study found that large-scale endoscopic screening was the best mean to detect and identify gastric cancer patients (Citation47), actually, endoscopic screening was more costly and less receptive by the testee of health checkup. Similarly, those reasons were why it was difficult to organize large-scale endoscopic screening in China. Additionally, in recent years, the Hp-GCS has been considered in the scheme of health checkup in many Chinese institutes, but Hp-GCS is still not mandatory in the governmental program, due to the insufficiency of robust evidence. In addition, it was reported in the United States that serological examination of Hp was low in sensitivity and cannot detect precancerous lesions (Citation48). The 14-C UBT was used to diagnose Hp infection in this study, but some studies suggested that the cost of enzyme-linked immunosorbent assay was lower (Citation49). Along with our findings, either UBT or serology might not be suitable as an independent screening test.
Hp-GCS may potentially consider several serologic factors of virulent Hp strains, while Hp-GCS can combine certain tumor biomarkers or precancerous lesion-associated biomarkers (Citation50). Cytotoxin-associated gene A (CagA), vacuolating cytotoxin A (VacA), helicobacter cysteine-rich protein C (HcpC), and chaperonin (GroEL), etc, which might be informative factors of virulent Hp strains in predicting gastric cancer (Citation28, Citation51). Pepsinogens and gastrin-17 were associated with precancerous lesions of gastric cancer (Citation16). Studies found the serologic pepsinogen test could be used as a supplementary screening test for high-risk individuals, but might not be ideal for gastric cancer itself (Citation52, Citation53). Gastrin-17 was a serologic marker, which could reflect the function of gastric mucosa and predict gastric cancer by increasing level along with decreasing pepsinogen levels (Citation54, Citation55). There were many reports on gastric cancer screening related to tumor biomarkers (Citation56, Citation57). Commonly, higher levels of serologic CEA, CA724, CA125, and CA199, etc, were associated with gastric cancer risk, which could be considered as supplementary tests in the gastric cancer screening (Citation34, Citation58). Besides Hp, some other pathogens might be associated with gastric cancer risk, such as Epstein-Barr virus (EBV), hepatitis B virus (HBV), human cytomegalovirus (CMV), human papillomavirus (HPV), and John Cunningham virus (JCV) (Citation59–61). All these factors can be investigated on the effectiveness and cost-effectiveness in gastric cancer screening.
There were certain limitations to be considered with caution. First, the study design was a single-hospital-based cross-sectional study, so the representativeness might be impaired to some extent in the simulative manner and sampling errors could not be waived out. Second, the detected cases were not classified by the TNM stage, and the role of Hp-GCS in early detection was kept unclear. Third, this study has an important limitation, that is, extrapolating a cross-sectional study to make conclusions on population-based cost-effectiveness, a major methodological limitation which has to be acknowledged. Cost-effectiveness analysis on screening cohort or whole-life health checkup might be more practicable for organized massive screening. Finally, the cost only contained direct medical cost during single round screening, while the cost of Hp eradication and endoscopic surveillance would better be considered.
In conclusion, UBT had moderate diagnostic strength in massive gastric cancer screening, and might be cost-effective in middle-aged population (40–59 years). More robust Hp-GCS protocol needs further investigate in test methods and individual biologic features.
Acknowledgments
The authors thank the substantial work of Volunteer Team of Gastric Cancer Surgery (VOLTGA), West China Hospital, Sichuan University, China.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Cavatorta O, Scida S, Miraglia C, Barchi A, Nouvenne A, Leandro G, Meschi T, De’ Angelis GL, Di Mario F. Epidemiology of gastric cancer and risk factors. Acta Biomed. 2018;89(8-s):82–7.
- Wang R, Chen XZ. High mortality from hepatic, gastric and esophageal cancers in mainland China: 40 years of experience and development. Clin Res Hepatol Gastroenterol. 2014;38(6):751–6. doi:https://doi.org/10.1016/j.clinre.2014.04.014
- Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen W, Li X, Wang L, Wang L, Liu Y, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;394(10204):1145–58. doi:https://doi.org/10.1016/S0140-6736(19)30427-1
- Zhang T, Chen H, Yin X, He Q, Man J, Yang X, Lu M. Changing trends of disease burden of gastric cancer in China from 1990 to 2019 and its predictions: findings from global burden of disease study. Chin J Cancer Res. 2021;33(1):11–26. doi:https://doi.org/10.21147/j.issn.1000-9604.2021.01.02
- The global, regional, and national burden of stomach cancer in 195 countries. 2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 1990;5(1):42–54.
- Suzuki H, Oda I, Abe S, Sekiguchi M, Mori G, Nonaka S, Yoshinaga S, Saito Y. High rate of 5-year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer. 2016;19(1):198–205. doi:https://doi.org/10.1007/s10120-015-0469-0
- Isobe Y, Nashimoto A, Akazawa K, Oda I, Hayashi K, Miyashiro I, Katai H, Tsujitani S, Kodera Y, Seto Y, et al. Gastric cancer treatment in Japan: 2008 annual report of the JGCA nationwide registry. Gastric Cancer. 2011;14(4):301–16. doi:https://doi.org/10.1007/s10120-011-0085-6
- Goto R, Hamashima C, Mun S, Lee W-C. Why screening rates vary between Korea and Japan-differences between two national healthcare systems. Asian Pac J Cancer Prev. 2015;16(2):395–400. doi:https://doi.org/10.7314/apjcp.2015.16.2.395
- Yang L, Ying X, Liu S, Lyu G, Xu Z, Zhang X, Li H, Li Q, Wang N, Ji J, et al. Gastric cancer: epidemiology, risk factors and prevention strategies. Chin J Cancer Res. 2020;32(6):695–704. doi:https://doi.org/10.21147/j.issn.1000-9604.2020.06.03
- Trédaniel J, Boffetta P, Buiatti E, Saracci R, Hirsch A. Tobacco smoking and gastric cancer: review and meta-analysis. Int J Cancer. 1997;72(4):565–73. doi:https://doi.org/10.1002/(SICI)1097-0215(19970807)72:4<565::AID-IJC3>3.0.CO;2-O
- Woo HD, Park S, Oh K, Kim HJ, Shin HR, Moon HK, Kim J. Diet and cancer risk in the Korean population: a meta-analysis. Asian Pac J Cancer Prev. 2014;15(19):8509–19. doi:https://doi.org/10.7314/apjcp.2014.15.19.8509
- Yang G-H, Li Q, Wang C-X, Hsia J, Yang Y, Xiao L, Yang J, Zhao L-H, Zhang J, Xie L, et al. Findings from 2010 Global Adult Tobacco Survey: implementation of MPOWER policy in China. Biomed Environ Sci. 2010;23(6):422–9. doi:https://doi.org/10.1016/S0895-3988(11)60002-0
- Guo H, Quan G. Tobacco control in China and the road to Healthy China 2030. Int J Tuberc Lung Dis. 2020;24(3):271–7. doi:https://doi.org/10.5588/ijtld.19.0106
- Wang S-S, Lay S, Yu H-N, Shen S-R. Dietary guidelines for Chinese residents (2016): comments and comparisons. J Zhejiang Univ Sci B. 2016;17(9):649–56. doi:https://doi.org/10.1631/jzus.B1600341
- Zeng H, Sun K, Cao M, Zheng R, Sun X, Liu S, Zhang Z, Liu Y, Guo G, Song G, et al. Initial results from a multi-center population-based cluster randomized trial of esophageal and gastric cancer screening in China. BMC Gastroenterol. 2020;20(1):398. doi:https://doi.org/10.1186/s12876-020-01517-3
- Ji L, Liu Z, Zhou B, Cai Y, An F, Wang L, Lv Z, Xia M, Yang J, Yuan J, et al. Community-based pilot study of a screening program for gastric cancer in a Chinese population. Cancer Prev Res (Phila). 2020;13(1):73–82. doi:https://doi.org/10.1158/1940-6207.CAPR-19-0372
- Lü YL, et al. Comparison of two gastric cancer screening schemes in a high-risk population. Zhonghua Zhong Liu Za Zhi. 2013;35(5):394–7.
- Cai Q, Zhu C, Yuan Y, Feng Q, Feng Y, Hao Y, Li J, Zhang K, Ye G, Ye L, et al. Development and validation of a prediction rule for estimating gastric cancer risk in the Chinese high-risk population: a nationwide multicentre study. Gut. 2019;68(9):1576–87. doi:https://doi.org/10.1136/gutjnl-2018-317556
- Dan YY, So JB, Yeoh KG. Endoscopic screening for gastric cancer. Clin Gastroenterol Hepatol. 2006;4(6):709–16. doi:https://doi.org/10.1016/j.cgh.2006.03.025
- Chen XZ, Zhang WH, Hu JK. A difficulty in improving population survival outcome of gastric cancer in mainland China: low proportion of early diseases. Med Oncol. 2014;31(12):315. doi:https://doi.org/10.1007/s12032-014-0315-y
- Papenfuss WA, Kukar M, Oxenberg J, Attwood K, Nurkin S, Malhotra U, Wilkinson NW. Morbidity and mortality associated with gastrectomy for gastric cancer. Ann Surg Oncol. 2014;21(9):3008–14. doi:https://doi.org/10.1245/s10434-014-3664-z
- Eghdami A, Ostovar R, Jafari A, Palmer AJ, Bordbar N, Ravangard R. Economic burden of gastric cancer: a case of Iran. Cancer Control. 2019;26(1):1073274819837185. doi:https://doi.org/10.1177/1073274819837185
- Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7–14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1–241.
- Leja M, Axon A, Brenner H. Epidemiology of Helicobacter pylori infection. Helicobacter. 2016; 21(Suppl 1):3–7. doi:https://doi.org/10.1111/hel.12332
- Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VWS, Wu JCY, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017;153(2):420–9. doi:https://doi.org/10.1053/j.gastro.2017.04.022
- Tang HR, Fan YJ, Liu S. Helicobacter pylori infection and associated risk factors in Chengdu. Sichuan Da Xue Xue Bao Yi Xue Ban. 2014;45(5):823–6.
- Waldum HL, Kleveland PM, Sørdal Ø F. Helicobacter pylori and gastric acid: an intimate and reciprocal relationship. Therap Adv Gastroenterol. 2016;9(6):836–44. doi:https://doi.org/10.1177/1756283X16663395
- Chen X-Z, Schöttker B, Castro FA, Chen H, Zhang Y, Holleczek B, Brenner H. Association of helicobacter pylori infection and chronic atrophic gastritis with risk of colonic, pancreatic and gastric cancer: a ten-year follow-up of the ESTHER cohort study. Oncotarget. 2016;7(13):17182–93. doi:https://doi.org/10.18632/oncotarget.7946
- Wang R, Bai D, Xiang W, Zhang Y-F, Ba K-Y, Chen X-Z, SIGES Research Group. Helicobacter pylori prevalence in the Southwest of China: a protocol for systematic review. Medicine (Baltimore). 2020;99(11):e19369. doi:https://doi.org/10.1097/MD.0000000000019369
- Pasechnikov V, Chukov S, Fedorov E, Kikuste I, Leja M. Gastric cancer: prevention, screening and early diagnosis. World J Gastroenterol. 2014;20(38):13842–62. doi:https://doi.org/10.3748/wjg.v20.i38.13842
- Lansdorp-Vogelaar I, Sharp L. Cost-effectiveness of screening and treating Helicobacter pylori for gastric cancer prevention. Best Pract Res Clin Gastroenterol. 2013;27(6):933–47. doi:https://doi.org/10.1016/j.bpg.2013.09.005
- Malfertheiner P, Megraud F, O’Morain CA, Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton J, Graham DY, European Helicobacter and Microbiota Study Group and Consensus panel, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66(1):6–30. doi:https://doi.org/10.1136/gutjnl-2016-312288
- Wang R, et al. Risk population of Helicobacter pylori infection among Han and Tibetan ethnicities in western China: a cross-sectional, longitudinal epidemiological study. Lancet. 2016;388:17.
- Chen X-Z, Zhang W-H, Yang K, Zhang B, Chen Z-X, Chen J-P, Zhou Z-G, Hu J-K. Quantitative comparisons of summary receiver operating characteristics (sROC) curves among conventional serological tumor biomarkers for predicting gastric cancer in Chinese population. Tumour Biol. 2014;35(9):9015–22. doi:https://doi.org/10.1007/s13277-014-1986-x
- Wang R, Chen XZ. Prevalence of atrophic gastritis in southwest China and predictive strength of serum gastrin-17: a cross-sectional study (SIGES). Sci Rep. 2020;10(1):4523. doi:https://doi.org/10.1038/s41598-020-61472-7
- Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014;345(2):196–202. doi:https://doi.org/10.1016/j.canlet.2013.08.016
- Zhang X, Xue L, Xing L, Wang J, Cui J, Mi J, Xing X, Wang J, Du Z, Misumi J, et al. Low serum pepsinogen I and pepsinogen I/II ratio and Helicobacter pylori infection are associated with increased risk of gastric cancer: 14-year follow up result in a rural Chinese community. Int J Cancer. 2012;130(7):1614–9. doi:https://doi.org/10.1002/ijc.26172
- Parsonnet J, Harris RA, Hack HM, Owens DK. Modelling cost-effectiveness of Helicobacter pylori screening to prevent gastric cancer: a mandate for clinical trials. Lancet. 1996;348(9021):150–4. doi:https://doi.org/10.1016/S0140-6736(96)01501-2
- Lee Y-C, Wu H-M, Chen TH-H, Liu T-Y, Chiu H-M, Chang C-C, Wang H-P, Wu M-S, Chiang H, Wu M-C, et al. A community-based study of Helicobacter pylori therapy using the strategy of test, treat, retest, and re-treat initial treatment failures. Helicobacter. 2006;11(5):418–24. doi:https://doi.org/10.1111/j.1523-5378.2006.00432.x
- Watabe H, Mitsushima T, Yamaji Y, Okamoto M, Wada R, Kokubo T, Doi H, Yoshida H, Kawabe T, Omata M, et al. Predicting the development of gastric cancer from combining Helicobacter pylori antibodies and serum pepsinogen status: a prospective endoscopic cohort study. Gut. 2005;54(6):764–8. doi:https://doi.org/10.1136/gut.2004.055400
- Hamashima C, Sasazuki S, Inoue M, Tsugane S, JPHC Study Group. Receiver operating characteristic analysis of prediction for gastric cancer development using serum pepsinogen and Helicobacter pylori antibody tests. BMC Cancer. 2017;17(1):183. doi:https://doi.org/10.1186/s12885-017-3173-0
- Kakiuchi T, Matsuo M, Endo H, Nakayama A, Sato K, Takamori A, Sasaki K, Takasaki M, Hara M, Sakata Y, et al. A Helicobacter pylori screening and treatment program to eliminate gastric cancer among junior high school students in Saga Prefecture: a preliminary report. J Gastroenterol. 2019;54(8):699–707. doi:https://doi.org/10.1007/s00535-019-01559-9
- Lee Y-C, Chen TH-H, Chiu H-M, Shun C-T, Chiang H, Liu T-Y, Wu M-S, Lin J-T. The benefit of mass eradication of Helicobacter pylori infection: a community-based study of gastric cancer prevention. Gut. 2013;62(5):676–82. doi:https://doi.org/10.1136/gutjnl-2012-302240
- Mason J, Axon ATR, Forman D, Duffett S, Drummond M, Crocombe W, Feltbower R, Mason S, Brown J, Moayyedi P, Leeds HELP Study Group, et al. The cost-effectiveness of population Helicobacter pylori screening and treatment: a Markov model using economic data from a randomized controlled trial. Aliment Pharmacol Ther. 2002;16(3):559–68. doi:https://doi.org/10.1046/j.1365-2036.2002.01204.x
- Mason JM, Moayyedi P, Young PJ, Duffett S, Crocombe W, Drummond MF, Axon ATR, For the Leeds H. pylori Study Group. Population-based and opportunistic screening and eradication of Helicobacter pylori. An analysis using trial baseline data. Leeds H. pylori Study Group. Int J Technol Assess Health Care. 1999;15(4):649–60. doi:https://doi.org/10.1017/S0266462399015445
- Leung WK, Wu M-s, Kakugawa Y, Kim JJ, Yeoh K-g, Goh KL, Wu K-c, Wu D-c, Sollano J, Kachintorn U, Asia Pacific Working Group on Gastric Cancer, et al. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol. 2008;9(3):279–87. doi:https://doi.org/10.1016/S1470-2045(08)70072-X
- Tashiro A, Sano M, Kinameri K, Fujita K, Takeuchi Y. Comparing mass screening techniques for gastric cancer in Japan. World J Gastroenterol. 2006;12(30):4873–4.
- Kim GH, Liang PS, Bang SJ, Hwang JH. Screening and surveillance for gastric cancer in the United States: is it needed? Gastrointest Endosc. 2016;84(1):18–28. doi:https://doi.org/10.1016/j.gie.2016.02.028
- Vakil N, Rhew D, Soll A, Ofman JJ. The cost-effectiveness of diagnostic testing strategies for Helicobacter pylori. Am J Gastroenterol. 2000;95(7):1691–1698. doi:https://doi.org/10.1111/j.1572-0241.2000.02193.x
- Dinis-Ribeiro M, Yamaki G, Miki K, Costa-Pereira A, Matsukawa M, Kurihara M. Meta-analysis on the validity of pepsinogen test for gastric carcinoma, dysplasia or chronic atrophic gastritis screening. J Med Screen. 2004;11(3):141–7. doi:https://doi.org/10.1258/0969141041732184
- Holleczek B, Schottker B, Brenner H. Helicobacter pylori infection, chronic atrophic gastritis and risk of stomach and esophagus cancer: results from the prospective population-based ESTHER cohort study. Int J Cancer. 2020;146(10):2773–2783. doi:https://doi.org/10.1002/ijc.32610
- Miki K. Gastric cancer screening using the serum pepsinogen test method. Gastric Cancer. 2006;9(4):245–53. doi:https://doi.org/10.1007/s10120-006-0397-0
- Castro C, Dinis-Ribeiro M, Rodrigues ANG, Calheiros T, Santos J, Pereira P, Ramos M, Cunha H, Andrade M, Costa A, et al. Western long-term accuracy of serum pepsinogen-based gastric cancer screening. Eur J Gastroenterol Hepatol. 2018;30(3):274–277.
- Sipponen P, Ranta P, Helske T, Kääriäinen I, Mäki T, Linnala A, Suovaniemi O, Alanko A, Härkönen M. Serum levels of amidated gastrin-17 and pepsinogen I in atrophic gastritis: an observational case-control study. Scand J Gastroenterol. 2002;37(7):785–791. doi:https://doi.org/10.1080/gas.37.7.785.791
- Cao Q, Ran ZH, Xiao SD. Screening of atrophic gastritis and gastric cancer by serum pepsinogen, gastrin-17 and Helicobacter pylori immunoglobulin G antibodies. J Dig Dis. 2007;8(1):15–22. doi:https://doi.org/10.1111/j.1443-9573.2007.00271.x
- Shen M, Wang H, Wei K, Zhang J, You C. Five common tumor biomarkers and CEA for diagnosing early gastric cancer: a protocol for a network meta-analysis of diagnostic test accuracy. Medicine (Baltimore). 2018;97(19):e0577. doi:https://doi.org/10.1097/MD.0000000000010577
- Chae H-D, Kim I-H, Lee GH, Shin I-H, Suh H-S, Jeon C-H. Gastric cancer detection using gastric juice pepsinogen and melanoma-associated gene RNA. Am J Clin Pathol. 2013;140(2):209–14. doi:https://doi.org/10.1309/AJCPOHXRM5IYXVOC
- Chen X-Z, Zhang W-K, Yang K, Wang L-L, Liu J, Wang L, Hu J-K, Zhang B, Chen Z-X, Chen J-P, et al. Correlation between serum CA724 and gastric cancer: multiple analyses based on Chinese population. Mol Biol Rep. 2012;39(9):9031–9. doi:https://doi.org/10.1007/s11033-012-1774-x
- Chen X-Z, Chen H, Castro FA, Hu J-K, Brenner H. Epstein-Barr virus infection and gastric cancer: a systematic review. Medicine (Baltimore). 2015;94(20):e792. doi:https://doi.org/10.1097/MD.0000000000000792
- Chen XZ, Wang R, Hu JK. Hepatitis B virus infection and gastric cancer risk: pitfalls in the potential association. Br J Cancer. 2015;112(11):1844. doi:https://doi.org/10.1038/bjc.2015.161
- Wang H, Chen X-L, Liu K, Bai D, Zhang W-H, Chen X-Z, Hu J-K, SIGES Research Group. Associations between gastric cancer risk and virus infection other than Epstein-Barr virus: a systematic review and meta-analysis based on epidemiological studies. Clin Transl Gastroenterol. 2020;11(7):e00201. doi:https://doi.org/10.14309/ctg.0000000000000201