Abstract
Background
The relationship between the dynamic alterations of nutritional indexes before and after surgery, and the prognosis of non-small-cell lung cancer (NSCLC) after radical surgery are unclear. Methods: This study enrolled 100 NSCLC patients in stages I–III who received radical surgery. The preoperative and postoperative 6-month levels of nine nutrition-related indicators were assessed in patients. Survival was analyzed using Kaplan–Meier curves as well as Cox regression models.
Results
Patients had better disease-free survival (DFS) with baseline total protein (TP) >76.66 g/L (75% vs. 50%, P = .027), baseline albumin (ALB) >37.7 g/L (60% vs. 26.7%, P = .002), baseline albumin to globulin ratio (AGR) >1.31 (63.5% vs. 40.5%, P = .006), or baseline globulin (GLOB) <31.42 g/L (39.4% vs. 62.7%, P = .037). Moreover, patients with increased hematocrit (HCT) (69.8% vs. 43.9% P = .013) and mean corpuscular volume (MCV) (73.2% vs. 42.4%, P = .014) at the postoperative 6-month examination had superior DFS. Cox proportional hazards regression analyses demonstrated that age >65 years, adenocarcinoma (pathological type), higher baseline TP, and post-surgery elevated HCT independently predicted favorable DFS.
Conclusion
Lower baseline TP and decreased postoperative HCT levels are independent predictors of prognosis in NSCLC following radical surgical procedures.
Introduction
Globally, lung cancer has one of the highest incidence rates and one of the highest cancer‐related mortality rates among cancers (Citation1). Non‐small‐cell lung cancer (NSCLC) accounts for 85% of all lung cancers (Citation2). The typical treatment for NSCLC patients without distant metastasis is surgery (Citation3); the 5-year survival rate for patients with early-stage NSCLC who undergo surgery is low, at 40% or less (Citation4). Many indications, such as Neuron-Specific Enolase (NSE), Carcinoembryonic Antigen (CEA) are used for NSCLC screening and evaluating the chances of cancer relapse, but they have limitations in terms of their accuracy in predicting prognosis (Citation5–Citation7). The best clinical prognostic indicator for cancer is the tumor node metastasis (TNM) stage; however, significant differences in survival have been reported for patients with the same TNM stage (Citation8). Therefore, robust and accessible prognostic biomarkers for predicting the risk of recurrence after radical resection need to investigated and identified.
Nutritional markers can be determined by routine blood and biochemical tests, which are often used to evaluate patients’ prognosis and risk of recurrence. Evidence shows that the nutrition and inflammation status are related to the occurrence and progression of cancer (Citation9, Citation10). In particular, it is important to provide nutritional support to cancer patients undergoing treatment through the entire duration of the treatment. Malnutrition could gradually progress to cachexia and eventually result in cancer progression and malignancy and interfere with the efficiency of treatment (Citation11).
Serum albumin (ALB) and globulin (GLOB) are the major components of serum proteins. Baseline ALB levels are directly correlated with nutrition status and clinical outcomes in colorectal and ovarian cancers, among others (Citation12–Citation14). Moreover, a lower baseline ALB-to-GLOB ratio (AGR) could independently predict prognosis in lung cancer and gastric cancer (Citation15, Citation16). However, baseline total protein (TP) has no predictive value for breast cancer, pancreatic cancer, or intrahepatic cholangiocarcinoma (Citation20–Citation22). In addition, one study indicated that high baseline blood values of red blood cell (RBC), hemoglobin (HGB), hematocrit (HCT), mean corpuscular volume (MCV), and mean corpuscular hemoglobin (MCH) could indicate good survival in colorectal cancer, osteosarcoma, and gastric cancer (Citation17–Citation19). On the contrary, in 2019, Jomrich et al. reported that high baseline MCV and MCH levels predict a poor outcome for patients with gastroesophageal adenocarcinoma (Citation23). However, the prognostic role of these nutritional indicators in early-stage lung cancer is uncertain. Given the above controversies, further study of the prognostic value of these baseline indicators levels is necessary.
Most of the reports focused on the impact of baseline nutrition-related indicators on cancer, but the association between postoperative nutrition-related indicators and survival of cancer is uncertain. Several studies have reported that nutritional indexes would be affected by surgery. For instance, Kiuchi et al. reported that in patients with severe postoperative complications, the levels of ALB (which is considered as an indicator of long-term postoperative nutritional status) are low; thus, ALB might have potential as a prognostic indicator in cancer patients who undergo surgery (Citation24). In addition, a study by Deng et al. suggested that dynamic reduction of serum AGR was related to poor survival in postoperative breast and lung cancer patients (Citation25). Moreover, one study indicated that postoperative HGB reduction has poor survival in colorectal cancer (Citation26). However, whether nutritional index fluctuations exist in NSCLC patients after suffering radical resection still remains unknown, and no previous studies have focused on the correlation of other postoperative nutritional indicator alterations with survival in NSCLC patients following surgery. Therefore, the purpose of this study was to assess the predictive value of baseline nutrition-related indexes and dynamical nutritional index fluctuations between baseline and post-surgery for survival in NSCLC patients.
We present the following article in accordance with the CONSORT reporting checklist.
Methods
Study Population
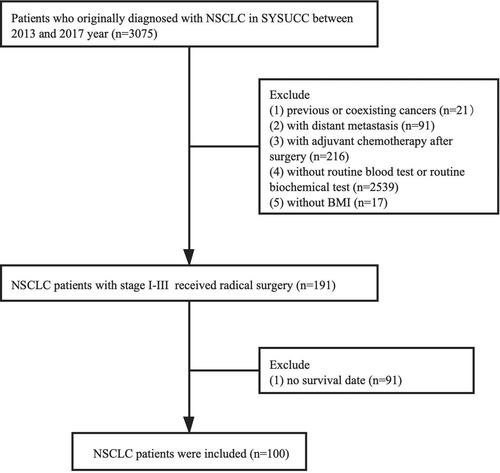
Patients selected for this retrospective research had histologically confirmed NSCLC between 2013 and 2017 at Sun Yat-sen University Cancer Center (SYSUCC) of China. The exclusion criteria were as follows: 1) previous or coexisting cancers, 2) no routine blood or biochemical test results before and at postoperative 6 mo, 3) with distant metastasis, 4) with adjuvant chemotherapy after surgery, 5) without body mass index (BMI), and 6) lost follow-up. Consequently, a total of 100 NSCLC patients in stages I–III were identified (). Patient data were collected from the Sun Yat-sen University Cancer Center (SYSUCC) of China hospital records.
Data Collection
Research information was collected for age, gender, TNM stage, pathologic type, BMI, smoking status, drinking status, complication of diabetes, complication of hypertension, radiotherapy history, baseline level, and levels of TP, ALB, GLOB, AGR, RBC, HGB, MCV, HCT, MCH at 6 mo, post-surgery.
X-Tile™
The X-Tile software (Yale University, New Haven, CT, US) was used to divide the study participants into two groups based on whether they had “low” or “high” levels of an indicator via a precise statistical estimation. The X-Tile software was used to evaluate the most suitable cutoff value of serum baseline nutritional indicators (Citation27).
Follow-up
Follow-up was performed every 3 mo, for the first 2 years after surgery, and every 6 mo, thereafter, or until death. The last follow-up evaluation was conducted for all existing patients in April 2020. Disease-free survival (DFS) was considered as the period starting from the day of the procedure and ending on the day of the first recurrence or the last follow-up appointment.
Statistical Analyses
We used the X-Tile software to determine the optimal cutoff values. A paired t-test was used to compare the nutrition-related index levels at two different time points. Survival was analyzed using the Kaplan–Meier method, and the log–rank test was used to compare survival. Univariate and multivariate analyses were conducted to determine the prognostic value of potential factors. Variables with P values <.15 according to the results of univariate analysis were included in the multivariate Cox regression model, together with 95% confidence intervals (CIs). The associations between patient characteristics and indexes related to nutrition and prognosis were analyzed, all tests were two-sided, and P < .05 was considered statistically significant for all analyses. Statistical analyses were performed using SPSS25 (IBM, Armonk, NY, USA), X-Tile software (Version 3.6.1), and Graphpad Prism 8 (Graphpad Software, CA, USA).
Results
Demographic and Clinical Features of the Cohort
The present cohort included 100 patients with histologically confirmed NSCLC who met the inclusion criteria. Among them, 66 (66%) were male and 34 (34%) were female. The median age was 60.5 years (interquartile range 22–81 years). The median BMI was 22.9 kg/m2 (interquartile range 17.2–31.2 kg/m2). In addition, 53 (53%) patients had non-adenocarcinoma and 47 (47%) patients had adenocarcinoma. There were 59 (59%) smokers and 41 (41%) nonsmokers. A total of 68 (68%) patients were nondrinkers and 32 (32%) patients were drinkers. There were nine (9%) patients with diabetes, and the number of hypertensive patients was low 16 (16%). Furthermore, the distribution of pathological stages was 19 (2.1%) patients in stage I and 81 (81%) in stages II–III. A total of three (3%) patients received radiotherapy and 97 (97%) did not receive radiotherapy ().
Table 1. Characteristics of all patients.
Comparison of Serum Levels of Nutrition-Related Indications Before and After Surgery
There were considerable changes in HGB and MCV at postoperative 6 mo: the baseline levels of HGB and MCV were higher than the levels at 6-month after surgery (P < .05) (). However, surgery had no effect on RBC, HCT, MCH, TP, ALB, GLOB, and AGR levels.
Table 2. The alterations of nutrition indexes in all patients.
Evaluation of Serum Nutrition-Related Indications Before Surgery by X-Tile
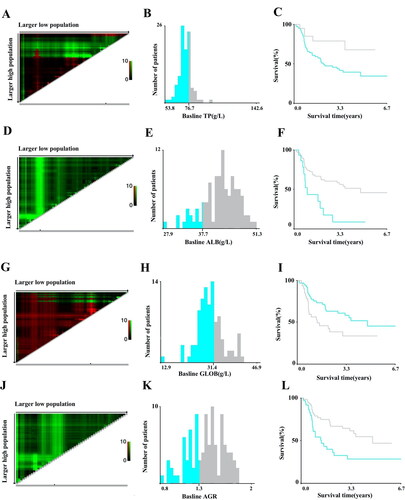
The appropriate cutoff values of preoperative TP, ALB, GLOB, and AGR levels conducted by X-Tile were 76.66 g/L, 37.7 g/L, 31.42 g/L, and 1.31, respectively (). However, the optimal cutoff levels of preoperative RBC, HGB, HCT, MCV, and MCH were not significantly different at 4.46 × 1012/L, 154 g/L, 44.7%, 94.2 fL, and 31.7 pg, respectively (data not shown).
Impact of Nutrition-Related Indicators on DFS
It was found that lower baseline TP, ALB, and AGR levels, and higher baseline GLOB levels in peripheral blood indicated poor DFS (). According to the Kaplan–Meier method, the DFS of patients who had baseline TP levels <76.66 g/L was significantly shorter than that of patients who had baseline TP levels >76.66 g/L (50% vs. 75%, P = .027; ; ). Patients with baseline ALB >37.7 g/L (60% vs. 26.7%, P = .002; ; ) and baseline AGR >1.31 (63.5% vs. 40.5%, P = .006; ; ) presented with better DFS than those with levels below their respective cutoff levels. However, patients with baseline GLOB levels less than 31.42 g/L had significantly higher DFS than those who had baseline GLOB levels higher than 31.42 g/L (62.7% vs. 39.4%, P = .037; ; ). Besides, patients with increasing HCT (69.8% vs. 43.9%, P = .013) and MCV (73.2% vs. 42.4%, P = .014; F); ) at the 6th month after surgery exhibited better DFS. No significant differences of baseline RBC, HGB, HCT, MCV, and MCH levels and postoperative RBC, HGB, MCH, TP, ALB, GLOB, and AGR levels could be found for DFS in any of the groups.
Figure 3. Survival curves according to the cutoff levels of baseline TP, ALB, GLOB, and AGR determined by X-Tile, and survival curves according to fluctuations in HCT and MCV levels. (A) Significant difference in DFS between patients with baseline TP higher than 76.66 g/L and those with TP lower than 76.66 g/L (75% vs. 50%, P = .027). (B) Significant difference in DFS between patients with baseline ALB higher than 37.7 g/L and those with ALB lower than 37.7 g/L (60% vs. 26.7%, P = .002). (C) Significant difference in DFS between patients with baseline GLOB higher than 31.42 g/L and those with GLOB lower than 31.42 g/L (62.7% vs. 39.4%, P = .037). (D) Significant difference in DFS between patients with baseline AGR higher than 1.31 and those with AGR lower than 1.31 (63.5% vs. 40.5%, P = .006). (E) Significant difference in DFS between patients with HCT elevation and those with HCT reduction after surgery compared to the baseline (69.8% vs. 43.9%, P = .013). (F) Significant difference in DFS between patients with MCV elevation and those with MCV reduction after surgery compared to the baseline (73.2% vs. 42.4%, P = .014).
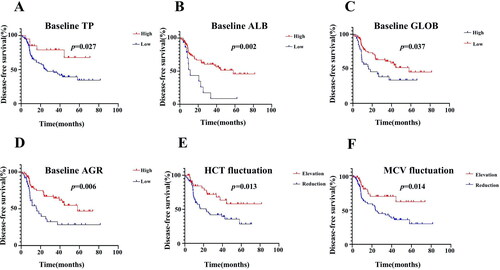
Table 3. The baseline nutrition-related indications for DFS.
Table 4. The nutrition-related indications alterations for DFS.
Univariate and Multivariate Analyses
The results of univariate and multivariate analysis of all the prognostic factors are summarized in . Univariate Cox regression analysis revealed the following risk factors for DFS: age (≥65 years vs. <65 years: hazard ratio [HR] [95% CI] = 1.00 [ref] vs. 0.540 [0.295–0.987], P = .045), histological type (non-adenocarcinoma vs. adenocarcinoma: HR [95% CI] = 1.00 [ref] vs. 0.412 [0.224–0.757], P = .004), baseline TP (high vs. low: HR [95% CI] = 1.00 [ref] vs. 2.736 [1.079–6.941], P = .034), baseline ALB (high vs. low, HR [95% CI] = 1.00 [ref] vs. 2.890 [1.451–5.754], P = .003), baseline GLOB (high vs. low, HR [95% CI] = 1.00 [ref] vs. 0.540 [0.299–0.974], P = .041), baseline AGR (high vs. low, HR [95% CI] = 1.00 [ref] vs. 2.229 [1.238–4.012], P = .008), postoperative HCT (elevation vs. reduction, HR [95% CI] = 1.00 [ref] vs. 2.218 [1.162–4.235], P = .016), and postoperative MCV (elevation vs. reduction, HR [95% CI] = 1.00 [ref] vs. 2.289 [1.160–4.519], P = .017).
Table 5. Predictive factors for DFS by univariate and multivariate analysis.
When the above variables were entered into the multivariate Cox proportional hazards model, the following independent predictive factors emerged: age (≥65 years vs. <65 years: HR [95% CI] = 1.00 [ref] vs. 0.479 [0.239–0.961], P = .038), histological (non-adenocarcinoma vs. adenocarcinoma: HR [95% CI] = 1.00 [ref] vs. 0.456 [0.229–0.907], P = .025), baseline TP (high vs. low, HR [95% CI] = 1.00 [ref] vs. 3.015 [1.055–8.610], P = .039), and postoperative HCT (elevation vs. reduction: HR [95% CI] = 1.00 [ref] vs. 2.417 [1.214–4.811], P = .012).
Nevertheless, baseline RBC, HGB, MCV, HCT, and MCH levels, and postoperative RBC, HGB, MCH, TP, ALB, GLOB, and AGR levels were not related to prognosis in univariate or multivariate analysis.
Discussion
At present, the clinical outcomes of NSCLC are heterogeneous, and TNM staging of this cancer has limitations in terms of predicting prognosis (Citation8, Citation28, Citation29). Several studies have developed survival predictors of surgically resected NSCLC patients; in particular, several molecular indicators with better predictive abilities have been identified (Citation30–Citation32). However, most of these methods are expensive, complicated, and not easily accessible (Citation33). Thus, there is a need for the evaluation and identification of simple and cost‐efficient predictors for prognosis. Jagoe et al. found that nutritional status was an important predictor of postoperative death and complications in patients with lung cancer (Citation34). In this study, we evaluated the prognostic value of pre- and postoperative serum nutritional indicators in NSCLC patients who underwent radical surgical resection procedures. HGB and MCV had significantly lower postoperative values compared to their baseline values. It was also evident that patients with lower baseline TP, ALB, AGR, and higher baseline GLOB levels may have adverse outcomes. In addition, univariate analysis revealed that in patients <65 years old, adenocarcinomas and higher baseline TP, ALB, AGR, and lower baseline GLOB were associated with superior DFS. Moreover, multivariate Cox analysis indicated that in patients <65 years old, adenocarcinoma and higher baseline TP exhibited favorable DFS. Interestingly, it was also found that patients with declining postoperative HCT and MCV after operation presented with a poorer prognosis. However, although both HCT and MCV showed a significant association with better survival in univariate analysis, in the multivariate Cox proportional hazards analysis, only postoperative decrease in HCT was found to be an independent prognostic factor for DFS.
ALB and GLB are vital components of blood in human beings: ALB is involved in the transport of various molecules and the regulation of plasma osmotic pressure. It is an important indicator of both nutritional status and the inflammation levels of the body (Citation35). Inflammatory processes result in increased levels of cytokines like IL-6 and TNF, which have an inhibitory effect on ALB production in hepatic cells (Citation36). Chronic inflammation may eventually result in damage to the vascular endothelium and an increase in vascular permeability; this would cause an increase in ALB levels in the interstitial fluid and a subsequent decrease in serum ALB levels (Citation37). GLB, which is also an indicator of nutrition and immune status, is produced by immune-related organs (Citation11, Citation38). Inflammation leads to a rapid increase in serum GLB levels (Citation37). In this study, the predictive values of baseline TP, ALB, GLOB, and AGR levels conflict with those reported in other studies. However, the findings are still consistent with some previous results which show that lower baseline ALB levels are an indicator of poor nutrition and poor prognosis in colorectal and ovarian cancers (Citation12–Citation14). However, one conflicting study indicated that lower preoperative serum ALB levels are associated with better survival in colon cancer (Citation39). Moreover, some studies have demonstrated that lower baseline AGR can serve as an independent prognostic indicator in lung and gastric cancers, which is consistent with our research (Citation15, Citation16). Interestingly, these study findings revealed that lower baseline TP was an independent predictor of survival in NSCLC patients. However, the predictive value of baseline TP was not found in breast cancer, pancreatic cancer or intrahepatic cholangiocarcinoma (Citation17–Citation19). In summary, in elderly NSCLC patients, the preoperative TP, ALB, GLOB, and AGR levels should be closely monitored and nutritional status should be improved.
As clinical detection indexes, RBC, HGB, MCV, HCT, and MCH were the main indexes in routine blood examination. Unfortunately, this study failed to show the baseline level of the above indexes as significant prognostic factors for NSCLC patients treated with surgery. There is still controversy about the predictive effects of RBC, HGB, MCV, HCT, and MCH. Most previous studies have confirmed that high baseline blood values of RBC, HGB, HCT, MCV, and MCH are related to good survival in colorectal cancer, osteosarcoma, and gastric cancer (Citation20–Citation22). In contrast, several studies have reported that high baseline MCV and MCH levels predict poor outcomes in gastroesophageal adenocarcinoma, head and neck cancer, and esophageal squamous cell carcinoma (Citation13, Citation40, Citation41). Therefore, the prognostic value of baseline nutritional indicators needs to be investigated.
Considering the previously reported strong correlation of prognosis with fluctuations in the levels of serum nutritional indicators (Citation24–Citation26), we investigated whether the dynamic changes in nutritional indicators after radical resection for NSCLC have an effect on disease recurrence. This study observed that postoperative HCT and MCV elevation plasma levels could indicate better DFS, and that postoperative elevated HCT is an independent prognostic factor for DFS. However, no previous study has investigated the correlation of postoperative HCT and MCV with survival in cancer patients. To the best of our knowledge, this study, for the first time, reported the association of postoperative HCT and MCV and NSCLC after radical surgery. HCT indicates the RBC volume of blood, and some studies show that low baseline HCT correlates with poor survival in gastric and colorectal cancers (Citation20, Citation21). When HCT levels are low, the oxygen-carrying capacity of blood is reduced and oxygen transport decreases. This results in hypoxia, which could increase the metastatic potential of cancer via various proteomic and genomic mechanisms (Citation42–Citation44). Gustavo et al. observed that residents at higher altitude have an obvious prolonged longevity with a normal increase of HCT, which indicates that higher HCT may be associated with better survival for residents of higher altitudes (Citation50, Citation51). Furthermore, another experimental research found that people at high altitude reduce the extra energy consumption of hyperventilation and tachycardia as feedback responses to increased HCT (Citation52), which could explain the underlying mechanism of the association between higher HCT and better survival. The other indicator MCV represents the average volume of RBCs. Additionally, it is a marker of folate and vitamin B12 deficiency, which along with macrocytic anemia, may have prognostic potential in various malignant cancers (Citation45–Citation47). Recently, higher MCV levels were found to correlate with poor prognosis in patients with colorectal, esophageal, liver, and head and neck cancer (Citation40, Citation41, Citation48, Citation49). However, these previous results are not consistent with the present findings. That is, postoperative MCV was identified as a prognostic factor, but its direct effect on the proliferation of lung cancer was unclear. Additionally, the molecular mechanisms of the association between MCV and DFS were also not evident from the findings. Therefore, more studies are required to confirm these findings and investigate the underlying mechanisms.
Nevertheless, the predictive value of postoperative TP, ALB, GLOB, and AGR levels was not observed in our study. A study by Kiuchi et al. showed that lower levels of postoperative ALB were associated with a poor prognosis in patients with severe postoperative complication (Citation25). In addition, a study by Deng et al. suggested that dynamic reduction of serum AGR was related to poor survival in postoperative breast and lung cancer patients (Citation26). Therefore, in view of the conflicting literature regarding the value of postoperative nutritional indicators on prognosis, we recommend that future studies investigate this area.
The present study has several limitations, and one of these is its retrospective nature. Another limitation is that it was conducted at a single center, as this limits the applicability of the findings. A third big limitation is the small sample size, which was not sufficient to investigate different surgical resection methods. Lastly, the overall survival of patients was not mature enough to be analyzed and only DFS analysis was performed. Despite these limitations, we identified two new prognostic indexes, lower baseline TP and higher postoperative MCV, which detrimentally affected survival. However, the exact underlying mechanisms are unknown and the findings need to be confirmed with a larger prospective analysis that also investigates the mechanisms underlying the connection between TP, MCV index, and NSCLC survival.
Conclusions
To the best of our knowledge, this is the first study to investigate the association between serum nutrition-related indicators and prognosis in NSCLC patients in stages I–III undergoing radical resection. Surgery significantly changed the levels of several nutritional indicators. Lower baseline TP, ALB, AGR, and higher baseline GLOB in peripheral blood levels were related to poorer DFS. Moreover, increases in HCT and MCV at postoperative 6 mo, were associated with better DFS. Importantly, both univariate and multivariate analyses demonstrated that lower baseline TP and declining levels of HCT after surgery were independent prognostic factors for NSCLC patients in stages I–III receiving radical resection. Improving pre- and postoperative nutritional status in patients with NSCLC may contribute to a better prognosis. Furthermore, the specific mechanism of declining HCT levels after surgery and the prognosis of patients with NSCLC need to be investigated under both in vitro and in vivo settings.
Ethical Standards
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The Ethics Committee approved the study protocol of the Sun Yat-sen University Cancer Center (B2021-056-01). Informed consent was obtained from each patient included in the study. This study was conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from each patient included in the study.
Author Contributions
Hongyun Zhao, Li Zhang took charge of designing the study and revising the paper. Jiaxin Cao, Fan Luo, Kangmei Zeng were the main writer of the paper, tested the entire index for all samples, and were responsible for the statistical analysis. Feiteng Lu, Wenjuan Ma, Yan Huang collected the clinical data and followed up the patients. All authors agree to the final approval of the version to be published.
| Abbreviations | ||
| NSCLC | = | non-small-cell lung cancer |
| TP | = | total protein |
| ALB | = | albumin |
| GLOB | = | globulin |
| AGR | = | albumin to globulin ratio |
| RBC | = | red blood cell |
| HGB | = | hemoglobin |
| MCV | = | mean corpuscular volume |
| MCH | = | mean corpuscular hemoglobin |
| HCT | = | hematocrit |
| DFS | = | disease-free survival |
| NLR | = | neutrophil to lymphocyte ratio |
| LDL-C | = | low-density lipoprotein cholesterol |
| HDL-C | = | high-density lipoprotein cholesterol |
| TNM | = | tumor node metastasis |
| SYSUCC | = | Sun Yat-sen University Cancer Center |
| BMI | = | body mass index |
Conflicts of Interest
The authors have no conflicts of interest to declare.
Additional information
Funding
References
- Chen W. Cancer statistics: updated cancer burden in China. Chin J Cancer Res. 2015;27(1):1. doi: 10.3978/j.issn.1000-9604.2015.02.07.
- Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584–94. doi: 10.4065/83.5.584.
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–49. doi: 10.3322/CA.2007.0010.
- Lüchtenborg M, Riaz SP, Lim E, Page R, Baldwin DR, Jakobsen E, Vedsted P, Lind M, Peake MD, Mellemgaard A, et al. Survival of patients with small cell lung cancer undergoing lung resection in England, 1998–2009. Thorax. 2014;69(3):269–73. doi: 10.1136/thoraxjnl-2013-203884.
- Duan X, Cui Y, Li H, Shi G, Wu B, Liu M, Chang D, Wang T, Kong Y. High preoperative and postoperative levels of carcinoembryonic antigen and CYFRA 21-1 indicate poor prognosis in patients with pathological Stage I nonsmall cell lung cancer. Indian J Cancer. 2015;52(7):158–63. doi:10.4103/0019-509X.186564
- Li S, Cao L, Wang X, Wang F, Wang L, Jiang R. Neuron-specific enolase is an independent prognostic factor in resected lung adenocarcinoma patients with anaplastic lymphoma kinase gene rearrangements. Med Sci Monit. 2019;25:675–90. doi: 10.12659/MSM.913054.
- Maeda R, Suda T, Hachimaru A, Tochii D, Tochii S, Takagi Y. Clinical significance of preoperative carcinoembryonic antigen level in patients with clinical stage IA non-small cell lung cancer. J Thorac Dis. 2017;9(1):176–86. doi: 10.21037/jtd.2017.01.30.
- Thrumurthy SG, Chaudry MA, Chau I, Allum W. Does surgery have a role in managing incurable gastric cancer? Nat Rev Clin Oncol. 2015;12(11):676–82. doi: 10.1038/nrclinonc.2015.132.
- Gomes de Lima KV, Maio R. Nutritional status, systemic inflammation and prognosis of patients with gastrointestinal cancer. Nutr Hosp. 2012;27:707–14. doi:10.3305/nh/2012.27.3.5567
- McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12(3):223–6. doi: 10.1097/MCO.0b013e32832a7902.
- Zhang Y, Zhu JY, Zhou LN, Tang M, Chen MB, Tao M. Predicting the prognosis of gastric cancer by albumin/globulin ratio and the prognostic nutritional index. Nutr Cancer. 2020;72(4):635–44. doi: 10.1080/01635581.2019.1651347.
- Ataseven B, du Bois A, Reinthaller A, Traut A, Heitz F, Aust S, Prader S, Polterauer S, Harter P, Grimm C, et al. Pre-operative serum albumin is associated with postoperative complication rate and overall survival in patients with epithelial ovarian cancer undergoing cytoreductive surgery. Gynecol Oncol. 2015;138(3):560–5. doi:10.1016/j.ygyno.2015.07.005
- Chiang JM, Chang CJ, Jiang SF, Yeh CY, You JF, Hsieh PS, Huang HY. Pre-operative serum albumin level substantially predicts post-operative morbidity and mortality among patients with colorectal cancer who undergo elective colectomy. Eur J Cancer Care. 2017;26(2):e12403. Epub 2015 Nov 3. doi:10.1111/ecc.12403
- Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Shibuya N, Kubota K. Clinical significance of the c-reactive protein to albumin ratio for survival after surgery for colorectal cancer. Ann Surg Oncol. 2016;23(3):900–7. doi:10.1245/s10434-015-4948-7
- Duran AO, Inanc M, Karaca H, Dogan I, Berk V, Bozkurt O, Ozaslan E, Ucar M, Eroglu C, Ozkan M, et al. Albumin–globulin ratio for prediction of long-term mortality in lung adenocarcinoma patients. Asian Pac J Cancer Prev. 2014;15(15):6449–53. doi:10.7314/APJCP.2014.15.15.6449
- Liu J, Chen S, Geng Q, Liu X, Kong P, Zhan Y, Xu D. Prognostic value of pretreatment albumin–globulin ratio in predicting long-term mortality in gastric cancer patients who underwent D2 resection. OTT. 2017;ume 10:2155–62. doi:10.2147/OTT.S99282
- Feng L, Gu S, Wang P, Chen H, Chen Z, Meng Z, Liu L. Pretreatment values of bilirubin and albumin are not prognostic predictors in patients with advanced pancreatic cancer. Cancer Med. 2018;7(12):5943–51. doi: 10.1002/cam4.1848.
- Shi H, Wang XH, Gu JW, Guo GL. Development and validation of nomograms for predicting the prognosis of triple-negative breast cancer patients based on 379 Chinese patients. Cancer Manag Res. 2019;11:10827–39. doi: 10.2147/CMAR.S234926.
- Zhang C, Wang H, Ning Z, Xu L, Zhuang L, Wang P, Meng Z. Serum liver enzymes serve as prognostic factors in patients with intrahepatic cholangiocarcinoma. Onco Targets Ther. 2017;10:1441–9. doi: 10.2147/OTT.S124161.
- Li Y, Wu H, Xing C, Hu X, Zhang F, Peng Y, Li Z, Lu T. Prognostic evaluation of colorectal cancer using three new comprehensive indexes related to infection, anemia and coagulation derived from peripheral blood. J Cancer. 2020;11(13):3834–45. doi:10.7150/jca.42409
- Lin J-X, Lin J-P, Xie J-W, Wang J-B, Lu J, Chen Q-Y, Cao L-L, Lin M, Tu R, Zheng C-H, et al. Preoperative hematocrit (HCT) is a novel and simple predictive marker for gastric cancer patients who underwent radical gastrectomy. Ann Surg Oncol. 2019;26(12):4027–36. doi: 10.1245/s10434-019-07582-7.
- Tu J, Wen L, Huo Z, Wang B, Wang Y, Liao H, Liu W, Zhong X, Kong J, Wang M, et al. Predictive value of dynamic change of haemoglobin levels during therapy on treatment outcomes in patients with Enneking stage IIB extremity osteosarcoma. BMC Cancer. 2018;18(1):428. doi: 10.1186/s12885-018-4279-8.
- Jomrich G, Hollenstein M, John M, Ristl R, Paireder M, Kristo I, Asari R, Schoppmann SF. High mean corpuscular volume predicts poor outcome for patients with gastroesophageal adenocarcinoma. Ann Surg Oncol. 2019;26(4):976–85. doi: 10.1245/s10434-019-07186-1.
- Kiuchi J, Komatsu S, Kosuga T, Kubota T, Okamoto K, Konishi H, Shiozaki A, Fujiwara H, Ichikawa D, Otsuji E, et al. Long-term postoperative nutritional status affects prognosis even after infectious complications in gastric cancer. Anticancer Res. 2018;38(5):3133–8. doi: 10.21873/anticanres.12575.
- Deng Y, Ma J, Tang D, Zhang Q. Dynamic biomarkers indicate the immunological benefits provided by Ganoderma spore powder in post-operative breast and lung cancer patients. Clin Transl Oncol. 2021;23(7):1481–90. doi: 10.1007/s12094-020-02547-9.
- Dru RC, Curtis NJ, Court EL, Spencer C, El Falaha S, Dennison G, Dalton R, Allison A, Ockrim J, Francis NK, et al. Impact of anaemia at discharge following colorectal cancer surgery. Int J Colorectal Dis. 2020;35(9):1769–76. doi:10.1007/s00384-020-03611-0
- Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 2004; 10(21):7252–9.
- Lowczak A, Kolasinska-Cwikla A, Ćwikła JB, Osowiecka K, Palucki J, Rzepko R, Glinka L, Doboszyńska A. Outcomes of patients with clinical stage I–IIIA large-cell neuroendocrine lung cancer treated with resection. JCM. 2020;9(5):1370. doi:10.3390/jcm9051370
- Ost D, Goldberg J, Rolnitzky L, Rom WN. Survival after surgery in stage IA and IB non-small cell lung cancer. Am J Respir Crit Care Med. 2008;177(5):516–23. doi:10.1164/rccm.200706-815OC
- Liu L, Shi M, Wang Z, Lu H, Li C, Tao Y, Chen X, Zhao J. A molecular and staging model predicts survival in patients with resected non-small cell lung cancer. BMC Cancer. 2018;18(1):966. doi: 10.1186/s12885-018-4881-9.
- Mecklenburg I, Sienel W, Schmid S, Passlick B, Kufer P. A threshold of systemic MAGE-A gene expression predicting survival in resected non-small cell lung cancer. Clin Cancer Res. 2017;23(5):1213–9. doi:10.1158/1078-0432.CCR-16-0557
- Schneider MA, Christopoulos P, Muley T, Warth A, Klingmueller U, Thomas M, Herth FJF, Dienemann H, Mueller NS, Theis F, et al. AURKA, DLGAP5, TPX2, KIF11 and CKAP5: five specific mitosis-associated genes correlate with poor prognosis for non-small cell lung cancer patients. Int J Oncol. 2017;50(2):365–72. doi: 10.3892/ijo.2017.3834.
- Li T, Kung HJ, Mack PC, Gandara DR. Genotyping and genomic profiling of non-small-cell lung cancer: implications for current and future therapies. J Clin Oncol. 2013;31(8):1039–49. doi: 10.1200/JCO.2012.45.3753.
- Jagoe RT, Goodship TH, Gibson GJ. Nutritional status of patients undergoing lung cancer operations. Ann Thorac Surg. 2001;71(3):929–35. doi: 10.1016/s0003-4975(00)02005-1.
- Arroyo V, Garcia-Martinez R, Salvatella X. Human serum albumin, systemic inflammation, and cirrhosis. J Hepatol. 2014;61(2):396–407. doi: 10.1016/j.jhep.2014.04.012.
- Pfensig C, Dominik A, Borufka L, Hinz M, Stange J, Eggert M. A new application for albumin dialysis in extracorporeal organ support: characterization of a putative interaction between human albumin and proinflammatory cytokines IL-6 and TNFα. Artif Organs. 2016;40(4):397–402. doi: 10.1111/aor.12557.
- Mao M-J, Wei X-L, Sheng H, Wang X-P, Li X-H, Liu Y-J, Xing S, Huang Q, Dai S-Q, Liu W-L, et al. Clinical significance of preoperative albumin and globulin ratio in patients with gastric cancer undergoing treatment. Biomed Res Int. 2017;2017:3083267. doi: 10.1155/2017/3083267.
- Ballow M. Mechanisms of action of intravenous immune serum globulin in autoimmune and inflammatory diseases. J Allergy Clin Immunol. 1997;100(2):151–7. doi: 10.1016/s0091-6749(97)70217-3.
- Jiang Z, Li Y, Han G, Zhang J, Li Z, Wang D, Liu Y. Association of serum albumin level with clinicopathologic features and prognosis in colon cancer. Zhonghua Wei Chang Wai Ke Za Zhi. 2016;19:80–3.
- Borsetto D, Polesel J, Tirelli G, Menegaldo A, Baggio V, Gava A, Nankivell P, Pracy P, Fussey J, Boscolo-Rizzo P, et al. Pretreatment high MCV as adverse prognostic marker in nonanemic patients with head and neck cancer. Laryngoscope. 2021;131(3):E836–45. doi: 10.1002/lary.28882.
- Zheng YZ, Dai SQ, Li W, Cao X, Li Y, Zhang LJ, Fu JH, Wang JY. Prognostic value of preoperative mean corpuscular volume in esophageal squamous cell carcinoma. WJG. 2013;19(18):2811–7. doi:10.3748/wjg.v19.i18.2811
- Habler OP, Messmer KF. Tissue perfusion and oxygenation with blood substitutes. Adv Drug Deliv Rev. 2000;40(3):171–84. doi: 10.1016/s0169-409x(99)00048-4.
- Harrison L, Blackwell K. Hypoxia and anemia: factors in decreased sensitivity to radiation therapy and chemotherapy? Oncologist. 2004;9(S5):31–40. doi:10.1634/theoncologist.9-90005-31
- Vaupel P. The role of hypoxia-induced factors in tumor progression. Oncologist. 2004;9(S5):10–7. doi:10.1634/theoncologist.9-90005-10
- Espinosa E, Feliu J, Zamora P, González Barón M, Sánchez JJ, Ordón ez A, Espinosa J. Serum albumin and other prognostic factors related to response and survival in patients with advanced non-small cell lung cancer. Lung Cancer. 1995;12(1-2):67–76. doi: 10.1016/0169-5002(95)00407-r.
- Jomrich G, Hollenstein M, John M, Baierl A, Paireder M, Kristo I, Ilhan-Mutlu A, Asari R, Preusser M, Schoppmann SF, et al. The modified glasgow prognostic score is an independent prognostic indicator in neoadjuvantly treated adenocarcinoma of the esophagogastric junction. Oncotarget. 2018;9(6):6968–76. doi:10.18632/oncotarget.24087
- Oñate-Ocaña LF, Aiello-Crocifoglio V, Gallardo-Rincón D, Herrera-Goepfert R, Brom-Valladares R, Carrillo JF, Cervera E, Mohar-Betancourt A. Serum albumin as a significant prognostic factor for patients with gastric carcinoma. Ann Surg Oncol. 2007;14(2):381–9. doi: 10.1245/s10434-006-9093-x.
- Nagai H, Yuasa N, Takeuchi E, Miyake H, Yoshioka Y, Miyata K. The mean corpuscular volume as a prognostic factor for colorectal cancer. Surg Today. 2018;48(2):186–94. doi:10.1007/s00595-017-1575-x
- Yoon HJ, Kim K, Nam YS, Yun JM, Park M. Mean corpuscular volume levels and all-cause and liver cancer mortality. Clin Chem Lab Med. 2016;54:1247–57. doi:10.1515/cclm-2015-0786
- Zubieta-Calleja GR, Zubieta-DeUrioste NA. Extended longevity at high altitude: benefits of exposure to chronic hypoxia. BLDE Univ J Health Sci. 2017;2(2):80–90. doi:10.4103/bjhs.bjhs_7_17
- León-Velarde F, Gamboa A, Chuquiza JA, Esteba WA, Rivera-Chira M, Monge CC. Hematological parameters in high altitude residents living at 4,355, 4,660, and 5,500 meters above sea level. High Alt Med Biol. 2000;1(2):97–104. doi:10.1089/15270290050074233
- Zubieta-Calleja G, Zubieta-DeUrioste N. The oxygen transport triad in high-altitude pulmonary edema: a perspective from the high Andes. IJERPH. 2021;18(14):7619. doi:10.3390/ijerph18147619