Abstract
Purpose: The present study aims to evaluate the effect of prognostic nutrition index (PNI) on the response and prognosis of patients with metastatic biliary tract cancer (BTC) patients treated with immunotherapy.Methods: The outcomes of 83 patients with metastatic BTC were evaluated retrospectively. Among them, 51 received immune checkpoint inhibitors (ICIs) treatment (ICIs cohort) and 32 patients received first-line chemotherapy (chemotherapy cohort). According to the optimal cutoff value of PNI, patients in ICIs cohort were divided into low PNI group (PNI < 44.30) and high PNI group (PNI≥ 44.30).Results: Patients received first-line immunotherapy-based combination antitumor therapy in ICIs cohort showed significant longer median PFS and OS contrast with first-line chemotherapy cohort. In ICIs cohort, median PFS and OS were significantly longer in the high PNI group. In addition, multivariate Cox regression analysis showed that high PNI was an independent risk factor for median PFS (hazard ratio (HR), 0.474, 95% CI, 0.246–0.910; P = 0.025) and median OS (HR, 0.229, 95% CI, 0.097–0.539; P = 0.001) in ICIs cohort, respectively. Conclusion: Our study provides preliminary evidence that immunotherapy for metastatic BTC is effective and safe. PNI was an independent prognostic indicator of median PFS and OS in patients with metastatic BTC receiving immunotherapy.
Introduction
Biliary tract cancers (BTC) are a group of rare but highly fatal carcinomas originating from the epithelial cells of the bile duct (Citation1). Depending on their anatomical location within the biliary tree, BTC are divided into gallbladder cancer, intrahepatic cholangiocarcinoma (CCA) and extrahepatic CCA (including perihilar and distal CCA) (Citation2–4). Globally, biliary tract cancer is the second most common primary liver cancer after hepatocellular carcinoma, and its incidence rate is increasing (Citation5–7).
Radical surgery is still the only potential curative therapeutic option to treat BTC, but unfortunately, the majority of BTC patients initially suffered from advanced disease and lost the chance to receive R0 surgery (Citation8). In addition, even after surgical resection with negative margin, the distant and local recurrence rates are still high (Citation9). Thus, the prognosis for advanced BTC remains poor as the 5 year overall survival rate is only approximately 5% (Citation10,Citation11). Systemic treatments represent the main treatment for metastatic BTC. The phase III ABC-02 trial, a milestone clinical study published in 2010, established the GEMCIS regimen (gemcitabine plus cisplatin) as the standard first-line chemotherapy treatment in advanced BTC for more than a decade, although with limited efficacy (OS: 11.7 mo,) (Citation12). Second-line treatment options are limited.
In the past decade, the clinical application of immune checkpoint inhibitors (ICIs) has revolutionized the treatment mode of multiple malignancies (Citation13–16). There have been a series of studies exploring the role of ICIs in BTC, including ICIs monotherapy, combined with anti-angiogenic agents or combined with chemotherapy (Citation17–22). Recently, a randomized phase III TOPAZ-1 trial (NCT03875235) evaluated the efficacy and safety of durvalumab (a PD-L1 inhibitor) plus GEMCIS as first-line treatment in advanced BTC (Citation23). Compared with standard GEMCIS chemotherapy alone, Durvalumab combined with GEMCIS chemotherapy significantly improved the survival rate. Meanwhile, addition of ICIs to GEMCIS was safe and well tolerated. This study has made a milestone breakthrough in the treatment of advanced BTC and changed the current situation of limited treatment options for BTC.
Malnutrition tends to occur in cancer patients and is closely related to the disease progression of cancer patients (Citation24–26). Prognostic nutritional index (PNI) represents a simple method to evaluate the immune nutritional status of cancer patients (Citation27). A series of studies have shown that PNI is closely related to the prognosis of various malignant tumors (Citation28–32). However, whether PNI is associated with clinical outcomes of metastatic biliary tract cancer patients treated with immunotherapy remains unclear. In addition, data on the efficacy of immunotherapy in BTC is still limited and unsatisfactory.
Therefore, in this study, we aimed to investigate the efficacy and safety of immune checkpoint inhibitors in Chinese patients with metastatic biliary tract cancer. Moreover, our study evaluated the value of PNI in predicting the response and prognosis in metastatic biliary tract cancer patients treated with immunotherapy.
Materials and Methods
Patients and Methods
We respectively analyzed 107 patients with metastatic biliary tract cancers at the Zhongnan hospital of Wuhan University between March 2017 and April 2022. Among them, 59 patients received immune checkpoint inhibitors (ICIs) treatment (ICIs cohort) and 48 received first-line chemotherapy (chemotherapy cohort). Clinical data were collected and searched by electronic medical records. Eligible patients were: (i) over 18 years old; (ii) histologically diagnosed with biliary tract cancers; (iii) underwent at least two cycles of chemotherapy or immunetherapy (PD-1 inhibitor or PD-L1 inhibitor) treatment; (iv) and there is at least one measurable disease. This study was conducted based on ethical guidelines of the Helsinki Declaration (revised in 2013). Informed consent was taken from all the patients and this study was approved by the Ethics Committee of Zhongnan Hospital of Wuhan University (approval number:20220127K).
Treatment and Toxicity
In ICIs cohort, patients received ICIs therapy as a monotherapy or combined with chemotherapy or anti-angiogenic therapy. Specifically, 22 patients received camrelizumab, 17 received sintilimab, five received nivolumab, and three received pembrolizumab. Treatment related toxicity was classified according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0 (CTCAE 5.0).
Efficacy and Survival Outcomes
All patients in the present study were followed up until May 30, 2022. According to response evaluation criteria in solid tumors (RECIST1.1), tumor response rate was evaluated every 4–8 weeks. Primary outcomes of the analysis included objective response rate (ORR) and progression-free survival (PFS). Overall survival and safety were secondary endpoints.
Calculation of Prognostic Nutritional Index
The PNI was calculated using the following formula: PNI = 5 × peripheral lymphocyte count (109/L) +serum albumin (g/L). The latest blood test results within 7 days before immunotherapy were selected for analysis.
Statistical Analysis
Continuous data was represented by median, and categorical data was represented by frequency (percentage). Receiver operating characteristic (ROC) analysis was established to determine the optimal cutoff value of PNI. Kaplan-Meier method and the Log rank test were used to compare the progression-free and overall survival of patients. Multivariate analysis using the COX regression method was used to identify the independent prognostic factors. The level of statistical significance was determined at P < 0.05. All statistical analysis was conducted using SPSS software version 25 (IBM, NC, USA).
Results
Clinical Characteristics
The outcomes of 107 patients with metastatic biliary tract cancer were evaluated retrospectively. In chemotherapy cohort, 48 patients with metastatic biliary tract cancers received first-line chemotherapy treatment at our center during the study period from March 2017 to April 2022. Of these, seven patients had no post-baseline assessment, and 9 had no follow-up data, leaving 32 eligible patients. Meanwhile, in ICIs cohort, 59 patients with metastatic biliary tract cancers received ICIs treatment at our center during the same study period. Of these, two patients had no post-baseline assessment, and 6 had no follow-up data, leaving 51 eligible patients. 31 patients received first-line immunotherapy. Detailed patient characteristics are presented in . The median follow-up time was 12 mo, (range 2–60 mo).
Table 1. Baseline clinical characteristics of first-line ICIs cohort and Chemotherapy cohort.
Treatment Efficacy between First-Line ICIs Cohort and Chemotherapy Group
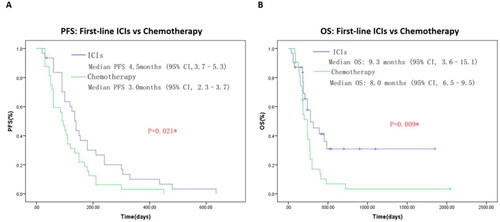
As shown in , patients received first-line immunotherapy-based combination antitumor therapy in ICIs cohort showed significant longer median PFS (4.5 vs 3.0 mo,; P = 0.021) and median OS (9.3 vs 8.0 mo,; P = 0.009) contrast with first-line chemotherapy group. Immunotherapy appear to be beneficial, effective and safe in metastatic biliary tract cancer. Our study provides preliminary evidence that Immunotherapy for metastatic BTC is effective and safe. Clinical characteristics of the patients included in this part are summarized in .
Antitumor Activity in ICIs Cohort
In ICIs cohort, at the final follow-up date of May 30, 2022, one patient achieved a CR, nine patients showed a PR, and 28 patients had a SD, with an ORR of 20.0% and a DCR of 74.5% (). The median PFS was 3.9 mo, (95% CI, 2.8–5.1). The median OS was 9.1 mo, (95% CI, 6.8–11.4) ().
Figure 2. Kaplan–Meier plot of progression-free survival (A) and overall survival (B) of whole ICIs cohort in this study.
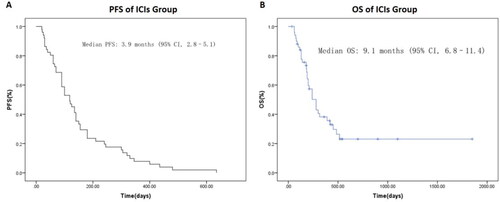
Table 2. Relationship Between Clinical Factors and Objective Response Rate in ICIs cohort.
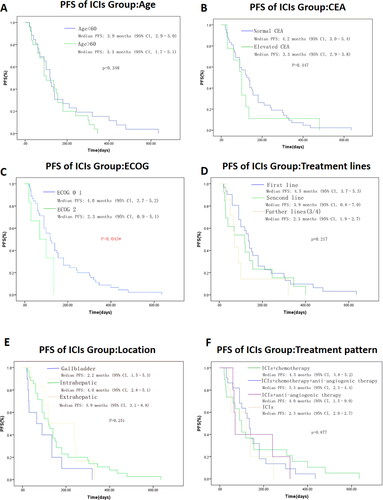
In order to explore the clinical factors that may affect the efficacy of ICIs treatment, relationship between clinical characteristics with ORR is shown in . The ORR is significantly different in patients with PNI ≥ 44.30 vs PNI < 44.30 (ORR: 36.8% vs 9.4%, P = 0.017). In addition, patients in ECOG 0–1 group showed a significant longer median PFS (ECOG 0–1 vs ECOG 2: 4.0 vs 2.3 mo, P = 0.045). We also found a tendency for longer PFS in patients with first line treatment, age < 60, intrahepatic cholangiocarcinoma, anti-PD-1 + chemotherapy and normal CEA level but without statistical significance (). There was no significant effect of gender, baseline CA19-9 level, and PD-L1 status on ORR or PFS.
Figure 3. Kaplan–Meier plot of progression-free survival of ICIs cohort in different groups of age(A), CEA level(B), ECOG performance status (C), treatment lines (D), location of primary tumor (E), and treatment pattern (F), respectively. Value with * indicate p-value is statistically significant. Abbreviations: CEA, carcinoembryonic antigen; ECOG, Eastern Cooperative Oncology Group; PFS, progression-free survival. OS, overall survival; PNI, prognostic nutrition index; PD-L1, programmed cell death ligand 1; ICIs, immune checkpoint inhibitors.
Subgroup Analysis of Relationship between PNI and Survival Outcomes in ICIs Cohort
PNI was calculated based on the serum albumin level and peripheral lymphocyte count. The median PNI in ICIs cohort was 43.30 in this study. Receiver operating characteristic (ROC) analysis was established to determine the optimal cutoff value of PNI. In ICIs cohort, the optimal cutoff value of PNI is 44.30 (sensitivity, 73.70%; specificity 75.00%). The area under curve (AUC) is 0.738 (95% CI = 0.597 ∼ 0.880, P = 0.005). Then patients in ICIs cohort were divided into low PNI group (PNI <44.30) and high PNI group (PNI ≥ 44.30) based on the optimal cutoff value of PNI determined by the receiver operating characteristic (ROC) analysis. As shown in , the patients in the high PNI group (PNI ≥44.30) had a significant longer median PFS (PNI≥ 44.30 vs PNI < 44.30: 5.10 vs 2.96 mo, P = 0.006) and median OS (PNI≥ 44.30 vs PNI < 44.30: not reached vs 6.25 mo, P = 0.000). Moreover, multivariate analysis using the COX regression method indicated that high PNI was an independent risk factor for median PFS (hazard ratio (HR), 0.474, 95% CI, 0.246–0.910; P = 0.025) and median OS (HR, 0.229, 95% CI, 0.097–0.539; P = 0.001), respectively ( and ).
Figure 4. Progression-free survival (A) and overall survival (B) of ICIs cohort according to different PNI (prognostic nutrition index) status. Value with * indicate p-value is statistically significant.
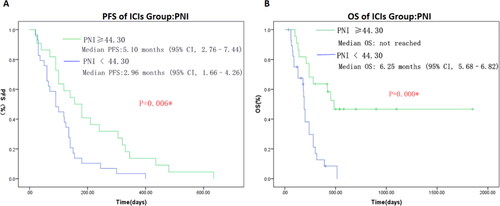
Table 3. Univariable analysis and multivariate analysis of PFS in ICIs cohort.
Table 4. Univariable analysis and multivariate analysis of OS in ICIs cohort.
Subgroup Analysis of Relationship between PDL1 and Survival Outcomes in ICIs Cohort
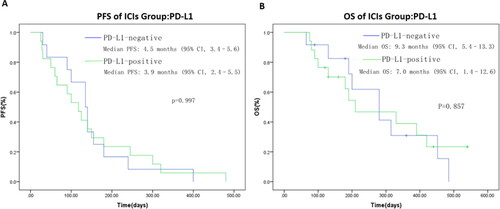
PD-L1 expression assessed by immunohistochemistry has been confirmed to associated with response to ICIs in multiple tumor types. However, to date, there is little data on the efficacy of ICIs treatment and PD-L1 expression in BTC patients. In our study, PD-L1 expression was not significantly associated with ORR or survival outcomes. ORR in PD-L1-positive and PD-L1-negative patients were 16.7% and 23.5%, respectively (). Median PFS in PD-L1-positive and PD-L1-negative patients were 3.9 and 4.5 mo, (P = 0.997), respectively (). Median OS in PD-L1-positive and PD-L1-negative patients were 7.0 and 9.3 mo, (P = 0.857), respectively ().
Adverse Events in ICIs Cohort
As shown in , the frequency of any adverse events (AEs) was 80.4% (n = 41) and most adverse events were grade 1–2. Most common grade 3–4 adverse events were thrombocytopenia (19.6%), neutropenia (7.8%), hepatic dysfunction (3.9%) and anemia (3.9%). The most common immune-related adverse events were hepatic dysfunction (25.5%) and hypothyroidism (29.4%). 3 patients (2.9%) experienced grade 3–4 immune-related adverse events, including two with hepatitis dysfunction and one with immune pneumonia. No drug-related death occurred. Adverse events associated with treatment were well tolerated. Thus, immune checkpoint inhibitors seem safe for biliary tract cancer.
Table 5. Adverse events occurring in patients in ICIs cohort.
Discussion
In this retrospective study, we aimed to investigate the efficacy and safety of immune checkpoint inhibitors (ICIs) in metastatic BTC. Herein, our study showed that immune checkpoint inhibitors appear to be beneficial, effective and safe in metastatic biliary tract cancer. Our study provides preliminary evidence that immunotherapy for metastatic BTC is effective and safe. Moreover, high pretreatment prognostic nutrition index (PNI) was an independent prognostic marker for favorable ORR, PFS and OS.
Gemcitabine based chemotherapy represent the main treatment for metastatic BTC in the past decade. Gemcitabine plus cisplatin (GEMCIS) was compared with gemcitabine alone in the phase III ABC-02 trial (Citation12). The GEMCIS group showed superior survival outcomes, although the median OS was only less than 1 year (11.7 mo, vs. 8.1 mo, p < 0.001). A randomized phase II BT22 study in Japan also showed a median OS improvement in the GEMCIS group compared with gemcitabine monotherapy (11.2 mo, vs. 7.7 mo, p < 0.001) (Citation33). Many clinical trials tried to develop a new first line treatment strategy, taking into account the limited benefit of the GEMCIS scheme. However, many studies based on gemcitabine or platinum chemotherapy regimens failed to further improve survival in advanced BTC. Phase III FUGA-BT clinical trial demonstrated that the survival outcomes of gemcitabine plus S-1 were similar with GEMCIS in advanced BTC (Citation34). In the phase II/III PRODIGE 38 AMEBICA trial, modified FOLFIRINOX (irinotecan, 5-FU and oxaliplatin) regimen also did not improve the 6-month PFS (44.6% vs. 47.3%) comparing with GEMCIS regimen (Citation35).
In recent years, the clinical application of immune checkpoint inhibitors has revolutionary changed the treatment mode of multiple malignancies, including BTC. The addition of durvalumab to GEMCIS lead to higher ORR (26.7% vs. 18.7%), PFS (7.2 vs. 5.7 mo,; HR 0.75, p = 0.001) and OS (12.8 vs. 11.5 mo,; HR 0.80, p = 0.021) compared with GEMCIS alone in TOPAZ-1 trial (Citation23). Based on the encouraging positive results of TOPAZ-1 trial, the NCCN clinical guidelines(2022v1) recommend durvalumab plus GEMCIS regimen as a new standard scheme for first-line systematic treatment for advanced BTC. This marks a key change in the first-line treatment pattern after the ABC-02 trial since 2010.
In the present study, we respectively analyzed the clinical outcomes of 83patients with metastatic biliary tract cancers who received ICIs therapy or first-line chemotherapy in our center. Patients received first-line immunotherapy in ICIs cohort showed significant longer median PFS (4.5 vs 3.0 mo,; P = 0.021) and median OS (9.3 vs 8.0 mo,; P = 0.009) contrast with first-line chemotherapy group.
In ICIs cohort, the ORR and DCR were 20.0% and 74.5%, respectively. Median PFS was 3.9 mo, (95% CI, 2.8–5.1) while the median OS was 9.3 mo, (95% CI, 6.9–11.7). Patients in ECOG 0–1 group had a significant longer median PFS compared to ECOG 2 group. A trend for longer PFS was observed in patients with first line treatment, age < 60, intrahepatic cholangiocarcinoma, anti-PD-1 + chemotherapy and normal CEA level but without statistical significance. Moreover, multivariate analysis indicated that PNI ≥ 44.30 was associated with a better prognosis (ORR, PFS and OS) (P < 0.05).
A series of studies have showed that nutritional status and immunity were closely associated with the prognosis of patients with malignant tumors (Citation28–32). PNI represents a simple method to evaluate the immune nutritional status of cancer patients. PNI was first proposed by Buzby et al in 1980. It was initially used to evaluate the surgical risk, preoperative nutritional status and postoperative complications of gastrointestinal surgery. In many subsequent studies, PNI was found to be significantly associated with the prognosis of a variety of cancers. Median PNI was 43.30 in this study. The patients were divided into low PNI group (PNI < 44.30) and high PNI group (PNI≥ 44.30) based on the optimal cutoff value of PNI determined by the receiver ROC analysis.
Our study reported that high pretreatment PNI value was an independent prognostic marker for favorable ORR, PFS and OS in metastatic BTC. Moreover, it was clarified that nutrition intervention could lower the risk of death of many cancer patients. A randomized study revealed that nutritional administration of whole-course could improve the therapeutic resistance and short-term prognosis of patients with esophageal cancer receiving concurrent radiochemotherapy (Citation36). Early nutritional and psychological intervention can reduce mortality in patients with advanced esophageal cancer by 32%, according to a randomized controlled clinical trial (Citation37). Therefore, our study suggests that screening high-risk patients with poor prognosis through PNI in the whole process management and further guiding clinicians to formulate individualized treatment and nutritional support strategies may be of great significance to improve the prognosis of patients with metastatic BTC.
PD-L1 expression detected using immunohistochemistry has been confirmed to associated with response to ICIs in multiple cancer types, including non-small cell lung cancer (NSCLC), urothelial carcinoma and gastric cancer (Citation38–40). However, to date, there is little data on the efficacy of ICIs treatment and PD-L1 expression in BTC patients. One study evaluated the correlation between PD-L1 expression and clinical outcomes in 54 intrahepatic cholangiocarcinoma patients (Citation41). This study found that PD-L1-positive group had worse survival outcomes compared to PD-L1-negative group. In the KEYNOTE-158 phase II trial, pretreated BTC patients with advanced disease were treated with pembrolizumab (Citation17). The ORR was only 5.8%. The median PFS and OS were 2.0 and 7.4 mo, respectively. In the subgroup analysis, ORR in PD-L1-positive (n = 61) and PD-L1-negative (n = 34) patients were 6.6% and 2.9%, respectively. A phase II clinical trial evaluated the efficacy and safety of nivolumab as second-line treatment for advanced BTC. The median PFS and OS in this study were 3.7 and 14.2 mo, respectively. PD-L1-positive patients has a significantly longer median PFS compared to PD-L1-negative patients (10.4 mo, vs 2.3 mo,; p < 0.001) (Citation19). In our study, PD-L1 expression was not significantly associated with ORR or survival outcomes in ICIs cohort. Overall, the correlation between PD-L1 expression level and ICIs treatment response remains to be determined.
The frequency of any adverse events (AEs) was 80.4% (n = 41) and most adverse events were grade 1–2 in ICIs cohort. Most common treatment-related AEs were neutropenia, thrombocytopenia, hypothyroidism, and liver dysfunction. Adverse events associated with treatment were well tolerated. Thus, immune checkpoint inhibitors seem safe in biliary tract cancer.
This study has limitations. This retrospective study was only a single center study, involving relatively few patients with metastatic biliary cancer. The results of this study should be further confirmed by a prospective study of large samples.
Conclusion
In this study, we found that ICIs appear to be beneficial, effective and safe in metastatic biliary tract cancer. Our study provides preliminary evidence that Immunotherapy for metastatic BTC is effective and safe. Moreover, high pretreatment prognostic nutrition index (PNI) was an independent prognostic marker for favorable ORR, PFS and OS. However, like the results of other studies, our study also showed that the prognosis of patients with metastatic BTC was very poor. Thus, more effective treatments still need to be explored.
Ethics approval and consent to participate
Informed consent was taken from all the patients and this study was approved by the Ethics Committee of Zhongnan Hospital of Wuhan University (approval number:20220127K).
Authors’ contributions
Conception and design: Lei Yang and Fuxiang Zhou; Administrative support: Juan Zhong; Provision of study materials or patients: Lei Yang and Juan Zhong; Collection and assembly of data: Juan Zhong and Wenbo Wang; Data analysis and interpretation: Lei Yang and Wenbo Wang; Manuscript writing: All authors; Final approval of manuscript: All authors. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Valle JW, Kelley RK, Nervi B, Oh D-Y, Zhu AX. Biliary tract cancer. Lancet. 2021;397(10272):428–444.
- Kam AE, Masood A, Shroff RT. Current and emerging therapies for advanced biliary tract cancers. Lancet Gastroenterol Hepatol. 2021;6(11):956–969.
- Razumilava N, Gores GJ. Classification, diagnosis, and management of cholangiocarcinoma. Clin Gastroenterol Hepatol. 2013;11(1):13–21.e1. quiz e3-4.
- Forner A, Vidili G, Rengo M, Bujanda L, Ponz-Sarvisé M, Lamarca A. Clinical presentation, diagnosis and staging of cholangiocarcinoma. Liver Int. 2019;39(Suppl 1):98–107.
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA A Cancer J Clin. 2021;71(1):7–33.
- Florio AA, Ferlay J, Znaor A, Ruggieri D, Alvarez CS, Laversanne M, Bray F, McGlynn KA, Petrick JL. Global trends in intrahepatic and extrahepatic cholangiocarcinoma incidence from 1993 to 2012. Cancer. 2020;126(11):2666–2678.
- Massarweh NN, El-Serag HB. Epidemiology of hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Cancer Control. 2017;24(3):1073274817729245.
- Neumann UP, Schmeding M. Role of surgery in cholangiocarcinoma: from resection to transplantation. Best Pract Res Clin Gastroenterol. 2015;29(2):295–308.
- Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: systematic review and meta-analysis. JAMA Surg. 2014;149(6):565–574.
- Tariq NU, McNamara MG, Valle JW. Biliary tract cancers: current knowledge, clinical candidates and future challenges. Cancer Manag Res. 2019;11:2623–2642.
- Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, Lind GE, Folseraas T, Forbes SJ, Fouassier L, et al. Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol. 2016;13(5):261–280.
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. New Eng J Med. 2010;363(2):198–
- Cheng M, Wang H, Zhao Y, Li G. Efficacy and prognostic factors for response to PD-1 inhibitors in advanced cervical carcinoma: a retrospective study. Drug Des Devel Ther. 2022;16:887–897.
- Ren S, Xiong X, You H, Shen J, Zhou P. The combination of immune checkpoint blockade and angiogenesis inhibitors in the treatment of advanced non-small cell lung cancer. Front Immunol. 2021;12:689132.
- Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin. 2021;71(3):264–279.
- Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16:223–249.
- Piha-Paul SA, Oh D-Y, Ueno M, Malka D, Chung HC, Nagrial A, Kelley RK, Ros W, Italiano A, Nakagawa K, et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: results from the KEYNOTE-158 and KEYNOTE-028 studies. Int J Cancer. 2020;147(8):2190–2198.
- Woods E, Le D, Jakka BK, Manne A. Changing landscape of systemic therapy in biliary tract cancer. Cancers (Basel). 2022;14(9):2137.
- Kim RD, Chung V, Alese OB, El-Rayes BF, Li D, Al-Toubah TE, Schell MJ, Zhou J-M, Mahipal A, Kim BH, et al. A phase 2 multi-institutional study of nivolumab for patients with advanced refractory biliary tract cancer. JAMA Oncol. 2020;6(6):888–894.
- Rizzo A, Ricci AD, Brandi G. PD-L1, TMB, MSI, and other predictors of response to immune checkpoint inhibitors in biliary tract cancer. Cancers (Basel). 2021;13(3):558.
- Klein O, Kee D, Nagrial A, Markman B, Underhill C, Michael M, Jackett L, Lum C, Behren A, Palmer J, et al. Evaluation of combination nivolumab and ipilimumab immunotherapy patients with advanced biliary tract cancers: subgroup analysis of a phase 2 nonrandomized clinical trial. JAMA Oncol. 2020;6(9):1405–1409.
- Sahai V, Griffith KA, Beg MS, Shaib WL, Mahalingam D, Zhen DB, Deming DA, Dey S, Mendiratta-Lala M, Zalupski M, et al. A multicenter randomized phase II study of nivolumab in combination with gemcitabine/cisplatin or ipilimumab as first-line therapy for patients with advanced unresectable biliary tract cancer (BilT-01). JCO. 2020;38(15_suppl):4582–
- Oh D-Y, He AR, Qin S, Chen L-T, Okusaka T, Vogel A, Kim JW, Suksombooncharoen T, Lee MA, Kitano M, et al. A phase 3 randomized, double-blind, placebo-controlled study of durvalumab in combination with gemcitabine plus cisplatin (GemCis) in patients (pts) with advanced biliary tract cancer (BTC): TOPAZ-1. JCO. 2022;40(4_suppl):378–
- Ravasco P. Nutrition in cancer patients. JCM. 2019;8(8):1211.
- Song C, Cao J, Zhang F, Wang C, Guo Z, Lin Y, Shi Y, Hu W, Ba Y, Xu H, et al. Nutritional risk assessment by scored patient-generated subjective global assessment associated with demographic characteristics in 23,904 common malignant tumors patients. Nutr Cancer. 2019;71(1):50–60.
- Li Y, Wang W-B, Yang L, Wang Q-Y, Dai J, Xia L, Peng J, Zhou F-X, Wei Y-C, Shi H-P, et al. The combination of body composition conditions and systemic inflammatory markers has prognostic value for patients with gastric cancer treated with adjuvant chemoradiotherapy. Nutrition. 2022;93:111464.
- Buzby GP, Mullen JL, Matthews DC, Hobbs CL, Rosato EF. Prognostic nutritional index in gastrointestinal surgery. Am J Surg. 1980;139(1):160–167.
- Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. 1984;85(9):1001–1005.
- Okadome K, Baba Y, Yagi T, Kiyozumi Y, Ishimoto T, Iwatsuki M, Miyamoto Y, Yoshida N, Watanabe M, Baba H, et al. Prognostic nutritional index, tumor-infiltrating lymphocytes, and prognosis in patients with esophageal cancer. Ann Surg. 2020;271(4):693–700.
- Hu Y, Shen J, Liu R, Feng Z, Zhang C, Ling L, Chen L. Prognostic value of pretreatment prognostic nutritional index in non-small cell lung cancer: A systematic review and meta-analysis. Int J Biol Markers. 2018;33(4):372–378.
- Wang D, Hu X, Xiao L, Long G, Yao L, Wang Z, Zhou L. Prognostic nutritional index and systemic immune-inflammation index predict the prognosis of patients with HCC. J Gastrointest Surg. 2021;25(2):421–427.
- Dai Y, Fu X, Li T, Yao Q, Su L, Su H, Li J. Long-term impact of prognostic nutritional index in cervical esophageal squamous cell carcinoma patients undergoing definitive radiotherapy. Ann Transl Med. 2019;7(8):175.
- Fukutomi A, Furuse J, Okusaka T, Miyazaki M, Taketsuna M, Koshiji M, Nimura Y. Effect of biliary drainage on chemotherapy in patients with biliary tract cancer: an exploratory analysis of the BT22 study. HPB (Oxford). 2012;14(4):221–227.
- Morizane C, Okusaka T, Mizusawa J, Katayama H, Ueno M, Ikeda M, Ozaka M, Okano N, Sugimori K, Fukutomi A, et al. Combination gemcitabine plus S-1 versus gemcitabine plus cisplatin for advanced/recurrent biliary tract cancer: the FUGA-BT (JCOG1113) randomized phase III clinical trial. Ann Oncol. 2019;30(12):1950–1958. doi: 10.1093/annonc/mdz402.
- Phelip JM, Desrame J, Edeline J, Barbier E, Terrebonne E, Michel P, Perrier H, Dahan L, Bourgeois V, Akouz FK, PRODIGE 38 AMEBICA Investigators/Collaborators, et al. Modified FOLFIRINOX Versus CISGEM chemotherapy for patients with advanced biliary tract cancer (PRODIGE 38 AMEBICA): a randomized phase II study. J Clin Oncol. 2022;40(3):262–271.
- Lyu J, Shi A, Li T, Li J, Zhao R, Zhu S, Wang J, Xing L, Yang D, Xie C, et al. Effects of enteral nutrition on patients with oesophageal carcinoma treated with concurrent chemoradiotherapy: a prospective, multicentre, randomised, controlled study. Front Oncol. 2022;12:839516.
- Lu Z, Fang Y, Liu C, Zhang X, Xin X, He Y, Cao Y, Jiao X, Sun T, Pang Y, et al. Early interdisciplinary supportive care in patients with previously untreated metastatic esophagogastric cancer: a phase III randomized controlled trial. J Clin Oncol. 2021;39(7):748–756.
- Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. 2019;37(7):537–546.
- Powles T, Walker J, Andrew Williams J, Bellmunt J. The evolving role of PD-L1 testing in patients with metastatic urothelial carcinoma. Cancer Treat Rev. 2020;82:101925.
- Rizzo A, Mollica V, Ricci AD, Maggio I, Massucci M, Rojas Limpe FL, Fabio FD, Ardizzoni A. Third- and later-line treatment in advanced or metastatic gastric cancer: A systematic review and meta-analysis. Future Oncol. 2020;16(2):4409–4418.
- Gani F, Nagarajan N, Kim Y, Zhu Q, Luan L, Bhaijjee F, Anders RA, Pawlik TM. Program death 1 immune checkpoint and tumor microenvironment: implications for patients with intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2016;23(8):2610–2617.