Abstract
Malnutrition and cachexia occur commonly in patients with advanced gastric cancer (AGC). This study elucidated the effect of nutritional support (NS) on survival outcomes among patients with AGC undergoing chemotherapy. We retrospectively evaluated new AGC cases at our institute between January 2015 and January 2021. Inclusion criteria were unresectable or recurrent chemotherapy-treated gastric adenocarcinoma, ECOG performance status (PS) 0–2, and adequate organ function. Time to treatment failure (TTF) and overall survival (OS) were evaluated, and univariate and multivariate analyses identified prognostic factors. A total of 103 eligible patients were separated into groups: 69 patients (67%) into NS and 34 (33%) into routine care (RC). The median follow-up time was 11.0 mo, (0.5–92). NS was offered to patients with poorer PS (p = 0.03), Glasgow prognostic score (GPS) positivity (p = 0.001), and high neutrophil-to-lymphocyte ratios (cut-off ≤ 3, p = 0.02). Median OS and TTF in the RC and NS groups were 11.6 and 10.4 mo, (p = 0.99) and 4.2 and 5.5 mo, (p = 0.07), respectively. Multivariate analyses identified NS (hazard ratio [HR] = 0.53, p = 0.01) and GPS positivity for TTF, and low body mass index (HR = 2.03, p = 0.007) and GPS positivity (HR = 2.25, p = 0.001) for OS as significant prognostic factors. Thus, NS with chemotherapy is a potentially effective intervention for AGC.
Introduction
Gastric cancer (GC) is a significant health problem worldwide and is the fourth leading cause of cancer-related death globally (Citation1). The standard of care for advanced gastric cancer (AGC) is systemic chemotherapy and supportive care. Currently, the preferred first-line therapies are oxaliplatin plus fluoropyrimidine plus nivolumab for human epidermal growth factor receptor-2 (HER2)-negative AGC (Citation2,Citation3) and fluoropyrimidine plus platinum-based chemotherapeutic agents (oxaliplatin or cisplatin) plus pembrolizumab plus trastuzumab for HER2 overexpressing AGC (Citation4). Although chemotherapy and immunotherapy improve prognosis, the median overall survival is limited to less than 14–16 mo, for HER2 negative AGC.
Current studies suggest that cancer-associated malnutrition, cachexia, and loss of skeletal muscle volume (sarcopenia) are associated with poor prognosis in upper gastrointestinal tract cancer (Citation5–7). Malnutrition is quite prevalent among patients with AGC. A prospective study of 1913 cancer patients revealed that half of gastric and esophageal cancer patients suffered from malnutrition (Citation8). Approximately 40–80% of patients with AGC develop cachexia, which has a negative impact not only on survival outcomes but also on the patient’s quality of life (Citation9–11). The pathophysiology of cancer-related cachexia is complicated. It is a multifactorial syndrome and is now considered to be caused by metabolic, immunological, and neurological abnormalities rather than mere nutritional abnormalities (Citation12). Metabolic abnormalities (loss of adipose tissue and skeletal muscle) and anorexia, represented by increased catabolism, are common phenomena in AGC, and cachexia is an important pathological aspect of GC, which needs to be addressed for comprehensive assessment and timely management of this potentially life-threatening condition (Citation13).
NS is recognized as an essential part of intervention for patients with advanced cancer exhibiting cachexia. Evidence-based guidelines recommend active nutritional assessment and intervention for advanced cancer patients with malnutrition or cachexia (Citation14–16). Many studies have been conducted examining nutritional therapy for patients with GC in the perioperative setting, and meta-analyses suggest it is a promising intervention for those receiving gastrectomy (Citation17). Several randomized controlled trials found that intensive nutritional intervention improved nutritional status and chemotherapy adherence in resectable cancer (Citation18–20).
Although some studies suggest that nutritional support is effective in patients with gastrointestinal cancer treated with chemotherapy (Citation21,Citation22), its impact on advanced, metastatic, and recurrent GC is not clear. The present study was conducted to evaluate whether NS provided by specialized dieticians and integrative teams and designed for patients with malnutrition or cachexia could improve outcomes in patients with AGC.
Materials and Methods
Study Population
A total of 684 patients, newly diagnosed with gastric or esophagogastric junction cancer at our medical center between January 2015 and January 2021 were screened. The inclusion criteria were age >18 years, unresectable or recurrent gastric adenocarcinoma, treatment with fluoropyrimidine monotherapy or fluoropyrimidine containing regimen as first-line therapy (trastuzumab was allowed for HER2 positive type), and an Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0–2. Patients with recurrence after curative resection during adjuvant therapy or within 6 mo, after completion of adjuvant therapy and those with poor organ function were excluded.
This study was approved by the Institutional Review Board in Nagoya Medical Center (No. 2020-046) and was conducted according to the principles of the Declaration of Helsinki. Informed consent was obtained in the form of opt-out.
Outcome Measures and Statistical Analysis
The treatment plan was chosen by the physician based on guidelines and conference discussions. Treatment consisted of chemotherapy and the appropriate supportive care, including standard antiemetic drugs. Nutritional care was provided at the discretion of the physician by a registered dietitian specializing in treating cancer cases. The patients were grouped according to whether they received routine care (RC) or nutritional support (NS). The relationship between clinical and pathological factors with NS were analyzed using a chi-square test. The overall response rate (ORR) was evaluated using the Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 guidelines (Citation23). Adverse events were assessed using the Common Terminology Criteria for Adverse Events (CTCAE) v5.0 guidelines (Citation24). Patients were classified into three groups according to the Glasgow prognostic score (GPS): patients with both elevated serum creatinine protein (CRP) (>10 mg/l) and hypoalbuminemia (<35 g/l) were given a score of 2; patients with only CRP >10 mg/l or hypoalbuminemia (<35 g/l) were allocated a score of 1; and patients with neither of these abnormalities were assigned a score of 0 (Citation25). The prognostic nutritional index (PNI) was calculated as follows: Albumin (g/l) + 5 × total lymphocyte count × 109/l (Citation26). Time to treatment failure (TTF) was calculated from the date of the first chemotherapy treatment to the date of treatment discontinuation. Overall survival (OS) was calculated from the date of first chemotherapy treatment to date of death or the last visit. To analyze the effect of NS on body weight (BW) change during first-line therapy, percent BW loss (%BWL) per month (%BWL/m) was examined. %BWL was calculated as BW (kg) at the beginning of 1st line therapy – (BW at the end of 1st line therapy) × 100/BW at baseline. %BWL/m was calculated as %BWL/duration of treatment (months). Treatment exposures were evaluated by relative dose intensity (RDI). RDI was defined as the actual dose divided by the standard dose in each of the five regimens: FOLFOX (5-fluorouracil, leucovorin, oxaliplatin), CAPOX (capecitabine plus oxaliplatin), SOX (S-1 plus oxaliplatin), SP (S-1 plus cisplatin), and S-1 monotherapy. Survival curves were estimated using Kaplan–Meier curves and compared with the log-rank test. Univariate and multivariate Cox regression analyses were performed to determine whether NS affected TTF or OS, and variables with p ≤ 0.1 in univariate analyses and NS were subjected to multivariate analyses. Two-sided p-values of ≤0.05 were considered statistically significant. All statistical analyses were performed using EZR (The R Foundation for Statistical Computing, Vienna, Austria) (Citation27).
Nutritional Assessment and Intervention
Initially, clinical nutritionists evaluated energy requirements (25–30 kcal/kg/day) and assessed energy intake using a 24-hour recall food questionnaire. Cancer-associated cachexia was assessed by the GPS. Subsequently, nutritionists provided dietary counseling to patients and evaluated the cancer-associated weight loss and nutrition impact symptoms (NIS, for example, anorexia, nausea, vomiting, oral mucositis, constipation, diarrhea, dysphagia, and malaise) at baseline. Consequently, nutritionists recommended individualized meal plans (amount of food, form of food, addition of protein or fat ingredients, and dietary supplements) and recipe suggestions. The duration of the first assessment and counseling was 20–30 mins. After completion of the first session of nutritional intervention and starting chemotherapy, the nutritionists continually reevaluated the patient’s energy intake, nutritional status (serum albumin, CRP, electrolyte), body weight, and NIS in collaboration with a medical oncologist, oncology nurse, and pharmacist. In the second evaluation session, the percentage of calorie requirement fulfillment was assessed, and further advice was given if there were any deficiencies. The assessments and interventions by nutritionists were provided in-person in the examination rooms or outpatient chemotherapy units during infusion. The second or further assessment and intervention were performed on the same day as the patients’ chemotherapy visit. During in-patient care, a NS team consisting of nutritionists, gastroenterologists, dentists, dental hygienists, laboratory technicians, and rehabilitation technicians were responsible for nutritional assessment and intervention. The evaluations and interventions mentioned above were conducted according to the Japanese Society for Clinical Nutrition and Metabolism and the European Society for Clinical Nutrition and Metabolism guidelines (Citation14).
Results
Patient Characteristics
Of 684 total newly registered AGC cases in our medical center between January 2015 and January 2021, 103 patients met the inclusion criteria and were analyzed after excluding patients with early-stage or resectable cancer, advanced cancer but not received chemotherapy, poor performance score (PS), or impaired organ function (). Of the 103 patients who met the inclusion criteria, 69 (67%) and 34 (33%) patients were classified into the RC and NS group, respectively. The patients’ characteristics are shown in . The overall median age was 71 (range, 26–92) years. The median follow-up time was 11.0 (0.5–92) months. Of the patients, 86% received platinum-based chemotherapeutic agents plus fluoropyrimidine (27% and 73% of patients were administered cisplatin and oxaliplatin, respectively), and the others were treated with fluoropyrimidine monotherapy. There were significant differences in PS (p = 0.03), prior gastrectomy (p = 0.01), and chemotherapy regimens (p = 0.03) between the two groups.
Figure 1. Study flow chart of inclusion and exclusion criteria. BSC, best supportive care; PS, performance status.
Table 1. Patient characteristics.
Nutritional Status
Nutritional status is shown in . Body mass index (<18.5, 18.5–25, or ≥25 kg/m2) in the RC and NS groups were 27.5, 62.3, or 10.1 and 14.7, 76.5, or 9.8%, respectively (p = 0.33). GPS positivity (score 1 and 2, p = 0.001), hypoalbuminemia (cut-off ≤35 g/l, p = 0.05), low PNI (cut-off ≤40, p = 0.02), and high neutrophil-to-lymphocyte ratio (cut-off ≤3, p = 0.01) were associated with NS. The average absolute body weights, before commencing chemotherapy and at the end of the first-line chemotherapy, and the weight change (mean % BWL/m) are presented in Supplementary Table S1. Weight change per time during the first-line chemotherapy was favorable in the GPS 1 and 2 groups and the hypoalbuminemia group, respectively, indicating that patients who received NS tended to maintain their body weight under first-line chemotherapy. Changes in albumin levels before and after the start of chemotherapy were analyzed by student’s t-test: the RC group had a mean of −2.5 mg/l and the NS group had a mean of −0.05 mg/l (p = 0.14), which was not statistically significant, but there was a trend toward the preservation of albumin levels in the NS group.
Treatment Outcomes
The ORR and disease control rate (DCR) were not significantly different in the entire cohort, RC group, and NS group with 51, 55.1, and 40.9% of ORR (p = 0.44) and 69, 73.5, and 59.1% of DCR (p = 0.44) for the entire cohort, RC, and NS groups respectively. Median OS and TTF in the RC and NS groups were 11.6 and 10.4 mo, (p = 0.99) and 4.2 and 5.5 mo, (p = 0.07), respectively (). Among the GPS-negative patients, median OS and TTF in the RC and NS groups were 20.0 mo, and unattained (p = 0.11) and 7.0 and 11.3 mo, (p = 0.04), respectively (). Among the GPS-positive patients, median OS and TTF in the RC and NS groups were 6.1 and 9.5 mo, (p = 0.49) and 2.3 and 5.3 mo, (p = 0.07), respectively (). Among the patients with hypoalbuminemia (cut-off ≤35 g/l), median OS and TTF in the RC and NS groups were 6.3 and 10.2 mo, (p = 0.33) and 2.3 and 5.3 mo, (p = 0.04), respectively. Among the elevated CRP cases (cut-off ≤10 mg/l), median OS and TTF in the RC and NS groups were 6.1 and 7.4 mo, (p = 0.81) and 2.2 and 5.3 mo, (p = 0.39), respectively. As calculated by the multivariate analyses, NS (hazard ratio [HR] = 0.53, 95% CI = 0.33–0.84, p = 0.01) and GPS positivity (HR = 1.78, 95% CI = 1.09–2.90, p = 0.01) for TTF and low body mass index (cut-off ≤18.5 [HR = 2.03, 95% CI = 1.20–3.41, p = 0.007]) and GPS positivity (HR = 2.25, 95%CI = 1.21–4.17, p = 0.001) for OS were significant prognostic factors (). Second-line therapy was given to a similar percentage of the patients (61.2 and 65.4% for the RC and NS groups, respectively; p = 0.81). Similarly, a comparison between the groups with additional lines of therapy, third line (35.8 and 31% for the RC and NS groups, respectively; p = 0.81), and fourth-line or subsequent-line of therapy (19% and 20.7% for the RC and NS groups, respectively; p = 1.00) was also done. We analyzed subsequent chemotherapy in sub-groups of the GPS 1 and 2 groups and hypoalbuminemia group, and no significant inter-group differences were observed (data not shown). No significant inter-group differences were noted between the RC and NS groups in terms of relative dose intensity (Supplementary Table S2) or grade three or higher hematologic and non-hematologic toxicities (). Any grade lymphocytopenia was significantly more common in the NS group than in the RC group (p = 0.01).
Figure 2. Kaplan–Meier survival curves for overall survival and time to treatment failure for the entire cohort (A), Glasgow prognostic score 0 cohort (B), and Glasgow prognostic score 1 and 2 cohort (C). NS, nutritional support; OS, overall survival; RC, routine care; TTF, time to treatment failure.
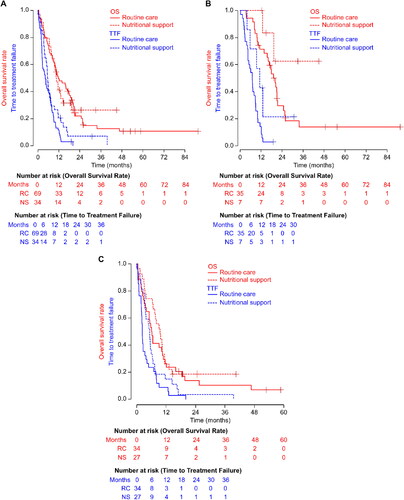
Table 2. Univariate and multivariate analyses of factors associated with TTF and OS.
Table 3. Adverse events.
Discussion
This study evaluated the impact of NS on oncological outcomes in patients with AGC receiving chemotherapy. In this retrospective study, patient characteristics of RC and NS groups were found to be different for several clinical factors. We analyzed the prognostic impact of some of the significant factors, including ECOG PS, prior gastrectomy, the types of platinum-based chemotherapeutic agent administered, serum albumin level, PNI (Citation28), NLR (Citation26), and GPS (Citation29). We found that PS, PNI, NLR, and GPS were negative prognostic factors in AGC cases; therefore, patients in the NS group would have a worse prognosis than those in the RC group. Despite differences in the background, median OS and TTF in the RC vs. NS groups were comparable. Moreover, in the subgroups of GPS 0, GPS 1-2, and serum hypoalbuminemia, TTF and OS tended to be better in the NS group than in the RC group. Multivariate analysis revealed that NS was an independent prognostic factor in TTF. These results imply that active NS by specialists can improve the prognosis of patients with AGC treated with chemotherapy. This study did not find a significant difference in ORR, adverse event rate, treatment exposure, or subsequent therapy. Body weight loss during chemotherapy is a negative prognostic factor in these patients (Citation30–32). In the presented data, body weight loss in the NS group was less severe than in the RC group. We assume that maintaining body weight through nutritional interventions is a core mechanism for favorable prognosis in nutritional care. Some randomized controlled trials investigating the effect of NS on prognosis in advanced cancer patients receiving chemotherapy failed to show its significant efficacy (Citation33,Citation34). Because these trials included patients with advanced cancer regardless of cancer type and chemotherapy regimen, it was difficult to evaluate the impact of NS on outcomes. A randomized phase three trial recently demonstrated that early active supportive care, including nutritional and psychological care, provided better outcomes than RC in advanced and esophageal carcinoma patients treated with chemotherapy (Citation35). From the results of these trials, it seems appropriate that studies examining the impact of supportive care on prognosis be conducted for the same cancer type and treatment.
Currently, a programmed cell death protein 1 (PD-1) pathway inhibitor is widely used in first-line or later-line therapy for AGC (Citation2,Citation3,Citation36). Most of the studies on GC and NS cited here were conducted before the immunotherapy era. Some studies reported that the efficacy of anti-PD-1 pathway inhibitors in AGC is influenced by NS and muscle mass (Citation37,Citation38). Weight loss in patients with GC is common and is associated with poor survival rates and reduced quality of life. NS activates the immune response in clinically ill and cancer patients (Citation22,Citation39). To maximize the efficacy of systemic therapy with the presently available immunotherapeutic agents, further research on the integration of chemo-immunotherapy and nutritional care is essential to develop an appropriate therapeutic regimen for patients with GC.
The present study assessed the effect of dietary advice and/or oral nutritional supplements on survival, nutritional endpoints, and quality of life in patients with weight loss receiving palliative chemotherapy for AGC. Although international guidelines of nutritional care and medical oncology recommend that advanced cancer cases should be assessed and supported by a clinical dietician (Citation14–16), a previous study revealed a lack of awareness regarding malnutrition or cachexia among many oncologists (Citation40). Only 34% of AGC cases received nutritional assessment and intervention in the study (Citation40). Marshall et al. (Citation8) reported that the prevalence of malnutrition in upper gastrointestinal cancer was 61%. Therefore, it is assumed that NS was not adequately provided to the patients who needed it. With approaches other than chemotherapy, it has been reported that early palliative care improved the quality of life and OS in patients with advanced lung cancer (Citation41). It is quite possible that integrated care, including palliative care, nutritional therapy, and psychotherapy, can improve the prognosis of patients with advanced AGC. These findings suggest that physicians should carefully consider both chemotherapy and early and appropriate supportive care for patients undergoing anticancer treatment.
The present study has several limitations. First, this study is a non-randomized, retrospective study with a small sample size. Second, because of the difference in patient characteristics, analyses of oncological outcomes are complicated. However, despite the NS group having a worse oncological background compared with the RC group, TTF and OS were comparable between the two groups. A third limitation is that the content of nutritional therapy and the length of time it was provided varied from patient to patient. Finally, the patients in this analysis were treated with a variety of treatment regimens, which might have influenced the outcomes.
Further studies need to be conducted with appropriate eligibility criteria that include the chemotherapy regimen and the content of nutritional therapy to more clearly elucidate the significance of nutritional therapy. In conclusion, NS for patients with AGC under first-line chemotherapy may improve survival outcomes.
Author Contributions
Keiji Sugiyama contributed to the conception and the design of the study; Keiji Sugiyama, Kazuhiro Shiraishi, Takuya Motohashi, and Shinpei Onoda collected, analyzed, and interpreted the data; Keiji Sugiyama, Kazuhiro Shiraishi, Mariko Sato, Kyoko Kato grafted the manuscript; Masashi Hattori, Masaya Suenaga, Noboru Hirashima, Masaaki Shimada, Masato Kataoka, Chiyoe Kitagawa revised the manuscript. All authors agree to be fully accountable for ensuring the integrity and accuracy of the work and have read and approved the final manuscript.
Supplemental Material
Download MS Word (23.5 KB)Supplemental Material
Download MS Word (25.5 KB)Acknowledgments
The authors thank Editage (www.editage.jp) for English language editing. The authors also thank all the nutritionists, medical assistants (M.H), and medical information manager (M.Y) at Nagoya medical center.
Disclosure Statement
The authors have no conflict of interest to declare regarding this study.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. doi: 10.3322/caac.21660. Cited in: PMID: 33538338.
- Kang Y-K, Chen L-T, Ryu M-H, Oh D-Y, Oh SC, Chung HC, Lee K-W, Omori T, Shitara K, Sakuramoto S, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23(2):234–47. doi: 10.1016/S1470-2045(21)00692-6. Cited in: PMID: 35030335. 10.1016/S1470-2045(21)00692-6
- Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Campos Bragagnoli A, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398(10294):27–40. doi:10.1016/S0140-6736(21)00797-2. Cited in: PMID: 34102137 10.1016/S0140-6736(21)00797-2
- Janjigian YY, Kawazoe A, Yañez P, Li N, Lonardi S, Kolesnik O, Barajas O, Bai Y, Shen L, Tang Y, et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature. 2021;600(7890):727–30. doi: 10.1038/s41586-021-04161-3. Cited in: PMID: 34912120.
- Sugiyama K, Narita Y, Mitani S, Honda K, Masuishi T, Taniguchi H, Kadowaki S, Ura T, Ando M, Tajika M, et al. Baseline sarcopenia and skeletal muscle loss during chemotherapy affect survival outcomes in metastatic gastric cancer. Anticancer Res. 2018;38(10):5859–66. doi: 10.21873/anticanres.12928. Cited in: PMID: 30275211.
- Dijksterhuis WPM, Pruijt MJ, van der Woude SO, Klaassen R, Kurk SA, van Oijen MGH, van Laarhoven HWM. Association between body composition, survival, and toxicity in advanced esophagogastric cancer patients receiving palliative chemotherapy. J Cachexia Sarcopenia Muscle. 2019;10(1):199–206. doi: 10.1002/jcsm.12371. Cited in: PMID: 30666831.
- Kubo Y, Miyata H, Sugimura K, Shinno N, Asukai K, Hasegawa S, Yanagimoto Y, Yamada D, Yamamoto K, Nishimura J, et al. Prognostic implication of postoperative weight loss after esophagectomy for esophageal squamous cell cancer. Ann Surg Oncol. 2021;28(1):184–93. doi: 10.1245/s10434-020-08762-6. Cited in: PMID: 32591956. 10.1245/s10434-020-08762-6
- Marshall KM, Loeliger J, Nolte L, Kelaart A, Kiss NK. Prevalence of malnutrition and impact on clinical outcomes in cancer services: a comparison of two time points. Clin Nutr. 2019;38(2):644–51. doi: 10.1016/j.clnu.2018.04.007. Cited in: PMID: 29789167.
- Li Y-F, Nie R-C, Wu T, Li S-M, Chen S, Wang W, Chen X-J, Chen G-M, Chen Y-B, Zhou Z-W, et al. Prognostic value of the Nutritional Risk Screening 2002 Scale in metastatic gastric cancer: a large-scale cohort study. J Cancer. 2019;10(1):112–9. doi: 10.7150/jca.27729. Cited in: PMID: 30662531.
- Hsieh M-C, Wang S-H, Chuah S-K, Lin Y-H, Lan J, Rau K-M. A prognostic model using inflammation- and nutrition-based scores in patients with metastatic gastric adenocarcinoma treated with chemotherapy. Medicine (Baltimore). 2016;95(17):e3504. doi: 10.1097/MD.0000000000003504. Cited in: PMID: 27124056.
- Guo ZQ, Yu JM, Li W, Fu ZM, Lin Y, Shi YY, Hu W, Ba Y, Li SY, Li ZN, Investigation on the Nutrition Status and Clinical Outcome of Common Cancers (INSCOC) Group, et al. Survey and analysis of the nutritional status in hospitalized patients with malignant gastric tumors and its influence on the quality of life. Support Care Cancer. 2020;28(1):373–80. doi: 10.1007/s00520-019-04803-3. Cited in: PMID: 31049672.
- Argilés JM, Busquets S, Stemmler B, López-Soriano FJ. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer. 2014;14(11):754–62. doi: 10.1038/nrc3829. Cited in: PMID: 25291291.
- Ongaro E, Buoro V, Cinausero M, Caccialanza R, Turri A, Fanotto V, Basile D, Vitale MG, Ermacora P, Cardellino GG, et al. Sarcopenia in gastric cancer: when the loss costs too much. Gastric Cancer. 2017;20(4):563–72. doi: 10.1007/s10120-017-0722-9. Cited in: PMID: 28477106.
- Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, Fearon K, Hütterer E, Isenring E, Kaasa S, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36(1):11–48. doi: 10.1016/j.clnu.2016.07.015. Cited in: PMID: 27637832.
- August DA, Huhmann MB, American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors A.S.P.E.N. clinical guidelines: nutrition support therapy during adult anticancer treatment and in hematopoietic cell transplantation. JPEN J Parenter Enteral Nutr. 2009;33(5):472–500. doi: 10.1177/0148607109341804. Cited in: PMID: 19713551.
- Arends J, Strasser F, Gonella S, Solheim TS, Madeddu C, Ravasco P, Buonaccorso L, de van der Schueren MAE, Baldwin C, Chasen M, ESMO Guidelines Committee. Electronic address: [email protected], et al. Cancer cachexia in adult patients: ESMO Clinical Practice Guidelines. ESMO Open. 2021;6(3):100092. doi: 10.1016/j.esmoop.2021.100092. Cited in: PMID: 34144781.
- Rinninella E, Cintoni M, Raoul P, Pozzo C, Strippoli A, Bria E, Tortora G, Gasbarrini A, Mele MC. Effects of nutritional interventions on nutritional status in patients with gastric cancer: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr ESPEN. 2020;38:28–42. doi: 10.1016/j.clnesp.2020.05.007. Cited in: PMID: 32690170.
- Xie F-L, Wang Y-Q, Peng L-F, Lin F-Y, He Y-L, Jiang Z-Q. Beneficial effect of educational and nutritional intervention on the nutritional status and compliance of gastric cancer patients undergoing chemotherapy: a randomized trial. Nutr Cancer. 2017;69(5):762–71. doi: 10.1080/01635581.2017.1321131. Cited in: PMID: 28524705.
- Kim H, Suh EE, Lee H-J, Yang H-K. The effects of patient participation-based dietary intervention on nutritional and functional status for patients with gastrectomy: a randomized controlled trial. Cancer Nurs. 2014;37(2):E10–20. doi: 10.1097/NCC.0b013e31829193c8. Cited in: PMID: 23632471.
- Hur H, Kim SG, Shim JH, Song KY, Kim W, Park CH, Jeon HM. Effect of early oral feeding after gastric cancer surgery: a result of randomized clinical trial. Surgery. 2011;149(4):561–8. doi: 10.1016/j.surg.2010.10.003. Cited in: PMID: 21146844.
- Trestini I, Carbognin L, Sperduti I, Bonaiuto C, Auriemma A, Melisi D, Salvatore L, Bria E, Tortora G. Prognostic impact of early nutritional support in patients affected by locally advanced and metastatic pancreatic ductal adenocarcinoma undergoing chemotherapy. Eur J Clin Nutr. 2018;72(5):772–9. doi: 10.1038/s41430-018-0155-5. Cited in: PMID: 29581564.
- Chen F, Fang J, Wang H, Song T, Zhu W, Wu M, Wu Y. Effects of nutritional support on short-term clinical outcomes and immune response in unresectable locally advanced oesophageal squamous cell carcinoma. Eur J Cancer Care (Engl). 2018;27(2):e12818. doi: 10.1111/ecc.12818. Cited in: PMID: 29345017.
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. doi: 10.1016/j.ejca.2008. Cited in: PMID: 19097774.
- U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES. CTCAE v5.0 Quick Reference 5x7. National Cancer Institute Division of Cancer Treatment and Diagnosis; 2017. [accessed October 26 2021]. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf.
- Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br J Cancer. 2003;89(6):1028–30. doi: 10.1038/sj.bjc.6601242. Cited in: PMID: 12966420.
- Li Q-Q, Lu Z-H, Yang L, Lu M, Zhang X-T, Li J, Zhou J, Wang X-C, Gong J-F, Gao J, et al. Neutrophil count and the inflammation-based glasgow prognostic score predict survival in patients with advanced gastric cancer receiving first-line chemotherapy. Asian Pac J Cancer Prev. 2014;15(2):945–50. doi: 10.7314/apjcp.2014.15.2.945. Cited in: PMID: 24568523.
- Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–8. doi: 10.1038/bmt.2012.244. Cited in: PMID: 23208313.
- Demirelli B, Babacan NA, Ercelep Ö, Öztürk MA, Kaya S, Tanrıkulu E, Khalil S, Hasanov R, Alan Ö, Telli TA, et al. Modified Glasgow Prognostic Score, prognostic nutritional index and ECOG performance score predicts survival better than sarcopenia, cachexia and some inflammatory indices in metastatic gastric cancer. Nutr Cancer. 2021;73(2):230–8. doi: 10.1080/01635581.2020.1749290. Cited in: PMID: 32270713.
- Sachlova M, Majek O, Tucek S. Prognostic value of scores based on malnutrition or systemic inflammatory response in patients with metastatic or recurrent gastric cancer. Nutr Cancer. 2014;66(8):1362–70. doi: 10.1080/01635581.2014.956261. Cited in: PMID: 25356861.
- Fukahori M, Shibata M, Hamauchi S, Kasamatsu E, Machii K. A retrospective cohort study to investigate the incidence of cancer-related weight loss during chemotherapy in gastric cancer patients. Support Care Cancer. 2021;29(1):341–8. doi: 10.1007/s00520-020-05479-w. Cited in: PMID: 32361831.
- Mizukami T, Hamaji K, Onuki R, Yokomizo A, Nagashima Y, Takeda H, Umemoto K, Doi A, Arai H, Hirakawa M, et al. Impact of body weight loss on survival in patients with advanced gastric cancer receiving second-line treatment. Nutr Cancer. 2022;74(2):539–45. doi: 10.1080/01635581.2021.1902542. Cited in: PMID: 33754895.
- Ock C-Y, Oh D-Y, Lee J, Kim T-Y, Lee K-H, Han S-W, Im S-A, Kim T-Y, Bang Y-J. Weight loss at the first month of palliative chemotherapy predicts survival outcomes in patients with advanced gastric cancer. Gastric Cancer. 2016;19(2):597–606. doi: 10.1007/s10120-015-0481-4. Cited in: PMID: 25749718.
- Baldwin C, Spiro A, McGough C, Norman AR, Gillbanks A, Thomas K, Cunningham D, O’Brien M, Andreyev HJN. Simple nutritional intervention in patients with advanced cancers of the gastrointestinal tract, non-small cell lung cancers or mesothelioma and weight loss receiving chemotherapy: a randomised controlled trial. J Hum Nutr Diet. 2011;24(5):431–40. doi: 10.1111/j.1365-277X.2011.01189.x. Cited in: PMID: 21733143.
- Bourdel-Marchasson I, Blanc-Bisson C, Doussau A, Germain C, Blanc J-F, Dauba J, Lahmar C, Terrebonne E, Lecaille C, Ceccaldi J, et al. Nutritional advice in older patients at risk of malnutrition during treatment for chemotherapy: a two-year randomized controlled trial. PLoS One. 2014;9(9):e108687. doi: 10.1371/journal.pone.0108687. Cited in: PMID: 25265392.
- Lu Z, Fang Y, Liu C, Zhang X, Xin X, He Y, Cao Y, Jiao X, Sun T, Pang Y, et al. Early Interdisciplinary supportive care in patients with previously untreated metastatic esophagogastric cancer: a phase III randomized controlled trial. J Clin Oncol. 2021;39(7):748–56. doi: 10.1200/JCO.20.01254. Cited in: PMID: 33417481.
- Kang Y-K, Boku N, Satoh T, Ryu M-H, Chao Y, Kato K, Chung HC, Chen J-S, Muro K, Kang WK, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10111):2461–71. doi: 10.1016/S0140-6736(17)31827-5. Cited in: PMID: 28993052. 10.1016/S0140-6736(17)31827-5
- Kim Y-Y, Lee J, Jeong WK, Kim ST, Kim J-H, Hong JY, Kang WK, Kim K-M, Sohn I, Choi D. Prognostic significance of sarcopenia in microsatellite-stable gastric cancer patients treated with programmed death-1 inhibitors. Gastric Cancer. 2021;24(2):457–66. doi:10.1007/s10120-020-01124-x. Cited in: PMID: 32970267.
- Fujii H, Makiyama A, Iihara H, Okumura N, Yamamoto S, Imai T, Arakawa S, Kobayashi R, Tanaka Y, Yoshida K, et al. Cancer cachexia reduces the efficacy of nivolumab treatment in patients with advanced gastric cancer. Anticancer Res. 2020;40(12):7067–75. doi: 10.21873/anticanres.14734. Cited in: PMID: 33288604.
- Quiroz-Olguín G, Gutiérrez-Salmeán G, Posadas-Calleja JG, Padilla-Rubio MF, Serralde-Zúñiga AE. The effect of enteral stimulation on the immune response of the intestinal mucosa and its application in nutritional support. Eur J Clin Nutr. 2021;75(11):1533–9. doi: 10.1038/s41430-021-00877-7. Cited in: PMID: 33608653.
- De Waele E, Demol J, Caccialanza R, Cotogni P, Spapen H, Malbrain ML, De Grève J, Pen JJ. Unidentified cachexia patients in the oncologic setting: Cachexia UFOs do exist. Nutrition. 2019;63-64:200–4. doi: 10.1016/j.nut.2019.02.015. Cited in: PMID: 31029048.
- Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, Dahlin CM, Blinderman CD, Jacobsen J, Pirl WF, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733–42. doi: 10.1056/NEJMoa1000678. Cited in: PMID: 20818875.
