Abstract
This study intends to conduct a meta-analysis based on existing research results to further investigate their relationship between artificial sweetener exposure and breast cancer risk. An electronic database literature search was performed up to July 2022, using PubMed, Web of Science, Ovid and Scopus. The relationship between artificial sweetener exposure and breast cancer (BC) incidence was evaluated by odds ratio (OR) and 95% confidence interval (CI). Among the five studies (two case-control studies and three cohort studies) that met the inclusion criteria, 314,056 participants were recruited in the cohort study, 4,043 cancer cases and 3,910 controls were recruited in the case-control study. It was found that exposure of artificial sweeteners was not related to the risk of BC (OR = 0.98, 95% CI = [0.94-1.03]). Subgroup analysis showed that compared with the non-exposure/very-low-dose group, the exposure to low, medium and high doses of artificial sweeteners were not associated with the risk of BC, which were OR = 1.01, 95% CI = [0.95-1.07], OR = 0.98, 95% CI = [0.93-1.02], OR = 0.88, 95% CI = [0.74-1.06], respectively. This study confirmed that there was no relationship between the exposure of artificial sweeteners and the incidence of BC.
Introduction
With an estimated 2.3 million new cases in 2020, breast cancer (BC) has surpassed lung cancer as the leading cause of global cancer incidence. Meanwhile, it is the fifth leading cause of cancer mortality in the world, accounting for 685,000 deaths. BC accounts for 1 in every 4 cancer cases and 1 in every six cancer deaths in women (Citation1). The incidence of BC is rising, so how to effectively prevent the occurrence of BC is extremely urgent. According to some researchers, breastfeeding, reducing alcohol consumption and preventing obesity can be implemented to lower the incidence of BC (Citation2).
Artificial sweetener is a term used to describe a substance that can be added to foods and beverages in place of sugar (Citation3). Currently, artificial sweeteners including saccharin, aspartame, cyclamate, and acesulfame potassium are widely used (Citation4). High sugar intake may increase the risk of type 2 diabetes (Citation5) and long-term weight gain (Citation6) by encouraging excessive calorie intake. Obesity and type 2 diabetes have been shown to be risk factors for BC in recent years (Citation7–9). Given the deleterious health effects of excess sugar intake, the World Health Organization (WHO) recommends limiting sugar consumption to less than 10% of daily energy intake (Citation10). People’s desire for less sugar and better health, as well as their desire for flavor, collide, promoting the use of artificial sweeteners (Citation11, Citation12). Some studies believed that artificial sweeteners can reduce energy consumption, promote weight loss and maintain weight (Citation5, Citation13–15), and further speculate that they may reduce the incidence of BC. But some scholars disagreed, believing commonly used artificial sweeteners may not be able to reduce weight (Citation16). Furthermore, because the artificial sweetener is a compound, its own toxicity is concerning (Citation17). An Italian study from 2007 revealed the incidence of BC was negatively correlated with the intake of artificial sweeteners (Citation18), while a French study in 2022 showed that compared with non-consumers, consumers of total artificial sweeteners, particularly aspartame and acesulfame potassium, were associated with an increased risk of BC (Citation12). Up to now, there is no systematic review on the relationship between artificial sweeteners and the risk of BC. In order to explore the relationship between exposure to artificial sweeteners and the incidence of BC, this study focuses on analyzing and synthesizing the data on these substances and BC.
Methods
Search Strategy
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyzes (PRISMA) guidelines (Citation19). To identify the relevant articles which reported the exposure of artificial sweeteners and the risk of BC were included, all literature up to July 2022 was searched in “PubMed”, “Web of Science”, “Ovid”, “Scopus”, using the following keywords: (((((((((((((Agent, Sweetening) OR (Agents, Sweetening)) OR (Sweetening Agent)) OR (Sweeteners)) OR (Sweetener)) OR (Sugar Substitutes)) OR (Substitute, Sugar)) OR (Substitutes, Sugar)) OR (Sugar Substitute)) OR (Artificial Sweeteners)) OR (Artificial Sweetener)) OR (Sweetener, Artificial)) OR (Sweeteners, Artificial)) AND (((((((((Breast Neoplasm) OR (Neoplasm, Breast)) OR (Breast Tumors)) OR (Breast Tumor)) OR (Tumor, Breast)) OR (Tumors, Breast)) OR (Neoplasms, Breast)) OR (Breast Cancer)) OR (Cancer, Breast)). References in primary articles and related reviews were also checked manually to avoid omitting any potentially relevant studies.
Inclusion Criteria and Exclusion Criteria
Studies were included if the following criteria were met: (1) participants without BC (cohort study) at the time of recruitment or previous history of BC (case-control study). (2) The exposed group was exposed to any type or dose of artificial sweeteners, and the non-exposed group was not exposed to artificial sweeteners. (3) The incidence of BC was taken as the result. (4) The study is a case-control, prospective or retrospective cohort study. The exclusion criteria are as follows: (1) Unable to get the full text. (2) Articles not published in English. (3) Unable to extract data. (4) If the cohort or participant is duplicated, included the articles with the most complete information or the latest data.
Artificial sweetener is a kind of food additive with high sweetness but little energy, including aspartame, saccharin, neotame, acesulfame K, sucralose, etc. The exposure group was classified into three groups according to the dose: the low-dose group was 1–3 times/month; the middle-dose group was >1 time/week and <7 times/week; the high-dose group was ≥1 time/day. The intake of non-exposure group or very-low-dose group was <1 time/month (Citation20, Citation21).
Quality Assessment and Data Extraction
Case-control studies and cohort studies were evaluated by using Newcastle-Ottawa Scale (Citation22). The assessment contents include selection, comparability, outcome, or exposure. When the score is more than 6, it belongs to high-quality articles. If there are differences, the third researcher will participate in discussing and analyzing together.
Two authors independently extracted the articles’ characteristics using a pre-designed data extraction form. These significant data included author, year, country, number of BC cases, number of participants, study type, number of people exposed to artificial sweeteners, dose and duration of exposure, age at recruitment, median follow-up time, and adjusting parameters.
Statistical Analysis
All statistical analyses of data were performed using Stata12.0 software. The relationship between exposure to artificial sweeteners and the incidence of BC was evaluated by the odd ratio (OR) and 95% confidence interval (CI). The Chi-square test was used to ascertain potential heterogeneity among studies; it is considered that there is a high degree of heterogeneity when I2 > 50% (Citation23). Considering the complexity and variety of exposure factors, including various artificial sweetener kinds, exposure durations, follow-up intervals in multiple cohorts, etc. In order to improve the reliability of the results, the included studies were selected by the random effect model, and the random effect model determined the proportion of each study. At the same time, subgroup analysis was carried out to explore the source of heterogeneity. If the number of articles included in the study is more than 10, publication bias can be assessed by Begg’s and Egger’s test (Citation24), and sensitivity analysis can be used to assess the stability of the results. P < 0.05 was considered statistically significant.
Results
Literature Search
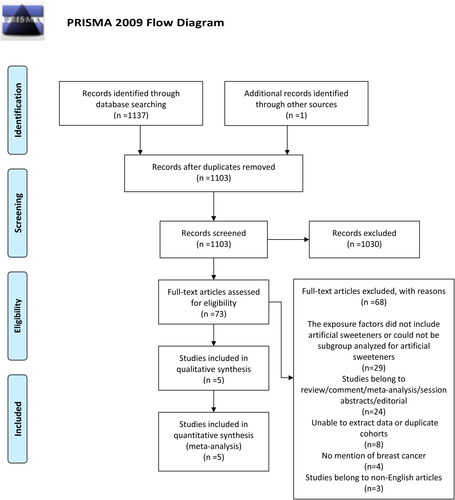
The study selection process according to PRISMA guidelines is reported in . In total, 1,138 potential publications were identified from the electronic databases (PubMed, Web of Science, Ovid, Scopus) and other sources. After removing duplicates, 1,103 articles were selected, of which 1,030 were excluded according to the title and abstract. Among 73 eligible articles, 68 were excluded due to the following reasons: 29 the exposure factors did not include artificial sweeteners or could not be subgroup analyzed for artificial sweeteners, 24 studies belong to reviews, comments, meta-analyses, session abstracts, editorials, eight unable to extract or duplicate cohorts, 4 no mention of BC, three studies belong to non-English articles. Finally, 5 studies (Citation12, Citation18, Citation20, Citation21, Citation25) were included in the systematic review, 2 case-control studies and three cohort studies.
Study Characteristics and Quality Evaluation
In five studies on the relationship between exposure to artificial sweeteners and the incidence of BC, one study each was conducted in these countries, Denmark, Italy, Australia, America, and France. The time of the experiment was from 1984 to 2021, and the articles were published between 1990 and 2022. The exposure assessment was conducted by questionnaire or interview. The cohort studies were conducted in MCCS, NHS, NHSII and NutriNet-Santé and enrolled 314,056 participants in total, of which 13,304 suffered from the BC. In case-control studies, they included 4,043 cancer cases and 3,910 controls. In this five studies, the recruitment age ranged from 18 to 70 and the median follow-up time ranged from 7.8 to 11.6 (Supplementary Table 1). Age adjustment was a factor in every analysis, and the remaining were mainly related to the place of residence, SEIFA, smoking, alcohol drinking, physical activity, BMI, total energy intake, consumption of hot beverages, family history of BC, etc. Other adjustment factors are detailed in .
Table 1. Characteristics of included observational studies in the meta-analysis.
The quality of all the above-mentioned case-control studies and cohort studies were assessed using the Newcastle-Ottawa Scale. The result met the quality requirements of the meta-analysis (Supplementary Tables 2 and 3).
Artificial Sweeteners and Risk of Breast Cancer
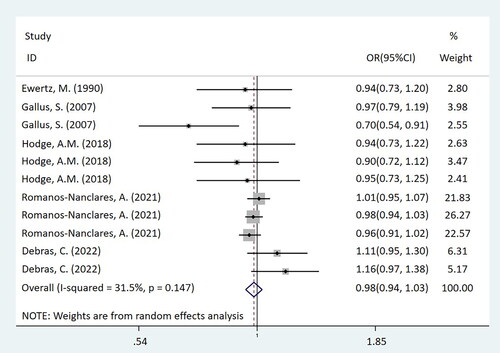
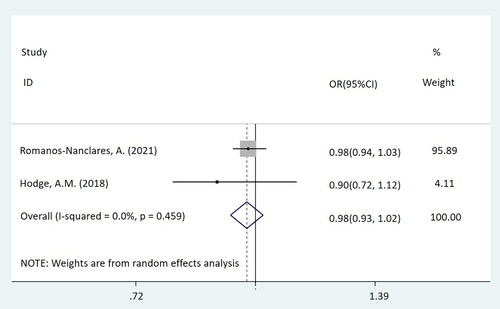
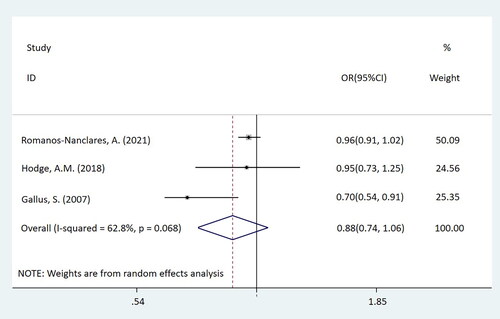
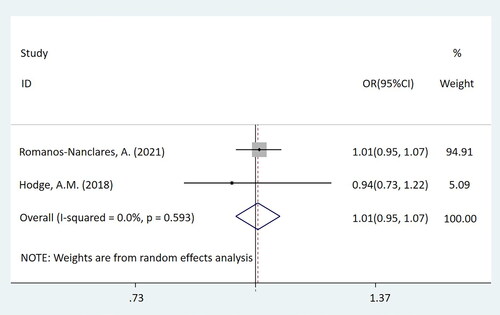
In this mate-analysis, the participant exposed to artificial sweeteners had no different incidence of BC from the non-exposure group (OR = 0.98, 95%CI = [0.94-1.03], p = 0.450) (). According to the dose of artificial sweeteners used, the study was separated into three subgroups. The low and middle dose groups were included in two cohort studies, while the high-dose group was included in three studies, which were two cohort studies and one case-control study respectively. Subgroup analysis showed that compared with the non-exposure/very-low-dose group, the incidence of BC had no difference in the population exposed to low-dose artificial sweeteners (OR = 1.01, 95%CI = [0.95-1.07], p = 0.831) (). In the middle-dose group, there was no difference in the incidence of BC between the exposed and the non-exposed/very-low-dose group (OR = 0.98, 95%CI = [0.93-1.02], p = 0.299) (). Compared with the non-exposure/very-low-dose group, the incidence of BC also had no difference in the population exposed to high-dose artificial sweeteners (OR = 0.88, 95%CI = [0.74-1.06], p = 0.185) ().
Figure 3. Forest plot of low-dose artificial sweeteners exposure and incidence of breast cancer (p = 0.831).
Discussion
This study included five studies (two case-control studies and three cohort studies), to evaluate the relationship between artificial sweeteners and BC. According to the statistical results, there was no statistical significance link between artificial sweeteners and BC. Furthermore, the results of subgroup analysis revealed no relationship between the exposure dose and the prevalence of BC.
Artificial sweeteners, such as aspartame, are calorie-free sweeteners that have been promoted as a healthier alternative to regular sugar. Artificial sweetener consumption is strongly associated with less weight gain (Citation26) and a lower risk of type 2 diabetes when compared to sucrose. This may be because reducing sugar consumption will lead to lower insulin and satiety, which will prevent weight gain and type 2 diabetes. According to current studies, desserts, sweets, and diets high in refined sugar may increase the risk of BC in women (Citation27–32). Potischman (Citation28) found a 32% increase in the incidence of BC among those who consumed the most sweets, as opposed to those who consumed the least, in a population-based case-control study of BC among American women under the age of 45. A case-control study based on an Italian hospital in 2005 (Citation29) also showed a 19% increase among those with the highest tertile of intake of dessert foods. This can be the case because eating sweet food in excess will lead to being fat and overweight. The relevant mechanisms of sweet food and sweetness and obesity include insulin resistance, hyperinsulinemia, increased bioavailability of steroid hormones, oxidative stress, and inflammation (Citation33). Together, these metabolic alterations produce a setting that favors the development and spread of malignancies. High sugar consumption have been demonstrated to independently induce chronic activation of the insulin signaling system, as well as elevation of indicators of oxidative stress and inflammation, which together raise the risk of BC (Citation34). This is in addition to their effect on body adiposity. Obesity increases the risk of BC in women with different menstrual conditions. While obesity does not significantly increase the incidence of BC in premenopausal women, studies have shown that postmenopausal overweight women have a 30%-60% higher chance of developing the disease than slim women (Citation35). Furthermore, another meta-analysis showed that the risk of BC in patients with type 2 diabetes increased by 23%, and it was also reported that there was a positive correlation between type 2 diabetes and the incidence rate of BC during the 10-year follow-up (Citation36). To summarize, obesity caused by high sugar intake and type 2 diabetes are both linked to BC.
From the molecular point of view, BC is divided into four different subtypes according to the differences in hormone expression, estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER-2) (Citation37). HER-2 and hyperglycemia and insulin resistance (IR) have been linked in clinical investigations to date (Citation38, Citation39), and type 2 diabetes patients appear to have considerably higher circulating HER-2 concentrations. Additionally, according to some research, the normal levels of insulin-link growth factor-1 (IGF-1) and insulin-link growth factor-2 (IGF-2) in overweight or obese people as well as the rise in insulin levels may cause an over-activation of the IR and IGF-1R pathways. Because the signal transduction mediated by IR/IGF-1R will further boost HER-2 synthesis, encourage cancer cell proliferation and spread, and hinder cancer cell death, the risk of BC will increase (Citation40). Because adipose tissue takes over as a primary source of estrogen synthesis after menopause, obesity may raise the risk of BC in postmenopausal women. Obesity caused by high glucose also means having more adipose tissue, and estrogen-sensitive breast tissue will receive more estrogen stimulation, while estradiol (E2) stimulates cell growth through the role of estrogen receptor, and causes replication errors during mitosis to cause mutations (Citation41).
Derived from the above mechanism, artificial sweeteners seem to reduce the incidence of BC, but our research results show that there is no relationship between artificial sweeteners and BC. Over the past few decades, a large number of human studies have been conducted to examine the effects of each artificial sweetener that the Food and Drug Administration (FDA) has approved on a variety of nonmalignant and primarily metabolic health parameters, including weight gain and glucose metabolism. Although differences in study designs and populations make direct comparison difficult, no artificial sweetener has been consistently linked to negative health consequences, and according to FDA inspection, all are considered safe for consumption (Citation42). Although the carcinogenic risk of artificial sweeteners is still debatable, certain of its by-products, such as formaldehyde, a by-product of aspartame metabolism, are known carcinogens. Formaldehyde, which can result in DNA damage, chromosomal abnormalities, and mitotic mistakes (Citation43, Citation44), is one biological mechanism that has currently been established to be acceptable through experiment research. Furthermore, it is generally known that individuals with BRCA2 mutations have a 69% chance of developing BC. Formaldehyde is a high-risk factor for triggering BC because it can break down the BRCA2 protein, which was initially a tumor suppressor protein, and impair DNA damage repair mechanisms (Citation45).
Artificial sweeteners and the risk of BC have conflicting findings. Most studies showed no association, but some studies showed that the risk was reduced (Citation15). Aspartame and potassium acetylsulfate were the two specific artificial sweeteners that were linked to an increased risk of BC, according to the most recent cohort study from France conducted in 2022. But there was no statistically significant relationship between the total amount of artificial sweeteners and BC risk (Citation14). There is no relationship between artificial sweeteners and BC, according to the findings of our systematic review, which are in line with those of the majority of research.
Advantages and Limitations of Current Data
As far as we know, this is the first systematic review and meta-analysis to study the relationship between the exposure of artificial sweeteners and BC risk. Due to the heterogeneity between the included studies, such as different types of artificial sweeteners, different exposure times, and different follow-up times of each cohort, the random effect model was used for analysis to improve the reliability of the results. However, this study also has some limitations, it is still necessary to acknowledge that this study has a large heterogeneity, which may be due to differences in methods, populations, BC types, artificial sweeteners and their intake dose. Because the number of documents included in this study is limited, there is no way to further explore. The system review includes North American countries, Nordic countries and Australia. Therefore, it may not be appropriate to extrapolate this research results to other geographical regions. Although we have grouped the doses, measurement errors will likely occur when collecting the artificial sweeteners intake dose data due to the use of self-reported questionnaires. Some scholars believed that the link between artificial sweeteners and BC risk was due to their high blood glucose index (Citation46) and obesity-inducing pathway (Citation6). However, these variables were not fully integrated as confounding factors in all studies. So far, it has been clarified that there are some independent risk factors for BC, including age, family history, gene, etc. But these independent risk factors were not fully considered when the cohort study and case-control study were designed.
Conclusion
This study confirmed that there is no relationship between the exposure of artificial sweeteners and the incidence of BC. However, due to the limitation of this study, more large-scale studies are needed to further explore.
Authors’ Contributions
All authors had read and approved the manuscript. Xia Ye and Yeyuan Zhang writing manuscript; Yujing He and Mingyuan Sheng performing procedures and data analysis; Jianing Huang and Yeyuan Zhang contribution to writing the manuscript; Wenzhu Lou contribution to drafting conception and design.
| Abbreviations | ||
| BC | = | breast cancer |
| OR | = | odds ratio |
| SEIFA | = | Socio-Economic Indexes for Areas |
| BMI | = | body mass index |
| NHS | = | Nurses’ Health Study |
| NHSII | = | Nurses’ Health Study II |
| WHO | = | the World Health Organization |
| PRISMA | = | the Preferred Reporting Items for Systematic Reviews and Meta-Analyzes |
| NOS | = | Newcastle-Ottawa Scale |
| CI | = | confidence interval |
| ER | = | estrogen receptor |
| PR | = | progesterone receptor |
| HER-2 | = | human epidermal growth factor receptor-2 |
| IR | = | insulin resistance |
| IGF-1 | = | insulin-link growth factor-1 |
| IGR-2 | = | insulin-link growth factor-2 |
| E2 | = | estradiol |
| FDA | = | Food and Drug Administration |
Supplemental Material
Download MS Word (14.8 KB)Supplemental Material
Download MS Word (12.9 KB)Supplemental Material
Download MS Word (14 KB)Supplemental Material
Download MS Word (1.5 MB)Disclosure Statement
No potential conflict of interest was reported by the authors.
Availability of Data and Materials
The datasets supporting this article’s conclusions are included within the article and its additional files.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2021;71(3):209–49. doi: 10.3322/caac.21660
- McTiernan A, Porter P, Potter JD. Breast cancer prevention in countries with diverse resources. Cancer. 2008;113(S8):2325–30. doi: 10.1002/cncr.23829
- Weihrauch MR, Diehl V. Artificial sweeteners–do they bear a carcinogenic risk? Ann Oncol. 2004;15(10):1460–5. doi: 10.1093/annonc/mdh256
- Kamenickova A, Pecova M, Bachleda P, Dvorak Z. Effects of artificial sweeteners on the AhR- and GR-dependent CYP1A1 expression in primary human hepatocytes and human cancer cells. Toxicol in Vitro. 2013;27(8):2283–8. doi: 10.1016/j.tiv.2013.10.001
- Imamura F, et al. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. BMJ. 2015;351:h3576.
- Te Morenga L, Mallard S, Mann J. Dietary sugars and body weight: systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ. 2012;346:e7492–e7492. doi: 10.1136/bmj.e7492
- Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4(8):579–91. doi: 10.1038/nrc1408
- Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG, Yee D. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33(7):1674–85. doi: 10.2337/dc10-0666
- Elliott SS, Keim NL, Stern JS, Teff K, Havel PJ. Fructose, weight gain, and the insulin resistance syndrome. Am J Clin Nutr. 2002;76(5):911–22
- WHO guidelines approved by the guidelines review committee, in guideline: Sugars intake for adults and children. Geneva: World Health Organization; 2015. Copyright © World Health Organization, 2015..
- Sakurai M, Nakamura K, Miura K, Takamura T, Yoshita K, Nagasawa SY, Morikawa Y, Ishizaki M, Kido T, Naruse Y, et al. Sugar-sweetened beverage and diet soda consumption and the 7-year risk for type 2 diabetes mellitus in middle-aged Japanese men. Eur J Nutr. 2014;53(4):1137–8. doi: 10.1007/s00394-014-0681-4
- Debras C, Chazelas E, Srour B, Druesne-Pecollo N, Esseddik Y, Szabo de Edelenyi F, Agaësse C, De Sa A, Lutchia R, Gigandet S, et al. Artificial sweeteners and cancer risk: Results from the NutriNet-Santé population-based cohort study. PLoS Med. 2022;19(3):e1003950. doi: 10.1371/journal.pmed.1003950
- Scharf RJ, DeBoer MD. Sugar-sweetened beverages and children’s health. Annu Rev Public Health. 2016;37(1):273–93. doi: 10.1146/annurev-publhealth-032315-021528
- Bucher Della Torre S, Keller A, Laure Depeyre J, Kruseman M. Sugar-sweetened beverages and obesity risk in children and adolescents: A systematic analysis on how methodological quality may influence conclusions. J Acad Nutr Diet. 2016;116(4):638–59. doi: 10.1016/j.jand.2015.05.020
- Vos MB, Kaar JL, Welsh JA, Van Horn LV, Feig DI, Anderson CA, Patel MJ, Cruz Munos J, Krebs NF, Xanthakos SA, et al. Added sugars and cardiovascular disease risk in children: A scientific statement from the American Heart Association. Circulation. 2017;135(19):e1017–e1034. doi: 10.1161/CIR.0000000000000439
- Mooradian AD, Smith M, Tokuda M. The role of artificial and natural sweeteners in reducing the consumption of table sugar: A narrative review. Clin Nutr ESPEN. 2017;18:1–8. doi: 10.1016/j.clnesp.2017.01.004
- Qurrat Ul A, Khan SA. Artificial sweeteners: safe or unsafe? J Pak Med Assoc. 2015;65(2):225–7.
- Gallus S, Scotti L, Negri E, Talamini R, Franceschi S, Montella M, Giacosa A, Dal Maso L, La Vecchia C. Artificial sweeteners and cancer risk in a network of case-control studies. Ann Oncol. 2007;18(1):40–4. doi: 10.1093/annonc/mdl346
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1
- Hodge AM, Bassett JK, Milne RL, English DR, Giles GG. Consumption of sugar-sweetened and artificially sweetened soft drinks and risk of obesity-related cancers. Public Health Nutr. 2018;21(9):1618–26. doi: 10.1017/S1368980017002555
- Romanos-Nanclares A, Collins LC, Hu FB, Willett WC, Rosner BA, Toledo E, Eliassen AH. Sugar-sweetened beverages, artificially sweetened beverages, and breast cancer risk: Results from 2 prospective US cohorts. J Nutr. 2021;151(9):2768–79. doi: 10.1093/jn/nxab172
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. doi: 10.1007/s10654-010-9491-z
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
- Peters JL. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295(6):676–80. doi: 10.1001/jama.295.6.676
- Ewertz M, Gill C. Dietary factors and breast-cancer risk in Denmark. Int J Cancer. 1990;46(5):779–84. doi: 10.1002/ijc.2910460505
- Tandel KR. Sugar substitutes: Health controversy over perceived benefits. J Pharmacol Pharmacother. 2011;2(4):236–43.
- Augustin LSA, Dal Maso L, La Vecchia C, Parpinel M, Negri E, Vaccarella S, Kendall CWC, Jenkins DJA, Franceschi S. Dietary glycemic index and glycemic load, and breast cancer risk: a case-control study. Ann Oncol. 2001;12(11):1533–8. doi: 10.1023/A:1013176129380
- Potischman N, Coates RJ, Swanson CA, Carroll RJ, Daling JR, Brogan DR, Gammon MD, Midthune D, Curtin J, Brinton LA, et al. Increased risk of early-stage breast cancer related to consumption of sweet foods among women less than age 45 in the United States. Cancer Causes Control. 2002;13(10):937–46. doi: 10.1023/A:1021919416101
- Tavani A, Giordano L, Gallus S, Talamini R, Franceschi S, Giacosa A, Montella M, La Vecchia C. Consumption of sweet foods and breast cancer risk in Italy. Ann Oncol. 2006;17(2):341–5. doi: 10.1093/annonc/mdj051
- Witte JS, Ursin G, Siemiatycki J, Thompson WD, Paganini-Hill A, Haile RW. Diet and premenopausal bilateral breast cancer: a case-control study. Breast Cancer Res Treat. 1997;42(3):243–51. doi: 10.1023/A:1005710211184
- Lubin JH, Burns PE, Blot WJ, Ziegler RG, Lees AW, Fraumeni JF. Dietary factors and breast cancer risk. Int J Cancer. 1981;28(6):685–9. doi: 10.1002/ijc.2910280605
- Seely S, Horrobin DF. Diet and breast cancer: the possible connection with sugar consumption. Med Hypotheses. 1983;11(3):319–27. doi: 10.1016/0306-9877(83)90095-6
- Roberts DL, Dive C, Renehan AG. Biological mechanisms linking obesity and cancer risk: new perspectives. Annu Rev Med. 2010;61(1):301–16. doi: 10.1146/annurev.med.080708.082713
- Klement RJ, Kämmerer U. Is there a role for carbohydrate restriction in the treatment and prevention of cancer? Nutr Metab (Lond). 2011;8(1):75. doi: 10.1186/1743-7075-8-75
- Sparano JA, Wang M, Zhao F, Stearns V, Martino S, Ligibel JA, Perez EA, Saphner T, Wolff AC, Sledge GW, et al. Obesity at diagnosis is associated with inferior outcomes in hormone receptor-positive operable breast cancer. Cancer. 2012;118(23):5937–46. doi: 10.1002/cncr.27527
- De Bruijn KMJ, Arends LR, Hansen BE, Leeflang S, Ruiter R, van Eijck CHJ. Systematic review and meta-analysis of the association between diabetes mellitus and incidence and mortality in breast and colorectal cancer. Br J Surg. 2013;100(11):1421–9. doi: 10.1002/bjs.9229
- Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, et al. Race, breast cancer subtypes, and survival in the Carolina breast cancer study. JAMA. 2006;295(21):2492–502. doi: 10.1001/jama.295.21.2492
- Memon AA, Bennet L, Zöller B, Wang X, Palmer K, Sundquist K, Sundquist J. Circulating human epidermal growth factor receptor 2 (HER2) is associated with hyperglycaemia and insulin resistance. J Diabetes. 2015;7(3):369–77. doi: 10.1111/1753-0407.12184
- Fernández-Real JM, et al. Serum HER-2 concentration is associated with insulin resistance and decreases after weight loss. Nutr Metab (Lond). 2010;7:14.
- O'Donovan N, Crown J. EGFR and HER-2 antagonists in breast cancer. Anticancer Res. 2007;27(3A):1285–94.
- Yue W, Yager JD, Wang J-P, Jupe ER, Santen RJ. Estrogen receptor-dependent and independent mechanisms of breast cancer carcinogenesis. Steroids. 2013;78(2):161–70.
- Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 2005;97(22):1679–87.
- Yılmaz S, Uçar A. A review of the genotoxic and carcinogenic effects of aspartame: does it safe or not? Cytotechnology. 2014;66(6):875–81.
- Rycerz K, Jaworska-Adamu JE. Effects of aspartame metabolites on astrocytes and neurons. Folia Neuropathol. 2013;51(1):10–7.
- Tan SLW, Chadha S, Liu Y, Gabasova E, Perera D, Ahmed K, Constantinou S, Renaudin X, Lee M, Aebersold R, et al. Class of environmental and endogenous toxins induces BRCA2 haploinsufficiency and genome instability. Cell. 2017;169(6):1105–18.e15. doi 10.1016/j.cell.2017.05.010
- Sieri S, Krogh V. Dietary glycemic index, glycemic load and cancer: An overview of the literature. Nutr Metab Cardiovasc Dis. 2017;27(1):18–31. doi 10.1016/j.numecd.2016.09.014