Abstract
Colorectal cancer (CRC) is one of the major causes of cancer-related mortality worldwide. Despite advances in treatment modalities, its prevalence continues to rise, notably among younger populations. Unhealthy dietary habits, sedentary routines, and obesity have been identified as one of the key contributors to the development of colorectal cancer, apart from genetic and epigenetic modifications. Recognizing the profound impact of diet and lifestyle on the intricate gut microbiota ecosystem offers a promising avenue for understanding CRC development and its treatment. Gut dysbiosis, characterized by imbalances favoring harmful microbes over beneficial ones, has emerged as a defining feature of CRC. Changes in diet and lifestyle can profoundly alter the composition of gut microbes and the metabolites they produce, potentially contributing to CRC onset. Focusing on recent evidence, this review discussed various dietary factors, such as high consumption of red and processed meats and low fiber intake, and lifestyle factors, including obesity, lack of physical activity, smoking, and excessive alcohol consumption, that influence the gut microbiome composition and elevate CRC risk.
Introduction
Colorectal cancer (CRC) ranks third in terms of incidence and second in fatalities among recognized cancers (Citation1). CRC accounts for 10% of worldwide cancer incidence and 9.4% of cancer deaths (Citation2). By 2030, it is estimated that there will be 2.2 million new cases of CRC and 1.1 million fatalities worldwide (Citation3). Both environmental and genetic factors influence CRC. The increased exposure to environmental risk factors brought on by the modification in lifestyle and diet is primarily responsible for the rise in CRC incidence (Citation4). Due to enhanced colonoscopy screenings, the incidence and death rates of CRC have been gradually dropping in the past few decades for people over age 65; however, the opposite has happened in adults under the age of 50 (Citation5, Citation6). It’s hypothesized that the surge in young-onset colorectal cancer is largely attributable to obesity and lifestyle changes, although the precise causes remain elusive. The persistence of colorectal cancer reinforces the urgent need to develop novel preventative techniques beyond screening, especially among young individuals. The etiology of CRC is highly complex and includes hereditary and environmental components. According to findings from twin and family-based research studies, only a small percentage (5–10%) of colorectal cancers are genetically linked, which include Familial Adenomatous Polyposis (FAP), Lynch syndrome, Peutz-Jeghers syndrome, etc, (Citation7). CRC are typically random or non-inherited. Dietary habits, characterized by high consumption of red and processed meat alongside low dietary fiber intake and certain lifestyles marked by lack of physical activity, weight gain and obesity, smoking, and heavy alcohol consumption, are external factors that notably contribute to the sporadic progression of CRC. Studies show that 50–60% of the cases of colorectal cancer can be avoided by modifying one’s lifestyle (Citation8).
Multiple studies propose that the gut microbiota could serve as a holistic mechanism influencing the connection between environmental factors and the onset of CRC. The large intestine contains an enormous microbial community (≥1014), which encodes 150 times more distinct genes than our genome (Citation9). Environmental factors, particularly food habits and ways of life, appear to impact this gut ecosystem’s structure and function significantly (Citation10). Alterations in immunological and metabolic signals induced by gut microbiota-derived metabolites may also impact the development of colorectal cancer (Citation11). Further, the microbiota also generates several toxic compounds that might harm the behavior of gut epithelial cells. A balanced and healthy gut microbiota maintains intestinal homeostasis and exhibits anti-cancer properties. However, gut dysbiosis, characterized by disruptions in the typical microbiota balance, is often observed in individuals with CRC. Gut dysbiosis arises from an imbalance between beneficial and opportunistic microbes, resulting in compositional and functional changes. This imbalance manifests in three main ways: the reduction of beneficial microbes, the proliferation of pathogenic ones, and a decrease in overall microbial diversity (Citation12).
This review gives an overview of how changes in diet and lifestyle may cause CRC by altering gut microbiota-associated mechanisms. We focused on the factors that significantly impact the composition and function of gut microbiota and are strongly linked to colorectal cancer. These include dietary habits, fiber intake, consumption of red and processed meats, overweight and obesity, levels of physical activity, alcohol consumption, and cigarette smoking. We also provided a brief overview of the effect of gut microbiota-derived metabolites, along with therapeutic approaches that can reduce CRC via restoring gut dysbiosis.
Search Strategy
Over the past three decades, relevant research concerning the dysbiosis of the gut microbiota and its correlation with colorectal carcinogenesis has mostly been found and retrieved using university library catalogs, Google Scholar, and the PubMed database. Searches were conducted using keywords such as “colon and rectal cancer,” “gut microbiota,” “diet,” and “lifestyle.” Articles considered for inclusion in this literature review as full-text, published between 1951 and the present, and written in English. Based on the title, abstract, full text, and publication date, the articles were taken into consideration for writing this narrative review.
Gut Microbiota Alteration and CRC
The gut microbiota is vital in maintaining normal gut functions, including digestion, vitamin synthesis, heat generation, immune response, and gut balance (Citation13–17). With approximately 40 trillion microorganisms, the gut microbiota varies among individuals but remains relatively stable within each person (Citation18, Citation19). Lifestyle, diet, and genetics influence the number and diversity of gut bacteria (Citation20, Citation21). While Firmicutes, Proteobacteria, Bacteroidetes, Actinobacteria and Fusobacteria are the dominant phyla in the human gut, their composition varies across different gut regions (Citation22). In the colon, Bacteroidetes (Bacteroidota) and Actinobacteria (Actinomycetota) make up over 90% of bacterial phyla, whereas Firmicutes dominates the small intestine, representing 40% of bacterial phyla (Citation23, Citation24). The proximal gut harbors a lower microbe count, mainly composed of Bacteroidetes (Bacteroidota) and Clostridiales. In contrast, the large intestine hosts a significantly higher microbe which includes Bacteroidetes (Bacteroidota), Verrucomicrobia (Verrucomicrobiota), Proteobacteria (Pseudomonadota), Actinobacteria (Actinomycetota), and Firmicutes (Bacillota) (Citation25). The ratio of Firmicutes to Bacteroidetes is crucial for gut health and colorectal cancer (CRC) progression, with changes linked to inflammatory bowel diseases (IBDs), which elevate CRC risk (Citation26–29).
Research on humans has revealed distinct differences in the gut microbiota between individuals with colorectal cancer (CRC) and those without, characterized by a decrease in beneficial bacteria and an increase in potentially harmful bacteria. Key bacterial species that are associated with colorectal carcinogenesis are Fusobacterium nucleatum, E. coli, Bacteroides fragilis, Streptococcus bovis/gallolyticus, Clostridium septicum, Enterococcus faecalis, and Peptostreptococcus anaerobius (Citation30–41). Meta-analyses have identified a core set of seven bacterial species enriched in CRC, including Fusobacterium nucleatum, Thermanaerovibrio acidaminovorans, Prevotella intermedia, Bacteroides fragilis, Parvimonas micra, Porphyromonas asaccharolytica, and Alistipes finegoldii, (Citation42). Moreover, a 2019 study identified 29 core species enriched in CRC across various geographic regions (Citation43). Specific bacterial species such as Bacteroides fragilis, Enterococcus, Escherichia/Shigella, Klebsiella, Streptococcus, and Peptostreptococcus were found to be significantly more abundant in the gut microbiota of CRC patients, while beneficial bacteria like Roseburia and other butyrate-producing bacteria were less abundant (Citation39).
Differences in the gut microbiota between CRC patients and healthy individuals were observed at the genus level, suggesting potential diagnostic significance, particularly in early CRC stages. Sheng et al. conducted a study involving 67 CRC patients and 30 healthy controls, analyzing fecal samples using 16S rRNA gene sequencing. They found notable increases in the genera Peptostreptococcus, Collinsella, and Ruminococcus in stage I CRC patients compared to controls. At the same time, Alistipes was enriched in stage III compared to stage IV patients (Citation44). Additionally, regardless of CRC stage, differences in microbiota composition compared to healthy controls were evident, with variations in the relative abundance of dominant genera, including Escherichia-Shigella, Bacteroides, Faecalibacterium, Prevotella, and Akkermansia (Citation44). Another study on a Japanese cohort revealed that Fusobacterium nucleatum levels begin to rise at stage 0 and increase with cancer progression. Atopobium parvulum and Actinomyces odontolyticus are elevated in cases with multiple polypoid adenomas and/or stage 0 but plateau in advanced stages, while Peptostreptococcus anaerobius, Peptostreptococcus stomatis, and Parvimonas micra increase in later stages, stage I/II and stage III/IV (Citation45).
The Interplay of Diet and Lifestyle on Gut Microbiota
Impact of Dietary Factors
Dietary Patterns
Significant variations in the metabolism and gut microbial community among people consuming various types of food indicate that the gut microbiota might significantly alter the correlations between dietary habits and the risk of CRC. For example, a quick change in the microbial abundance and composition, as well as an elevated Ki-67 level in colon cells, occurs when an individual changes his dietary pattern from a traditional African diet (rich in plant polysaccharides, fiber, low in fat and processed meat) to a Western diet that contains less amount of plant polysaccharides, fiber and high amount of fat, processed meat, and sugar (Citation10, Citation46). Species from Prevotella, which are generally involved in the degradation of starch, xylan, and hemicellulose, dominate the gut of rural Africans. In contrast, the Bacteroides genus is more common in Americans’ microbiota and has more detrimental Proteobacteria, such as Escherichia and Acinetobacter (Citation47). These differences in microbial structural composition are associated with a decreased incidence of CRC in African countries compared to Western countries. Similar variations can be identified in the profile of feces metabolites, with native Africans exhibiting higher amounts of SCFAs (Short Chain Fatty Acids) and African Americans showing more significant quantities of secondary bile acids (Citation48). These conclusions are supported by evidence from an intervention trial which demonstrated that when African Americans switched to a diet high in fiber and low in fat for two weeks, it resulted in increased production of SCFAs, reduced synthesis of secondary bile acids, and decreased markers of colonic mucosal inflammation and cell growth associated with cancer (Citation46).
Recent mechanistic research reveals that a Western diet may interact with the gut bacteria, weakening the colonic mucus layer and potentially promoting cancer growth. Billions of gut bacteria remain separated from the host via this mucus layer, which acts as a layer of defense (Citation49, Citation50). A Western diet decreases mucus production, making the colonic mucus barrier more permeable. These effects are associated with modifications in the microbial ecosystem, characterized by an increase in Firmicutes and a gradual drop in Bifidobacterium and the Bacteroidales family S24–7, which are the generators of SCFAs (Citation49). Fusobacterium nucleatum has been studied the most for CRC, and clinical data consistently connects this bacterium to this disease (Citation51). According to a recent dietary crossover study, shifting African Americans to a high-fiber, low-fat diet reduces stool F. nucleatum levels while switching rural Africans to a low-fiber, high-fat diet causes a significant rise in the levels (Citation10, Citation46). The first evidence that F. nucleatum promotes myeloid cell infiltration into intestinal tumors in Apc Min/+ mice and increases the expression of pro-inflammatory genes such as Ptgs2 (COX-2), Scyb1(IL8), IL6, Tnf (TNFα), and Mmp3 was provided by Kostic et al. (Citation16). Later studies using quantitative polymerase chain reaction (qPCR) and fluorescent in situ hybridization (FISH) confirmed that the colonic mucosa, tissues, and feces of people with adenomas or adenocarcinomas had higher levels of F. nucleatum than those without. These results are similar across different ethnic groups and stages of colorectal malignancies (CRCs) (Citation52, Citation53). Research on human colonic tissues has uncovered the intricate mechanisms by which F. nucleatum fosters a pro-tumorigenic environment. Yang et al. conducted experiments where CRC cell lines were exposed to F. nucleatum in mice, observing changes in microRNA (miRNA) expression (Citation54). Their findings revealed that F. nucleatum increases inflammatory factors and miRNA21 via toll-like receptors.
Moreover, F. nucleatum affects downstream targets of miR21, including the oncoprotein RAS P21 Protein Activator 1 (RASA1) and tumor suppressor programmed cell death protein 4 (Pdcd4) (Citation54). Evidence suggests that the abnormal expression of RASA1 in CRC activates the RAS-mitogen-activated protein kinase (MAPK) cascade. Consequently, F. nucleatum’s upregulation of miR21 correlates strongly with MAPK cascade activation, potentially contributing to CRC development.
The composition of gut microbes is also influenced by different dietary patterns such as the Vegan/Vegetarian Diet, high-fat diet, and Mediterranean diet. Vegans, a subgroup of vegetarians, go a step further by excluding animal products like eggs, milk, dairy products, and honey from their diet (Citation55). Research studies have indicated that compared to omnivores, vegetarians and vegans tend to have higher ratios of Bacteroides/Prevotella, Bacteroides thetaiotaomicron, and lower ratios of Clostridium cluster XIVa and Bilophila wadsworthia (Citation56). Another study showed that vegans and vegetarians have lower numbers of Bifidobacterium and Bacteroides species, but there is no significant difference in fecal SCFA levels between vegans and omnivores (Citation57). The Mediterranean diet, which emphasizes fruits, vegetables, legumes, olive oil, nuts, and whole grains, increases Bifidobacteria and Bacteroides and decreases Enterobacteria (Citation58).
Intake of Red and Processed Meat
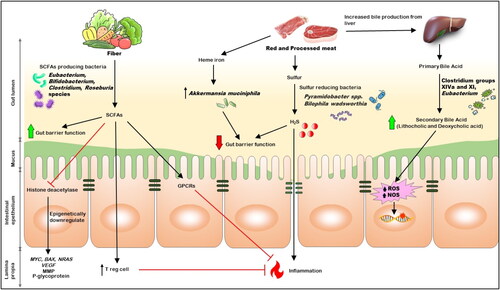
Red meat and processed meat were listed as carcinogens in 2015 by the IARC, a division of the World Health Organization (WHO) and the foremost organization for studying carcinogens. Over 800 epidemiological studies evaluating the connection between red meat and cancer worldwide were assessed by the IARC panel, which included 22 specialists from 10 different nations. After doing so, they classified processed meat as being “carcinogenic to humans” in Group 1 and fresh red meat as being “probably carcinogenic to humans” in Group 2 A (Citation59). Processed meat is defined as “meat that has been transformed through salting, curing, fermentation, smoking, or other processes to enhance flavor or improve preservation (Citation60). According to the IARC, 100 g of fresh red meat consumed daily increases the risk of CRC by 17%, whereas 50 g of processed red meat increases the risk of CRC by 18%. White meat, on the other hand, doesn’t seem to be linked to a higher risk of CRC (Citation60). Specific minerals present in meats, such as heme iron, sulfur, and saturated fat, along with preservatives like nitrates and nitrites used in red and processed meats, as well as compounds such as polycyclic aromatic hydrocarbons and heterocyclic amines, which are formed during meat processing and cooking have all been associated with promoting cancer. Recent research has shown that gut microbiota is important for modulating the cancer-causing substances in red meat. For instance, the detrimental effect of heme iron on the epithelium cells of the colonic surface layer depends on the gut microbiota. Heme iron resulted in an eight-fold rise in the total number of the microbe Akkermansia muciniphila, which breaks down mucin, disrupting the function of the mucus barrier. Antibiotics can reverse the harm caused by heme and its subsequent carcinogenic effects, including compensated hyperproliferation, hyperplasia, and different expressions of tumor suppressors and proto-oncogenes (Citation61).
Apart from directly impacting the gut microbes, compounds connected to a high red meat intake can also act as substrates for gut microorganisms’ metabolism and encourage CRC growth. The liver produces even more bile acids to emulsify the fat present in red meat since it contains a large amount of saturated fat. Although most bile acids are reabsorbed in the small intestine, anaerobic bacteria (such as Clostridium groups XIVa and XI as well as Eubacterium) transform those that reach the colon into secondary bile acids like Lithocholic acids and Deoxycholic acids. Secondary bile acids release ROS and NOS, resulting in DNA damage and apoptosis resistance (). The detrimental effects of Secondary bile acids in colonic epithelial cells are discussed later in this review.
Sulfur is another common element in red and processed meat (Citation62). Sulfur-reducing bacteria can generate hydrogen sulfide, a compound linked to colorectal cancer. They do so by processing both inorganic sulfur, commonly found in processed meat as a preservative, and organic sulfur, present in amino acids like cysteine and methionine in red meat (Citation63). People with colitis or colorectal cancer have higher hydrogen sulfide levels in their feces than healthy controls (Citation64). Due to its carcinogenic properties, raised hydrogen sulfide formation in the colon may harm DNA, impair the nutrition of colon cells by preventing butyrate’s β-oxidation, decrease the integrity of the mucus layer, result in epithelial hyperproliferation, and worsen inflammation due to altered immune cell numbers and functions (Citation62). The research revealed that people with colorectal cancer had higher sulfur-reducing bacteria in their intestinal epithelium than those without the condition. These bacteria included Pyramidobacter spp. and Bilophila wadsworthia (Citation65).
Xiaoyan et al. conducted a recent study to assess the impact of a diet high in red meat on mice’s gut flora. This study selected a control and red meat group of mice based on their diet history. The mice were sacrificed after eight weeks, and the contents of their intestines were taken for 16S rRNA sequencing and bioinformatics analysis. Results showed that Bacteroidetes were more prevalent in the red meat groups, whereas Firmicutes were less prevalent (Citation66).
While the evidence suggests that changes in microbial composition caused by the consumption of red meat may contribute to cancer development, the correlations between red meat and carcinogenesis are weak. According to an analysis of 35 prospective studies by Alexander et al. (Citation67), the association between red meat consumption and colon cancer was insignificant. The highest relative risk observed was less than 1, and did not reach statistical significance. An alternative argument is that certain dietary combinations may change the impact of the colonic microbiome in either beneficial or detrimental ways. For instance, the gut metabolism of rats concurrently fed red meat and high amylose-resistant starch changed from being dominated by protein fermentation to one that included both protein and carbohydrate fermentation. This was linked to increased Ruminococcus bromii, Bifidobacteria, Turicibacteraceae, and Lactobacillaceae species (Citation68). Similar to this, processed meats supplemented with the prebiotic carbohydrate inulin led to an increase in the Blautia genus’ population, which in turn led to the rise in the production of protective SCFAs like propionate and butyrate (Citation69) and eventually decrease in colonic polyps in experimental rats (Citation70).
Intake of Fiber
Only plant-based foods, such as grains, fruits, soybeans, and vegetables, contain the dietary fiber essential for human health (Citation71). Polysaccharides (starch, pectin, cellulose, hemicellulose), oligosaccharides, and lignins are the major constituents that make up fiber. They are primarily divided into soluble and insoluble fibers. Although the proportions of each type of fiber vary, almost all plant-based foods include both soluble and insoluble fibers (Citation71). Dietary fiber can prevent several diseases, such as heart disease, type 2 diabetes, gastrointestinal problems, and various malignancies (Citation72). For example, Fructans, a type of soluble fiber found in garlic, has been proposed to impact gut microbiota, intestinal mucosa health, and epithelial permeability and can potentially reduce the risk of intestinal adenoma (IA) and CRC. It also has immunomodulatory effects as it produces SCFAs through their fermentation by colonic microbiota. According to a study, higher garlic consumption was correlated with a decrease in the abundance and prevalence of Family XI and Finegoldia genus within the Clostridia class and Clostridiales order and an increase in the Corynebacteriales order, Nocardiaceae family, and Rhodococcus genus within the Actinomycetia class (formerly Actinobacteria) (Citation73).
Increased fiber intake may help reduce the risk of CRC by two general mechanisms. First, the consumption of insoluble fiber speeds up the movement of food through the intestines, potentially reducing the exposure of colonic epithelial cells to ingested carcinogens, such as heterocyclic aromatic amines (HCAs) and polycyclic aromatic hydrocarbons (PAHs) found in charred or grilled meat. Second, some types of bacteria ferment soluble fiber to generate the SCFAs, a family of beneficial metabolites that includes butyrate, acetate, and propionate. According to research, increasing fiber intake increases the number of butyrate-producing bacteria in the gut, including Eubacterium, Bifidobacterium, Clostridium, and Roseburia species, and boosts SCFA production (Citation74). Studies confirm that SCFAs can reduce the risk of colorectal cancer, and their protective mechanism is described below in the metabolites section.
Studies employing gnotobiotic mice models have proven that dietary fiber inhibits histone deacetylase in a microbiota and butyrate-dependent manner to prevent colorectal carcinogenesis (Citation75). The incidence and multiplicity of colorectal tumors were decreased when a high-fiber diet was combined with the butyrate-producing bacterium Butyrivibrio fibrisolvens, although neither factor was protective on its own. The colon tissue’s energy equilibrium depends heavily on butyrate, a primary energy source for colonocytes (Citation76). Due to the Warburg effect, characterized by the increased conversion of glucose to lactate by tumor cells despite adequate oxygen levels, results in reduced butyrate metabolism within cancer cells. Consequently, butyrate accumulates within the nucleus of cancerous colonocytes. It acts as a histone deacetylase inhibitor and epigenetically downregulates the expression of various genes involved in tumor growth (such as MYC, BAX, and NRAS), angiogenesis (vascular endothelial growth factor), migration (matrix metalloproteinase family, the plasminogen-plasmin system), and chemoresistance (P-glycoprotein) (Citation77).
In addition to epigenetic regulation, the significance of butyrate and other SCFAs in maintaining gut-immune equilibrium through the regulation of immune system regulatory T cells (Tregs) has been supported by accumulating evidence (Citation78). Tregs are essential in controlling inflammatory and allergy reactions because they stop the growth of effector CD4+ T cells. Butyrate and propionate have been shown to induce extrathymic production, functional Treg differentiation, and the prevention of colitis (Citation79). Possible mechanisms include the inhibition of histone deacetylase, activation of GPR109a to promote the anti-inflammatory phenotype of colonic macrophages and dendritic cells and overexpression of the Foxp3 gene, an essential controller of Treg function. Several G protein-coupled receptors (GPRs), including GPR43 (Citation79,Citation80) and GPR109a, can be activated by butyrate at the surface of colonic epithelial cells to prevent inflammation and maybe even carcinogenesis (Citation81).
It’s interesting to note that the host’s genetic makeup may influence butyrate’s positive effects. According to a recent study, APCMin/+MSH2/mice, which serve as a model system for defective DNA mismatch repair, exhibited accelerated and aggressive development of adenoma and colorectal cancer (CRC) with microsatellite instability in hereditary nonpolyposis CRC (Lynch syndrome), were found to have CRC when exposed to butyrate (Citation82). Future research must evaluate the fiber CRC relationship with microsatellite instability status to determine whether these results can be extrapolated to humans.
Despite the well-established theory that a high-fiber diet may reduce the risk of colorectal cancer through minimizing exposure to gut cancer-causing agents, epidemiological investigations associating fiber from food consumption to a subsequently elevated risk of colorectal cancer have yielded inconsistent findings. This gap may be caused by variability among cancer subtypes, substantial differences in fiber ingestion between populations, variations in dietary sources of fiber, and variations in confounding control across studies (Citation62).
Impact of Lifestyle
Obesity and Weight Gain
Global westernization, which is characterized by excessive intake of readily available, calorie-dense foods and a sedentary lifestyle, is thought to be a significant contributor to the obesity pandemic. Several epidemiologic studies showed that increased body weight gain in adulthood is consistently linked to a higher risk of colorectal cancer. According to a meta-analysis, there is a 5% increase in the risk of CRC for every 5-kg/m2 increase in body mass index (BMI, defined as body weight in kilograms divided by square of height in meters) (Citation83). Numerous physiological and immunological parameters, such as insulin growth factor 1 signaling, sex hormones, adipokines, and systemic inflammation, have been related to the association between obesity and colorectal cancer. The gut microbiota, however, has emerged as a critical component that controls the host’s metabolism and has been proposed to be important in metabolic alterations linked to being overweight, including inflammation and resistance to insulin (Citation84).
The connection between being overweight and the gut microbiome works in both directions. People who are obese are more likely to have an imbalanced gut microbiota, with a decrease in the Bacteroidetes group and an increase in Firmicutes group. Enterobacter species and Clostridia, such as Clostridium leptum, and an increase in Firmicutes group. Enterobacteriaceae species and in the Bacteroidales genera, such as Bacteroides spp., Lactobacillus spp., Enterococcus spp., and Bifidobacterium spp., are all upregulated. However, this relationship is not straightforward, as the changes in the gut microbiota are not just a consequence of obesity. In experiments, mice that received a transplant of the gut microbiota from obese mice became overweight, even though they consumed less than normal. This suggests that the gut microbiota can play a role in causing obesity. Furthermore, using antibiotics in early life may disrupt the gut microbiota and increase the risk of obesity and other health problems, including colorectal cancer. However, the link between these two conditions needs to be clarified (Citation81).
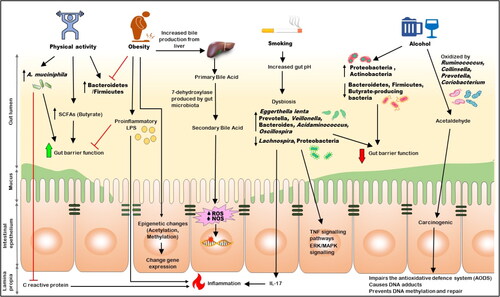
Changes in the gut microbiota brought on by obesity may potentially promote the development of cancer by altering pro-inflammatory chemicals (like lipopolysaccharide [LPS]) and metabolites (such as the reduced amount of butyrate and increased acetate) generated by microorganisms. These modifications can affect the function of the gut barrier and increase permeability, which might accelerate cancer development (Citation85), as shown in (). LPS-induced impairment of the intestinal barrier function, resulting in increased mucosal permeability, is thought to contribute to bacterial translocation (Citation86) significantly. Studies in animal model found three main pathways facilitate bacterial translocation. These mechanisms include (a) the intestinal mucosal barrier’s increased permeability, which permits gut microbial proliferation; (b) the host immune system’s deficiency; and (c) the disturbance of the ecological Gastrointestinal stability (Citation87). Together, these three pathways might promote the systemic spreading of gut-derived bacteria, leading to infections in different body parts (Citation88). The bacteria translocate through an intracellular pathway across the epithelial cells in animal models in which the intestinal barrier is not physically disrupted. They then migrate via the lymph to the mesenteric lymph nodes. When bacteria or their byproducts come into direct contact with the body, they trigger a response from immune cells through signaling pathways involving TLR4 and MyD88 in the lamina propria. This interaction, particularly with LPS derived from gut bacteria, initiates the secretion of proinflammatory mediators, contributing to the continuation of inflammation at the local level. Notably, studies have shown that administering TAK-242, an inhibitor of the LPS-TLR4 signaling pathway, via intraperitoneal injection can mitigate the effects of intestinal barrier disruption by dampening this inflammatory response (Citation89, Citation90). Bacterial LPS triggers lethal shock and organ failure. It does so by activating certain chemicals within the host, including eicosanoids, IL-1, IL-6, TNF-α, and nitric oxide, while also damaging epithelial cells. TNF-α is particularly crucial in the inflammatory response. Its activation leads to the stimulation of NF-κB signaling, which upregulates IL-6 and IL-1β.
Obesity can cause inflammation throughout the body by affecting the intestinal barrier function. This can lead to the leakage of LPS, which can cause metabolic endotoxemia (Citation91). People who are overweight tend to have higher levels of LPS and LPS-binding protein (LBP) in their blood, while losing weight can lower these levels (Citation92, Citation93).
Wade and his team’s research has shown evidence of a connection between colorectal cancer, gut bacteria, and obesity that may be altered by epigenetic changes (Citation94, Citation95). The scientists found that a diet heavy in fat led to broad modifications in the acetylation patterns in regions of DNA that control gene expression, which may help in colon cancer development. Animals fed the same diet but given bacteria from non-obese donors did not exhibit the same changes, which led researchers to conclude that the modifications were caused by gut bacteria (Citation95). When a high-fat diet and fecal transplantation were combined, identical gene expression patterns to those of human colorectal cancer were analyzed (Citation96). These results imply that relationships between adiposity and the gut microbiome may impact the host’s epigenome, raising the risk of colon cancer. Further research is necessary to determine the precise function of the gut microbiota in this process, which is still unidentified (Citation94).
Physical Activity
Numerous cross-sectional investigations have shown that physical activity impacts the composition and function of the gut microbiota. While there is no advantage for rectal cancer, people with higher levels of physical exercise had a 19% lower risk of the disease than those with the lowest physical exercise. Forty-six male controls and 40 elite rugby league players’ fecal metabolomes were compared (Citation97, Citation98). Since the athletes tended to have higher BMIs, the investigation contained two control groups, one with a BMI of 25 and the other with a BMI of >28, to lessen the impact of BMI. It was discovered that the athletes’ gut microbiomes were more diverse than the controls. Changes in the relative abundances of Akkermansia, Ruminococcus, Veillonella, and Lachnospira are frequently seen in the microbial community in response to exercise training in healthy persons. Additionally, there is proof that exercise can alter the relative abundances of Prevotella, Faecalibacterium, Roseburia, and other nonspecific butyrate makers. The Verrucomicrobia phylum member Akkermansia muciniphila can establish colonies in the mucous membrane layer and improve the immune system’s metabolism and responses by thickening the mucus. A. muciniphila is also essential for metabolic activity, resulting in advantageous short-chain fatty acids (SCFAs) (Citation99). C-reactive protein, a significant inflammation biomarker, and Akkermansia municiphila are antagonistic and may be associated with lowered CRC risk ().
The relative abundance of the gut microbiota at the genus and species levels was altered by the kind of exercise training and the athlete’s diet (Citation100). In a study conducted by Jang et al., it was observed that bodybuilders (n = 15) had significantly higher levels (p 0.05) of Faecalibacterium, Sutterella, Clostridium, Haemophilus, and Eisenbergiella than athletes (n = 15) and the non-athlete control group (n = 15). Contrarily, bodybuilders’ levels of Bifidobacterium and Parasutterella were considerably lower (p 0.05), likely due to their high-protein, high-fat, low-carbohydrate, and low-dietary fiber diets (Citation101).
According to recent animal studies, exercise may alter the gut microbiota composition and increase the synthesis of SCFAs (Citation101–103). Physical activity increased the ratio of Bacteroidetes to Firmicutes, which fell in mice with obesity, proportionally increased with the level of activity, and restored bacterial diversity in obese rats. Additionally, exercise increased the concentration and proportion of butyrate-producing bacteria in intestinal fluid (Citation104). The beneficial effects of butyrate have been mentioned in the metabolites section.
Alcohol Consumption
A dose-dependent link has been shown between drinking alcohol and an elevated risk of colorectal cancer. The majority of prospective cohort and case-control studies demonstrate that excessive alcohol consumption raises the risk of CRC, even though other data have shown inconsistent or weak relationships. A recent meta-analysis of 16 CRC studies showed a J-shaped association between alcohol consumption and CRC risk (Citation105). In comparison to individuals who do not drink or only drink occasionally (≤1 g/day), those who engage in light to moderate drinking (up to 2 drinks/day) demonstrated a reduced risk of colorectal cancer (OR: 0.92, 95% CI: 0.88–0.98, p = 0.005). However, heavy drinking (2–3 drinks/day) did not show a significant association with colorectal cancer risk (OR: 1.11, 95% CI: 0.99–1.24, p = 0.08), while consuming more than 3 drinks per day (hefty drinking) was found to be significantly associated with an increased risk (OR: 1.25, 95% CI: 1.11–1.40, p < 0.001) (Citation107).
Prolonged alcohol consumption can result in a dysbiotic colorectal microbiome. Alcohol consumption has been demonstrated to boost Proteobacteria and Actinobacteria while decreasing Bacteroidetes, Firmicutes, and butyrate-producing bacteria (Citation106). These shifts can result in altered bacterial community structure and metabolism of food products, colonic mucosal hyperpermeability, translocation of bacterial products from the mucosa to the blood, and chronic inflammation, all of which increase a person’s risk of developing cancer.
Moreover, the microbiome can independently oxidize ethanol outside the liver, leading to elevated acetaldehyde levels within the colorectal region. In the colon and rectum, under aerobic conditions, specific intestinal bacteria such as Ruminococcus, Collinsella, Prevotella, and Coriobacterium are linked to producing carcinogenic acetaldehyde from ethanol (Citation107). Acetaldehyde is a toxic, mutagenic, and carcinogenic substance that impairs the antioxidative defense system (AODS), causes DNA adducts, and prevents DNA methylation and repair (Figure 2). This implies that ethanol oxidation and intra-colorectal acetaldehyde levels may increase among heavy drinkers due to changes in the gut flora, possibly reaching the minimum quantities required to start carcinogenesis. Chronic ethanol consumption reduces intestinal motility and causes intestinal barrier dysfunction. This dysfunction leads to leaky gap junctions, allowing bacteria and their toxic byproducts (endotoxins) to cross the intestinal barrier. Consequently, immunogenic microbial substances are exposed to the lamina propria, where immune cells are located, causing intestinal inflammation. Furthermore, this condition increases the vulnerability of colorectal epithelial cells to carcinogens (Citation47).
It is unclear why alcohol intake could alter the composition of the gut microbiome. According to some research, alcohol may influence the intestinal microbiota by lowering gastrointestinal motility, dampening innate and adaptive immune responses, or blocking bactericidal protein production (Citation108).
Cigarette Smoking
Smoking affects the gut microbiota composition and raises the risk of developing colorectal cancer, with an extended latency period, based on the evidence from studies on animals and humans. Former smokers continued to have a higher risk than never-smokers for up to 25 years after quitting (Citation109).
A complicated chemical blend makes up cigarette smoke, which includes nicotine, aldehydes, polycyclic aromatic hydrocarbons (PAHs), nitrosamines, heavy metals, and other substances that are breathed as aerosol particles or accessible in a gaseous condition into the lungs (Citation110, Citation111). These toxic chemicals are then ingested into the GI tract and cause gut microbiota dysbiosis through various mechanisms, including antimicrobial activity and control of the intestinal microenvironment (Citation112). Exposure to cigarette smoke increases gut pH, which may help some bacteria flourish and lead to intestinal microbiota dysbiosis.
Cigarette smoking causes current smokers to have higher fecal levels of the Prevotella, Veillonella, Bacteroides, Acidaminococcus, and Oscillospira and lower levels of the phyla Firmicutes, to be more precise the genera Lachnospira and Proteobacteria compared to nonsmokers (Citation113–115). Turicibacteraceae and Peptococcaceae significantly increased in male mice exposed to nicotine by drinking water for 13 weeks at 60 mg/L, as reported by Chi et al. (Citation116). Additionally, they also discovered that male and female mice varied significantly. Targeted fluorescence in situ hybridization was used in a study that showed that smokers’ feces contained more Bacteroides and prevotella than nonsmokers. Contrarily, it has been shown that quitting smoking decreases the abundance of Bacteroidetes (Prevotella spp. and Bacteroides spp.) and Proteobacteria and enhances the numerous amounts of essential members of the phyla Firmicutes (Clostridium coccoides, E. rectale, and Clostridium leptum subgroup) and Actinobacteria (HGC bacteria and Bifidobacterium) (Citation114).
According to a recent study by Bai et al., mice exposed to smoking cigarettes after being given the carcinogen azoxymethane to induce colorectal cancer (CRC) had a significant incidence of colorectal cancer tumors. They had a higher growth of colonic epithelial cells than mice with the disease, who were only exposed to fresh air. Additionally, in this study, mice exposed to smoke had gut microbiota that were significantly enriched in Eggerthella lenta and Staphylococcus capitis and depleted in Parabacteroides distasonis and Lactobacillus species (Citation117). Increased fecal taurodeoxycholic acid (TDCA), diminished barrier function, elevated ERK/MAPK signaling activation, and inflammatory IL-17 and TNF signaling pathways in colon epithelium cells were all correlated with smoking-induced microbial dysbiosis (Citation114) (). Subsequently, when germ-free mice are given the stools of CRC animals exposed to smoking, the recipient mice have damaged colonic epithelium, higher levels of E. coli and TDCA, and more active MAPK/ERK and proinflammatory pathways (Citation117). These results suggest that smoking-induced dysbiosis of the microbial community contributes to the progression of CRC. It would be interesting to investigate the impact of colonizing specific bacterial species or metabolites diminished during smoking as potential preventative or therapeutic measures for CRC.
Metabolites Derived from Gut Microbiota and Their Role in CRC
A host’s lifestyle and dietary habits substantially impact the microbial population and the metabolites produced by these bacteria. This complex interplay between lifestyle, gut microorganisms, and metabolites in CRC establishes a significant link. Studies have demonstrated that whereas other microbial metabolites such as secondary bile acids, trimethylamine N-oxide (TMAO), and hydrogen sulfide (H2S) promote carcinogenesis, the short-chain fatty acids, including butyrate, acetate, and propionate, act in the reduction of inflammation and cancer.
Short-Chain Fatty Acids
Short-chain fatty acids, including acetate, butyrate, and propionate, which are produced by the anaerobic gut microbiota through fermentation of dietary fiber, are essential for maintaining the integrity of the colonic mucosa since they control cellular immunity and protect the intestinal barrier. SCFAs, through different pathways like lysosomal membrane permeabilization, which is related to mitochondrial dysfunction and degradation (Citation118), induce apoptotic cell death in CRC cells and reduce the proliferation of CRC cells by triggering T cell-mediated immunological responses (Citation119), generating epigenetic alterations such as methylation and deacetylation (Citation120), and activating intracellular signaling pathways (Citation121). SCFAs prevent the activation of NFATc3 and calcineurin, which helps to regulate protein acetylation and cancer cell growth (Citation122). Additionally, short-chain fatty acids vigorously promote cell cycle arrest and death via altering the expression of genes linked to TNF, NF-kB, CARD, and Bcl-2 signaling mechanisms in human colon cancer (Citation123). SCFAs may stimulate the free G protein-coupled receptors fatty acid receptor 2 (FFAR2) and fatty acid receptor 3 (FFAR3), as well as hydroxycarboxylic acid receptor 2 (HCAR2) receptors on intestinal epithelial cells and immunological cells. This may lead to a range of inflammatory and immune responses that preserve the integrity of the tissues and the host’s defense (Citation124, Citation125).
Among the various forms of SCFAs, Butyrate’s effectiveness in preventing CRC has been the subject of the most research. Compared to acetate and propionate, it was found to have a stronger inhibitory impact against the proliferation of cancer cells in the human colon (Citation123). Butyrate inhibits cell growth in CRC cells in a variety of ways. Butyrate reduces the expression of neuropilin-1, a vascular endothelial growth factor (VEGF) receptor frequently increased in colon cancer cells. The downregulation of neuropillin-1 inhibits the growth of colon cancer cells since VEGF is a crucial regulator of angiogenesis (Citation126, Citation127). Butyrate inhibits the proliferation and invasion of CRC cells by inducing apoptosis and controlling the expression of microRNAs such as miR-92a (Citation128) and miR-203 (Citation129). Butyrate also inhibits the Akt/ERK signaling, decreasing CRC cell metastasis (Citation130).
When butyrate has higher concentrations, it can inhibit the Histone deacetylase (HDACs) and enhance the response against inflammation in the gut of mice with CRC caused by azoxymethane/dextran sodium sulfate (AOM/DSS) (Citation131).
Propionate has the same anti-inflammatory effect as butyrate in the gut and on colorectal cancer. Propionate and butyrate may immediately boost the expression of genes relevant to Tc17 cells and CD8+ CTLs and promote antitumor effects based on accumulating in vitro and in vivo studies (Citation132). Tedelind et al. demonstrated one of the critical functions of propionate, demonstrating how it might reduce inflammation by blocking the NF-B pathway and limiting the production of IL-6 from colon cells (Citation133). Propionate has also been demonstrated to significantly regulate the development of tumorigenic lesions in colon cancer cells via the rise in histone acetylation (Citation134).
Acetate is the subject of significantly fewer studies than butyrate and propionate that evaluate its potential impact on CRC. Therefore, the precise mechanism of acetate’s anti-inflammatory effect on the development of CRC is unknown. According to one study, Acetate may prevent the progression of CRC by raising the levels of cytokines that suppress inflammation and decreasing the production of inflammatory cytokines and the NF-B pathway in CRC cells (Citation133).
Trimethylamine N-Oxide
Trimethylamine (TMA) produced by the gut microbiota and subsequently is converted to trimethylamine N-Oxide (TMAO) in the liver has drawn more attention due to its significant contribution to the growth of colorectal cancer. Initially, TMA is produced by intestinal microorganisms from dietary substances such as L-carnitine, choline, ergothioneine, betaine, phosphatidylcholine, and dimethylglycine that are abundant in animal proteins and fats such as red meat, dairy products, and eggs. TMA then enters the bloodstream and eventually converts into TMAO in the liver by the liver’s FMO3 and FMO1 (flavin-containing mono-oxygenases 3 and 1) enzymes. Furthermore, TMAO can be ingested directly through the gut and naturally exists in preformed states in fishes and seafood (Citation135).
In one of the earliest studies, Bae et al. investigated this relationship and found a positive correlation between plasma TMAO levels and CRC risks in American women (Citation136). The study discovered that the risk of developing rectal cancer was 3.4 times higher in those with the highest plasma TMAO content than in those with the lowest levels. Furthermore, a recent investigation revealed that colorectal cancer (CRC) patients exhibited elevated serum levels of TMAO compared to healthy individuals, suggesting a potential role for TMAO as a predictive biomarker for CRC (Citation137). These findings suggest that TMAO could contribute to CRC development, either directly or indirectly. In a study by Xu et al. (Citation138) two distinct epigenetic interaction networks (EINs) were constructed utilizing data on disease-gene interactions, chemical gene interactions, and protein-protein interactions. These networks aimed to explore the diseases associated with TMAO, mainly focusing on genetic pathways connecting TMAO with CRC pathogenesis. Analysis of data from both EINs highlighted CRC’s prominence among TMAO-related diseases. Additionally, the study revealed numerous shared genetic pathways between CRC and TMAO, including 52 pathways linked to OMIM (Online Mendelian Inheritance in Man) genes and 39 pathways associated with GWAS (Genome-Wide Association Studies), providing further evidence for the genetic association between TMAO and CRC.
Shuyan Yang et al. recently discovered that TMAO encourages cell growth and angiogenesis in CRC. After feeding long-term choline to tumor-bearing mice, levels of VEGF-A and CD31 significantly increased. Rising levels of circulating TMAO and increased tumor volume were also confirmed (Citation139).
DNA damage, Oxidative stress, inflammation, and disruption of protein folding are a few more potential pathways that have been discovered that might be essential in the development of colorectal cancer to TMAO (Citation135, Citation140). Furthermore, it has been suggested that TMAO plays a role in the molecular pathways that generate N-nitroso compounds (Citation141), which have been shown to damage DNA and may assist in the development of cancer (Citation142). Despite numerous findings correlating TMAO to CRC, more experimental mechanistic analysis is needed to prove TMAO’s role in the development of CRC.
Hydrogen Sulfide
Hydrogen sulfide (H2S), which is generated by the digestion of several sulfur-containing substrates (including taurine/cysteine/methionine/sulfoquinovose), both by the gut microbiota and endogenous enzymes, has been identified as an essential controller of intestinal health, especially in CRC (Citation143, Citation144). The activity of Sulfate-Reducing Bacteria (SRB) influences the synthesis of gut luminal H2S. In the human gut, the principal constituents among these SRBs are Desulfovibrio (D. piger, D. desulfuricans), Bilophila, and Desulfomicrobium (Citation145). SRB requires two vital substrates to generate H2S: sulfate and an electron donor for sulfate reduction. Diets rich in sulfate lead to heightened growth of D. piger and increased H2S production in the colon of both humans and mice (Citation146, Citation147). Sulfidogenic bacteria and H2S overexpression may be potential risk factors that promote the rapid development of CRC (Citation144, Citation148). An enhanced risk of distant CRC may originate from a dietary pattern that increases sulfidogenic bacteria in feces that persisted for a long time (Citation149).
In cancer, the biological impacts of H2S follow a characteristic bell-shaped concentration-response curve. Optimal concentrations of H2S can stimulate various cancer-related processes including cell growth, cellular energy production, blood vessel formation, dedifferentiation, invasion, metastasis, and resistance to chemotherapy. However, once the concentration of H2S surpasses a certain threshold, it switches to exerting tumor-suppressing effects. These effects include reducing cancer cell proliferation, migration, and cellular energy production, triggering apoptosis in cancer cells, sensitizing them to chemotherapy, and inducing a transition from mesenchymal to epithelial states (Citation150–152).
High concentrations of H2S in the colon, often linked to a high protein diet (HPD), can trigger an adaptive response in colon cells by boosting the expression of the sulfide quinone reductase (Sqr) gene. This suggests a potential protective mechanism for the epithelial layer against harmful levels of H2S (Citation153). However, in CRC, enzymes involved in the oxidation pathway of sulfide may be diminished in the epithelium (Citation154). Conversely, low levels of H2S can upregulate the expression of genes associated with inflammation, like iNos and Il-6, while inhibiting mitochondrial oxygen consumption in colon cells (Citation153). Recent studies indicate that low H2S concentrations impede CRC cell proliferation by influencing apoptosis and the cell cycle (Citation155, Citation156). The investigation of Du et al. (Citation157) describes individuals with normal colonoscopy findings exhaled a significantly greater quantity of H2S compared to patients with colorectal cancer (CRC). In CRC patients, the exhaled amount of H2S was associated with factors including gender, lesion location, and tumor characteristics such as lymphatic metastasis, depth of invasion, and TNM stage (Citation157). Wang et al. (Citation158) study among 214,797 participants shows the correlation between colorectal cancer probability and dietary patterns, specifically with the sulfur microbial diet (SMD). Particularly in older individuals, higher SMD scores are positively associated with traditional colorectal adenoma risk, emphasizing the significance of dietary factors in colorectal cancer development (Citation158).
Secondary Bile Acids
Cholesterol 7 α hydroxylase (CYP7A1) in hepatocytes synthesizes primary bile acids (BAs), which are expelled into the intestinal tract with the bile. 95% of the bile acids are returned to the hepatocytes through the portal vein circulation after absorption in the ileum. The other substances evade enterohepatic circulation and travel to the intestines, where intestinal bacteria dehydroxylate them into secondary BAs (Citation159). Secondary bile acids are usually left unconjugated by active enzymes called bile salt hydrolases, which may deconjugate both primary and secondary bile acids (Citation160). Eubacterium, Lactobacillus, Bifidobacterium, Bacteroides vulgatus, Clostridium perfringens, and Bacteroides fragilis are among the bacteria that can produce secondary bile acids. Deoxycholic acid (DCA) and lithocholic acid (LCA) are produced from conjugated bile acids such as cholic acid (CA) and chenodeoxycholic acid (CDCA) through 7-α-dehydroxylation (Citation161). Increased levels of secondary BAs, especially deoxycholic acid, are crucial to the carcinogenic development of colorectal adenomas, which may result from interactions between BAs and the gut microbiota (Citation145). Patients with colorectal adenoma exhibited significantly higher serum concentrations of DCA than healthy individuals (Citation162). For example, in one research, mice supplied with DCA in their diets developed greater colon tumors and malignancies than control mice (Citation163). An epidemiological study reported that a higher level of SBAs such as DCA, LCA, and UDCA was found in the fecal matter of CRC patients compared to normal control patients. Furthermore, it has been found that an increased concentration of SBAs is usually found in carnivorous people, but no significant changes in primary bile acids were observed in those people (Citation164).
APCmin/+ mice, in particular, when fed 0.2% DCA in the water, showed a greater tumor prevalence than control mice. This was linked to a significant improvement in intestinal cell proliferation because of Wnt signaling. In a human colon cancer cell line, DCA and, at higher levels, CDCA have also been demonstrated to stimulate apoptosis, which was proposed to be dependent on the synthesis of ROS (Citation165). Ursodeoxycholic acid (UDCA), another secondary bile acid, has received attention from researchers. Due to UDCA’s anti-inflammatory effects, an actual dose of UDCA significantly decreased the intestines’ severe inflammation in the DSS-induced colitis mouse model. This beneficial effect might be dependent on the subsequent metabolism of UDCA into LCA by the gut microbiota because a single UDCA analog (6-MUDCA), which exhibits an identical anti-inflammatory impact to that of UDCA in vitro but must be metabolized by the microbiota, does not exhibit this anti-inflammatory response in mice (Citation166). Therefore, it has been suggested that high-dose UDCA may help inhibit the growth of cancerous tumors, particularly in UC patients with PSC. In contrast, when UDCA with a high dose was added to the dietary pattern of the patients with ulcerative colitis (UC) and PSC, they usually considered that the risk of CRC neoplasia progression had increased in comparison to the control group, indicating the risk of increasing CRC pathogenesis due to the high dose of UDCA (Citation167) ().
Table 1. Function of gut microbiome-derived metabolites.
Therapeutic Approaches to Restore Gut Dysbiosis for the Treatment of CRC
Gut microbiota modulation, which aims to enhance gut microbial equilibrium, is being investigated as a possible approach for CRC prevention and therapies. These strategies include dietary modifications, probiotics, prebiotics, antibiotics, and FMT. The importance of gut biomodulators and microbe-based therapies as anticancer agents has been demonstrated through various experimental investigations; however, their specific practical impact on preventing CRC and treating it still needs to be explored.
Diet Modification
The management of CRC is significantly influenced by diet, which alters the microbiome. While there is substantial ongoing research into the impact of a high-fat diet on colorectal cancer and gut microbiota, the microbiome-level implications of diets rich in cereal grains, which are associated with a potential reduction in colorectal cancer risk, remain uncertain (Citation173). The capability of seven different types of grains to lower the risk of colorectal cancer in mice fed a high-fat diet has shown that the highest CRC reduction is due to glutinous rice, non-glutinous rice, and sorghum dietary intake. Specific modified genera connected with Colorectal cancer, such as Lactobacillus, Bacteroides, Acinebacter, and Ruminococcus, were stabilized by non-glutinous rice (Citation174).
In mice models of CRC and colitis caused by bacteria, the risk of cancer by Vitamin D supplements (Citation175). CRC was triggered by azoxymethane/dextran sodium sulfate, and high doses of vitamin D supplements were fed to mice, showing results with improved weight gain, reduced colon shortening, and decreased expression of pro-inflammatory cytokines like TNF-alpha and IL-6. In addition, Vitamin D has a crucial regulatory role in maintaining the integrity of the intestinal barrier, which is regulated by the mucin-using bacterium Akkermansia muciniphila (Citation176).
Different types of low-calorie consumption, especially fasting, show advantageous outcomes in carcinogenesis prevention and enhancing the efficiency of cancer treatments because there are insufficient regulations on the sorts of diets that may possess a major effect on cancer development (Citation177). The microbes in the gut play an important role in one of the fundamental mechanisms by which fasting results in improved metabolism. For example, fasting for an intermittent period changed the structure of the gut microbiome and raised the amount of fermentation products, such as lactate and acetate. Additionally, this dietary plan decreased Actinobacteria, Bacteroidetes, and Terericutes while increasing Firmicutes numbers and short-chain fatty acid synthesis (Citation178).
Notably, the mice fed with a high-fat diet food withdrawal reduced the amount of possibly harmful proteobacteria while increasing Akkermansia muciniphila (Citation179). Tumors cannot metabolize ketone bodies that act as a source of energy due to various abnormalities in either or both of the essential enzymes found in mitochondria. To prevent the formation of tumors, it might consequently be suitable to apply a ketogenic diet (KD) (Citation180).
Antibiotics
Antibiotic treatment is a reasonable method for treating cancer since it helps to eradicate the gut microbiota and repair harmful dysbiosis. Antibiotics are frequently used in in-vivo models to investigate the effects of the gut microbiota on cancer or other inflammatory disorders. They are usually delivered via gavage or drinking water. Multiple investigations have found that antibiotic-mediated microbiota reduction inhibits the development of CRC (Citation181, Citation182).
Treatment with Metronidazole eliminates Fusobacterium colonization in mice harboring CRC xenografts and decreases colorectal cancer growth (Citation183) highlighting antibiotics as an alternative treatment strategy for Fusobacterium-enriched colorectal cancer patients. Another investigation shows that the role of neutrophils in CRC revealed a unique microbiome composition found in mice, either with or without neutrophil reduction. In the meantime, Antibiotic therapy decreases the number of bacteria in the tumor and prevents cancer development (Citation184). When F. nucleatum colonization occurs in ApcMin/+ mice models, Berberine (BBR), an isoquinoline compound with antibiotic action, is used as a treatment. In addition to reversing the microbiome disequilibrium triggered by Fusobacterium nucleatum, Berberine also prevented the release of Interleukin-21, Interleukin-22, Interleukin-31, and CD40L, which are mucosal immune cytokines. The Janus kinase/signal transducer and activator of transcription (JAK/STAT) and the mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) pathway activation caused by F. nucleatum were also suppressed by this substance (Citation185).
Additionally, the BBR strategy significantly reversed the bacterial composition modification in Fn-infected mice, which suggests antimicrobial action as an alternate therapy for Fusobacterium nucleatum-associated colorectal cancer. Elevated levels of Tenericutes and Verrucomicrobia characterize this modification. In prior ETBF-inoculated animals, cefoxitin, a partially synthetic and wide-spectrum cephalosporin, stimulated an entire and permanent clearing of enterotoxigenic Bacteroides fragilis colonization with a simultaneous reduction in median adenoma development. An unexpected decrease followed the complete elimination of ETBF in colonic interleukin-17A levels, which is similar to the pro-tumorigenic Th17 immune system defense of Entero-toxigenic Bacteroides fragilis and indicates that other bacteria are involved in the IL-17 response (Citation186). In human CRC cells, erythromycin inhibits the gene expression of NF-B and the activator protein-1 (AP-1), and it also inhibits the activity of its downstream standards, Interleukin-6, and cyclooxygenase-2 (COX-2). In addition, it was shown that ApcMin/+ mice had fewer intestinal polyps and a decreased level of Interleukin-6 and Cox-2 expression (Citation187). Therefore, antibiotic administration is a highly rigorous method of altering gut microbiome composition and its applications in treating cancers.
Probiotics
Probiotics can prevent and treat CRC by enhancing quantitative and qualitative compositions as well as abundant diversification of the gut microbiota to maintain a balanced composition (Citation188). Intake of an adequate number of probiotic bacteria can reduce the non-beneficial disease-causing bacteria such as Klebsiella pneumonia and Clostridium perfringens, which are widely known as pathobionts and may potentially be symbiotic microbes in specific gut environment circumstances (Citation189). Certain probiotic bacteria are capable of producing antimicrobial compounds that either completely eliminate or significantly slow the growth of harmful bacteria in the colon. These compounds include reuterin, hydrogen peroxide, lactic acid, and bacteriocins (Citation190). Lactobacillus rhamnosus GG has been embraced for its inhibition influence on the gastrointestinal erroneous crypt foci. These impacts are caused by the alteration in the gut microbiome, increased levels of the protein genes that regulate Bax, IFN-, P53, IL-2, and caspase-3, as well as decreased levels of the expression of β catenin, COX-2, IL-10, Bcl-2, tumor necrosis factor (TNF), and NF-kB in rats provided with dimethylhydrazine (Citation191). The ingestion of Lactobacillus casei BL23 strain in an azoxymethane (AOM)/DSS-induced C57BL/6 mice model is currently shown to cure dysbiosis associated with CRC. It may inhibit CRC by decreasing the gene expression of cytokine IL-22 and increasing the production of caspase-7 and caspase-9 (Citation192).
Probiotics may assist in slowing the proliferation of colon cancer cells by secreting anti-inflammatory compounds and generating short-chain fatty acids (Citation193). A few experimental investigations of in-vivo models show Clostridium butyricum has inhibitory effects on CRC progression via modifying Wnt signaling, increasing the concentration of cecal SCFAs, decreasing the amount of SBA in feces, and activating the short-chain fatty acid-sensing receptors GPR109A and GPR43 (Citation193).
In HT29 human colon cancer cells, pro-apoptotic gene expression was enhanced and anti-apoptotic gene expression was decreased in the combination of TNF-related apoptosis-inducing ligand and Propionibacterium freudenreichii (Citation194). Metabolites generated by specific probiotic bacteria, which include Lactococcus, Lactobacillus, and Saccharomyces, may possess an anti-inflammatory impact on the epithelial cells of the intestine by inducing the production of cytokines and inhibiting the production of IL-8 and PGE-2. This is another example of an adjuvant that assists with cancer and inflammatory diseases (Citation195). Nozari et al. evaluated the possibility of chemotherapeutic consequences of Lactobacillus paracasei cell membrane protein fragments with the human CRC Caco-2 cell line using the MTT assay, cell viability analyses, and Annexin V-FITC/propidium iodide staining (Citation196). In-vivo investigation in rats revealed that Saccharomyces boulardii (S. boulardii) cell wall protein possessed an effective anticancer effect on CRC prevention. In general, the research results demonstrated that S. boulardii cell wall protein exhibits anticancer activity by reducing the number of abnormal crypt foci and altering the activity of the enzymes quinone reductase and β-glucuronidase activity (Citation197).
Lactobacillus strains (L. brevis PM150, L. plantarum PM153, L. brevis PM177, L. delbrueckii subsp. bulgarius BCRC10696, L. reuteri BCRC14625, L. salivarius BCRC14759, and L. johnsonii BCRC17010) were investigated by Chen et al. for their ability to prevent HT-29 cells from developing colorectal cancer. Based on this research, the pathogenic bacterial strain Lactobacillus johnsonii BCRC17010 increased lactate dehydrogenase release and proapoptotic activity in HT-29 cells, dramatically reducing HT-29 cell proliferation (Citation198).
Kahouli et al. discovered that the probiotic combination of Lactobacillus acidophilus ATCC 314 and Lactobacillus fermentum NCIMB 5221 affected CaCo-2 cells. Treatment using a probiotic combination greatly decreased the growth of cancer cells and caused CaCo-2 cells to undergo apoptosis (Citation199). Sambrani et al. investigated the impact of Saccharomyces cerevisiae on HT-29 cell proliferation, metastasis, and apoptosis. CFS from Saccharomyces cerevisiae demonstrated anti-proliferative activity in HT-29 cells after 24 h of treatment by upregulating the expression of Caspase-3 and PTEN while downregulating the expression of RelA and Bclxl (Citation200). Additionally, several probiotics are now using for clinical treatment of CRC patients such as Bifidobacterium and lactobacillus can prevent CRC development and growth by improving intestinal barrier functions via SCFAs secretion, inhibiting angiogenesis and inflammation in the gut (Citation201). A clinical study was conducted on CRC and polypectomized patients for 12 weeks, which was a random, double-blind, and placebo-controlled study with the administration of Lactobacillus rhamnosus GG, Bifidobacterium lactis Bb12 strains 105, and oligofructose-enriched inulin. Improvements in the function of the epithelial barrier, as well as a decrease in CRC proliferation and the ability of fecal water to cause necrosis in colonic cells, were noted (Citation202).
The advantageous outcomes of probiotic consumption vary based on the host biological functioning, the extent of the disease, the strain, the dosage, the period of the intervention, along with other dietary supplements, etc. Probiotic supplements enhanced CRC patients’ immune system and gut balance, improved antimicrobial defense, and destroyed carcinogenic substances. More research is strongly recommended to determine probiotics’ specific mechanism and understand the possible role in CRC prevention.
Prebiotics
Prebiotics are considered as non-digestible food substances that enhance host health by selectively promoting the proliferation and activity of particular bacteria in the gut (Citation203). Prebiotics should promote the growth of a few species of colonic bacteria and their specific metabolites, which might benefit anti-cancer therapies (Citation204). Prebiotics can improve colon health by stimulating specific bacteria to eliminate nutrient breakdown. Depending on the prebiotic, these nutrients either promote the bacterial groups’ decline or growth (Citation205).
Prebiotics may help in the reduction of cell proliferation by inducing apoptosis, inhibiting the formation of new blood vessels (known as angiogenesis), promoting metastasis processes to build an immune defense, and reducing the chances of CRC (Citation206, Citation207). Prebiotics may be grouped, based on the monomer linkage, such as disaccharide (Two monomers joined together), oligosaccharide (more than 3–10 monomers joined together), and polysaccharide (more than 10 monomers joined together). Effective standard types of oligosaccharides that have been proven to be valuable prebiotics, such as soybean oligosaccharides (SBOS), xylooligosaccharides (XOS), fructooligosaccharides (FOS), trans galactooligosaccharides (TOS), and isomaltooligosaccharides (IMO) (Citation208, Citation209). Certain polysaccharides such as starch, inulin, pectin, cellulose, and hemicellulose may also exhibit prebiotic properties (Citation210).
Additional studies in animals such as rats, mice, and humans have indicated the beneficial effect of prebiotics, and the potential mechanisms of prebiotics conferring colorectal cancer prevention have been discussed regarding their implications for gut microbiome structure and functions and bacterial metabolism generation in gastrointestinal circumstances.
Animal models highly validated prebiotics’ beneficial impacts on the burden of colorectal cancer, while human research is still in the early stages (Citation202). These substances changed the predominance of some commensal microbiota, such as (Bacteroides, Bifidobacterium, Escherichia coli, and Enterococcus). These contentious data imply that additional study is required to fully understand their therapeutic impact in lowering the burden of CRC at population-based levels (Citation211). Studies indicate that diets high inulin can increase the prevalence of significant propionate makers in populations of Bacteroidetes. The leading cause is a substantial increase in families like Prevotellacea, Porphyromonadaceae, and Bacteroidaceae (Citation70).
Lyophilized jaboticaba seed extract (LJE) is a notable reservoir of phenolic compounds, including castalagin, vescalagin, procyanidin A2, and ellagic acid. It exhibited cytotoxic effects on cancer cells, attributed to its pro-oxidant activity. However, LJE reduced reactive oxygen species (ROS) generation in a normal human cell line (IMR90), showcasing antioxidant properties. Incorporating LJE into yogurt enhanced the yogurt’s antioxidant capacity, and the final product received positive sensory acceptance (Citation212).
A significant supply of prebiotics like Yacon, which is made of FOS and synthesized through the cecum by bacteria of the particular species Bifidobacterium, might impact the immune system and raise secretory Immunoglobulin A(sIgA) levels (Citation213). Yacon treatment additionally exerts significant effects on the immune system, as shown by a decreased ratio of TNF-/IL-10, which balances cytokines that are pro- and anti-inflammatory. IL-10, produced by Th2 lymphocytes, prevents Th1 cells from producing macrophage-dependent cytokines like TNF-α (Citation214). The evidence consequently indicates the possible existence of an auto-regulatory cycle in which TNF-α stimulates the production of IL-10, further preventing the synthesis of TNF-α (Citation215). In the colons of BALB/c mice given treatment with a Yacon-based product containing FOS, the proportion of regulatory T cells (T reg), which also produce IL-10, increased. Immune system modulations are typically associated with increased levels of sIgA, an antibacterial defensin, and anti-inflammatory cytokines, particularly IL-10. The combination of the herbal tea Gynostemma pentaphyllum (GpS) and a mushroom called Ganoderma lucidum (Lingzhi) polysaccharides (GLP) significantly enhanced the mice’s inflamed intestinal barrier. This effect is accomplished by a reduction in polyps, an alteration in the colonic M1 to M2 macrophage ratio, a positive reverse of the E/N cadherin ratio, and a decrease in the expression of oncogenic signaling molecules (Citation216).
Prebiotic application is a prospective therapeutic strategy. Prebiotics include high fiber supplements that heavily alter the microbiota population, increase the number of bacteria that generate SCFA, enhance the functional pathways that produce SCFA, and increase the concentrations of its metabolites. Prebiotics consumption can reduce inflammation and colitis in the gut, modulate the immune response, prevent colorectal cancer, maintain gut homeostasis, and regulate the host’s energy metabolism. According to this perspective, the gut microbiota may be enriched and controlled through the consumption of prebiotics in the diet, especially those that emphasize physiologically active substances present in plant-based foods. These substances may help to prevent or slow the progression of CRC.
Fecal Microbiota Transplantation
Fecal Microbiota Transplantation (FMT) is the go-to treatment in Clostridiodes difficile infections (CDI) due to its impressive high efficacy, with cure rates being as high as 90% (Citation217). In most CDI cases, patients recover within a single treatment regime (Citation218). To restore the normal gut microbiota condition and functions referred to as normobiosis, healthy microbiota is transferred from a healthy donor to the patient. In some cases, though, endotoxins or infectious microbial organisms could also get integrated into the patient’s gut during transplantation (Citation219). Choosing specific microbes for the transplant could help resolve such complications (Citation220). In addition to treating gut dysbiosis, FMT improves the responsiveness of insulin levels and is increasingly being explored in treating cardiometabolic diseases (Citation221). It is also being studied to treat various other disorders, such as irritable bowel syndrome, ulcerative colitis, constipation, sclerosis, Parkinson’s disease, hepatitis, and cirrhosis (Citation222–225).
In a study, FMT inhibited tumor growth and restored the balance of the gut microbiota simultaneously (Citation226). In another study, compared to control mice with their bacteria, laboratory mice transplanted with intestinal microbiomes from wild mice demonstrated better resistance to CRC and reduced inflammation (Citation227). This finding supports the idea that FMT may hold potential therapeutic benefits for CRC. Despite the suppression of tumor progression in the CRC mouse model, FMT successfully maintained the equilibrium of the Gut microbiome. FMT plays a significant role in suppressing the levels of inflammation; interleukin-1, Interleukin-6, and TNF-α levels were diminishing, as interleukin-10 and TGF-β the anti-inflammatory cytokines were enhanced through the regulation of canonical NF-kB action and cell growth. Likewise, FMT therapy increases Tregs instead of Th1, Th2, and Th17 cells. The beneficial effects of FMT as a chemopreventive agent against FOLFOX-induced intestinal damage were examined. Further, it is important to note that FMT on the immune response of particular people is complex and difficult to predict and that there is always a chance that a new infection will spread.
Conclusion and Future Perspective
Alteration of gut microbiota composition can be a potential factor in the development of CRC. Diet and lifestyle factors are primarily responsible for shaping the composition of gut microbiota. The research investigation suggests that increasing dietary fiber consumption may reduce the risk of CRC; however, the high risk of CRC is associated with the consumption of red or processed meat in large amounts, alcohol consumption, smoking habits, a lack of physical activity, and high levels of obesity. The composition of gut microbiota exhibits substantial variation from person to person. Factors such as genetics, diet, age, geography, antibiotic usage, and birth method (vaginal delivery vs. cesarean section) contribute to this natural diversity. This inherent variation in gut microbial composition influences how individuals metabolize nutrients, respond to dietary changes, and even their susceptibility to certain diseases. Recognizing the significance of this natural variation underscores the importance of personalized treatment approaches that consider an individual’s unique gut microbial profile. Strategies aimed at reducing CRC risk through modulation of the gut microbiome, such as dietary modifications, probiotics, prebiotics, antibiotics, and fecal microbiota transplantation (FMT), hold promise but require further investigation to delineate their practical impact on CRC prevention and treatment. Moving forward, it is imperative to conduct comprehensive human clinical studies that consider personalized factors and delve into the intricate processes governing the efficacy of specific interventions in mitigating CRC risk and progression. In addition, the best-known study on the connection between lifestyle and gut microbiota is based on small cross-sectional or transient interventions. To ascertain the causal effect of gut microbiome dysbiosis, dietary habits, and lifestyle on the CRC’s progression and development, it is urgent to establish more well-designed prospective studies with detailed diet and lifestyle data that evaluate the composition of gut microbiota and gastrointestinal health status across different life stages.
Authors’ Contributions
SP, AKDR, AKR, and AB designed, wrote, and revised the manuscript. BS, RAT, and SA wrote the initial part of the manuscript and drew the figures.
Acknowledgement
We want to thank the Chettinad Academy of Research and Education, Chettinad Hospital, and Research Institute for providing the facilities to complete this work.
Disclosure Statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Hossain MS, Karuniawati H, Jairoun AA, Urbi Z, Ooi DJ, John A, Lim YC, Kibria KMK, Mohiuddin AKM, Ming LC, et al. Colorectal cancer: a review of carcinogenesis, global epidemiology, current challenges, risk factors, preventive and treatment strategies. Cancers (Basel). 2022;14(7):1732–56. 10.3390/cancers14071732
- Xi Y, Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol. 2021;14(10):101174. 10.1016/j.tranon.2021.101174
- Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019;14(2):89–103. 10.5114/pg.2018.81072
- Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16(12):713–32. 10.1038/s41575-019-0189-8
- Mannucci A, Zuppardo RA, Rosati R, Leo M, Di Perea J, Cavestro GM. Colorectal cancer screening from 45 years of age: thesis, antithesis and synthesis. World J Gastroenterol. 2019;25(21):2565–80. 10.3748/wjg.v25.i21.2565
- Constantinou V, Constantinou C. Focusing on colorectal cancer in young adults (Review). Mol Clin Oncol. 2024;20(1):8. 10.3892/mco.2023.2706
- Drewes JL, Housseau F, Sears CL. Sporadic colorectal cancer: microbial contributors to disease prevention, development and therapy. Br J Cancer. 2016;115(3):273–80. 10.1038/bjc.2016.189
- Islami F, Goding Sauer A, Miller KD, Siegel RL, Fedewa SA, Jacobs EJ, McCullough ML, Patel AV, Ma J, Soerjomataram I, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. 2018;68(1):31–54.
- Zhu B, Wang X, Li L. Human gut microbiome: the second genome of human body. Protein Cell. 2010;1(8):718–25. 10.1007/s13238-010-0093-z
- Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D, Costea PI, Godneva A, Kalka IN, Bar N, et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555(7695):210–5. 10.1038/nature25973
- Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474(7351):327–36. 10.1038/nature10213
- Petersen C, Round JL. Defining dysbiosis and its influence on host immunity and disease. Cell Microbiol. 2014;16(7):1024–33. 10.1111/cmi.12308
- Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915–20. 10.1126/science.1104816
- Ivanov II, Frutos RdL, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4(4):337–49. 10.1016/j.chom.2008.09.009
- Boleij A, Tjalsma H. Gut bacteria in health and disease: a survey on the interface between intestinal microbiology and colorectal cancer. Biol Rev Camb Philos Soc. 2012;287(3):701–30. 10.1111/j.1469-185X.2012.00218.x
- Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, Clancy TE, Chung DC, Lochhead P, Hold GL, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14(2):207–15. 10.1016/j.chom.2013.07.007
- Leung A, Tsoi H, Yu J. Fusobacterium and Escherichia : models of colorectal cancer driven by microbiota and the utility of microbiota in colorectal cancer screening. Expert Rev Gastroenterol Hepatol. 2015;9(5):651–7. 10.1586/17474124.2015.1001745
- Bogaert D, Keijser B, Huse S, Rossen J, Veenhoven R, van Gils E, Bruin J, Montijn R, Bonten M, Sanders E, et al. Variability and diversity of nasopharyngeal microbiota in children: a metagenomic analysis. PLoS One. 2011;6(2):e17035. 10.1371/journal.pone.0017035
- Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. Bacterial community variation in human body habitats across space and time. Science. 2009;326(5960):1694–7. 10.1126/science.1177486
- Hopkins MJ, Sharp R, Macfarlane GT. Age and disease related changes in intestinal bacterial populations assessed by cell culture, 16S rRNA abundance, and community cellular fatty acid profiles. Gut. 2001;48(2):198–205. 10.1136/gut.48.2.198
- Erwin G, Zoetendal ADLA. The host genotype affects the bacterial community in the human gastronintestinal tract. Microb Ecol Health Dis. 2001;13(3):129–34.
- Tap J, Mondot S, Levenez F, Pelletier E, Caron C, Furet J-P, Ugarte E, Muñoz-Tamayo R, Paslier DLE, Nalin R, et al. Towards the human intestinal microbiota phylogenetic core. Environ Microbiol. 2009;11(10):2574–84.
- Peterson DA, Frank DN, Pace NR, Gordon JI. Metagenomic approaches for defining the pathogenesis of inflammatory bowel diseases. Cell Host Microbe. 2008;3(6):417–27. 10.1016/j.chom.2008.05.001
- Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, Clemente JC, Knight R, Heath AC, Leibel RL, et al. The long-term stability of the human gut microbiota. Science. 2013;341(6141):1237439. 10.1126/science.1237439
- Walter J, Ley R. The human gut microbiome: ecology and recent evolutionary changes. Annu Rev Microbiol. 2011;65(1):411–29. 10.1146/annurev-micro-090110-102830
- Corr SC, Hill C, Gahan CGM. Advances in food and nutrition research. Elsevier, San Diego, US. 2009; 56:. p.1–15. https://doi.org/10.1016/S1043-4526(08)00601-3
- Sun J, Kato I. Gut microbiota, inflammation and colorectal cancer. Genes Dis. 2016;3(2):130–43. 10.1016/j.gendis.2016.03.004
- Podolsky DK. The current future understanding of inflammatory bowel disease. Best Pract Res Clin Gastroenterol. 2002;16(6):933–43. 10.1053/bega.2002.0354
- Sartor RB. Genetics and environmental interactions shape the intestinal microbiome to promote inflammatory bowel disease versus mucosal homeostasis. Gastroenterology. 2010;139(6):1816–9. 10.1053/j.gastro.2010.10.036
- Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14(2):195–206. 10.1016/j.chom.2013.07.012
- Martin HM, Campbell BJ, Hart CA, Mpofu C, Nayar M, Singh R, Englyst H, Williams HF, Rhodes JM. Enhanced Escherichia coli adherence and invasion in Crohn’s disease and colon cancer. Gastroenterology. 2004;127(1):80–93. 10.1053/j.gastro.2004.03.054
- Wu S, Morin PJ, Maouyo D, Sears CL. Bacteroides fragilis enterotoxin induces c-Myc expression and cellular proliferation. Gastroenterology. 2003;124(2):392–400. 10.1053/gast.2003.50047
- Toprak NU, Yagci A, Gulluoglu BM, Akin ML, Demirkalem P, Celenk T, Soyletir G. A possible role of Bacteroides fragilis enterotoxin in the aetiology of colorectal cancer. Clin Microbiol Infect. 2006;12(8):782–6. 10.1111/j.1469-0691.2006.01494.x
- Housseau F, Sears CL. Enterotoxigenic Bacteroides fragilis (ETBF)-mediated colitis in Min (Apc+/-) mice: a human commensal-based murine model of colon carcinogenesis. Cell Cycle. 2010;9(1):3–5. 10.4161/cc.9.1.10352
- Abdulamir AS, Hafidh RR, Bakar FA. The association of Streptococcus bovis/gallolyticus with colorectal tumors: the nature and the underlying mechanisms of its etiological role. J Exp Clin Cancer Res. 2011;30(1):11. 10.1186/1756-9966-30-11
- Hermsen JL, Schurr MJ, Kudsk KA, Faucher LD. Phenotyping Clostridium septicum infection: a surgeon’s infectious disease. J Surg Res. 2008;148(1):67–76. 10.1016/j.jss.2008.02.027
- Mirza NN, McCloud JM, Cheetham MJ. Clostridium septicum sepsis and colorectal cancer - a reminder. World J Surg Oncol. 2009;7:73. 10.1186/1477-7819-7-73
- Balamurugan R, Rajendiran E, George S, Samuel GV, Ramakrishna BS. Real-time polymerase chain reaction quantification of specific butyrate-producing bacteria, Desulfovibrio and Enterococcus faecalis in the feces of patients with colorectal cancer. J Gastroenterol Hepatol. 2008;23(8 Pt 1):1298–303. 10.1111/j.1440-1746.2008.05490.x
- Wang X, Yang Y, Moore DR, Nimmo SL, Lightfoot SA, Huycke MM. 4-Hydroxy-2-nonenal mediates genotoxicity and bystander effects caused by Enterococcus faecalis–infected macrophages. Gastroenterology. 2012;142(3):543–51.e7. 10.1053/j.gastro.2011.11.020
- Tsoi H, Chu ESH, Zhang X, Sheng J, Nakatsu G, Ng SC, Chan AWH, Chan FKL, Sung JJY, Yu J, et al. Peptostreptococcus anaerobius induces intracellular cholesterol biosynthesis in colon cells to induce proliferation and causes dysplasia in Mice. Gastroenterology. 2017;152(6):1419–33.e5. 10.1053/j.gastro.2017.01.009
- Long X, Wong CC, Tong L, Chu ESH, Ho Szeto C, Go MYY, Coker OO, Chan AWH, Chan FKL, Sung JJY, et al. Peptostreptococcus anaerobius promotes colorectal carcinogenesis and modulates tumour immunity. Nat Microbiol. 2019;4(12):2319–30. 10.1038/s41564-019-0541-3
- Dai Z, Coker OO, Nakatsu G, Wu WKK, Zhao L, Chen Z, Chan FKL, Kristiansen K, Sung JJY, Wong SH, et al. Multi-cohort analysis of colorectal cancer metagenome identified altered bacteria across populations and universal bacterial markers. Microbiome. 2018;6(1):70. 10.1186/s40168-018-0451-2
- Wirbel J, Pyl PT, Kartal E, Zych K, Kashani A, Milanese A, Fleck JS, Voigt AY, Palleja A, Ponnudurai R, et al. Meta-analysis of fecal metagenomes reveals global microbial signatures that are specific for colorectal cancer. Nat Med. 2019;25(4):679–89. 10.1038/s41591-019-0406-6
- Sheng Q, Du H, Cheng X, Cheng X, Tang Y, Pan L, Wang Q, Lin J. Characteristics of fecal gut microbiota in patients with colorectal cancer at different stages and different sites. Oncol Lett. 2019;18(5):4834–44. Sep 10; 10.3892/ol.2019.10841
- Mizutani S, Yamada T, Yachida S. Significance of the gut microbiome in multistep colorectal carcinogenesis. Cancer Sci. 2020;111(3):766–73. 10.1111/cas.14298
- O’Keefe SJD, Li JV, Lahti L, Ou J, Carbonero F, Mohammed K, Posma JM, Kinross J, Wahl E, Ruder E, et al. Fat, fibre and cancer risk in African Americans and rural Africans. Nat Commun. 2015;6:6342. 10.1038/ncomms7342
- Song M, Chan AT. Environmental factors, gut microbiota, and colorectal cancer prevention. Clin Gastroenterol Hepatol. 2019;17(2):275–89. 10.1016/j.cgh.2018.07.012
- Royston KJ, Adedokun B, Olopade OI. Race, the microbiome and colorectal cancer. World J Gastrointest Oncol. 2019;11(10):773–87.
- Schroeder BO. Fight them or feed them: how the intestinal mucus layer manages the gut microbiota. Gastroenterol Rep (Oxf). 2019;7(1):3–12. 10.1093/gastro/goy052
- Desai MS, Seekatz AM, Koropatkin NM, Kamada N, Hickey CA, Wolter M, Pudlo NA, Kitamoto S, Terrapon N, Muller A, et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. 2016;167(5):1339–53.e21. 10.1016/j.cell.2016.10.043
- Wang S, Liu Y, Li J, Zhao L, Yan W, Lin B, Guo X, Wei Y. Fusobacterium nucleatum acts as a pro-carcinogenic bacterium in colorectal cancer: from association to causality. Front Cell Dev Biol. 2021;9:710165. 10.3389/fcell.2021.710165
- Yachida S, Mizutani S, Shiroma H, Shiba S, Nakajima T, Sakamoto T, Watanabe H, Masuda K, Nishimoto Y, Kubo M, et al. Metagenomic and metabolomic analyses reveal distinct stage-specific phenotypes of the gut microbiota in colorectal cancer. Nat Med. 2019;25(6):968–76. 10.1038/s41591-019-0458-7
- Kostic AD, Gevers D, Pedamallu CS, Michaud M, Duke F, Earl AM, Ojesina AI, Jung J, Bass AJ, Tabernero J, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012;22(2):292–8. 10.1101/gr.126573.111
- Yang Y, Weng W, Peng J, Hong L, Yang L, Toiyama Y, Gao R, Liu M, Yin M, Pan C, et al. Fusobacterium nucleatum increases proliferation of colorectal cancer cells and tumor development in mice by activating toll-like receptor 4 signaling to nuclear factor-κB, and up-regulating expression of microRNA-21. Gastroenterology. 2017;152(4):851–66.e24. 10.1053/j.gastro.2016.11.018
- Matijašić BB, Obermajer T, Lipoglavšek L, Grabnar I, Avguštin G, Rogelj I. Association of dietary type with fecal microbiota in vegetarians and omnivores in Slovenia. Eur J Nutr. 2014;53(4):1051–64. 10.1007/s00394-013-0607-6
- Ruengsomwong S, La-Ongkham O, Jiang J, Wannissorn B, Nakayama J, Nitisinprasert S. Microbial community of healthy thai vegetarians and non-vegetarians, their core gut microbiota, and pathogen risk. J Microbiol Biotechnol. 2016;26(10):1723–35.
- Zimmer J, Lange B, Frick J-S, Sauer H, Zimmermann K, Schwiertz A, Rusch K, Klosterhalfen S, Enck P. A vegan or vegetarian diet substantially alters the human colonic faecal microbiota. Eur J Clin Nutr. 2012;66(1):53–60. 10.1038/ejcn.2011.141
- Rinninella E, Cintoni M, Raoul P, Lopetuso LR, Scaldaferri F, Pulcini G, Miggiano GAD, Gasbarrini A, Mele MC. Food components and dietary habits: keys for a healthy gut microbiota composition. Nutrients. 2019;11(10):2393–415. 10.3390/nu11102393
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Red Meat and Processed Meat. Lyon (FR): International Agency for Research on Cancer; 2018.
- Sasso A, Latella G. Role of heme iron in the association between red meat consumption and colorectal cancer. Nutr Cancer. 2018;70(8):1173–83. 10.1080/01635581.2018.1521441
- Ijssennagger N, Belzer C, Hooiveld GJ, Dekker J, van Mil SWC, Müller M, Kleerebezem M, van der Meer R. Gut microbiota facilitates dietary heme-induced epithelial hyperproliferation by opening the mucus barrier in colon. Proc Natl Acad Sci U S A. 2015;112(32):10038–43. 10.1073/pnas.1507645112
- Song M, Garrett WS, Chan AT. Nutrients, foods, and colorectal cancer prevention. Gastroenterology. 2015;148(6):1244–60.e16. 10.1053/j.gastro.2014.12.035
- Teigen LM, Geng Z, Sadowsky MJ, Vaughn BP, Hamilton MJ, Khoruts A. Dietary factors in sulfur metabolism and pathogenesis of ulcerative colitis. Nutrients. 2019;11(4):931–50. 10.3390/nu11040931
- Yao CK, Sarbagili-Shabat C. Gaseous metabolites as therapeutic targets in ulcerative colitis. World J Gastroenterol. 2023;29(4):682–91. 10.3748/wjg.v29.i4.682
- Yazici C, Wolf PG, Kim H, Cross T-WL, Vermillion K, Carroll T, Augustus GJ, Mutlu E, Tussing-Humphreys L, Braunschweig C, et al. Race-dependent association of sulfidogenic bacteria with colorectal cancer. Gut. 2017;66(11):1983–94. 10.1136/gutjnl-2016-313321
- Liu X, Tan F, Cui M, Li D, Yao P. Effects of red meat diet on gut microbiota in mice. Food Sci Technol. 2022;42. 10.1590/fst.28321
- Alexander DD, Cushing CA. Red meat and colorectal cancer: a critical summary of prospective epidemiologic studies. Obes Rev. 2011;12(5):e472–93. 10.1111/j.1467-789X.2010.00785.x
- Nielsen TS, Bendiks Z, Thomsen B, Wright ME, Theil PK, Scherer BL, Marco ML. High-amylose maize, potato, and butyrylated starch modulate large intestinal fermentation, microbial composition, and oncogenic miRNA expression in rats fed a high-protein meat diet. Int J Mol Sci. 2019;20(9). 10.3390/ijms20092137
- Fernández J, Ledesma E, Monte J, Millán E, Costa P, de la Fuente VG, García MTF, Martínez-Camblor P, Villar CJ, Lombó F, et al. Traditional processed meat products re-designed towards inulin-rich functional foods reduce polyps in two colorectal cancer animal models. Sci Rep. 2019;9(1):14783. 10.1038/s41598-019-51437-w
- Appunni S, Rubens M, Ramamoorthy V, Tonse R, Saxena A, McGranaghan P, Kaiser A, Kotecha R. Emerging evidence on the effects of dietary factors on the gut microbiome in colorectal cancer. Front Nutr. 2021;8:718389. 10.3389/fnut.2021.718389
- Marlett JA, McBurney MI, Slavin JL, American Dietetic Association. Position of the American Dietetic Association: health implications of dietary fiber. J Am Diet Assoc. 2002;102(7):993–1000. 10.1016/s0002-8223(02)90228-2
- Anderson JW, Baird P, Davis RH, Ferreri S, Knudtson M, Koraym A, Waters V, Williams CL. Health benefits of dietary fiber. Nutr Rev. 2009;67(4):188–205. 10.1111/j.1753-4887.2009.00189.x
- Speciani MC, Gargari G, Penagini R, Mutignani M, Ferraroni M, Natale A, Katsoulis M, Cintolo M, Leone P, Airoldi A, et al. Garlic consumption in relation to colorectal cancer risk and to alterations of blood bacterial DNA. Eur J Nutr. 2023;62(5):2279–92. 10.1007/s00394-023-03110-2
- So D, Whelan K, Rossi M, Morrison M, Holtmann G, Kelly JT, Shanahan ER, Staudacher HM, Campbell KL. Dietary fiber intervention on gut microbiota composition in healthy adults: a systematic review and meta-analysis. Am J Clin Nutr. 2018;107(6):965–83. 10.1093/ajcn/nqy041
- Donohoe DR, Holley D, Collins LB, Montgomery SA, Whitmore AC, Hillhouse A, Curry KP, Renner SW, Greenwalt A, Ryan EP, et al. A gnotobiotic mouse model demonstrates that dietary fiber protects against colorectal tumorigenesis in a microbiota- and butyrate-dependent manner. Cancer Discov. 2014;4(12):1387–97. 10.1158/2159-8290.CD-14-0501
- Donohoe DR, Garge N, Zhang X, Sun W, O’Connell TM, Bunger MK, Bultman SJ. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13(5):517–26. 10.1016/j.cmet.2011.02.018
- Encarnação JC, Abrantes AM, Pires AS, Botelho MF. Revisit dietary fiber on colorectal cancer: butyrate and its role on prevention and treatment. Cancer Metastasis Rev. 2015; Sep34(3):465–78. 10.1007/s10555-015-9578-9
- Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–73. 10.1126/science.1241165
- Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40(1):128–39. 10.1016/j.immuni.2013.12.007
- Tang Y, Chen Y, Jiang H, Robbins GT, Nie D. G-protein-coupled receptor for short-chain fatty acids suppresses colon cancer. Int J Cancer. 2011;128(4):847–56. 10.1002/ijc.25638
- Song M, Chan AT. Diet, gut microbiota, and colorectal cancer prevention: a review of potential mechanisms and promising targets for future research. Curr Colorectal Cancer Rep. 2017; Dec13(6):429–39. 10.1007/s11888-017-0389-y
- Belcheva A, Irrazabal T, Robertson SJ, Streutker C, Maughan H, Rubino S, Moriyama EH, Copeland JK, Surendra A, Kumar S, et al. Gut microbial metabolism drives transformation of MSH2-deficient colon epithelial cells. Cell. 2014;158(2):288–99. 10.1016/j.cell.2014.04.051
- Clinton SK, Giovannucci EL, Hursting SD. The world cancer research fund/american institute for cancer research third expert report on diet, nutrition, physical activity, and cancer: impact and future directions. J Nutr. 2020;150(4):663–71. 10.1093/jn/nxz268
- Khan MT, Nieuwdorp M, Bäckhed F. Microbial modulation of insulin sensitivity. Cell Metab. 2014;20(5):753–60. 10.1016/j.cmet.2014.07.006
- Cani PD, Jordan BF. Gut microbiota-mediated inflammation in obesity: a link with gastrointestinal cancer. Nat Rev Gastroenterol Hepatol. 2018;15(11):671–82. 10.1038/s41575-018-0025-6
- Berg RD. Mechanisms in the Pathogenesis of Enteric Diseases 2. Springer, Boston, MA. 1999;473: p.11–30. doi:10.1007/978-1-4615-4143-1_2
- Xiao Q, Lu W, Kong X, Shao YW, Hu Y, Wang A, Bao H, Cao R, Liu K, Wang X, et al. Alterations of circulating bacterial DNA in colorectal cancer and adenoma: a proof-of-concept study. Cancer Lett. 2021;499:201–8.
- Shi M, Zong X, Hur J, Birmann BM, Martinez-Maza O, Epeldegui M, Chan AT, Giovannucci EL, Cao Y. Circulating markers of microbial translocation and host response to bacteria with risk of colorectal cancer: a prospective, nested case-control study in men. EBioMedicine. 2023;91:104566. 10.1016/j.ebiom.2023.104566
- Horioka K, Tanaka H, Isozaki S, Konishi H, Fujiya M, Okuda K, Asari M, Shiono H, Ogawa K, Shimizu K, et al. Acute colchicine poisoning causes endotoxemia via the destruction of intestinal barrier function: the curative effect of endotoxin prevention in a murine model. Dig Dis Sci. 2020;65(1):132–40. 10.1007/s10620-019-05729-w
- Ghosh SS, Wang J, Yannie PJ, Ghosh S. Intestinal barrier dysfunction, LPS translocation, and disease development. J Endocr Soc. 2020;4(2):bvz039.
- Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–72. 10.2337/db06-1491
- Yang B, Bostick RM, Tran HQ, Gewirtz AT, Campbell PT, Fedirko V. Circulating biomarkers of gut barrier function: correlates and nonresponse to calcium supplementation among colon adenoma patients. Cancer Epidemiol Biomarkers Prev. 2016;25(2):318–26. 10.1158/1055-9965.EPI-15-0488
- Moreno-Navarrete JM, Ortega F, Serino M, Luche E, Waget A, Pardo G, Salvador J, Ricart W, Frühbeck G, Burcelin R, et al. Circulating lipopolysaccharide-binding protein (LBP) as a marker of obesity-related insulin resistance. Int J Obes (Lond). 2012;36(11):1442–9. 10.1038/ijo.2011.256
- Li R, Grimm SA, Mav D, Gu H, Djukovic D, Shah R, et al. Transcriptome and DNA methylome analysis in a mouse model of diet-induced obesity predicts increased risk of colorectal cancer. Cell Rep. 2018;22(3):624–37.
- Li R, Grimm SA, Chrysovergis K, Kosak J, Wang X, Du Y, Burkholder A, Janardhan K, Mav D, Shah R, et al. Obesity, rather than diet, drives epigenomic alterations in colonic epithelium resembling cancer progression. Cell Metab. 2014;19(4):702–11. 10.1016/j.cmet.2014.03.012
- Qin Y, Roberts JD, Grimm SA, Lih FB, Deterding LJ, Li R, Chrysovergis K, Wade PA. An obesity-associated gut microbiome reprograms the intestinal epigenome and leads to altered colonic gene expression. Genome Biol. 2018;19(1):7. 10.1186/s13059-018-1389-1
- Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499(7456):97–101. 10.1038/nature12347
- Ohtani N, Yoshimoto S, Hara E. Obesity and cancer: a gut microbial connection. Cancer Res. 2014;74(7):1885–9. 10.1158/0008-5472.CAN-13-3501
- Loo TM, Kamachi F, Watanabe Y, Yoshimoto S, Kanda H, Arai Y, Nakajima-Takagi Y, Iwama A, Koga T, Sugimoto Y, et al. Gut microbiota promotes obesity-associated liver cancer through PGE2-mediated suppression of antitumor immunity. Cancer Discov. 2017;7(5):522–38. 10.1158/2159-8290.CD-16-0932
- Clarke SF, Murphy EF, O’Sullivan O, Lucey AJ, Humphreys M, Hogan A, Hayes P, O’Reilly M, Jeffery IB, Wood-Martin R, et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. 2014;63(12):1913–20. 10.1136/gutjnl-2013-306541
- Jang LG, Choi G, Kim SW, Kim BY, Lee S, Park H. The combination of sport and sport-specific diet is associated with characteristics of gut microbiota: an observational study. J Int Soc Sports Nutr. 2019;16(1):21.
- Barton W, Penney NC, Cronin O, Garcia-Perez I, Molloy MG, Holmes E, Shanahan F, Cotter PD, O’Sullivan O. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut. 2018;67(4):625–33. 10.1136/gutjnl-2016-313627
- Aya V, Flórez A, Perez L, Ramírez JD. Association between physical activity and changes in intestinal microbiota composition: A systematic review. PLoS One. 2021;16(2):e0247039. 10.1371/journal.pone.0247039
- Matsumoto M, Inoue R, Tsukahara T, Ushida K, Chiji H, Matsubara N, Hara H. Voluntary running exercise alters microbiota composition and increases n-butyrate concentration in the rat cecum. Biosci Biotechnol Biochem. 2008;72(2):572–6.
- Evans CC, LePard KJ, Kwak JW, Stancukas MC, Laskowski S, Dougherty J, Moulton L, Glawe A, Wang Y, Leone V, et al. Exercise prevents weight gain and alters the gut microbiota in a mouse model of high fat diet-induced obesity. PLoS One. 2014;9(3):e92193. 10.1371/journal.pone.0092193
- Petriz BA, Castro AP, Almeida JA, Gomes CP, Fernandes GR, Kruger RH, Pereira RW, Franco OL. Exercise induction of gut microbiota modifications in obese, non-obese and hypertensive rats. BMC Genomics. 2014;15(1):511. 10.1186/1471-2164-15-511
- McNabb S, Harrison TA, Albanes D, Berndt SI, Brenner H, Caan BJ, Campbell PT, Cao Y, Chang-Claude J, Chan A, et al. Meta-analysis of 16 studies of the association of alcohol with colorectal cancer. Int J Cancer. 2020;146(3):861–73.
- Tsuruya A, Kuwahara A, Saito Y, Yamaguchi H, Tenma N, Inai M, Takahashi S, Tsutsumi E, Suwa Y, Totsuka Y, et al. Major anaerobic bacteria responsible for the production of carcinogenic acetaldehyde from ethanol in the colon and rectum. Alcohol Alcohol. 2016;51(4):395–401. 10.1093/alcalc/agv135
- Gong J, Hutter C, Baron JA, Berndt S, Caan B, Campbell PT, Casey G, Chan AT, Cotterchio M, Fuchs CS, et al. A pooled analysis of smoking and colorectal cancer: timing of exposure and interactions with environmental factors. Cancer Epidemiol Biomarkers Prev. 2012;21(11):1974–85. 10.1158/1055-9965.EPI-12-0692
- Lee SH, Yun Y, Kim SJ, Lee E-J, Chang Y, Ryu S, Shin H, Kim H-L, Kim H-N, Lee JH, et al. Association between cigarette smoking status and composition of gut microbiota: population-based cross-sectional study. J Clin Med. 2018;7(9):282–94. 10.3390/jcm7090282
- Gui X, Yang Z, Li MD. Effect of cigarette smoke on gut microbiota: state of knowledge. Front Physiol. 2021;12:673341. 10.3389/fphys.2021.673341
- Berkowitz L, Pardo-Roa C, Salazar GA, Salazar-Echegarai F, Miranda JP, Ramírez G, Chávez JL, Kalergis AM, Bueno SM, Álvarez-Lobos M, et al. Mucosal exposure to cigarette components induces intestinal inflammation and alters antimicrobial response in mice. Front Immunol. 2019;10:2289. 10.3389/fimmu.2019.02289
- Fluhr L, Mor U, Kolodziejczyk AA, Dori-Bachash M, Leshem A, Itav S, Cohen Y, Suez J, Zmora N, Moresi C, et al. Gut microbiota modulates weight gain in mice after discontinued smoke exposure. Nature. 2021;600(7890):713–9. 10.1038/s41586-021-04194-8
- Biedermann L, Brülisauer K, Zeitz J, Frei P, Scharl M, Vavricka SR, Fried M, Loessner MJ, Rogler G, Schuppler M, et al. Smoking cessation alters intestinal microbiota: insights from quantitative investigations on human fecal samples using FISH. Inflamm Bowel Dis. 2014;20(9):1496–501.
- Prakash A, Peters BA, Cobbs E, Beggs D, Choi H, Li H, Hayes RB, Ahn J. Tobacco Smoking and the Fecal Microbiome in a Large, Multi-ethnic Cohort. Cancer Epidemiol Biomarkers Prev. 2021; Jul30(7):1328–35. 10.1158/1055-9965.EPI-20-1417
- Chi L, Bian X, Gao B, Tu P, Ru H, Lu K. The effects of an environmentally relevant level of arsenic on the gut microbiome and its functional metagenome. Toxicol Sci. 2017;160(2):193–204. 10.1093/toxsci/kfx174
- Bai X, Wei H, Liu W, Coker OO, Gou H, Liu C, Zhao L, Li C, Zhou Y, Wang G, et al. Cigarette smoke promotes colorectal cancer through modulation of gut microbiota and related metabolites. Gut. 2022;71(12):2439–50. 10.1136/gutjnl-2021-325021
- Gomes SD, Oliveira CS, Azevedo-Silva J, Casanova MR, Barreto J, Pereira H, Chaves SR, Rodrigues LR, Casal M, Côrte-Real M, et al. The role of diet related short-chain fatty acids in colorectal cancer metabolism and survival: prevention and therapeutic implications. Curr Med Chem. 2020;27(24):4087–108. 10.2174/0929867325666180530102050
- Zhang M, Zhou Q, Dorfman RG, Huang X, Fan T, Zhang H, Zhang J, Yu C. Butyrate inhibits interleukin-17 and generates Tregs to ameliorate colorectal colitis in rats. BMC Gastroenterol. 2016;16(1):84. 10.1186/s12876-016-0500-x
- Pan P, Oshima K, Huang YW, Agle KA, Drobyski WR, Chen X, et al. Loss of FFAR2 promotes colon cancer by epigenetic dysregulation of inflammation suppressors. Int J Cancer. 2018;143(4):886–96.
- Lim SJ, Choi MK, Kim MJ, Lee CH. Effect of butyrate on the heregulin/ErbB-mediated proliferation of human colorectal cancer cells. Mol Med Rep. 2009;2(3):497–502. 10.3892/mmr_00000127
- Zagato E, Pozzi C, Bertocchi A, Schioppa T, Saccheri F, Guglietta S, Fosso B, Melocchi L, Nizzoli G, Troisi J, et al. Endogenous murine microbiota member Faecalibaculum rodentium and its human homologue protect from intestinal tumour growth. Nat Microbiol. 2020;5(3):511–24. 10.1038/s41564-019-0649-5
- Zeng H, Hamlin SK, Safratowich BD, Cheng WH, Johnson LK. Superior inhibitory efficacy of butyrate over propionate and acetate against human colon cancer cell proliferation via cell cycle arrest and apoptosis: linking dietary fiber to cancer prevention. Nutr Res. 2020;83:63–72. 10.1016/j.nutres.2020.08.009
- Carretta MD, Quiroga J, López R, Hidalgo MA, Burgos RA. Participation of short-chain fatty acids and their receptors in gut inflammation and colon cancer. Front Physiol. 2021;12:662739. 10.3389/fphys.2021.662739
- Moniri NH, Farah Q. Short-chain free-fatty acid G protein-coupled receptors in colon cancer. Biochem Pharmacol. 2021;186:114483. 10.1016/j.bcp.2021.114483
- Yu DCW, Waby JS, Chirakkal H, Staton CA, Corfe BM. Butyrate suppresses expression of neuropilin I in colorectal cell lines through inhibition of Sp1 transactivation. Mol Cancer. 2010;9(1):276. 10.1186/1476-4598-9-276
- Yu DC, Bury JP, Tiernan J, Waby JS, Staton CA, Corfe BM. Short-chain fatty acid level and field cancerization show opposing associations with enteroendocrine cell number and neuropilin expression in patients with colorectal adenoma. Mol Cancer. 2011;10(1):27. 10.1186/1476-4598-10-27
- Hu S, Liu L, Chang EB, Wang JY, Raufman JP. Butyrate inhibits pro-proliferative miR-92a by diminishing c-Myc-induced miR-17-92a cluster transcription in human colon cancer cells. Mol Cancer. 2015;14(1):180. 10.1186/s12943-015-0450-x
- Han R, Sun Q, Wu J, Zheng P, Zhao G. Sodium butyrate upregulates miR-203 expression to exert anti-proliferation effect on colorectal cancer cells. Cell Physiol Biochem. 2016;39(5):1919–29. 10.1159/000447889
- Li Q, Ding C, Meng T, Lu W, Liu W, Hao H, et al. Butyrate suppresses motility of colorectal cancer cells via deactivating Akt/ERK signaling in histone deacetylase dependent manner. J Pharmacol Sci. 2017;135(4):148–55.
- Alrafas HRD, Busbee BP, Nagarkatti P, Nagarkatti M. Effect of sodium butyrate supplementation on the gut microbiome during colorectal cancer. J Immunol. 2019;202(1_Suppl):191.10. 10.4049/jimmunol.202.Supp.191.10
- Luu M, Weigand K, Wedi F, Breidenbend C, Leister H, Pautz S, Adhikary T, Visekruna A. Regulation of the effector function of CD8+ T cells by gut microbiota-derived metabolite butyrate. Sci Rep. 2018;8(1):14430. 10.1038/s41598-018-32860-x
- Tedelind S, Westberg F, Kjerrulf M, Vidal A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: a study with relevance to inflammatory bowel disease. World J Gastroenterol. 2007;13(20):2826–32.
- Liu Z, Cao AT, Cong Y. Microbiota regulation of inflammatory bowel disease and colorectal cancer. Semin Cancer Biol. 2013;23(6 Pt B):543–52. 10.1016/j.semcancer.2013.09.002
- Jalandra R, Dalal N, Yadav AK, Verma D, Sharma M, Singh R, Khosla A, Kumar A, Solanki PR. Emerging role of trimethylamine-N-oxide (TMAO) in colorectal cancer. Appl Microbiol Biotechnol. 2021;105(20):7651–60. 10.1007/s00253-021-11582-7
- Bae S, Ulrich CM, Neuhouser ML, Malysheva O, Bailey LB, Xiao L, Brown EC, Cushing-Haugen KL, Zheng Y, Cheng T-YD, et al. Plasma choline metabolites and colorectal cancer risk in the Women’s Health Initiative Observational Study. Cancer Res. 2014;74(24):7442–52. 10.1158/0008-5472.CAN-14-1835
- Liu X, Liu H, Yuan C, Zhang Y, Wang W, Hu S, Liu L, Wang Y. Preoperative serum TMAO level is a new prognostic marker for colorectal cancer. Biomark Med. 2017;11(5):443–7. 10.2217/bmm-2016-0262
- Xu R, Wang Q, Li L. A genome-wide systems analysis reveals strong link between colorectal cancer and trimethylamine N-oxide (TMAO), a gut microbial metabolite of dietary meat and fat. BMC Genomics. 2015;16 Suppl 7(Suppl 7):S4. 10.1186/1471-2164-16-S7-S4
- Yang S, Dai H, Lu Y, Li R, Gao C, Pan S. Trimethylamine N-Oxide promotes cell proliferation and angiogenesis in colorectal cancer. J Immunol Res. 2022;2022:7043856–7. 10.1155/2022/7043856
- Chan CWH, Law BMH, Waye MMY, Chan JYW, So WKW, Chow KM. Trimethylamine-N-oxide as one hypothetical link for the relationship between intestinal microbiota and cancer - where we are and where shall we go? J Cancer. 2019;10(23):5874–82.
- Oellgaard J, Winther SA, Hansen TS, Rossing P, von Scholten BJ. Trimethylamine N-oxide (TMAO) as a new potential therapeutic target for insulin resistance and cancer. Curr Pharm Des. 2017;23(25):3699–712. 10.2174/1381612823666170622095324
- Robbiano L, Mereto E, Corbu C, Brambilla G. DNA damage induced by seven N-nitroso compounds in primary cultures of human and rat kidney cells. Mutat Res. 1996;368(1):41–7. 10.1016/s0165-1218(96)90038-5
- Kushkevych I, Cejnar J, Treml J, Dordević D, Kollar P, Vítězová M. Recent advances in metabolic pathways of sulfate reduction in intestinal bacteria. Cells. 2020;9(3):698. 10.3390/cells9030698
- Zhang W, An Y, Qin X, Wu X, Wang X, Hou H, Song X, Liu T, Wang B, Huang X, et al. Gut microbiota-derived metabolites in colorectal cancer: the bad and the challenges. Front Oncol. 2021;11:739648. 10.3389/fonc.2021.739648
- Kushkevych I, Dordević D, Vítězová M. Possible synergy effect of hydrogen sulfide and acetate produced by sulfate-reducing bacteria on inflammatory bowel disease development. J Adv Res. 2021;27:71–8. 10.1016/j.jare.2020.03.007
- Rey FE, Gonzalez MD, Cheng J, Wu M, Ahern PP, Gordon JI. Metabolic niche of a prominent sulfate-reducing human gut bacterium. Proc Natl Acad Sci USA. 2013;110(33):13582–7. 10.1073/pnas.1312524110
- Gibson GR, Macfarlane GT, Cummings JH. Occurrence of sulphate-reducing bacteria in human faeces and the relationship of dissimilatory sulphate reduction to methanogenesis in the large gut. J Appl Bacteriol. 1988;65(2):103–11. 10.1111/j.1365-2672.1988.tb01498.x
- Blachier F, Beaumont M, Kim E. Cysteine-derived hydrogen sulfide and gut health: a matter of endogenous or bacterial origin. Curr Opin Clin Nutr Metab Care. 2019;22(1):68–75. 10.1097/MCO.0000000000000526
- Nguyen LH, Ma W, Wang DD, Cao Y, Mallick H, Gerbaba TK, Lloyd-Price J, Abu-Ali G, Hall AB, Sikavi D, et al. Association between sulfur-metabolizing bacterial communities in stool and risk of distal colorectal cancer in men. Gastroenterology. 2020;158(5):1313–25. 10.1053/j.gastro.2019.12.029
- Wang RH, Chu YH, Lin KT. The hidden role of hydrogen sulfide metabolism in cancer. Int J Mol Sci. 2021;22(12):6562. 10.3390/ijms22126562
- Shackelford RE, Mohammad IZ, Meram AT, Kim D, Alotaibi F, Patel S, Ghali GE, Kevil CG. Molecular functions of hydrogen sulfide in cancer. Pathophysiology. 2021;28(3):437–56. 10.3390/pathophysiology28030028
- Khattak S, Rauf MA, Khan NH, Zhang Q-Q, Chen H-J, Muhammad P, Ansari MA, Alomary MN, Jahangir M, Zhang C-Y, et al. Hydrogen sulfide biology and its role in cancer. Molecules. 2022;27(11):3389. 10.3390/molecules27113389
- Beaumont M, Andriamihaja M, Lan A, Khodorova N, Audebert M, Blouin J-M, Grauso M, Lancha L, Benetti P-H, Benamouzig R, et al. Detrimental effects for colonocytes of an increased exposure to luminal hydrogen sulfide: the adaptive response. Free Radic Biol Med. 2016;93:155–64. 10.1016/j.freeradbiomed.2016.01.028
- Libiad M, Vitvitsky V, Bostelaar T, Bak DW, Lee H-J, Sakamoto N, Fearon E, Lyssiotis CA, Weerapana E, Banerjee R, et al. Hydrogen sulfide perturbs mitochondrial bioenergetics and triggers metabolic reprogramming in colon cells. J Biol Chem. 2019;294(32):12077–90. 10.1074/jbc.RA119.009442
- Sakuma S, Minamino S, Takase M, Ishiyama Y, Hosokura H, Kohda T, Ikeda Y, Fujimoto Y. Hydrogen sulfide donor GYY4137 suppresses proliferation of human colorectal cancer Caco-2 cells by inducing both cell cycle arrest and cell death. Heliyon. 2019;5(8):e02244. 10.1016/j.heliyon.2019.e02244
- Vitvitsky V, Libiad M, Bostelaar T, Maebius A, Lee H, Lyssiotis CA, Banerjee R. Reprogramming of colonic cell metabolism by H 2 S. FASEB J. 2019;33(S1):485–511. 10.1096/fasebj.2019.33.1_supplement.485.11
- Du P, Tseng Y, Liu P, Zhang H, Huang G, Hu C, Chen J. Role of exhaled hydrogen sulfide in the diagnosis of colorectal cancer. BMJ Open Gastroenterol. 2024;11(1):e001229. 10.1136/bmjgast-2023-001229
- Wang Y, Nguyen LH, Mehta RS, Song M, Huttenhower C, Chan AT. Association between the sulfur microbial diet and risk of colorectal cancer. JAMA Netw Open. 2021;4(11):e2134308. 10.1001/jamanetworkopen.2021.34308
- Jia W, Xie G, Jia W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol. 2018;15(2):111–28. 10.1038/nrgastro.2017.119
- Zeng H, Umar S, Rust B, Lazarova D, Bordonaro M. Secondary bile acids and short chain fatty acids in the colon: a focus on colonic microbiome, cell proliferation, inflammation, and cancer. Int J Mol Sci. 2019;20(5).
- Wise JL, Cummings BP. The 7-α-dehydroxylation pathway: an integral component of gut bacterial bile acid metabolism and potential therapeutic target. Front Microbiol. 2022;13:1093420. 10.3389/fmicb.2022.1093420
- Liu T, Song X, Khan S, Li Y, Guo Z, Li C, Wang S, Dong W, Liu W, Wang B, et al. The gut microbiota at the intersection of bile acids and intestinal carcinogenesis: an old story, yet mesmerizing. Int J Cancer. 2020;146(7):1780–90.
- Bernstein C, Holubec H, Bhattacharyya AK, Nguyen H, Payne CM, Zaitlin B, Bernstein H. Carcinogenicity of deoxycholate, a secondary bile acid. Arch Toxicol. 2011;85(8):863–71. 10.1007/s00204-011-0648-7
- Turjman N, Goodman GT, Jaeger B, Nair PP. Metabolism of bile acids. Am J Clin Nutr. 1984;40(4 Suppl):937–41. 10.1093/ajcn/40.4.937
- Ignacio Barrasa J, Olmo N, Pérez-Ramos P, Santiago-Gómez A, Lecona E, Turnay J, Antonia Lizarbe M. Deoxycholic and chenodeoxycholic bile acids induce apoptosis via oxidative stress in human colon adenocarcinoma cells. Apoptosis. 2011;16(10):1054–67. 10.1007/s10495-011-0633-x
- Ward JBJ, Lajczak NK, Kelly OB, O’Dwyer AM, Giddam AK, Ní Gabhann J, et al. Ursodeoxycholic acid and lithocholic acid exert anti-inflammatory actions in the colon. Am J Physiol Gastrointest Liver Physiol. 2017;312(6):G550–8.
- Eaton JE, Silveira MG, Pardi DS, Sinakos E, Kowdley KV, Luketic VAC, Harrison ME, McCashland T, Befeler AS, Harnois D, et al. High-dose ursodeoxycholic acid is associated with the development of colorectal neoplasia in patients with ulcerative colitis and primary sclerosing cholangitis. Am J Gastroenterol. 2011;106(9):1638–45. 10.1038/ajg.2011.156
- Liu J, Tan Y, Cheng H, Zhang D, Feng W, Peng C. Functions of gut microbiota metabolites, current status and future perspectives. Aging Dis. 2022;13(4):1106–26. 10.14336/AD.2022.0104
- Imdad S, Lim W, Kim JH, Kang C. Intertwined relationship of mitochondrial metabolism, gut microbiome and exercise potential. Int J Mol Sci. 2022;23(5):2679.
- Cao Y, Aquino-Martinez R, Hutchison E, Allayee H, Lusis AJ, Rey FE. Role of gut microbe-derived metabolites in cardiometabolic diseases: systems based approach. Mol Metab. 2022; Oct64:101557. 10.1016/j.molmet.2022.101557
- Buret AG, Allain T, Motta JP, Wallace JL. Effects of hydrogen sulfide on the microbiome: from toxicity to therapy. Antioxid Redox Signal. 2022;36(4–6):211–9. 10.1089/ars.2021.0004
- Yang R, Qian L. Research on gut microbiota-derived secondary bile acids in cancer progression. Integr Cancer Ther. 2022;21:15347354221114100. 10.1177/15347354221114100
- Perillo F, Amoroso C, Strati F, Giuffrè MR, Díaz-Basabe A, Lattanzi G, Facciotti F. Gut microbiota manipulation as a tool for colorectal cancer management: recent advances in its use for therapeutic purposes. Int J Mol Sci. 2020;21(15)
- Yang J, McDowell A, Kim EK, Seo H, Lee WH, Moon C-M, Kym S-M, Lee DH, Park YS, Jee Y-K, et al. Development of a colorectal cancer diagnostic model and dietary risk assessment through gut microbiome analysis. Exp Mol Med. 2019;51(10):1–15. 10.1038/s12276-019-0313-4
- Meeker S, Seamons A, Paik J, Treuting PM, Brabb T, Grady WM, Maggio-Price L. Increased dietary vitamin D suppresses MAPK signaling, colitis, and colon cancer. Cancer Res. 2014;74(16):4398–408. 10.1158/0008-5472.CAN-13-2820
- Zhou X, Chen C, Zhong YN, Zhao F, Hao Z, Xu Y, Lai R, Shen G, Yin X. Effect and mechanism of vitamin D on the development of colorectal cancer based on intestinal flora disorder. J Gastroenterol Hepatol. 2020;35(6):1023–31.
- Brandhorst S, Longo VD. Fasting and caloric restriction in cancer prevention and treatment. Recent Results Cancer Res. 2016;207:241–66. 10.1007/978-3-319-42118-6_12
- Li G, Xie C, Lu S, Nichols RG, Tian Y, Li L, Patel D, Ma Y, Brocker CN, Yan T, et al. Intermittent fasting promotes white adipose browning and decreases obesity by shaping the gut microbiota. Cell Metab. 2017;26(4):672–85.e4. 10.1016/j.cmet.2017.08.019
- Zheng X, Zhou K, Zhang Y, Han X, Zhao A, Liu J, Qu C, Ge K, Huang F, Hernandez B, et al. Food withdrawal alters the gut microbiota and metabolome in mice. FASEB J. 2018;32(9):4878–88. 10.1096/fj.201700614R
- Hao GW, Chen YS, He DM, Wang HY, Wu GH, Zhang B. Growth of human colon cancer cells in nude mice is delayed by ketogenic diet with or without omega-3 fatty acids and medium-chain triglycerides. Asian Pac J Cancer Prev. 2015;16(5):2061–8. 10.7314/apjcp.2015.16.5.2061
- Cao H, Xu M, Dong W, Deng B, Wang S, Zhang Y, Wang S, Luo S, Wang W, Qi Y, et al. Secondary bile acid-induced dysbiosis promotes intestinal carcinogenesis. Int J Cancer. 2017;140(11):2545–56.
- Zackular JP, Baxter NT, Chen GY, Schloss PD. Manipulation of the gut microbiota reveals role in colon tumorigenesis. mSphere. 2016;1(1):e00001-15. 10.1128/mSphere.00001-15
- Wang M, Li Y, Yang X, Liu Z, Wang K, Gong D, Li J. Effects of metronidazole on colorectal cancer occurrence and colorectal cancer liver metastases by regulating Fusobacterium nucleatum in mice. Immun Inflamm Dis. 2023; Nov11(11):e1067.
- Triner D, Devenport SN, Ramakrishnan SK, Ma X, Frieler RA, Greenson JK, Inohara N, Nunez G, Colacino JA, Mortensen RM, et al. Neutrophils restrict tumor-associated microbiota to reduce growth and invasion of colon tumors in mice. Gastroenterology. 2019;156(5):1467–82. 10.1053/j.gastro.2018.12.003
- Yu Y-N, Yu T-C, Zhao H-J, Sun T-T, Chen H-M, Chen H-Y, An H-F, Weng Y-R, Yu J, Li M, et al. Berberine may rescue Fusobacterium nucleatum-induced colorectal tumorigenesis by modulating the tumor microenvironment. Oncotarget. 2015;6(31):32013–26. 10.18632/oncotarget.5166
- DeStefano Shields CE, Van Meerbeke SW, Housseau F, Wang H, Huso DL, Casero RA, O’Hagan HM, Sears CL. Reduction of murine colon tumorigenesis driven by enterotoxigenic bacteroides fragilis using cefoxitin treatment. J Infect Dis. 2016;214(1):122–9. 10.1093/infdis/jiw069
- Hamoya T, Miyamoto S, Tomono S, Fujii G, Nakanishi R, Komiya M, Tamura S, Fujimoto K, Toshima J, Wakabayashi K, et al. Chemopreventive effects of a low-side-effect antibiotic drug, erythromycin, on mouse intestinal tumors. J Clin Biochem Nutr. 2017;60(3):199–207. 10.3164/jcbn.16-107
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–14. 10.1038/nrgastro.2014.66
- Vitetta L, Vitetta G, Hall S. Immunological tolerance and function: associations between intestinal bacteria, probiotics, prebiotics, and phages. Front Immunol. 2018;9:2240. 10.3389/fimmu.2018.02240
- Gamallat Y, Meyiah A, Kuugbee ED, Hago AM, Chiwala G, Awadasseid A, Bamba D, Zhang X, Shang X, Luo F, et al. Lactobacillus rhamnosus induced epithelial cell apoptosis, ameliorates inflammation and prevents colon cancer development in an animal model. Biomed Pharmacother. 2016;83:536–41. 10.1016/j.biopha.2016.07.001
- Gamallat Y, Ren X, Walana W, Meyiah A, Xinxiu R, Zhu Y, et al. Probiotic Lactobacillus rhamnosus modulates the gut microbiome composition attenuates preneoplastic colorectal Aberrant crypt foci. J Funct Foods. 2019;53:146–56.
- Jacouton E, Chain F, Sokol H, Langella P, Bermúdez-Humarán LG. Probiotic strain Lactobacillus casei BL23 prevents colitis-associated colorectal cancer. Front Immunol. 2017;8:1553. 10.3389/fimmu.2017.01553
- Chen D, Jin D, Huang S, Wu J, Xu M, Liu T, Dong W, Liu X, Wang S, Zhong W, et al. Clostridium butyricum, a butyrate-producing probiotic, inhibits intestinal tumor development through modulating Wnt signaling and gut microbiota. Cancer Lett. 2020;469:456–67.
- Cousin FJ, Jouan-Lanhouet S, Théret N, Brenner C, Jouan E, Le Moigne-Muller G, Dimanche-Boitrel M-T, Jan G. The probiotic Propionibacterium freudenreichii as a new adjuvant for TRAIL-based therapy in colorectal cancer. Oncotarget. 2016;7(6):7161–78. 10.18632/oncotarget.6881
- De Marco S, Sichetti M, Muradyan D, Piccioni M, Traina G, Pagiotti R, Pietrella D. Probiotic cell-free supernatants exhibited anti-inflammatory and antioxidant activity on human gut epithelial cells and macrophages stimulated with LPS. Evid Based Complement Alternat Med. 2018;2018:1756308–12. 10.1155/2018/1756308
- Nozari S, Faridvand Y, Etesami A, Ahmad Khan Beiki M, Miresmaeili Mazrakhondi SA, Abdolalizadeh J. Potential anticancer effects of cell wall protein fractions from Lactobacillus paracasei on human intestinal Caco-2 cell line. Lett Appl Microbiol. 2019;69(3):148–54. 10.1111/lam.13198
- Fortin O, Aguilar-Uscanga BR, Vu KD, Salmieri S, Lacroix M. Effect of Saccharomyces boulardii cell wall extracts on colon cancer prevention in male F344 rats treated with 1,2-dimethylhydrazine. Nutr Cancer. 2018;70(4):632–42. 10.1080/01635581.2018.1460672
- Chen ZY, Hsieh YM, Huang CC, Tsai CC. Inhibitory effects of Probiotic lactobacillus on the growth of human colonic carcinoma cell line HT-29. Molecules. 2017;22(1):107–18. 10.3390/molecules22010107
- Kahouli I, Malhotra M, Westfall S, Alaoui-Jamali MA, Prakash S. Design and validation of an orally administrated active L. fermentum-L. acidophilus probiotic formulation using colorectal cancer Apc Min/+ mouse model. Appl Microbiol Biotechnol. 2017;101(5):1999–2019. 10.1007/s00253-016-7885-x
- Sambrani R, Abdolalizadeh J, Kohan L, Jafari B. Saccharomyces cerevisiae inhibits growth and metastasis and stimulates apoptosis in HT-29 colorectal cancer cell line. Comp Clin Path. 2019;28(4):985–95.
- Chattopadhyay I, Dhar R, Pethusamy K, Seethy A, Srivastava T, Sah R, Sharma J, Karmakar S. Exploring the role of gut microbiome in colon cancer. Appl Biochem Biotechnol. 2021;193(6):1780–99. 10.1007/s12010-021-03498-9
- Rafter J, Bennett M, Caderni G, Clune Y, Hughes R, Karlsson PC, Klinder A, O’Riordan M, O’Sullivan GC, Pool-Zobel B, et al. Dietary synbiotics reduce cancer risk factors in polypectomized and colon cancer patients. Am J Clin Nutr. 2007;85(2):488–96. 10.1093/ajcn/85.2.488
- Pineiro M, Asp N-G, Reid G, Macfarlane S, Morelli L, Brunser O, Tuohy K. FAO Technical meeting on prebiotics. J Clin Gastroenterol. 2008;42 Suppl 3 Pt 2(Suppl 3):S156–S9. 10.1097/MCG.0b013e31817f184e
- Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14(8):491–502. 10.1038/nrgastro.2017.75
- Bach Knudsen KE. Microbial degradation of whole-grain complex carbohydrates and impact on short-chain fatty acids and health. Adv Nutr. 2015;6(2):206–13. 10.3945/an.114.007450
- Kotecha R, Takami A, Espinoza JL. Dietary phytochemicals and cancer chemoprevention: a review of the clinical evidence. Oncotarget. 2016;7(32):52517–29. 10.18632/oncotarget.9593
- Hosseini A, Ghorbani A. Cancer therapy with phytochemicals: evidence from clinical studies. Avicenna J Phytomed. 2015;5(2):84–97.
- Annison G, Illman RJ, Topping DL. Acetylated, propionylated or butyrylated starches raise large bowel short-chain fatty acids preferentially when fed to rats. J Nutr. 2003;133(11):3523–8. 10.1093/jn/133.11.3523
- Patterson JA, Burkholder KM. Application of prebiotics and probiotics in poultry production. Poult Sci. 2003;82(4):627–31. 10.1093/ps/82.4.627
- Baurhoo B, Letellier A, Zhao X, Ruiz-Feria CA. Cecal populations of lactobacilli and bifidobacteria and Escherichia coli populations after in vivo Escherichia coli challenge in birds fed diets with purified lignin or mannanoligosaccharides. Poult Sci. 2007;86(12):2509–16. 10.3382/ps.2007-00136
- Xie X, He Y, Li H, Yu D, Na L, Sun T, Zhang D, Shi X, Xia Y, Jiang T, et al. Effects of prebiotics on immunologic indicators and intestinal microbiota structure in perioperative colorectal cancer patients. Nutrition. 2019;61:132–42. 10.1016/j.nut.2018.10.038
- Fidelis M, Santos JS, Escher GB, Rocha RS, Cruz AG, Cruz TM, Marques MB, Nunes JB, do Carmo MAV, de Almeida LA, et al. Polyphenols of jabuticaba [Myrciaria jaboticaba (Vell.) O.Berg] seeds incorporated in a yogurt model exert antioxidant activity and modulate gut microbiota of 1,2-dimethylhydrazine-induced colon cancer in rats. Food Chem. 2021;334:127565. 10.1016/j.foodchem.2020.127565
- Pabst O. New concepts in the generation and functions of IgA. Nat Rev Immunol. 2012;12(12):821–32. 10.1038/nri3322
- Kumari R, Kumar S, Ahmad MK, Singh R, Pradhan A, Chandra S, Kumar S. TNF-α/IL-10 ratio: an independent predictor for coronary artery disease in North Indian population. Diabetes Metab Syndr. 2018;12(3):221–5. 10.1016/j.dsx.2017.09.006
- Gorosito Serrán M, Fiocca Vernengo F, Beccaria CG, Acosta Rodriguez EV, Montes CL, Gruppi A. The regulatory role of B cells in autoimmunity, infections and cancer: perspectives beyond IL10 production. FEBS Lett. 2015;589(22):3362–9. 10.1016/j.febslet.2015.08.048
- Verediano TA, Viana ML, das Graças Vaz Tostes M, de Oliveira DS, de Carvalho Nunes L, Costa NM. Yacón (Smallanthus sonchifolius) prevented inflammation, oxidative stress, and intestinal alterations in an animal model of colorectal carcinogenesis. J Sci Food Agric. 2020;100(15):5442–9. 10.1002/jsfa.10595
- Hrncir T. Gut microbiota dysbiosis: triggers, consequences, diagnostic and therapeutic options. Microorganisms. 2022;10(3):578. 10.3390/microorganisms10030578
- Brandt LJ, Borody TJ, Campbell J. Endoscopic fecal microbiota transplantation: “first-line” treatment for severe clostridium difficile infection? J Clin Gastroenterol. 2011;45(8):655–7. 10.1097/MCG.0b013e3182257d4f
- Xu H, Wang X, Feng W, Liu Q, Zhou S, Liu Q, Cai L. The gut microbiota and its interactions with cardiovascular disease. Microb Biotechnol. 2020;13(3):637–56. 10.1111/1751-7915.13524
- Tamburini S, Shen N, Wu HC, Clemente JC. The microbiome in early life: implications for health outcomes. Nat Med. 2016;22(7):713–22. 10.1038/nm.4142
- Mutalub YB, Abdulwahab M, Mohammed A, Yahkub AM, Al-Mhanna SB, Yusof W, Tang SP, Rasool AHG, Mokhtar SS. Gut microbiota modulation as a novel therapeutic strategy in cardiometabolic diseases. Foods. 2022;11(17):2575. 10.3390/foods11172575
- Cui J, Lin Z, Tian H, Yang B, Zhao D, Ye C, Li N, Qin H, Chen Q. Long-Term follow-up results of fecal microbiota transplantation for irritable bowel syndrome: a single-center, retrospective study. Front Med (Lausanne). 2021;8:710452. 10.3389/fmed.2021.710452
- Lopetuso LR, Ianiro G, Allegretti JR, Bibbò S, Gasbarrini A, Scaldaferri F, Cammarota G. Fecal transplantation for ulcerative colitis: current evidence and future applications. Expert Opin Biol Ther. 2020;20(4):343–51. 10.1080/14712598.2020.1733964
- Tian H, Ge X, Nie Y, Yang L, Ding C, McFarland LV, Zhang X, Chen Q, Gong J, Li N, et al. Fecal microbiota transplantation in patients with slow-transit constipation: a randomized, clinical trial. PLoS One. 2017;12(2):e0171308. 10.1371/journal.pone.0171308
- Xue L-J, Yang X-Z, Tong Q, Shen P, Ma S-J, Wu S-N, Zheng J-L, Wang H-G. Fecal microbiota transplantation therapy for Parkinson’s disease: a preliminary study. Medicine. 2020;99(35):e22035. 10.1097/MD.0000000000022035
- Wang Z, Hua W, Li C, Chang H, Liu R, Ni Y, Sun H, Li Y, Wang X, Hou M, et al. Protective role of fecal microbiota transplantation on colitis and colitis-associated colon cancer in mice is associated with treg cells. Front Microbiol. 2019;10:2498. 10.3389/fmicb.2019.02498
- Rosshart SP, Vassallo BG, Angeletti D, Hutchinson DS, Morgan AP, Takeda K, Hickman HD, McCulloch JA, Badger JH, Ajami NJ, et al. Wild mouse gut microbiota promotes host fitness and improves disease resistance. Cell. 2017;171(5):1015–28.e13. 10.1016/j.cell.2017.09.016