ABSTRACT
Chaetodactylus krombeini (Acariformes: Astigmata: Chaetodactylidae) mites are kleptoparasites of solitary bees in the genus Osmia and depend on bee hosts for harbourage, nutrition, and dispersal, with infestations increasing morbidity and larval mortality. The life history traits of C. krombeini are unclear, complicating the control efforts of mites in managed bee nests. Mites in the family Chaetodactylidae have a facultative life stage, the heteromorphic deutonymph, used for either dispersal or dormancy when food resources are sparse. The heteromorphic deutonymph life stage is hypothesized to consist only of females, requiring arrhenotokous parthenogenesis to reproduce. This study examined this hypothesis by determining the sex of the adults that developed from each deutonymph morph. Phoretic deutonymphs were isolated after overwintering either with or without a bee host; inert deutonymphs were isolated from field collections from various locations. Mites were individually observed for 14 days, and development was recorded. Mites that reached adulthood were sexed. After 14 days, 32–38% of heteromorphic deutonymphs that reached adulthood were male. These results disprove the previous hypothesis that heteromorphic deutonymphs are exclusively female. The survival and reproductive strategies of C. krombeini are now in question, specifically the role of true arrhenotoky in founding new mite populations.
Introduction
Chaetodactylus krombeini (Baker) is a kleptoparasitic mite species in the nests of solitary bees within the families Apidae and Megachilidae (Krombein Citation1962; Klimov et al. Citation2016). These mites feed on pollen provisions intended for developing bee larvae, causing the development of smaller adult bees and decreased fecundity (Krombein Citation1962; Bosch and Kemp Citation2001). Smaller Osmia bees are less efficient pollinators and often produce a smaller brood (Bosch and Kemp Citation2001). Chaetodactylid mites may also attack and kill developing bee larvae and eggs, destroying up to 50% of Osmia provisioned nests (van Lith Citation1957; Krombein Citation1962; Krunic et al. Citation2001).
Chaetodactylus krombeini uses multiple pathways for development to the adult stage based on environmental conditions (Baker Citation1962; Krombein Citation1962; Bosch and Kemp Citation2001; Qu et al. Citation2003; Klimov et al. Citation2016). Early season infested cells with plenty of food will allow a mite life cycle of egg, larva, protonymph, tritonymph, and adult () (Krombein Citation1962). However, to survive in temporary habitats with transitory food sources, Cheatodactylus mites possess a facultative heteromorphic deutonymph life stage, often referred to as a hypopus (Knülle Citation1987; Houck and OConnor Citation1991). The non-feeding hypopus life stage occurs after the protonymph stage; when the hypopus is established in a new bee nest with available resources, it will continue development and morph into a tritonymph and then an adult () (Krombein Citation1962, Citation1967; Knülle Citation1987; Eickwort Citation1994; Bosch and Kemp Citation2001; Krunić et al. Citation2005 Park et al. Citation2008). The heteromorphic deutonymph life stage can be precipitated by multiple environmental conditions, such as low food quality, reduction of humidity, mite overcrowding, or a synchronous life stage with its host (Krombein Citation1962; Knülle Citation1987). The two morphs of the C. krombeini deutonymph are a highly regressive inert form that stays in the nest waiting for a new host to reuse the nests, and a phoretic form that attaches to adult bees as they emerge () (Krombein Citation1962). Inert heteromorphic deutonymphs stay encased within the protective cast skin of protonymphs (Baker Citation1962; Krombein Citation1962; Krunić et al. Citation2005). They are smooth, round, have four pairs of greatly reduced legs, and are immobile () (Baker Citation1962; Klimov et al. Citation2016). This morph has the capacity to lay dormant for several months to years while waiting for a new bee host with similar nesting behaviour (Krombein Citation1962; Eickwort Citation1994; Krunić et al. Citation2005).
Figure 1. The life cycle of C. krombeini showing all of the possible developmental pathways. The solid arrows indicate development to adulthood when resources are readily available. The dashed arrows indicate development to adulthood when the heteromorphic deutonymph is necessary. The inert deutonymph can develop when there is a decrease in humidity or food resources. The phoretic deutonymph has synchronous development with the bee host and can develop as the be reaches later development stages, such as late larval or the pupa stages. (I) Egg (X500); (II) larva (X300); (III) protonymph (X200); (IV) heteromorphic deutonymph, (IVA) phoretic deutonymph, (IVB) inert deutonymph (X150); (V) tritonymph (X300); (VI) adults (VIA) adult male, (VIB) adult female (X120). Images are not to scale.
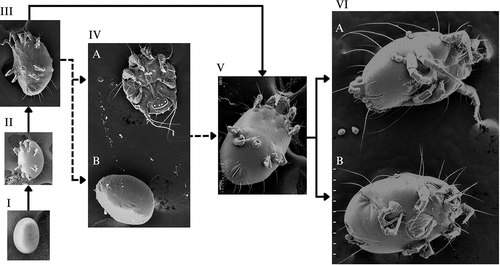
Figure 2. The heteromorphic deutonymphs of Chaetodactylus krombeini. (a) Dorsal view of phoretic deutonymph showing the propodosomal (solid arrow), hysterosomal shields (dashed arrow), and recurved empodial claw (blue arrow). The phoretic deutonymph is highly sclerotized and adapted for harsher environments outside the nest and on the bee host, this morph is non-feeding and has more sensory and attachment organs to secure a bee host for phoresy. (b) The inert deutonymph is a highly regressive morph that is non-feeding and has greatly reduced appendages. The inert deutonymph is adapted for the long latency period that occurs between one bee host generation vacating a nest typically between March and April, and the next generation reusing the nest the following spring.
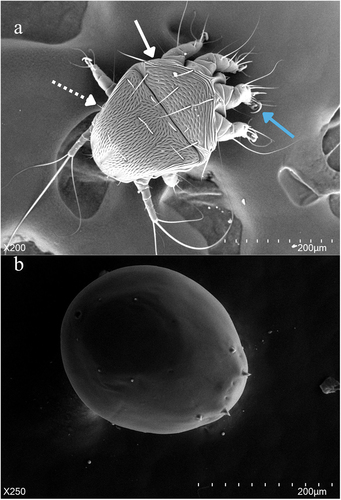
In contrast, the phoretic heteromorphic deutonymph is used for dispersal. Chaetodactylus krombeini, like other mites that are obligatorily associated with solitary bees, synchronize their development with their host, and when the bee offspring reaches the late larval or pupal stage the phoretic instar is produced, remaining in the brood cell until bee offspring emerges as an adult (Eickwort Citation1994). Phoretic deutonymphs are more sclerotized than other life stages and have a propodosomal and hysterosomal shield that gives them a brownish colour () (Baker Citation1962; Krombein Citation1962; Bosch and Kemp Citation2001). The first three pairs of legs have recurved empodial claws, while the last pair of legs have long whip-like setae (earning them the nickname “hairy-toed mite”) () (Baker Citation1962; Bosch and Kemp Citation2001). Phoretic deutonymphs also have a suctorial plate posteriorly on their ventral side (. IVA) (Baker Citation1962; Krombein Citation1962). These morphological adaptations likely aid with phoresy as they attach to the bee host (van Lith Citation1957; Krombein Citation1962; Eickwort Citation1994; OConnor Citation2009). When phoretic deutonymphs attach to adult bees they are most often located on the posterior thorax and anterior abdomen, with some mites randomly dispersed all over the bee’s body () (Krombein Citation1962; Eickwort Citation1994). Phoretic mites are often in locations that are not well groomed by their host (Eickwort Citation1994).
Figure 3. Osmia lignaria adult male pin mounted with phoretic deutonymphs (arrow) attached to posterior thorax. Phoretic deutonymphs primarily attach to their host in this location because it is difficult for their host to remove mites from this area when grooming. Phoretic deutonymphs were individually removed from the bee hosts with forceps or with a disposable mascara wand while using a dissecting microscope.

Chaetodactylus krombeini and other closely related mite species have been hypothesized to only produce adult females from the deutonymph stages (Krombein Citation1962; Krunić et al. Citation2005; McKinney and Park Citation2013). This life history trait would therefore require the use of arrhenotokous parthenogenesis, or the ability to produce male offspring from unfertilized eggs, likely followed by oedipal mating (Adamson and Ludwig Citation1993), to start new populations (Krombein Citation1962; Krunić et al. Citation2005; McKinney and Park Citation2013). Oedipal mating is a strategy where unmated female mites lay an unfertilized egg which hatches into a male (haploid), then the female mite mates with her son and lays fertilized eggs which will hatch into a female (diploid). Other chaetodactylids have been shown to produce both male and female heteromorphic deutonymphs (Qu et al. Citation2003), possibly indicating alternative reproductive strategies to arrhenotoky. Group dispersal prior to sexual maturity may point to sexual reproduction, requiring at least one individual of each sex to be present after phoresy (Mitchell Citation1970). The adult sex ratio within a mite population may also be indicative of the primary reproductive strategy (Nagelkerke and Sabelis Citation1998).
In this study, C. krombeini collected from O. lignaria Say and O. cornifrons Radoszkowski nests were reared under various laboratory conditions to determine the sex of inert and phoretic deutonymphs. This was accomplished by providing inert and phoretic deutonymphs with a quality food source and monitoring development into adulthood so each mite could be sexed. The sex ratios of mites that survived to adulthood were used to estimate the reproductive strategy of this mite species.
Materials and methods
Inert deutonymphs
Chaetodactylus krombeini samples were field collected from eight different sites across five cities in Oregon and Washington from commercial Osmia nests and shipped to Riverside, CA. Inert deutonymphs were removed from C. krombeini field collected samples and used in each 14-day sex determination assay (n = 80 total). For each assay, one inert deutonymph was placed into a 1.5 µl microcentrifuge tube (Genesee Scientific, El Cajon, CA, USA) (n = 10) with ca. 50 µl pollen provision consisting of 60% honey bee pollen, 16% honey, 20% DI water, and 4% fructose (). Each tube was examined under a dissecting microscope, and mite life stage (inert deutonymph, tritonymph, or adult) was recorded every 1–3 days for 14-days. Mites that moulted into adults were sexed by observation of an oviporus (female) or aedeagus (male) on the ventral surface (). All mites were maintained in an environmental chamber at 26°C ± 0.5°C during the assay (Fisher Scientific Model No: 146E).
Figure 4. Chaetodactylus krombeini female (a) and male (b) adults were cleared, and slide mounted in PVA mounting medium (100×). Genitalia are distinct on the ventral side of both sexes, with a cleavage-like oviporus present on females and a dark coloured aedeagus present on males.
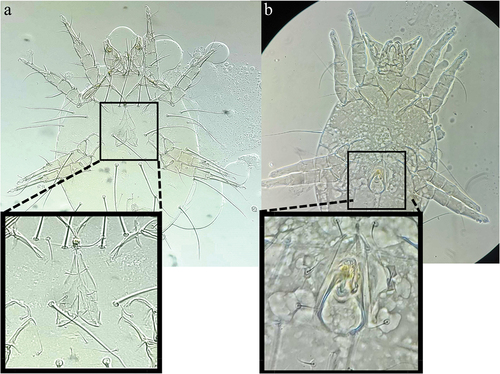
Table 1. Inert deutonymph sex determination. Location of collection site of mite infestations in commercial Osmia lignaria and O. cornifrons nests*. Ten inert deutonymphs were collected from each site (n = 80). Only mites that developed through the adult stage could be sexed. The number of each mite life stage, sex of the adults, and percentage (%) of total from each site is reported.
Phoretic deutonymphs overwintered with host
Phoretic deutonymphs were separated from collected C. krombeini (as above) and placed in several 1.5 µl microcentrifuge tubes with each tube containing several hundred mites. Bee cocoons were placed in microcentrifuge tubes containing phoretic deutonymphs for ca. five minutes to allow phoretic deutonymphs to attach to bee cocoons. Bee cocoons with phoretic deutonymphs were then individually placed in clear gel capsules (XPRS Nutra size: 000, Amazon, CA, USA) and then placed in a 4°C refrigerator from November 2022 to April 2023, allowing mites to overwinter with the host. Cocoons were moved to room temperature (23°C ± 1°C) on 13 April 2023, initiating adult bee emergence. Four female O. cornifrons, five male O. cornifrons, and one male O. lignaria were randomly selected, placed in a freezer for at least 30 min to kill the bee without killing mites, then mounted on a pin to remove mites (). Bees selected for this assay were the first to eclose. There was no observed mortality of phoretic deutonymphs on freeze-killed bee hosts, and all mites became mobile after removal from the freezer.
Visible phoretic deutonymphs were removed using forceps or small disposable mascara wands (Micro Eyebrow Brush with Cap, CANIPHA, Amazon.com), all other mite stages were discarded. Phoretic deutonymphs were placed individually into microcentrifuge tubes with ca. 50 µl of pollen provision (as above). Sample sizes of mites were dependent on how many phoretic deutonymphs were attached to each adult bee (6–46 deutonymphs). Every 2–5 days for 14-days tubes were examined, and the mite life stage (phoretic deutonymph, tritonymph, or adult) was recorded. Mites that moulted into adults were sexed as described above. All mites were maintained in an environmental chamber at (Fisher Scientific Model No: 146E) set to 26°C ± 0.5°C during the assay.
Phoretic deutonymph overwintered without host
Phoretic deutonymphs were separated from C. krombeini field collections and placed in 1.5 µl microcentrifuge tubes in the absence of bee cocoons, and placed in a refrigerator at 4°C in November 2022 to overwinter without a host. Mites were removed from 4°C refrigerators on 11 May 2023, and placed into individual microcentrifuge tubes with ca. 50 µl of pollen provision (as above) (n = 128). For each 14-day assay, tubes were visually examined under a dissecting scope, mite life stage (phoretic deutonymph, tritonymph, or adult) was recorded every 2–5 days for two weeks. All mites were maintained in an environmental chamber (Fisher Scientific Model No: 146E) set to 26°C ± 0.5°C during the assay.
Statistics
Statistical analyses were conducted in VassarStats (Lowry Citation2001–2023). A binomial probabilities test was performed to compare the observed sex ratio emergence to expected probabilities in mite populations with various reproduction strategies: average sex ratio (female: male) of populations that use sexual reproduction (1:1), arrhenotoky (9:1), and pseudo-arrhenotoky (7:3) (Nagelkerke and Sabelis Citation1998).
Results
Inert deutonymphs developed into adults from seven of the eight sites examined (). One of the eight sites grew mould resulting in no development past the deutonymph stage. Of the 80 inert deutonymphs that were observed, 32 developed into adults (n = 12 male, n = 20 female) and 3 inert deutonymphs only developed to the tritonymph stage before dying (; ). Both male and female adult mites developed from inert deutonymphs. Female mites were observed to develop from seven of the eight sites, with up to 100% of the adult mites from a single site being female. Males developed from inert deutonymphs from six of the eight sites, and the ratio of male to female adults was higher in two of the eight sites.
Figure 5. Percentage of each sex at emergence. (a) Inert deutonymphs percentage of each sex that developed into adults at each sample site (S1–S8). No adults emerged from site 4. (b) Phoretic deutonymph percentage of each sex that developed into adults from individual host bee (B3–B10). Adult mites did not emerge from bees 1, 2, 4, and 7.
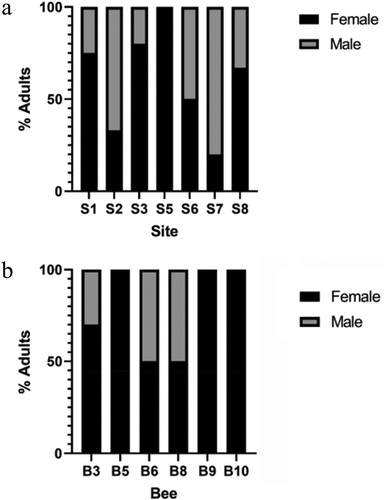
Both male and female adult mites developed from phoretic deutonymphs that overwintered with their bee host (). Of the 249 phoretic deutonymphs observed, 19 developed into adults (n = 6 male, n = 13 female) and 1 phoretic deutonymph morphed into a tritonymph before dying (; ). The remaining 230 phoretic deutonymphs either died within the 14-day assay or were still alive but did not develop past the deutonymph stage during the assay. Differences in infestation among bee sex or bee species were not observed.
Table 2. Phoretic deutonymph sex determination. Phoretic deutonymphs were collected from different Osmia spp. and sexes. Only mites that developed through the adult stage could be sexed. The number of each mite life stage, sex of the adults, and percentage (%) of total from each bee is reported.
None of the 128 phoretic deutonymphs that overwintered without a host developed into tritonymphs or adults. All died within the 14-day assay, indicated by mites becoming immobile and desiccating.
Because of the small sample size, the observed deutonymph sex ratio was pooled across sites or bees by type (inert or phoretic) and compared to the average sex ratio (female: male) of populations that use sexual reproduction (1:1), arrhenotoky (9:1), and pseudo-arrhenotoky (7:3) (Nagelkerke and Sabelis Citation1998). The proportion of males that developed from inert (k = 12, n = 32) and phoretic (k = 6, n = 19) deutonymphs with a host were compared to each mode of reproduction: sexual (inert: z-ratio = −1.24, p = 0.053; phoretic: z-ratio = −1.38, p = 0.052), arrhenotoky (inert: z-ratio = 4.89, p = 0.000027; phoretic: z-ratio = 2.75, p = 0.0069), and pseudo-arrhenotoky (inert: z-ratio = 0.73, p = 0.096; phoretic: z-ratio = −0.1, p = 0.19).
Discussion
This study showed that C. krombeini heteromorphic deutonymphs do not exclusively develop into adult females. In this study, a larger proportion of inert deutonymphs (40%) developed to adulthood than phoretic deutonymphs (<8%). Only phoretic deutonymphs that overwintered with a host (male or female bee) developed into adults. It is possible that inert deutonymphs need the increased relative humidity provided by a new food source as a necessary cue to continue development, whereas phoretic deutonymphs need at least one cue from the bee host to further develop (Krombein Citation1962). Phoretic deutonymphs have sensory organs that can identify an adult bee and a host cell that has or will have a food source () (Eickwort Citation1994).
High mite mortality was observed in these assays, specifically with the phoretic deutonymph, with survival ranging from ca. 2% to 24%, and mites from four of the ten host bees exhibiting no survival. This may have been caused by damage during the removal of mites from their original host with forceps. In addition, all of the heteromorphic deutonymphs were shipped from Washington or Oregon to California, which may have damaged the mites or exposed them to detrimental temperatures. The phoretic deutonymphs were then introduced to a new bee host cocoon from either California, Oregon, or Washington field collected bees that may have been at different development stages within their cocoon. The 128 phoretic deutonymphs that overwintered without a bee host in this study did not continue development past the deutonymph life stage. Phoretic deutonymphs may need to remain in close contact with their bee host to be able to complete development.
Previously, researchers have hypothesized that the heteromorphic deutonymphs of C. krombeini are all female and that mite reproduction in new nests was achieved by arrhenotokous parthenogenesis followed by oedipal mating (Krombein Citation1962; Krunić et al. Citation2005). This hypothesis was originally developed by Krombein (Citation1962) who compared the life history traits of C. krombeini to Histiostoma mites associated with halictid bees, which had been observed to lay eggs that only develop into males. However, Histiostoma male offspring were not observed to mate with their mothers (Krombein Citation1962). Other closely related chaetodactylid mites (C. osmiae) have been shown to be haplo-diploid and capable of arrhenotokous parthenogenesis (Rożej-Pabijan and Witaliński Citation2018). However, arrhenotokous parthenogenesis may not be a necessary or primary survival strategy as both males and females were observed in this study to be present in the dormant and dispersal morphs of C. krombeini deutonymphs. Previous studies observed that copulation was required for egg production in C. osmiae mites, which are haplo-diploid (Chmielewski Citation1993; Rożej-Pabijan and Witaliński Citation2018). In the current study, following the 14-day assays some female mites were not killed in the freezer and were instead kept for observation. These unmated female mites were observed twice a week for 3 weeks, but egg production was not observed. Some of these unmated female mites were then placed together to see if the presence of other females would initiate oviposition as a strategy to avoid inbreeding depression, but female mites still did not lay eggs after a month of observation. In a host preference field experiment where host bees were inoculated with phoretic deutonymphs at the beginning of the study, there was a single adult female mite found in a provisioned cell that survived through the season, but she did not found a colony in the nest (Holquinn Citation2023). While it is possible that field nest conditions were not ideal for reproduction in the aforementioned field experiment, this may suggest that copulation or the presence of male mites is required for oviposition. This is additionally supported by comparing the observed and expected sex ratio of C. krombeini with different modes of reproduction. Adults developing from both inert and phoretic deutonymphs had no significant difference in sex ratios when compared to sexual reproduction and pseudo-arrhenotoky, however there was a significant difference in the sex ratio when compared to arrhenotoky (sexual reproduction 1 female: 1 male, arrhenotoky 9 females: 1 male, or pseudo-arrhenotoky 7 females: 3 males), though the overall high mite mortality observed may be skewing the observed sex ratios (Nagelkerke and Sabelis Citation1998).
Chaetodactylus krombeini inert and phoretic deutonymphs are often observed in groups on adult bee hosts or in bee nests, increasing the potential number of founders introduced to new nests (Krunić et al. Citation2005; J.A.H, personal observation). Inert deutonymphs from field collections and phoretic deutonymphs that overwintered with adults were both found in groups, giving mites that found new populations a higher chance to reproduce sexually (Mitchell Citation1970). Additionally, there is the possibility that phoretic deutonymphs will not successfully disperse with a sufficient number of mites that allows for mating (Mitchell Citation1970), in this case, arrhenotoky and oedipal mating would be required to found a new population. Mites that skip the heteromorphic deutonymph life stage generally occupy a desirable habitat and have access to mates, which would make arrhenotoky unnecessary. Finally, C. krombeini that inhabit an environment with sufficient resources can be observed engaging in mating (J.A.H, personal observation), further suggesting arrhenotoky may not be the primary mode of reproduction. In pseudo-arrhenotoky, males are produced from fertilized eggs but become haploid after the paternal chromosomes are inactivated (Nagelkerke and Sabelis Citation1998). This reproduction strategy is often seen in phytoseiid mites, with the overall production of daughters observed to be 69–76% of the mite population (Nagelkerke and Sabelis Citation1998). In the current study, 63% and 68% of inert and phoretic deutonymphs, respectively, developed into adult females.
This study documented that the heteromorphic deutonymphs of C. krombeini can develop into male and female adults. The current evidence suggests that C. krombeini does not rely on arrhenotoky reproduction, though further evidence is needed to clarify if sexual reproduction and/or pseudo-arrhenotoky are the primary reproductive strategy. Further research is needed to understand how mite life stage transitions are regulated in response to different stimuli and the role of the bee host in mite development. Understanding the survival strategies of these nest parasites, such as mode of reproduction and what genes are involved in life stage transitions, will inform commercial Osmia beekeepers and potentially lead to changes in management strategies that can disrupt C. krombeini life cycle.
Acknowledgments
We thank Dr Caleb Hubbard and Hannah Chu (UC Riverside) for their valuable comments on this manuscript. We also thank the commercial Osmia beekeepers in Oregon and Washington for their invaluable mite collection.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sets are available from J.A.H. upon request.
References
- Adamson M, Ludwig D. 1993. Oedipal mating as a factor in sex allocation in haplodiploids. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 341:195–202.
- Baker EW. 1962. Descriptions of the stages of Chaetodactylus krombeini, new species, a mite associated with the bee, Osmia lignaria say (Acarina: Chaetodactylidae). Proceedings of the Biological Society of Washington. Natural History of Plummers Island. Maryland. Lawrence, Kansas: The Allen Press; p. 227–236.
- Bosch J, Kemp W. 2001. How to manage the blue orchard bee as an orchard pollinator. Beltsville: Sustainable Agriculture Network National Agricultural Library.
- Chmielewski W. 1993. Biology of Chaetodactylus osmiae (Duf. 1866) (Acaridae, Chaetodactylidae) - polleneater phoretic on solitary bees (Apoidea) [solitariae]. Pszczelnicze Zeszyty Naukowe [Bee Science Notebooks]. 37:133–143.
- Eickwort GC. 1994. Evolution and life-history patterns of mites associated with bees. In: Houck M, editor. Mites: ecological and evolutionary analyses of life-history patterns. Boston: Springer US; p. 218–251.
- Holquinn JA. 2023. Life history traits of Chaetodactylus krombeini [ master’s thesis]. Riverside (CA): University of California, Riverside.
- Houck MA, OConnor BM. 1991. Ecological and evolutionary significance of phoresy in the Astigmata. Annu Rev Entomol. 36:611–636.
- Klimov P, OConnor B, Ochoa R, Bauchan G, Redford A, Scher J. 2016. Chaetodactylus. USA: Bee Mite ID; [accessed 2022 Mar 2]. https://idtools.org/bee_mite/index.cfm?packageID=1&entityID=88.
- Knülle W. 1987. Genetic variability and ecological adaptability of hypopus formation in a stored product mite. Exp & Appl Acarol. 3:21–32.
- Krombein KV. 1962. Biological notes on Chaetodactylus krombeini Baker, a parasitic of the Megachilid bee, Osmia (Osmia) lignaria say (Acarina: Chaetodacytidae). Proceedings of the Biological Society of Washington, Natural History of Plummers Island. Maryland. Lawrence, Kansas: The Allen Press; p. 237–250.
- Krombein KV. 1967. Trap-nesting wasps and bees: life histories, nests, and associates. Washington, D.C: Smithsonian Press (American Entomologist). doi: 10.1093/besa/13.3.246.
- Krunic M, Stanisavljevic L, Brajkovic M, Tomanovic Ž, Radovic I. 2001. Ecological studies of Osmia cornuta (latr.) (Hymenoptera, Megachilidae) populations in Yugoslavia with special attention to their diapause. Acta horticulturae. 297–301. doi: 10.17660/actahortic.2001.561.45.
- Krunić M, Stanisavljević L, Pinzauti M, Felicioli A. 2005. The accompanying fauna of Osmia cornuta and Osmia rufa and effective measures of protection. Bulletin of Insectology. 58:141–152.
- Lowry R. 2001–2023. Binomial probabilities. VassarStats. [accessed 2023 Aug 17]. http://vassarstats.net/binomialX.html.
- McKinney MI, Park YL. 2013. Distribution of Chaetodactylus krombeini (Acari: Chaetodactylidae) within Osmia cornifrons (Hymenoptera: Megachilidae) nests: implications for population management. Exp & App Acarol. 60:153–161.
- Mitchell R. 1970. An analysis of dispersal in mites. The Am Nat. 104:425–431.
- Nagelkerke CJ, Sabelis MW. 1998. Precise control of sex allocation in pseudo-arrhenotokous phytoseiid mites. J Evol Biol. 11:649–684.
- OConnor BM. 2009. Cohort Astigmatina. In: Krantz G, Walter D, editors. A manual of acarology. Lubbock: Texas Tech; p. 565–657.
- Park Y-L, Kondo V, White J, West T, McConnell B, McCutcheon T. 2008. Nest-to-nest dispersal of chaetodactylus krombeini (Acari, chaetodactylidae) associated with osmia cornifrons (hym., megachilidae). J Appl Entomol. 133:174–180. doi: 10.1111/j.1439-0418.2008.01351.x.
- Qu D, Maeta Y, Nakatsuka KJ, Kitamura K, Goubara M. 2003. Reproductive strategy in the two species of cleptoparasitic Astigmatid mites, Chaetodactylus nipponicus and Tortonia sp. (Acari: Chaetodactylidae and Suidasiidae), infesting Osmia cornifrons (Hymenoptera: Megachilidae) II. Life history, phoretic positions, development and reproductivity. Japanese J Entomol. 6:55–73. Japanese. doi: 10.20848/kontyu.6.2_55.
- Rożej-Pabijan E, Witaliński W. 2018. Gonads and gametogenesis in Chaetodactylus osmiae (Acariformes: Astigmata: Chaetodactylidae) a parasite of solitary bees. Acarologia. 58:801–824.
- van Lith JP. 1957. On the behavior of Chaetodactylus mites (Acar., Tyr.) in the nests of Osmia rufa L. and Chelostoma florisomne (L.) (Apidae. Megachilidae). Entomologische Bericheten. 19:197–198.
