Abstract
The plantar surfaces of the stonefly tarsomeres and pretarsus are examined chiefly using scanning electron microscopy (SEM) of 39 Plecoptera exemplar species representing each family within the two suborders Arctoperlaria and Antarctoperlaria. Features examined include the shape and size of the tarsomeres as well as external features of the plantar surfaces of the tarsomeres, unguitractor plate, claws, arolium and orbicula. Characteristics presumed to be part of the stonefly ground pattern were determined by mapping these features on a cladogram of stonefly phylogeny (Zwick Citation2000, Annual Review of Entomology 45:709–746). This analysis indicates that the following characters are part of the stonefly ground pattern: (1) cylindrical shape of the tarsomeres, (2) an elongate tarsomere 1, (3) a short tarsomere 2, (4) presence of hairy euplantulae on the plantar surfaces of tarsomeres 1 and 2, (5) absence of specialised attachment surfaces on the plantar area of tarsomere 3, (6) absence of setae on the arolium plantar surface, and (7) presence of setae on the orbicula surface. Hairs covering the euplantulae of tarsomeres 1 and 2 and the median longitudinal unscelerotised region of tarsomeres 1–3 are non-setal cuticular projections and appear to be acanthae. Hairy euplantulae are unusual and are only present in one other insect order, the Mantophasmatodea. Specialised tenent setae were not found. The presence of hairy euplantulae as part of the stonefly ground pattern contradicts the recent view (Beutel and Gorb Citation2006, Arthropod Systematics & Phylogeny 64:3–25) that stoneflies lack pad-like euplantulae and that the ancestral condition for tarsomeres 1 and 2 may be a narrow median longitudinal unsclerotised band such as that found in representatives of the Austroperlidae. The hypothesis that Plecoptera are the sister clade to the remaining Neoptera suggests that euplantulae might be a synapomorphy of both clades. On the other hand, placement of Plecoptera within a monophyletic Polyneoptera clade within the Neoptera suggests that euplantulae could be an autapomorphy of this subordinate clade.
Introduction
Two recent studies by Beutel and Gorb (Citation2001, Citation2006) that incorporated characters from tarsal attachment structures together with a large suite of other morphological features have contributed to an understanding of the evolution of hexapod tarsal attachment surfaces. Cladograms produced by both analyses support Zwick's (Citation1980) view that Plecoptera is the sister group of a clade comprising the rest of the Neoptera. This interpretation contrasts with the inclusion of stoneflies in a monophyletic Polyneoptera within the Neoptera proposed by Wheeler et al. (Citation2001) and Grimaldi and Engel (Citation2005). In their later analysis, Beutel and Gorb (Citation2006) examine the tarsi of two stonefly species belonging to the suborder Antarctoperlaria, the gripoterygid Trinotoperla irrorata Tillyard, 1924 and the austroperlid Acruroperla atra (Sámal, 1921), and conclude from this limited sample that pad-like tarsal attachment structures or euplantulae are absent in Plecoptera. Instead these workers view the narrow median longitudinal unsclerotised band of the plantar surfaces of tarsomeres 1 and 2 of A. atra as a form of the euplantula. Moreover, they postulate that if Plecoptera is the sister group to the remaining Neoptera, then the condition exemplified by A. atra could be an earlier stage in the transformation to the form of euplantulae found in other lower neopteran groups such as Blattodea, Mantodea, Ensifera, Caelifera, Phasmatodea, Mantophasmatodea and Grylloblattodea. This conjecture, however, rests on whether the form of the tarsal attachment surfaces exhibited by A. atra is actually part of the ground pattern (Wägele Citation2005) for Plecoptera.
The objective of the present paper is to characterise, within a phylogenetic framework, the ground pattern condition of Plecopteran attachment structures using both a comparative scanning electron microscope (SEM) study of the tarsal plantar surface ultrastructure and previously published analyses of the pretarsus. Once the ground pattern condition is determined, the modifications leading to the various forms of the plantar surfaces of the tarsomeres and pretarsus of the various groups and families of Plecoptera can be identified. In addition, the hypothesised ground pattern can be used to test Beutel and Gorb's (Citation2006) contention regarding the evolution of the euplantulae in the lower Neoptera.
Methods and terminology
A total of 39 exemplar species were selected from each of the 16 families and, where possible, 18 subfamilies comprising the two Plecopteran suborders Arctoperlaria and Antarctoperlaria. Metathoracic legs of ethanol preserved specimens were removed and placed in vials containing acetone, cleaned in an ultrasonic cleaner and then dehydrated through increasing ethanol concentrations to 99% and then placed in 1,1,1,3,3,3-hexamethyldisilazane. The legs were then attached to aluminium stubs and coated with gold using a Hummer IV (Anatech Ltd.) sputter-coater. Mounted specimens were examined and photographed using a JEOL JSM-T220A SEM.
Terms used for various tarsal and pretarsal structures of Plecoptera generally follow Hanson (Citation1946) and Dashman (Citation1953):
Tarsus. Leg region articulating with the distal end of the tibia and in stoneflies consisting of three segments or tarsomeres.
Euplantulae. Pad-like structures found on the plantar surfaces of the tarsomeres. Crampton (Citation1923) introduced this term using the blattodean Periplaneta americana (Linnaeus, 1758) as an exemplar. Beutel and Gorb (Citation2001) and Haas and Gorb (Citation2004) incorporated the absence of hairs into a definition of these structures. However, Beutel and Gorb (Citation2006) observed that the euplantulae surfaces of the mantophasmatodean Mantophasma zephyra Zompro, Klass, Kristensen and Addis, 2002 are not smooth but covered with long thin acanthae. Since euplantulae surfaces can either be smooth or bear hairs, it would be less confusing to retain the original description and not incorporate the surface profile condition as part of the definition.
Pretarsus. The terminal section of the leg comprised of the claws (ungues), arolium, orbicula, and unguitractor plate.
Claws (ungues). Two ventrally curving structures with sharply pointed apices located at the distal end of the pretarsus. Dorsally they articulate with the median process, the unguifer, of the distalmost tarsal segment.
Unguitractor plate. A broad sclerite situated on the ventral surface of the pretarsus proximal to the claws. In stoneflies it is widest distally and narrows to a bluntly rounded margin proximally. An unguitractoral tendon attaches to the midpoint of the proximal border of the plate.
Arolium. The cushion-like, somewhat triangular-shaped median lobe that arises between the claws.
Orbicula. Triangular-shaped plate on the dorsal surface surface of the arolium. It is widest distally and proximally narrows to articulate with the unguifer of the distalmost tarsal segment between the claws.
Abbreviations for terms on the photomicrographs are provided with the figure legends. The classification system used in this study follows the family-level arrangement of Zwick (Citation2000).
Determining the ground plan of the locomotory attachment structures was done by using the trace character function of MacClade 4.06 (Maddison and Maddison Citation2000) to map the structures on the phylogeny of Plecoptera produced by Zwick (Citation2000). This phylogeny and ones very similar to it (Zwick Citation1973, Citation1980; Nelson Citation1984; Terry and Whiting (in preparation)) have been the dominant paradigm for over 30 years.
Results
Tarsal and pretarsal surface ultrastructure
I. Arctoperlaria
I.1. Euholognatha
I.1.1. Nemouroidea
I.1.1.1. Nemouromorpha
I.1.1.1.1. Nemouridae (Nemourinae): Ostrocerca albidipennis (Walker, 1852)
Tarsus and pretarsus of this species are shown in . Tarsomeres 1, 2, and 3 cylindrical: ring or tube-like (). Tarsomeres 1 and 3 elongate: tarsomere 1 longer than tarsomere 3. Tarsomere 2 short: length approximately one-half that of tarsomere 3. Tarsomere surfaces uniformly covered with long, thin setae and the plantar surfaces lack specialised attachment structures ( and ). The unguitractor plate () bears overlapping scale-like microtrichia (Nelson Citation1991) and is similar to that described for various Odonata species by Gorb (Citation1996). A pair of inward curving setae, the setiform basipulvilii (), are found close to the base of the two lateral claws. The claws have smooth margins and lack setae (). Setae are not present on the arolium plantar surface and orbicula surface (Nelson Citation1991). Sharply-pointed spinulae are found on the orbicula and the dorsal bases of the claws ().
Figures 1–8. (1–4) Nemouridae: Nemourinae (Euholognatha): Ostrocerca albidipennis (Walker), (1) ventral view of tarsomeres 1–3 and pretarsus; (2) lateral view of tarsomeres 1 and 2; (3) ventral view of pretarsus; (4) dorsolateral view of pretarsus; (5) Notonemouridae (Euholognatha): Aphanicercopsis tabularis Barnard, ventrolateral view of tarsomeres 1 and 2. (6, 7) Leuctridae: Megaleuctrinae (Euholognatha): Megaleuctra complicata Claassen. (6) Ventrolateral view of tarsomeres 1 and 2; (7) ventrolateral view of tarsomere 3. (8) Leuctridae: Leuctrinae (Euholognatha): Leuctra grandis Banks, lateral view of tarsomeres 1–3. Abbreviations: ar, arolium; cl, claw; or, orbicula; sbp, setiform basipulvulus; ss, stout setae; t, tarsomeres; ut, unguitractor plate.
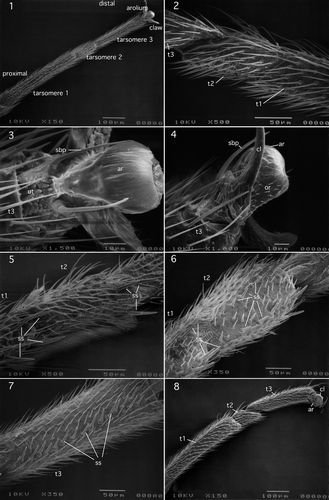
I.1.1.1.2. Nemouridae (Amphinemurinae): Amphinemura nigritta (Provancher, 1876)
Tarsus and pretarsus of this species closely resemble those of Ostrocerca albidipennis shown in .
I.1.1.1.3. Notonemouridae: Aphanicercopsis tabularis Barnard, 1934
Tarsus and pretarsus of this South African species shown in closely resemble those of the preceding two nemourid species. However, the length of tarsomere 2 is approximately two-thirds that of tarsomere 3. In addition to long, thin setae, several long, stout setae can be found near the distal plantar margins of tarsomeres 1 and 2.
I.1.1.1.4. Notonemouridae: Austronemura chilena Aubert, 1960
Tarsus and pretarsus of this South American species are very similar to those of Aphanicercopsis tabularis shown in .
I.1.1.1.5. Leuctridae (Megaleuctrinae): Megaleuctra complicata Claassen, 1937
Tarsus and pretarsus of this species are shown in and and are similar to the notonemourid Aphanicercopsis tabularis shown in , except that tarsomere 2 is approximately one-half the length of tarsomere 3. In addition the stout setae of the plantar surfaces are more numerous: present as two longitudinal rows on tarsomeres 1 and 2 () and as a single row on tarsomere 3 (). The claws bear a small number of setae (Nelson Citation1991).
I.1.1.1.6. Leuctridae (Leuctrinae): Leuctra grandis Banks, 1906
Tarsus and pretarsus of this species are shown in . Although the tarsus and pretarusus are similar to Megaleuctra complicata, they more closely resemble those of the nemourid Ostrocerca albidipennis shown in . Like these nemourids, L. grandis does not possess any stout setae on the plantar surfaces of tarsomeres 1–3 and lacks setae on the claws.
I.1.1.1.7. Leuctridae (Leuctrinae): Despaxia augusta (Banks, 1907)
Tarsus and pretarsus of this species are very similar to those of Leuctra grandis shown in .
I.1.1.1.8. Capniidae: Isocapnia grandis (Banks, 1907)
Tarsus and pretarsus of this species are shown in and are very similar to those of the Nemouridae and Leuctrinae described previously, except that tarsomere 1 is slightly shorter than tarsomere 3 and tarsomere 2 is very short, approximately one-third the length of tarsomere 3. Moreover, the setae of tarsomeres 1–3 surfaces are more numerous on the plantar surfaces.
Figures 9–16. (9) Capniidae (Euholognatha): Isocapnia grandis Banks, ventrolateral view of tarsomeres 1–3 and pretarsus; (10) Taeniopterygidae: Taeniopteryginae (Euholognatha); Taeniopteryx maura (Pictet), ventrolateral view of tarsomeres 1–3 and pretarsus; (11–12) Scopuridae (Euholognatha): Scopura bihamulata Uchida, (11) ventrolateral view of tarsomeres 1–3 and pretarsus; (12) ventral view of tarsomere 1; (13–16) Scopuridae (Euholognatha): Scopura bihamulata Uchida, (13) ventral view of tarsomere 2; (14) ventral view of tarsomere 3; (15) ventral view of arolium; (16) dorsal view of orbicula. Abbreviations: ar, arolium; cl, claw; or, orbicula; t, tarsomeres; ut, unguitractor plate.
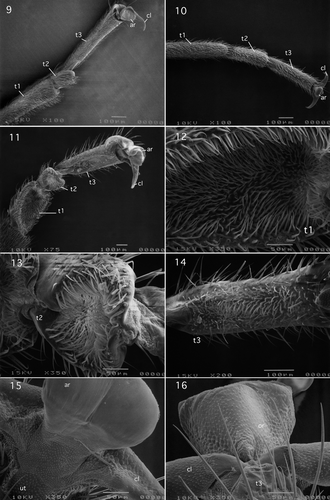
I.1.1.2. Taeniopterygidae
I.1.1.2.1. Taeniopterygidae (Taeniopteryginae): Taeniopteryx maura (Pictet, 1841)
Tarsus and pretarus of this species are shown in and are basically similar to the nemourid Ostrocerca albidipennis shown in . However, Tarsomere 2, like tarsomeres 1 and 3, is elongate, approximately four-fifths the length of tarsomere 3.
I.1.1.2.2. Taeniopterygidae (Brachypterinae): Strophopteryx limata (Frison, 1942)
Tarsus and pretarsus of this species closely resemble those of Taeniopteryx maura shown in .
I.1.2. Scopuridae
I.1.2.1. Scopuridae: Scopura bihamulata Uchida, 1987
Tarsus and pretarsus of this species are shown in . Tarsomeres 1 and 2 ventrally becoming slightly dilated (). Tarsomere 3 cylindrical. Tarsomeres 1 and 3 elongate: length of tarsomere 1 approximately three-quarters the length of tarsomere 3. Tarsomere 2 short: length approximately one-half length of tarsomere 3. Plantar surfaces of tarsomeres bear a dense covering of tapering flexible-appearing setae (). The setal-bearing surfaces are concave, suggesting that the cuticle is resilient. Together the setae and concave surfaces may maximise conformance to heterogeneous substrates. The unguitractor plate surface () bears scale-like microtrichia. Setiform basipulvilli are absent. The claws have smooth margins and lack setae. Setae are not present on the arolium plantar surface and orbicula ( and ).
I.2. Systellognatha
I.2.1. Pteronarcyoidea
I.2.1.1. Pteronarcyidae: Pteronarcella badia (Hagen, 1874)
Tarsus and pretarsus of this species are shown in . Tarsomeres 1, 2, and 3 cylindrical: ring or tube-like (). Tarsomeres 1 and 3 elongate: tarsomere 1 approximately three-fifths the length of tarsomere 3. Tarsomere 2 short: length slightly longer than one-third length of tarsomere 3. Plantar surfaces of tarsomeres 1–3 exhibit a median longitudinal unsclerotised region (); that on tarsomeres 1 and 2 is broadly expanded distally to form a pad-like euplantula (, ). The unsclerotised surfaces of the plantar regions of all three tarsomeres are hairy owing to being densely covered with sharply pointed cuticular projections approximately 5 μm in length (). These were referred to as specialised setae by Stark and Nelson (2000) who observed them on the tarsal plantar surfaces of the peltoperlid Viehoperla ada (Needham and Smith, 1916). However, these projections lack the sockets () that are associated with setae and are either acanthae or microtrichia (Richards and Richards Citation1979). The unguitractor plate surface () bears scale-like microtrichia. Setiform basipulvilli are absent. The two lateral claws bear a small number of setae, have smooth margins and gradually curve to a sharp point distally. The arolium is greatly expanded laterally and its plantar surface bears two clusters of setae as well as non-setal cuticular projections (, ). The orbicula bears several setae ().
Figures 17–24. Pteronarcyidae (Systellognatha): Pteronarcella badia (Hagen). (17) Ventral view of tarsomeres 1–3 and pretarsus; (18) ventrolateral view of tarsomeres 1–2; (19) lateral view of tarsomere 2; (20) ventral view of tarsomere 3; (21) cuticular projections of tarsomere 2; (22) ventrolateral view of unguitractor and arolium; (23) setae and cuticular projections of arolium; (24) dorsolateral view of orbicula. Abbreviations: ar, arolium; cl, claw; cp, non-setal cuticlar projections; eu, euplantula; mb, median longitudinal unsclerotised band; or, orbicula; s, setae; t, tarsomeres; ut, unguitractor plate.
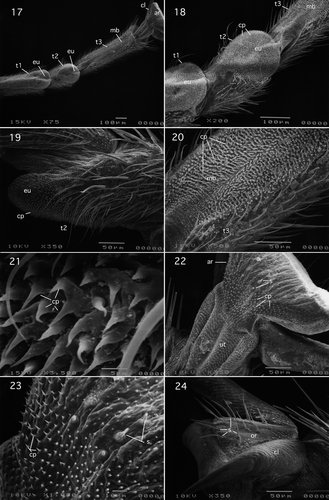
I.2.1.2. Pteronarcyidae: Pteronarcys californica Newport, 1851
Tarsus and pretarsus of this species shown in are similar to Pteronarcella badia shown in . However, the euplantulae lack hairs and the surface profiles are smooth. In addition the claws lack setae.
Figures 25–32. (25–27) Pteronarcyidae (Systellognatha): Pteronarcys californica Newport, (25) ventral view of tarsomeres 1–3 and pretarsus; (26) ventral view of tarsomere 2; (27) lateral view of tarsomere 2; (28) Styloperlidae (Systellognatha): Cerconychia livida Klapalek, ventral view of tarsomeres 1–3 and pretarsus; (29) Peltoperlidae; Peltoperlinae (Systellognatha): Yoraperla brevis (Banks), ventral view of tarsomeres 1–3; (30) Peltoperlidae: Peltoperlinae (Systellognatha): Tallaperla maria (Needham & Smith), ventral view of tarsomeres 1–3 and pretarsus; (31) Perlodidae: Perlodinae (Systellognatha): Megarcys signata (Hagen), ventral view of tarsomeres 1–3; (32) Perlidae; Perlinae (Systellognatha): Claassenia sabulosa (Banks), ventral view of tarsomeres 1–3. Abbreviations: ar, arolium; cl, claw; eu, euplantula; mb, median longitudinal unsclerotised band; t, tarsomeres; ut, unguitractor plate.
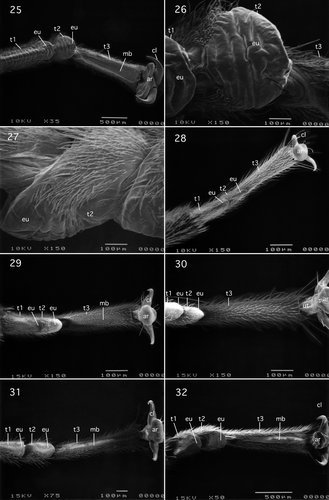
I.2.1.3. Styloperlidae: Cerconychia livida Klapalek, 1913
Tarsus and pretarsus of this representative of the Asian family shown in are similar to the pteronarcyid Pteronarcys califiornica shown in , except that tarsomeres 1 and 2 are both short: tarsomere 1 is approximately two-fifths the length of tarsomere 3. The presence of a short tarsomere 1 and 2 was also noted for Nearctic representatives of the families Peltoperlidae, Perlodidae, Perlidae, and Chloroperlidae by Stark and Nelson (2000). Moreover, tarsomere 3 lacks a hairy median longitudinal unsclerotised band on its plantar surface, but instead is uniformly covered with unspecialised setae. The arolium is normal, not greatly expanded laterally, and bears two setae on its plantar surface (Nelson Citation1991). The orbicula bears only three setae (Nelson Citation1991).
I.2.1.4. Peltoperlidae (Peltoperlinae): Yoraperla brevis (Banks, 1907)
I.2.1.5. Peltoperlidae (Peltoperlinae): Sierraperla cora (Needham and Smith, 1916)
I.2.1.6. Peltoperlidae (Peltoperlinae): Soliperla campanula (Jewett, 1954)
The tarsus and pretarsus of these representatives of three western Nearctic Peltoperlinae genera are closely similar. Like Cerconychia livida, tarsomeres 1 and 2 of these species are short. The euplantulae surfaces of tarsomeres 1 and 2, as shown for Yoraperla brevis in , are hairy. Tarsomere 3, like that of the pteronarcyid species described previously, exhibits a hairy median longitudinal unsclerotised band on its plantar surface. The lateral claws each bear two setae, the normal-sized arolium bears two setae on its plantar surface, and the orbicula bears several setae (Nelson Citation1991).
I.2.1.7. Peltoperlidae (Peltoperlinae): Tallaperla maria (Needham and Smith, 1916)
I.2.1.8. Peltoperlidae (Peltoperlinae): Peltoperla arcuata Needham, 1905
I.2.1.9. Peltoperlidae (Peltoperlinae): Viehoperla ada (Needham and Smith, 1916)
The tarsus and pretarsus of these representatives of three eastern Nearctic Peltoperlinae genera closely resemble each other and those of the western Nearctic genera described above. However, the eastern representatives differ from the western forms in that tarsomere 3, as shown for Talloperla maria in , does not exhibit a specialised attachment surface but instead is uniformally covered with setae.
1.2.1.10. Peltoperlidae (Microperlinae): Microperla brevicauda Kawai, 1958
Tarsomere plantar surfaces of this representative of the Asian peltoperlid subfamily are basically identical to those of the styloperlid Cerconychia livida shown in . However, tarsomere 1 of M. brevicauda, approximately one-third the length of tarsomere 3, is slightly shorter than that of C. livida. The euplantulae surfaces are smooth and there is no specialised attachment region on tarsomere 3. The lateral claws are also similar to C. livida in lacking setae (Nelson Citation1991). The arolium plantar surface and orbicula each bear two setae (Nelson Citation1991).
I.2.2. Perloidea
I.2.2.1. Perlodidae (Perlodinae): Megarcys signata (Hagen, 1874)
The tarsus and pretarsus of this species shown in closely resemble those of the previously described peltoperlid Yoraperla brevis shown in . However, tarsomere 1 is approximately one-half the length of tarsomere 3. Setae are absent from the claws of M. signata as well as from claws of species comprising two other genera, Skwala and Arcynopteryx, belonging to the tribe Arcynopterygini (Nelson Citation1991). In contrast, setae are found on the claws of species belonging to genera in the tribes Perlodini and Diploperlini (Nelson Citation1991). The arolium of M. signata bears four setae on the plantar surface and the orbicula bears a number of setae.
I.2.2.2. Perlodidae (Isoperlinae): Clioperla clio (Newman, 1839)
Tarsomeres 1–3 of this species closely resemble the Perlodinae Megarcys signata shown in . Tarsomere 1, like that of the latter species, is short, approximately two-fifths the length of tarsomere 3. The claws lack setae, the arolium bears two setae on the plantar surface and the orbicula bears a number of setae (Nelson Citation1991).
I.2.2.3. Perlidae (Perlinae): Claassenia sabulosa (Banks, 1900)
Tarsus and pretarsus of this species shown in resemble the previously described perlodid Megarcys signata shown in , except that tarsomere 1 is approximately one-third the length of tarsomere 3 and the arolium plantar surface bears a variable number of setae.
I.2.2.4. Perlidae (Perlinae): Neoperla osage Stark and Lentz, 1988
Tarsus and pretarsus of this species shown in are very similar to that previously described for Claassenia sabulosa shown in except that the euplantulae of tarsomeres 1 and 2 are reduced to two hair-covered mounds. Moreover, tarsomere 3 lacks a specialised attachment surface, but instead is uniformly covered with setae. The arolium plantar surface and the orbicula each bear two setae.
Figures 33–40. (33) Perlidae: Perlinae (Systellognatha): Neoperla osage Stark & Lentz, ventral view of tarsomeres 1–3; (34) Perlidae: Acroneurinae (Systellognatha): Perlesta adena Stark, ventral view of tarsomeres 1–3; (35) Chloroperlidae: Paraperlinae (Systellognatha): Paraperla frontalis (Banks), ventral view of tarsomeres 1–3 and pretarsus; (36) Chloroperlidae: Chloroperlinae (Systellognatha): Sweltsa lamba (Needham & Claassen), ventral view of tarsomeres 1–3 and pretarsus; (37, 38) Gripopterygidae: Antarctoperlinae (Gripopterygoidea): Antarctoperla michaelseni (Klapalek), (37) lateral view of tarsomeres 1–3; (38) ventrolateral view of pretarsus; (39, 40) Gripopterygidae: Gripopteryginae (Gripopterygoidea): Illiesoperla australis (Tillyard); (39) ventrolateral view of tarsomeres 1–3; (40) ventrolateral view of tarsomeres 1 and 2. Abbreviations: ar, arolium; cl, claw; eu, euplantula; mb, median longitudinal unsclerotised band; t, tarsomeres; ut, unguitractor plate.
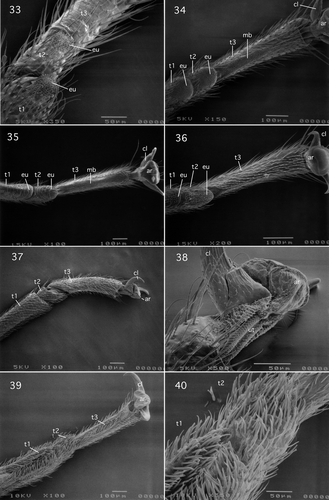
I.2.2.5. Perlidae (Acroneurinae): Perlesta adena Stark, 1989
Tarsus and pretarsus of this species shown in closely resemble those of the Perlinae Claassenia sabulosa shown in , except that it bears two setae on its plantar surface and four setae on the orbicula.
I.2.2.6. Perlidae (Acroneurinae): Acroneuria abnormis (Newman, 1838)
The tarsus and pretarsus of this species closely resemble those of Perlesta adena, except, like that of the perlodid Megarcys signata, it bears four setae on the arolium plantar surface and a number of setae on the orbicula.
I.2.2.7. Chloroperlidae (Paraperlinae): Paraperla frontalis (Banks, 1902)
The tarsus of this species shown in is very similar to those of Styloperlidae and Peltoperlidae in that tarsomere 1 is short, approximately two-fifths the length of tarsomere 3. The euplantulae of tarsomeres 1 and 2 resemble those of the pteronarcyid Pteronarcys californica shown in and in that their surface profile is smooth. The plantar region of tarsomere 3 is identical to those of Pteronarcella badia (Pteronarcyidae) shown in , Yoraperla brevis (Peltoperlidae) shown in , Megarcys signata (Perlodidae) shown in , and Claassenia sabulosa shown in in exhibiting a specialised attachment surface consisting of a hairy median longitudinal unscelerotised band. The claws of P. frontalis lack setae. However, there are two setae on the arolium plantar surface and as many as four setae on the orbicula.
I.2.2.8. Chloroperlidae (Paraperlinae): Kathroperla takhoma Stark and Surdick, 1987
Tarsus and pretarsus of this species similar to those of Paraperla frontalis shown in , except that the euplantulae surfaces are hairy.
I.2.2.9. Chloroperlidae (Chloroperlinae): Sweltsa lamba (Needham and Claassen, 1925)
The tarsus and pretarsus of this species shown in are similar to those of Paraperla frontalis shown in , except that the euplantulae, like those of Kathroperla takhoma, are hairy. Moreover, tarsomere 3 of this species differs from the two Paraperlinae species described above in lacking a specialised attachment region on its plantar surface. Instead the tarsomere is uniformly covered with setae. However, the lateral claws are similar to Paraperla frontalis in lacking setae. The arolium plantar surface and orbicula each bear two setae.
I.2.2.10. Chloroperlidae (Chloroperlinae): Alloperla usa Ricker, 1952
I.2.2.11. Chloroperlidae (Chloroperlinae): Suwallia starki Alexader and Stewart, 1999
I.2.2.12. Chloroperlidae (Chloroperlinae): Triznaka signata (Banks 1935)
Tarsus and pretarsus of representatives of these three genera are closely similar to those of Sweltsa lamba shown in , except that the euplantulae, like those of Paraperla frontalis described above, have a smooth surface profile.
II. Antarctoperlaria
II.1. Gripopterygoidea
II.1.1. Gripopterygidae (Antarctoperlinae): Antarctoperla michaelseni (Klapalek, 1904)
The tarsus and pretarsus of this species shown in and are similar to those described for the Nemouroidea of the Suborder Arctoperlaria. Tarsomeres 1–3 cylindrical: ring or tube-like (). Tarsomeres 1 and 3 elongate: tarsomere 1 longer than tarsomere 3. Tarsomere 2 short: length approximately one-third that of tarsomere 3. Tarsomere surfaces uniformly covered with setae and plantar surfaces lack specialised attachment structures (). The unguitractor plate is comprised of scale-like microtrichia (). Setiform basipulvilli are absent. The claws and arolium plantar surface lack setae () and the orbicula bears two setae.
II.1.2. Gripopterygidae (Paragripopteryginae): Dinotoperla evansi Kimmins, 1951
The tarsus and pretarsus of this species closely resemble those of the Antarctoperlinae Antarctoperlaria michaelseni described above. However, tarsomere 1 is slightly shorter than tarsomere 3.
II.1.3. Gripopterygidae (Gripopteryginae): Illiesoperla australis (Tillyard, 1924)
The tarsus and pretarsus of this species are shown in . Tarsomeres 1–3 cylindrical: ring or tube-like (). Tarsomeres 1 and 3 elongate: length of tarsomere 1 is slightly shorter than that of tarsomere 3. Tarsomere 2 short: length is approximately one-third that of tarsomere 3. Tarsomere plantar surface somewhat similar to the scopurid Scopura bihamulata of the Arctoperlaria shown in in that they bear a dense covering of setae (). The surfaces of tarsomeres 1 and 3 appear concave (), suggesting that the cuticle is flexible and helps in maximising adherence to the substrate. The pretarsus is closely similar to that described for Antarctoperla michaelseni shown in .
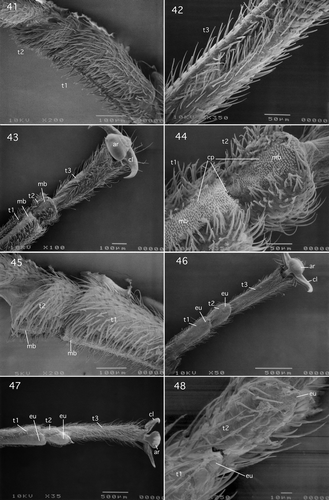
II.1.4. Austroperlidae: Austroperla cyrene (Newman, 1845)
The tarsus and pretarsus of this species are shown in . Tarsomeres 1, 2, and 3 cylindrical: ring or tube-like (). Tarsomeres 1 and 3 elongate: length of tarsomere 1 approximately two-thirds length of tarsomere 3. Tarsomere 2 short: length approximately one-fourth length of tarsomere 3. Plantar surfaces of tarsomeres 1 and 2 similar to that described for Acruroperla atra (Samal) by Beutel and Gorb (Citation2006) in that each exhibits a hairy narrow median longitudinal unsclerotised band that does not form a pad-like euplantuala (, ). The pretarsus is closely similar to that described for Antarctoperla michaelseni shown in , except that the orbicula bears a number of setae (Nelson Citation1991).
Figures 41–48. (41, 42) Gripopterygidae: Gripopteryginae (Gripopterygoidea): Illiesoperla australis (Tillyard), (41) lateral view of tarsomeres 1 and 2; (42) ventrolateral view of tarsomere 3; (43, 44) Austroperlidae (Gripopterygoidea) Austroperla cyrene (Newman), (43) ventrolateral view of tarsus and pretarsus; (44) ventrolateral view of tarsomeres 1 and 2; (45) Austroperlidae (Gripopterygoidea): Austroperla cyrene (Newman), lateral view of tarsomeres 1 and 2; (46) Eustheniidae (Eusthenoidea): Stenoperla prasina (Newman), ventral view of tarsus and pretarsus; (47) Diamphipnoidae (Eusthenoidea): Diamphipnopsis samali Illies, ventral view of tarsus and pretarsus. (48) Embiidina, Oligotomidae: Oligotoma nigra Hagen, ventral view of tarsomeres 1 and 2. Abbreviations: ar, arolium; cl, claw; cp, non-setal cuticular projections; eu, euplantula; mb, median longitudinal unsclerotised band; t, tarsomeres.
II.2. Eusthenioidea
II.2.1. Eustheniidae (Stenoperlinae): Stenoperla prasina (Newman, 1845)
Tarsus and pretarsus of this species are shown in . Tarsomeres 1, 2, and 3 cylindrical: ring or tube-like. Tarsomeres 1 and 3 elongate: length of tarsomere 1 approximately two-thirds the length of tarsomere 3. Tarsomere 2 short: length approximately one-third length of tarsomere 3. Plantar surfaces of tarsomeres 1 and 2 closely resemble those of previously described representatives of the Systellognatha of the Arctoperlaria in that each exhibits a median longitudinal unsclerotised region that forms a euplantula bearing a hairy surface. However, tarsomere 3 lacks a specialised attachment surface, but instead is uniformly covered with setae. The unguitractor plate is comprised of scale-like microtrichia. Each claw has smooth margins and bears two to three setae (Nelson Citation1991). The arolium lacks setae on the plantar surface and the orbicula bears a number of setae (Nelson Citation1991).
II.2.2. Diamphipnoidae: Diamphipnopsis samali Illies, 1962
Tarsus and pretarsus of this species shown in are similar to those of Stenoperla prasina shown in , except that the claw margins are serrated (Nelson Citation1991).
Discussion
Evolution of stonefly tarsal and pretarsal structures
The following tarsal and pretarsal attributes were mapped onto a cladogram of the Plecoptera phylogeny of Zwick (Citation2000):
Tarsomeres 1 and 2 shape and length
Tarsomeres 1 and 2 shape. Analysis of the mapping of this character on the cladogram of stonefly phylogeny indicates that the slight ventral expansion of tarsomeres 1 and 2 of the Scopuridae () is an autapomorphy of this family. The cylindrical shape for the tarsomeres exhibited by the remaining stonefly families is a stonefly ground pattern character.
Figures 49–52. (49) Evolution of tarsomeres 1 and 2 shape (L = 1, CI = 1.00, RI = 0.0). (50) Evolution of tarsomere 1 length relative to tarsomere 3 length (L = 4, CI = 0.50, RI = 0.70). (51) Evolution of tarsomere 2 length relative to tarsomere 3 length (L = 1, CI = 1.00, RI = 0.0). (52) Evolution of tarsomeres 1 and 2 plantar surfaces (L = 4, CI = 0.75, RI = 0.80).
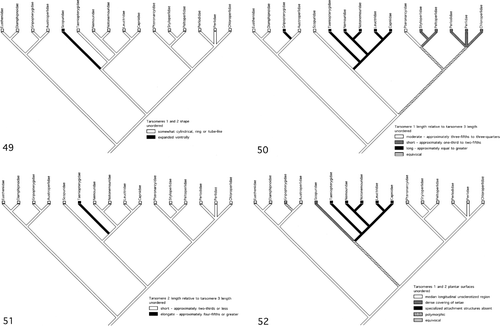
Tarsomere 1 length. In stoneflies the moderate length of tarsomere 1, exhibited by members of the Eustheniidae, Diamphipnoidae, Austroperlidae, Scopuridae and Pteronarcyidae, is the ground pattern character of the order (). A long tarsomere 1 appears convergently in Gripopterygidae and families belonging to the Nemouroidea clade: Taeniopterygidae, Nemouridae, Notonemouridae, Leuctridae and Capniidae. The presence of a short tarsomere 1 in two of the families comprising the Pteronarcyoidea clade, Styloperlidae and Peltoperlidae, as well as in the families comprising the Perloidea clade, Perlodidae, Perlidae, and Chloroperlidae, could be interpreted as having appeared independently in both these sister clades. On the other hand a short tarsomere 1 could be part of the ground pattern of the Systellognatha and the presence of a tarsomere 1 of moderate length in members of the Pteronarcyidae of the Pteronarcyoidea a reversal to the stonefly ground pattern condition. Still another possibility is that the short tarsomere 1 could be a synapomorphic feature that unites the Peltoperlidae and Styloperlidae together with the Perloidea in a single monophyletic clade – a topological arrangement that had been earlier considered by Zwick (Citation1973) and Nelson (Citation1984). More recently Zwick (Citation2000) suggested that this view still merited attention.
Tarsomere 2 length. In stoneflies tarsomere 2 typically is the shortest of the three tarsal segments. Mapping of this character onto the stonefly phylogeny cladogram indicates that it is part of the ground pattern of the order (). An elongate tarsomere 2 subequal to both tarsomere 1 and tarsomere 3 is an autapomorphy of the Taeniopterygidae (Zwick Citation2000).
Tarsomeres 1–3 plantar surfaces
Tarsomeres 1 and 2 plantar surfaces
Three distinct conditions of the plantar surfaces of tarsomeres 1 and 2 are recognised here: (i) the presence of a median longitudinal unsclerotised region, (ii) the presence of a dense covering of setae, and (iii) the absence of a specialised attachment surface with the entire tarsomere bearing a uniform covering of setae. Mapping this character onto the stonefly phylogeny cladogram () indicates that condition (i), the median longitudinal unsclerotised region, is the ground pattern condition for the Plecoptera. Transformation of this condition for tarsomeres 1 and 2 occurred independently in the Gripopterygidae and the Euholognatha clade (Scopuridae, Taeniopterygidae, Nemouridae, Notonemouridae, Leuctridae, Capniidae). For both clades, the cladogram indicates that either condition (ii), a dense covering of setae, or condition (iii), the absence of specialised attachment surfaces, could be part of the ground pattern. Within the Euholognatha, however, the cladogram identifies the condition (iii), the absence of unspecialised attachment surfaces, as part of the ground pattern of the Nemouroidea clade (Taeniopterygidae, Nemouridae, Notonemouridae, Leuctridae, Capniidae).
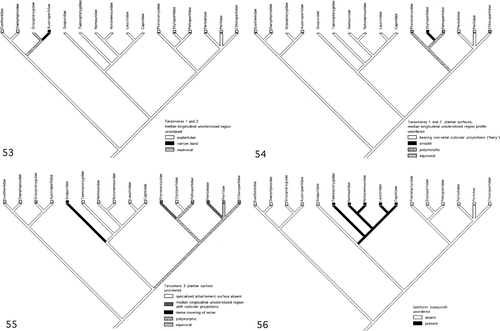
Development of Tarsomeres 1 and 2 median longitudinal unsclerotised region. Contrary to Beutel and Gorb (Citation2006), the presence of the median longitudinal unsclerotised region as euplantulae on the plantar surfaces of tarsomeres 1 and 2 is the ground pattern condition for stoneflies (). The narrow median longitudinal unsclerotised band on tarsomeres 1 and 2 of the Austroperlidae is an apomorphic condition for this family.
Figures 53–56. (53) Evolution of the median longitudinal unsclerotised region of tarsomeres 1 and 2 (L = 1, CI = 1.00, RI = 0.0). (54) Evolution of the surface profile of the median longitudinal unsclerotised region of tarsomeres 1 and 2 (L = 4, CI = 1.00, RI = 0.0). (55) Evolution of tarsomere 3 plantar surface (L = 7, CI = 0.86, RI = 0.0). (56) Evolution of the setiform basipulvilli of the pretarsus (L = 1, CI = 1.00, RI = 1.00).
If, as Zwick (Citation1980) and Beutel and Gorb (Citation2006) assert, Plecoptera is the sister group to the rest of the Neoptera, then the presence of euplantulae could be a synapomorphy of both groups. In this scenario the euplantulae were lost in various lineages of the rest of the Neoptera (Isoptera, the hemipteroid orders, and Endopterygota) and retained in others (Blattodea, Mantodea, Ensifera, Caelifera, Phasmatodea, Mantophasmatodea and Grylloblattodea). Modification of the euplantulae may have occurred in two other lower Neopteran groups, Dermaptera and Embiidina. In the former the euplantulae are longitudinal median distally extended membranous flaps termed plantulae by Haas and Gorb (Citation2004). There is some controversy as to whether these structures are homologous to euplantulae and even whether they are part of the Dermaptera ground pattern (Haas and Gorb Citation2004; Beutel and Gorb Citation2006). In Embiidina the euplantulae have been considered to be absent (Beutel and Gorb Citation2001, Citation2006). However, the tarsomeres bear small membranous mounds termed papillae () that Ross (Citation2000) notes have been considered to be homologous to euplantulae. Moreover, these structures in Embiidina exhibit sexual dimorphism (Ross Citation2000) in that the euplantulae of males have a smooth surface profile and those of females bear peg-like setae (possibly acanthae or microtrichea). Ross (Citation2000) considers that the peg-like setae facilitate females' turning movements in the narrow tunnels where they reside. Males do not live in tunnels and, thus, do not require setae to assist in making turning movements.
An alternative phylogenetic hypothesis to that presented by Beutel and Gorb (Citation2006) would place the Plecoptera and Embiidina along with Dermaptera, Blattodea, Isoptera, Mantodea, Ensifera, Caelifera, Phasmatodea, Mantophasmatodea and Grylloblattodea in a monophyletic assemblage, the Polyneoptera (Wheeler et al. Citation2001, Grimaldi and Engel Citation2005). If this placement is correct the euplantulae may be an autapomorphic feature of the Polyneoptera and in this scenario were lost in Isoptera and never present in the hemipteroid orders and Endopterygota.
Tarsomeres 1 and 2 median longitudinal unsclerotised region surface profile. Mapping this character on the stonefly phylogeny cladogram () indicates that a hairy surface resulting from presence of non-setal cuticular projections on the median longitudinal unsclerotised region of tarsomeres 1 and 2 is part of plecopteran ground pattern. Euplantulae or narrow median longitudinal unsclerotised plantar bands bearing hairs is unusual in insects and has previously only been reported for the euplantulae of the order Mantophasmatodea (Beutel and Gorb Citation2006). In Plecoptera euplantulae with a smooth surface profile appear multiple times in families belonging to the Systellognatha (Pteronarcyidae, Styloperlidae, Peltoperlidae, Chloroperlidae). However, the cladogram indicates that this condition is apparently not part of the ground pattern of this clade.
Tarsomere 3 plantar surface. Mapping this feature on the stonefly phylogeny indicates that the absence of any specialised attachment structures on the tarsomere 3 plantar surface is part of the ground pattern of the order (). Excepting Styloperlidae, a median longitudinal unsclerotised band bearing non-setal cuticular projections is a specialised attachment surface found on tarsomere 3 in some members of every family within the Pteronarcyidae, Peltoperlidae, Perlidae, Perlodidae, and Chloroperlidae of the Systellognatha clade. However, without a more complete understanding of the phylogenetic relationships of the species within the families of this clade, it is not possible to determine whether this feature is part of the ground pattern of the Systellognatha or arose multiple times independently. The condition of a dense covering of unspecialised setae on the plantar surface of tarsomere 3 appears to have originated independently in Scopuridae and Illiesoperla of the Gripopterygidae.
Pretarsus
Setiform basipulviilli. The cladogram of stonefly phylogeny indicates that the absence of setiform basipulvilli is the ground pattern condition for Plecoptera (). The presence of setiform basipulvilli, as indicated by Nelson (Citation1991) and Zwick (Citation2000), is a synapomorphic feature of the Nemouroidea clade (Taeniopterygidae, Nemouridae, Notonemouridae, Leuctridae, Capniidae).
Claw setae. The present topology of stonefly phylogeny does not permit determination of whether the presence or absence of claw setae is part of the Plecoptera ground pattern ().
Figures 57–60. (57) Evolution of the claw setae (L = 2, CI = 0.50, RI = 0.67). (58) Evolution of the arolium width of the pretarsus (L = 1, CI = 1.00, RI = 0.0). (59) Evolution of the arolium setae of the pretarsus (L = 1, CI = 1.00, RI = 0.0). (60) Evolution of the orbicula setae of the pretarsus (L = 1, CI = 1.00, RI = 0.0).
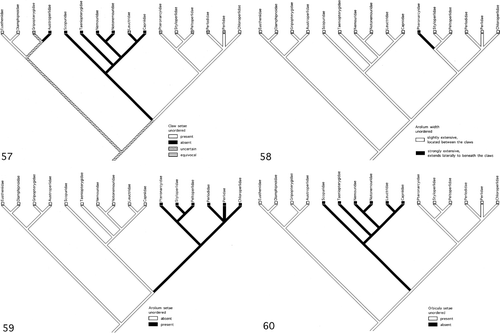
Arolium width. The stonefly phylogeny cladogram indicates that the somewhat triangular-shaped arolium is the ground pattern condition for the order (). The laterally expanded arolium, present in the Pteronarcydae, is a derived feature and is part of the ground pattern of this family (Nelson Citation1991).
Arolium plantar surface setae. Mapping of this character on the stonefly phylogeny cladogram indicates that absence of setae on the arolium plantar surface is part of the stonefly ground pattern (). The presence of setae on the arolium plantar surface is a synapomorphy for the families comprising the Systellognatha: Pteronarcyidae, Peltoperlidae, Styloperlidae, Perlidae, Perlodidae, Chloroperlidae (Nelson Citation1991).
Orbicula surface setae. The cladogram of stonefly phylogeny () indicates that the presence of setae on the orbicula is part of the stonefly ground pattern. The absence of seta on this structure is a synapomorphy for the families comprising the Euholognatha: Scopuridae, Taeniopterygidae, Nemouridae, Notonemouridae, Leuctridae, Capniidae (Nelson Citation1991).
Functional significance
Although specific information on the preferred substrates for individual stonefly species is largely lacking, Stewart (Citation1994) listed a number of stonefly mating encounter sites and identified a variety of different substrates associated with these sites: stones, algae-covered rocks, moss, grass, bushes, tree stems, flowers, leaves, streamside leaf litter, streamside vegetation debris, bridges, posts, ice, and snow. Gorb (Citation2001) has indicated that in order to traverse diverse substrates, insects evolved both smooth and hairy locomotory attachment surfaces. In Plecoptera both types of attachment surfaces are present and very likely facilitate horizontal and vertical movement on a multiplicity of substrates by serving to increase the area of contact between the attachment and substrate surfaces (Beutel and Gorb Citation2001). The hairy system of Plecoptera is restricted, however, in that specialised tenent setae with thickened or spatulate tips, characteristic of the locomotory surfaces of various insects (Beutel and Gorb Citation2001, Haas and Gorb Citation2004), are absent. Nelson (Citation1991) suggested that the claws of the stonefly pretarsus facilitate movement on rough surfaces. During locomotion the position of the claws is locked in place with the assistance of the unguitractor plate (Gorb Citation1996). Flexure of the claws results from contraction of the unguitractor tendon. The scale-like microtrichia of the plate then engage the facing microtrichia of the distalmost inner surface of tarsomere 3. This action maintains the retraction of the claws as they grip the substrate. Detachment of the claws from the substrate occurs when the unguitractor tendon relaxes allowing the microtrichia of the plate and tarsomere surfaces to disengage. For many insects mechanical attachment is facilitated by epidermal chemical secretions (Gorb et al. Citation2002). Although this is probably the case for stoneflies, such chemicals have yet to be identified.
Acknowledgements
I am indebted to the following individuals for the loan or gift of material: R.M. Duffield (Howard University), O.S. Flint (United States National Museum), Y. Isobe (Nara Women's University), B. Kondratieff (Colorado State University), R. Prins (Western Kentucky University), D.M. Stevens (University of Cape Town) and S. Uchida (Aichi Institute of Technology). I thank B.P. Stark (Mississippi College) for making available SEM photomicrographs of various representatives of the Systellognatha. I also thank R. Bell for technical assistance with the SEM. T. Gaudin and S. Chatzimanolis provided valuable comments and suggestions on this manuscript.
References
- Beutel , R. G. and Gorb , S. N. 2001 . ‘Ultrastructure of attachment specializations of hexapods (Arthropoda): evolutionary patterns inferred from a revised ordinal phylogeny’ . Journal of Zoological Systematics and Evolutionary Research , 39 : 177 – 207 .
- Beutel , R. G. and Gorb , S. N. 2006 . ‘A revised interpretation of the evolution of attachment structures in Hexapoda with special emphasis on Mantophasmatodea’ . Arthropod Systematics & Phylogeny , 64 : 3 – 25 .
- Crampton , G. C. 1923 . ‘Preliminary note on the terminology applied to parts of an insect's leg’ . The Canadian Entomologist , 55 : 126 – 132 .
- Dashman , T. 1953 . ‘Terminology of the pretarsus’ . Annals of the Entomological Society of America , 46 : 56 – 62 .
- Gorb , S. N. 1996 . ‘Design of insect unguitractor apparatus’ . Journal of Morphology , 230 : 219 – 230 .
- Gorb , S. N. 2001 . Attachment Devices of Insect Cuticle , Dordrecht : Kluwer Academic Publishers .
- Gorb , S. N. , Beutel , R. G. , Gorb , E. V. , Jiao , Y. , Kastner , V. , Niederegger , S. , Popov , V. L. , Scherge , M. , Schwarz , U. and Vötsch , W. 2002 . ‘Structural design and biomechanics of friction-based releasable attachment devices in insects’ . Integrated Comparative Biology , 42 : 1127 – 1139 .
- Grimaldi , D. and Engel , M. S. 2005 . Evolution of the Insects , Cambridge, New York, Melbourne, Madrid, Cape Town, Singapore, Sao Paulo : Cambridge University Press .
- Haas , F. and Gorb , S. N. 2004 . ‘Evolution of locomotory attachment pads in the Dermaptera (Insecta)’ . Arthropod Structure & Development , 33 : 45 – 66 .
- Hanson , J. F. 1946 . ‘Comparative morphology and taxonomy of the Capniidae (Plecoptera)’ . The American Midland Naturalist , 35 : 193 – 249 .
- Maddison , D. R. and Maddison , W. P. 2000 . MacClade 4.0. Analysis of phylogeny and character evolution, Version 4.0 , Sunderland , MA : Sinauer Associates .
- Nelson , C. H. 1984 . ‘Numerical cladistic analysis of phylogenetic relationships in Plecoptera’ . Annals of the Entomological Society of America , 77 : 466 – 473 .
- Nelson , C. H. 1991 . “ ‘Preliminary note on the comparative morphology of the stonefly pretarsus (Plecoptera)’ ” . In Overview and Strategies of Ephemeroptera and Plecoptera , Edited by: Alba-Tercedor , J. and Sanchez-Ortega , A. 135 – 156 . Gainesville , FL : Sandhill Crane Press .
- Richards , A. G. and Richards , P. A. 1979 . ‘The cuticular protuberances of insects’ . International Journal of Insect Morphology & Embryology , 8 : 143 – 157 .
- Ross , E. S. 2000 . ‘Embia: Contributions to the biosystematics of the insect Order Embiidina. Part 1. Origin, relationships and integumental anatomy of the Insect Order Embiidina. Part 2. A review of the Biology of Embiidina’ . Occasional Papers of the California Academy of Sciences , No. 149, vi + Part 1:1–53 + Part 2: 1–36
- Stark , B. P. and Nelson , C. R. 2000 . “ ‘The nearctic plecopteran families: morphology and systematics’ ” . In The stoneflies (Plecoptera) of eastern North America, Vol. I. Peltoperlidae, Pteronarcyidae, and Taeniopterygidae Edited by: Stark , B. P. and Armitage , B. J. Bulletin of the Ohio Biological Survey, 14, 11–28
- Stewart , K. W. 1994 . ‘Theoretical considerations of mate finding and other adult behaviors of Plecoptera’ . Aquatic Insects , 16 : 95 – 104 .
- Wägele , J.-W. 2005 . Foundations of Phylogenetic Systematics. Translated from the German second edition by C. Stefen, J.-W. Wägele, and revised by B. Sinclair , München : Verlag Dr. Friedrich Pfeil .
- Wheeler , W. C. , Whiting , M. , Wheeler , Q. D. and Carpenter , J. M. 2001 . ‘The phylogeny of the extant hexapod orders’ . Cladistics , 17 : 113 – 169 .
- Zwick , P. 1973 . `Insecta: Plecoptera Phylogenetisches System und Katalog' . Das Tierreich , 94, I–XXXII : 1 – 465 .
- Zwick , P. 1980 . ‘Plecoptera (Steinfliegen)’ . Handbuch der Zoologie , 4, 2/7 : 1 – 115 .
- Zwick , P. 2000 . ‘Phylogenetic system and zoogeography of the Plecoptera’ . Annual Review of Entomology , 45 : 709 – 746 .