Abstract
There is no consensus about the relations of Plecoptera with other insects. Very different sistergroup relationships have been proposed in the literature, several of which are discussed. The phylogenetic analysis is hampered by the diversity among Plecoptera. In the literature, traits of particular subgroups of Plecoptera have sometimes been mistaken as typical of the entire order. A plea for revived interest in plecopteran morphology is made in order to document the existing diversity and to establish the ground pattern of important structures for meaningful comparisons with other insects. A possible sistergroup relationship between Plecoptera and the remaining Neoptera, on the one hand, and a sistergroup relationship with the Polyneoptera on the other hand, seem most likely. Suggested close relationships of Plecoptera with Embioptera and Phasmatodea are refuted. Molecular data so far also failed to provide robust phylogenetic hypotheses for the placement of Plecoptera.
Introduction
Establishing the identity of Plecoptera and distinguishing them from other insects was the cardinal problem when early students began to perceive the multitude of insects and tried to order the astounding diversity. There was enormous progress from the earliest presentation of a stonefly as a nameless beautiful natural object (Hoefnagel Citation1592) to the detailed works by Burmeister (Citation1839) and Pictet (Citation1841) who were familiar with many details of adult and larval morphology, ecology and behaviour of Plecoptera. Following their studies, Plecoptera were no longer confused with other insects.
However, the question where in the insect system the Plecoptera belong, what their closest relatives are, remains unanswered today. I do also not know but would like to discuss some suggested relations. The literature offers different answers to the above question but only work applying sound phylogenetic methodology (use of only homologous characters, conscientious distinction of plesiomorphic from apomorphic character states, conclusions based on only the latter) are relevant. The result of such work is a hypothesis of relations which becomes increasingly probable as efforts to falsify it fail, but it can never be proven.
The various phylogenetic relations of Plecoptera proposed in the past were discussed by, for example, Kristensen (Citation1975), Willmann (Citation2003, Citation2005), and Grimaldi and Engel (Citation2005). New, previously unavailable tools of analysis recently provided by molecular genetics and the recent discovery of a new insect order, the Mantophasmatodea, aroused new interest in insect phylogeny and some of the relevant papers also cast light on the position of Plecoptera in the insect system. However, recent opinions on plecopteran affinities are not based on new evidence concerning Plecoptera. Instead, stoneflies are only shoved around as other taxa are studied and views of their interrelations change. In this process, characters of some Plecoptera have sometimes been mistaken as typicaI of stoneflies in general. For example, Cranston and Gullan (2003) considered only Antarctoperlaria and Nemouroidea, omitting the Systellognatha. In contrast, Wheeler, Whiting, Wheeler and Carpenter (Citation2001) actually examined several stoneflies, but only from one single superfamily, Perloidea. Zompro (Citation2004, Citation2005) took the systellognathan egg for the ground pattern of plecopteran eggs, which is almost certainly incorrect.
I will not consider evidently unsupported relations of Plecoptera with the “higher” Neoptera (Paraneoptera and Endopterygota or Holometabola) that were once suggested (see discussions in Kristensen Citation1975; Grimaldi and Engel Citation2005). Most students today place Plecoptera as the possible sistergroup of, or somewhere among, the Polyneoptera.
To improve the present situation we ourselves, the Plecoptera experts, need to establish explicitly what the plecopteran ground pattern is, for as many structures or organ systems as possible. The clearer the statements, the more likely will the information be perceived by those who consider phylogenetic relationships between the insect orders. My presentation is a plea for renewed interest in morphology and phylogeny of stoneflies.
Plecoptera as sistergroup of the other Neoptera?
Hennig (Citation1969) suggested Plecoptera might be the sistergroup of all other Neoptera, because of uniquely primitive traits. Finding more uniquely primitive traits of stoneflies I felt the same (Zwick Citation1973). In Kristensen's synopsis (1991) the position of Plecoptera among the Lower Neoptera remained unresolved.
Plesiomorphies allow no definite conclusions, and Grimaldi and Engel (Citation2005) were ironic about Hennig's reasoning. However, if stoneflies were indeed sistergroup to the other Neoptera, circumstantial evidence is all that can logically be expected:
| • | Additional synapomorphies of all Neoptera | ||||
| • | More synapomorphies supporting the monophyly of Plecoptera | ||||
| • | Synapomorphies of Neoptera, exclusive of the plesiomorphic Plecoptera | ||||
The tracheate condition of Hexapoda and of the primarily wingless insects documents the terrestrial origin of the entire group. Strikingly, however, the most primitive Pterygota, namely Ephemeroptera, Odonata, and Plecoptera have aquatic larvae. A causal relation between aquatic mode of life and possession of wings has been suggested.
The origin of wings from rigid paranota used to glide was the leading theory until Kukalová-Peck (Citation1978) combined several earlier ideas and proposed the origin of wings from flapping gills, clearly inspired by one particular type of mayfly gill. Whether that type represents the ground pattern of mayfly gills was not discussed. In brief, wingless tracheate hexapods invaded water. A suite of functional steps was described, starting from protective covers over open spiracles which developed into gills whose size eventually necessitated ventilation by active movement. Rowing with gills was a by-product of ventilation movements, and propulsion by gill beat was ultimately used to fly when late instar larvae or adults returned to an aerial life. This szenario assumed an intimate connection between spiracles, gills, and finally wings (Kukalová-Peck Citation1978).
Later, the same author (Kukalová-Peck Citation1991, Citation2008) assumed the origin of gills from leg exites and thereby abandoned the intimate connection between gills and spiracles. In the relevant illustrations, the shape of gills was clearly borrowed from Plecoptera: Eustheniidae. Apparently the author regards gills of mayflies and stoneflies as homologous. Gills of the two orders are mentioned in one breath also by Haas (Citation2006), as though they were closely similar. Actually, they are not, and I am not certain of their homology.
Whether segmental abdominal gills are part of the Plecoptera ground pattern is doubtful. Sinichenkova (Citation1997) found no gill-bearing fossil Plecoptera larvae. Many Plecoptera, also large ones, lack gills, but others have gills on almost any part of the body (Zwick Citation1973, Citation1980). Abdominal gills occur only in a minority of Plecoptera, mainly large-bodied taxa. While abdominal mayfly gills are mostly in dorsolateral position, stonefly gills are always ventrolateral and have, by their tracheation and position on the body, a close relation to the rudimentary larval spiracles. Unlike many mayflies no stonefly is capable of vigorous movements of abdominal gills.
Aquatic insect life requires respiratory adaptations but in the dilute freshwater environment osmoregulation is also mandatory. Wichard and co-authors studied the osmoregulatory organs of mayflies and stoneflies (for example, 1971, 1972, 1973a, b, 1974, 1979). Both groups possess unicellular chloride cells as well as pluricellular osmoregulatory organs. The former are typical normal transport cells exhibiting a strong similarity to chloride cells in fish (Wichard and Komnick Citation1971). This type has probably been developed several times, also among insects. In contrast, pluricellular organs are found only in Ephemeroptera and Plecoptera. Their complex porous plates agree closely, down to the ultrastructural level. Enlargement of surface area of the porous plates even led to similar cuticular structures on the body surface.
Wichard (Citation1997) is certainly right that the pluricellular osmoregulatory organs of mayflies and stoneflies are homologous, and symplesiomorphic. Odonata seem to have lost them secondarily, having moved their respiratory and osmoregulatory functions to the hind gut, where complex cuticular apparatuses are neither needed nor possible. Also, as predators they obtain some of the needed ions from their prey. Advanced freshwater insects requiring osmoregulation achieve it by single chloride cells.
The point made by Wichard (Citation1997) was that the aquatic larval life of the most primitive Pterygota and the presence of a unique homologous kind of osmoregulatory organs in Ephemeroptera and Plecoptera among the Neoptera is in line with the proposed origin of wings when insects invaded water, possibly from gills. My point is that replacement of the complex pluricellular osmoregulatory organs by all Neoptera except the stoneflies points at the possible sistergroup relation of Plecoptera with the remaining Neoptera.
According to the above, Pterygota primitively have aquatic larvae. In contrast, Grimaldi and Engel (Citation2005) who see Plecoptera as members of a polyneopteran clade suggest that an aquatic larval life may be an apomorphy supporting the monophyly of Plecoptera.
Polyneopteran relations
Wing venation
The concept of Polyneoptera in part relies on wing characters: the transformation of front wings into semi-hard tegmina (see below) and the presence of a large anal fan in the hind wing which at rest is folded longitudinally. The latter caused Burmeister (Citation1839) to call the stoneflies “Plecoptera”, Umschlagfalter, that is: part of the wing is flipped over when folded. Because of this, Burmeister thought stoneflies to be closely related to Orthoptera, but also to the Megaloptera and Trichoptera which have similar hind wings. Apart from size of the anal fan the largely unbranched anal veins distinguish the Polyneoptera. The closely parallel course of veins Cu and A1 is mentioned in the literature, Plecoptera show it clearly. However, the fact that some Holometabola also have a large hind wing anal fan (as noticed by Burmeister Citation1839) weakens support of Polyneoptera by this character.
Haas and Kukalová-Peck (Citation2001) assigned importance to functional aspects, placing Plecoptera among the most primitive Neoptera. A fold separating the remigium (which is the anterior portion of wing providing the main thrust for flight) from the less efficient posterior wing portion may run between veins Cu and A, separating the entire anojugal lobe from the remigium, or alternatively between some branches of A, functionally including part of the anal fan in the remigium. Plecoptera, Embioptera and the Orthoneoptera (grashoppers and allies) share the former primitive condition. Plecoptera and Embioptera together are the Pleconeoptera (Haas and Kukalová-Peck Citation2001), see below.
All other insects share the derived condition (Haas and Kukalová-Peck Citation2001), that is the former Polyneoptera are heterogenous. The authors are not explicit if this advanced functional trait developed independently among Polyneoptera, and again in the ancestor(s) of Hemineoptera and Endoneoptera, or whether they actually regard the Blattoneoptera from among the former Polyneoptera to be phylogenetically more closely related to bugs plus Holometabola than to the more primitive members of Polyneoptera.
When polyneopteran wing venation is discussed, some large stoneflies with rich venation, like Eustheniidae or Pteronarcyidae, are usually illustrated. However, from paleontological evidence, Sinichenkova (in her presentation at the Lausanne International Symposium, 1995) concluded that in the ground pattern stoneflies are neither large nor do they have a rich venation. Willmann (Citation2005) believes the same.
A large anal fan is certainly a ground pattern of Plecoptera occurring also in small species without rich venation, across both suborders and in most families. Reduction of the hindwing anal area took place only in a scattered minority of Plecoptera, and clearly several times independently, for example in most Leuctridae, a few Notonemouridae, exceptional Capniidae, and the Chloroperlidae.
Bethoux (2005) analysed and compared Plecoptera wing venation patterns in detail and concluded that “no other taxon can reliably be pointed out as potential sister group”.
Ontogeny of wings in Plecoptera
During stonefly ontogenesis, the appearance of rigid non-articulated (contra suggestions in Kukalová-Peck Citation1991) dorsolateral extensions of the pterothorax visibly indicates wing development. Wing pads increase at moults, three morphologically well defined steps seem to be standard in the order, except two in Pteronarcyidae (Townsend and Pritchard Citation1998; Zwick and Teslenko Citation2002).
The only published study addressing events inside the body during wing formation concerns Pteronarcys (Holdsworth Citation1942). He used reconstructions from serial body cross-sections for early, and of whole mounts of wing pads of late instars to describe the stepwise change of lacunae and tracheae across several instars. Holdsworth found wing development to begin in the earliest instars and continue during all larval life.
However, late steps of development in Pteronarcys described by Holdsworth differ markedly from what is seen in late instars of Nemouroidea. Wing pads of late instar stonefly larvae usually have some pattern resembling wing venation, and some like Protonemura meyeri (Pictet) even show mottled pigmentation, the same as later appears in the adult wing. However, the pattern is caused by external structures on the wing pad surface, mainly minute serial setation. There are no visible tracheae inside the wing pads of hundreds of perfectly transparent larvae of Leuctridae and Nemouridae mounted in Euparal which I studied. Wing pads are empty, except for the thin epithelium forming the cuticle, and probably some hemolymph. Just before a moult, the epithelium produces the wing pad of the next instar which is again empty, also in the last instar.
During the last instar, wing pads of Nemouroidea undergo visible change. Nemurella pictetii (Klapálek) and Nemoura cinerea (Retzius) were studied (my own unpublished data). 53 larvae kept singly in sprinkler tray units (Zwick and Teslenko Citation2002; Zwick and Hohmann Citation2003) were every 12 hours inspected with a dissecting microscope (6 x or higher) until the adult emerged. At about 14°C, the last instar lived on average for 26 days.
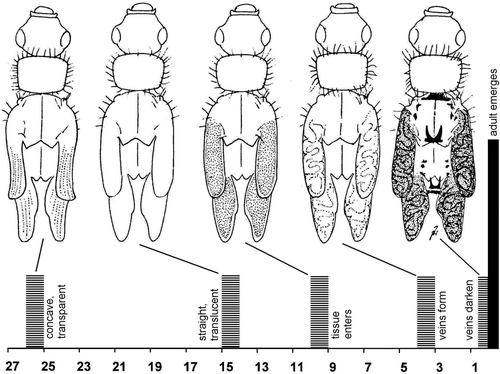
After the moult to last instar, wing pads curled up for several days () and were glass clear: every sand grain on the ground under the animals was perfectly visible across the wing pads. After larvae resumed feeding, wing pads straightened, remained translucent, and empty. Nine to 10 days before adult emergence, tissue budded from the larval trunk into the wing pad which then turned milky. Visible structures formed not earlier than four days before emergence. Veins in the developing wings were strongly undulating from the start; straight veins or other straight structures as Holdsworth illustrated them in Pteronarcys never appeared. Adults emerged within 12 hours after wings began to darken.
Figure 1. Phases of wing development in last instar Nemouroidea. Top, aspect in Leuctra sp. (modified from Zwick, Citation1991). Bottom, temporal sequence (abscissa: number of days before adult emergence) of the same developmental phases in Nemoura cinerea and Nemurella pictetii. Bars mark onset of the respective condition (original).
A study is needed if Plecoptera wings form in different ways in different subgroups, and how the ontogeny of wings compares with other primitive Pterygota.
Tarsal attachment structures
Among tarsal attachment structures, the arolium, a large soft pad between the tarsal claws, was thought to be distinctive of Polyneoptera, However, it was recently shown (Beutel and Gorb Citation2006) to be widespread among insects and not distinctive of Polyneoptera. The large arolium of adult Plecoptera was studied in detail (Nelson Citation1991).
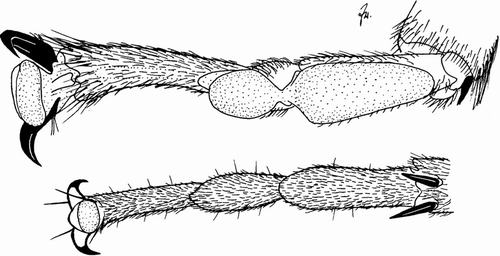
In contrast, euplantulae (soft tarsal soles protruding sometimes in an almost cushion-like form) which occur in only few taxa, also Eusthenia (Beutel and Gorb Citation2006), seem to have promise to define a monophyletic group, a clade possibly including Plecoptera. However, Plecoptera are not uniform in this respect. Soft, bulging soles are obvious in many large representatives like Eustheniidae, Pteronarcyidae (including the soft midline of the third segment), Perlidae, and Perlodidae. In contrast, there is no trace of euplantulae in Nemouroidea. This is not simply a matter of body size because the minute chloroperlid Siphonoperla has soft soles to its tiny basal tarsal segments, and so do some small Dinotoperla while some large Trinotoperla, both of them Gripopterygidae, have no trace of euplantulae. They have narrow tarsal segments with hairy soles, like Taeniopterygidae, for example Brachyptera (). Nelson (Citation2009) provides detailed information on tarsal attachment structures in all families of Plecoptera.
Relations with Embioptera
When friends returning from holidays in tropical countries bring back a presumed stonefly, it usually actually is a winged termite, or a male webspinner. There is resemblance in habitus, but there are many profound differences in structure as well as mode of life (embiopteran silk glands, gula, very different internal genitalia, sub-social life with brood care, etc.). Several authors assumed a close relationship between webspinners and stoneflies (for example, Wheeler et al. Citation2001; Haas and Kukalová-Peck Citation2001; Cranston and Gullan 2003). Grimaldi and Engel (Citation2005) recognise the Plecopterida, with Plecoptera as basal sistergroup of Embioptera + Zoraptera. The main uniting feature would be the complete loss of an ovipositor. However, three-segmented tarsi which are occasionally suggested in support would have arisen independently in each of the groups (Grimaldi and Engel Citation2005). I will not address characters brought forward in support of the sistergroup relationship between Embioptera and Zoraptera but only the relations between webspinners and stoneflies. Although placed among Polyneoptera, Embioptera have very narrow wings with only a minute anojugal lobe, instead of the large polyneopteran vannus, and also lack an arolium and euplantulae. This is usually interpreted as reductions.
Haas and Kukalová-Peck (Citation2001) recognise Pleconeoptera for stoneflies and webspinners because a fold separates the full anojugal lobe from the remigium, and because the medial sectors MP and MP originate from a long common stem. Actually, many Embioptera have no fork of M. Anyway, both of these characters are shared with Orthoneoptera, and no character convincingly supporting monophyly of Pleconeoptera is presented. The loosely articulated thorax, especially the freely movable prothorax of Plecoptera and Embioptera and their resemblance regarding the trochantin is archaic (Bitsch and Ramond Citation1970) and also provides no proof of a close relationship. In contrast, Grimaldi and Engel (Citation2005) think details of trochantin structure may support their Plecopterida.
Embioptera, Plecoptera and Grylloblattodea are the only insects with unpaired ventral excurrent ostia of the dorsal vessel. Because number and location on body segments do not agree between the orders (Pass et al. Citation2006), the significance of this resemblance is uncertain.
Typical Polyneoptera have complex ovipositors and primary male copulatory organs. Both are lacking in Plecoptera as well as Embioptera who use other structures to mate. Indeed, male clasping organs from the 10th abdominal tergum making good for the suppression of phallomeres and male styli is an alleged synapomorphy of webspinners and Plecoptera. It is true that tergite 10 may also be involved in mating in some of the Plecoptera, but even then actual structures and modes of mating in the two orders are strikingly different.
Male Embioptera have a divided, asymmetrical tergite 10, asymmetrical paraprocts and a modified left cercus with some kind of claw, while the right cercus is normal. In her unpublished thesis (1968) our late friend Christa Sattler gave a detailed account of webspinner mating: the mating position reminds one of Plecoptera but the asymmetry causes Embiidae to always sit on the right-hand side of the female. Embiid clasping organs seize the female abdominal tip and squeeze it so strongly that the genital opening becomes briefly exposed. At that moment, the male deposits a spermatophore next to it. In the species studied by Sattler mating lasted only 30–60 seconds but the strong male grip left imprints on the female body for some time.
Nothing similar occurs in stoneflies. The alleged similarity in the involvement of tergite 10 in mating is actually an imaginative creation. In Plecoptera, tergite 10 is involved only in Systellognatha, and in different ways in different taxa. In the systellognathan ground pattern, tergite 10 is split into hemitergites, which when hook-shaped may lift the female subgenital plate. In many more, the split of tergite 10 mainly provides a ‘garage’ for the complex epiproct sunk in a cowl between the hemitergites. Stonefly genitalia provide outstanding taxonomic characters; structure and function are amazingly diverse and attracted early attention. Klapálek (Citation1896) excellently described and illustrated several European taxa. Brinck's work (1956) in which he studied some of the same plus additional ones, focused on mating and sperm transfer, and was a milestone. Known structures and functions were summarised (Zwick Citation1973, Citation1980, Citation2000) – but many more await description.
Of course, differences between Plecoptera and Embioptera do not disprove their possible close relationship, but there is no trustworthy supporting evidence of it. I suspect the greatest agreement between the two orders is that students do not know where else to place them.
If students of insect phylogeny are not to be misled by incomplete or confusing information, we need to establish the ground pattern of stonefly genitalia and mating, or else document the full diversity in detail.
Plecoptera – close relatives of Phasmatodea?
A close affinity between stick insects and stoneflies suggested by Matsuda (Citation1970) was refuted by Kristensen (Citation1975). However, the idea was recently revived by Zompro. In connection with his work on Phasmatodea and Mantophasmatodea he dealt with the Polyneoptera in general (Zompro Citation2004, Citation2005) and recognised two clades among them. The first is the Orthopteromorpha (including Grylloblattodea, Dermaptera, Mantophasmatodea, Ensifera, Caelifera, and the cockroaches, termites, and mantids). The second polyneopteran clade, the Phasmatomorpha, takes its name from the Phasmatodea which are the species-rich sister group of the Plecopteriformia. The latter include the Plecoptera, and the Timematodea (with the single North American genus Timema, which other students regard as a phasmid; all of which, and only they, have paired prothoracic glands) plus the webspinners, Embioptera.
Zompro's proposal of a close relationship between Plecoptera and phasmids is based on several characters. He thinks that Plecoptera front wings are typical tegmina. Haas (Citation2006) also made a point of hardened plecopteran front wings but there is no clear definition when to call a wing a tegmen. To me, the plecopteran front wing is only little harder than the hind wing, as is normal in insects which superimpose their wings at rest, for example also Megaloptera and Trichoptera. In the latter groups, the difference between wings is much more pronounced than in Plecoptera. This character is of no use in the search for Plecopteran affinities.
Mate finding via abdominal drumming is, according to Zompro, also common to Phasmatodea and Plecopteriformia, at least among “basal taxa”. Details are not presented; I have no information about drumming in stick insects. In any case, vibrational communication is so widespread among insects that this agreement alone is insufficient to support the proposed relationship. At a conference a few years ago (Dresden, Germany, 2003; Klass Citation2003), U. Aspöck, specialist of Neuroptera and Raphidioptera, even questioned whether drumming can document the monophyly of Arctoperlarian stoneflies. Considering the related behavioural and structural details, it certainly does.
Zompro (Citation2004) believes the existence of an area apicalis on the tibia, the reduced male styli and egg structure support his Phasmatomorpha. I see no special area apicalis in any stonefly (). Zompro further emphasises the ability of stick insects and stoneflies to regenerate (often imperfectly) lost limbs during larval moults which to him also suggests a close relationship of Phasmatodea and Plecopteriformia. Characters supporting the latter are “femora and tibiae strongly depressed laterally” which is simply not the case in most Plecoptera, and the trimerous tarsi. Zompro's main point is an alleged close agreement in eggs between the orders of Phasmatomorpha. However, all he presented in support are some poor habitus photos of eggs to underline their similarity, especially the detachable operculum.
Phasmatodean eggs have very diverse, taxonomically useful shapes but the basic structure is uniform (). The hard-shelled eggs serve distribution; the flightless females often vigorously throw each as far as possible. A button-like top, the capitulum on the operculum, seems to increase the attractiveness of eggs to ants, which often pick eggs up and transport them. Down the egg perimeter is a micropylar plate, with a single micropyle – which is internally closed (Hinton Citation1981)!
Figure 3. Eggs of Plecoptera, Phasmatodea, and Embioptera. Top, from left: Perla, Timema, Embia, Agathemera; modified from Zompro (Citation2005). Arrowheads identify opercular pole; note that the Perla egg is upside down. Bottom, from left, eggs of Neoperla fallax Klapálek, 1910, with smooth chorion, easily seen micropyles, and visible opercular suture (modified from Zwick Citation1983), Extatosoma (Phasmatodea; from Key, 1991) and Embia (from Kaltenbach Citation1968), in morphologically similar orientation. m = micropyle. Not to scale.
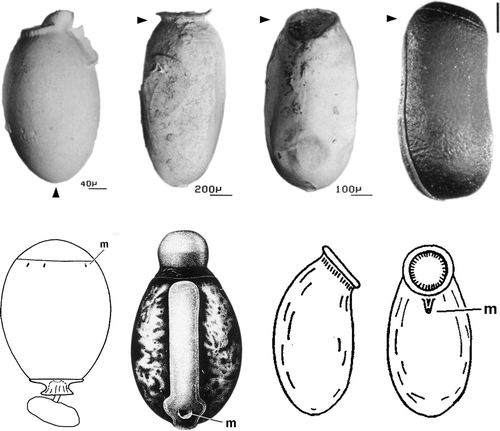
Eggs of Embioptera also have a single micropyle, directly below the operculum. Females lay eggs singly, attaching them to the bottom, side or roof of their retreat but always so that the operculum is directed away from the substratum. Females may carry the eggs around, arrange them in clusters, and tend and clean them. Some even provide the young larvae with food (Sattler Citation1968).
A note on the side regarding the development of Embioptera is in place. Literature contains a bizarre error regarding the number of larval instars, which would be only four! This is repeated in the famous Traité de Zoologie (Denis Citation1949), in Handbuch der Zoologie (Kaltenbach Citation1968) and in present-day textbooks (Klausnitzer Citation1996; Günther Citation2005). Günther (Citation2005) even states that neotenous specimens may be sexually mature in the second instar! Apparently, the wing-bearing stages described by Mills (Citation1932) have been mistaken for the total number of instars. Christa Sattler (Citation1968) reared many individuals singly, documenting their development. She confirms wing polymorphism and that sexual maturity can be attained by brachypterous specimens, but on average there are 10 instars in males, and 12 in females!
The similarity between Perla- and Phasmatoptera eggs which impressed Zompro results only from the upside-down position of the stonefly egg in his illustration. What reminded Zompro of the operculum plus capitulum of stick insect eggs are the plecopteran collar and anchor which, however, are at the opposite egg pole than the operculum. All stonefly eggs have several (internally open) micropyles. Egg structure certainly provides no support for the idea of a close relationship between stoneflies, webspinners, and stick insects.
This curious misconception underlines the urgent need to establish the ground pattern of Plecoptera eggs. Even eggs of Systellognatha are far from uniform, as was documented early (for example, Brinck Citation1949; Knight, Nebeker and Gaufin Citation1965a, Citationb) and has since been additionally illustrated by excellent SEM micrographs in many papers. The hard eggs of many Antarctoperlaria are of various shapes, details of structure are known only in a few but they never exhibit the traits of Systellognathan eggs.
Do hard-shelled eggs represent the ground pattern of stonefly eggs, or are the soft, structureless glutinous egg shells of Nemouroidea closer to it? E. Rosciszewska (Citation1996) discovered and illustrated the complex and characteristic chorion structure and micropyles of the nemourid egg which differs much from other Plecoptera. From such egg studies I expect additional proof of nemouroidean monophyly, and means of checking on the position of Scopura. Footnote1
Molecular data
Numerous recent studies of animal relationships are based entirely or in part on evidence from molecular data, also in insects. Conflict with opinions based on morphological evidence is not rare, as seen in Wheeler et al. (Citation2001) or Klass (Citation2003).
Terry (Citation2003) studied the Polyneoptera, with a focus on Plecoptera and Mantophasmatodea. He found no support for the monophyly of Polyneoptera and inferred “a relatively basal placement of Plecoptera” in the complex. None of the other molecular studies focused on the phylogenetic placement of Plecoptera, and the three major lineages within the order (Antarctoperlaria, Systellognatha, and Euholognatha) were rarely all sampled. Hypotheses on the placement of the order Plecoptera differ strongly between and occasionally within studies (e.g. Carapelli, Liò, Nardi, Wath and Frati Citation2007: Plecoptera + Diptera; Flook and Rowell Citation1998: ((Nemoura + Dermaptera) + Grylloblattodea), Hassanin Citation2006: Pteronarcys + several Diptera, Coleoptera, and Sternorrhyncha; Terry and Whiting Citation2005: Plecoptera + (Dermaptera + Zoraptera)), but share the lack of even moderately strong statistical support values. Conversely, congruence between seemingly independent studies that place Plecoptera as the sistergroup to Dermaptera or Dermaptera + Zoraptera (Flook and Rowell Citation1998; Kjer 2004; Terry and Whiting Citation2005; Misof et al. Citation2007) can most probably be attributed to a shared data basis, namely the ribosomal gene 18S and the repeated usage of identical sequences from Genbank as a replacement for own data.
Conclusion
I have no definite answer to the question I raised. There is more evidence suggesting a sistergroup relation between Plecoptera and all other Neoptera than recognised in recent reviews of the subject. At the same time, there is some evidence that Plecoptera may be sistergroup of the other Polyneoptera (whatever ultimately remains in them). However, there is no convincing support for suggested close relations of stoneflies with Embioptera or Phasmatodea. To resolve the present undecided situation many additional ground pattern features of Plecoptera need be established, and compared with other insects.
Published molecular data consist almost exclusively of ribosomal and mitochondrial DNA sequences and, similar to morphological data, have failed so far to provide robust phylogenetic hypotheses for the placement of Plecoptera. It remains to be seen if the use of multiple, protein-coding nuclear markers, careful data analysis and improved sampling across all major plecopteran lineages will yield strongly supported phylogenetic hypotheses.
Acknowledgements
I sincerely thank my son, Andreas Zwick, for help with the molecular studies and an anonymous reviewer for useful suggestions.
Notes
1. I regret to be unable to read Kishimoto (Citation1997).
References
- Béthoux , O. 2005 . ‘Wing venation pattern of Plecoptera (Insecta: Neoptera)’ . Illiesia , 1 : 52 – 81 .
- Beutel , R. G. and Gorb , S. N. 2006 . ‘A revised interpretation of the evolution of attachment structures in Hexapoda with special emphasis on Mantophasmatodea’ . Arthropod Systematics & Phylogeny , 64 : 3 – 25 .
- Bitsch , J. and Ramond , S. 1970 . ‘Étude du squélette et de la musculature prothoraciques d'Embia ramburi R.-K. (Insecta Embioptera). Comparaison avec la structure du prothorax des autres Polynéoptères et des Aptérygotes’ . Zoologisches Jahrbuch Anatomie , 87 : 63 – 93 .
- Brinck , P. 1949 . ‘Studies on Swedish stoneflies (Plecoptera)’ . Opuscula Entomologica , : 1 – 250 .
- Brinck , P. 1956 . ‘Reproductive system and mating in Plecoptera’ . Opuscula Entomologica , 21 : 57 – 127 .
- Burmeister , H. C.C. 1839 . Handbuch der Entomologie (Plecoptera: Vol. 2) , 863 – 881 . Berlin : T.C.F. Enslin .
- Carapelli , A. , Liò , P. , Nardi , F. , Wath , E. V.D. and Frati , F. 2007 . ‘Phylogenetic analysis of mitochondrial protein coding genes confirms the reciprocal paraphyly of Hexapoda and Crustacea’ . BMC Evolutionary Biology , 7 ( Suppl. l2 ) S8, 1–13
- Cranston , P. S. and Gullan , P. J. 2003 . “ ‘Phylogeny of insects’ ” . In Encyclopedia of Insects , Edited by: Resh , V. H. and Cardé , R. 882 – 898 . Amsterdam : Academic Press .
- Denis , R. 1949 . “ ‘Ordre des Embioptères’ ” . In Traité de Zoologie Anatomie, Systématique, Biologie , Edited by: Grassé , P.-P. Vol. 9 , 744 Paris : Masson et Cie .
- Flook , P. K. and Rowell , C. H.F. 1998 . ‘Inferences about orthopteroid phylogeny and molecular evolution from small subunit nuclear ribosomal DNA sequences’ . Insect Molecular Biology , 7 : 163 – 178 .
- Grimaldi , D. and Engel , M. S. 2005 . Evolution of the Insects , New York : Cambridge University Press .
- Günther , K. K. 2005 . “ ‘11. Ordnung Embioptera, Tarsenspinner, Spinnfüßer, Embien’ ” . In Lehrbuch der Speziellen Zoologie, Band I: Wirbellose Tiere, 5. Teil Insecta , Edited by: Dathe , H. H. Heidelberg , , Berlin : Spektrum Akademischer Verlag, 2nd ed . corrected new printing, pp. 167–172
- Haas , F. 2006 . ‘Evidence from Folding and Functional Lines of Wings on Inter-ordinal Relationships in Pterygota’ . Arthropod Systematics & Phylogeny , 64 : 149 – 158 .
- Haas , F. and Kukalová-Peck , J. 2001 . ‘Dermaptera hindwing structure and folding: New evidence for familial, ordinal and superordinal relationships within Neoptera (Insecta)’ . European Journal of Entomology , 98 : 445 – 509 .
- Hassanin , A. 2006 . ‘Phylogeny of Arthropoda inferred from mitochondrial sequences: Strategies for limiting the misleading effects of multiple changes in pattern and rates of substitution’ . Molecular Phylogenetics and Evolution , 38 : 100 – 116 .
- Hennig , W. 1969 . Die Stammesgeschichte der Insekten , 49 Frankfurt : Waldemar Kramer, Senckenberg-Bücher .
- Hinton , H. E. 1981 . “ ‘16. Plecoptera and Embioptera’ ” . In Biology of Insect Eggs , Vol. 2 , 508 – 512 . Oxford : Pergamon Press .
- Hoefnagel , G. 1592 . ‘Archetypa studiaque Patris Georgii Hoefnagelii Jacobus F: genio duce ab ipso scalpta omnibus philomusis amice D: ac perbenigne communicat’ Frankfurt/Main
- Holdsworth , R. P. 1942 . ‘The wing development of Pteronarcys proteus Newman (Pteronarcidae: Plecoptera)’ . Journal of Morphology , 70 : 431 – 462 .
- Kaltenbach , A. 1968 . ‘Embiodea (Spinnfüßer)’ . Handbuch der Zoologie , IV ( 22/8 ) : pp. 1 – 29 .
- Key , K. H.L. 1991 . “ ‘25. Phasmatodea (Stick-insects)’ ” . In The insects of Australia. A Textbook for Students and Research Workers , Edited by: CSIRO, Division of Entomology . 394 – 404 . Carlton : Melbourne University Press .
- Kishimoto , T. 1997 . ‘Egg envelopes of a stonefly Scopura montana Maruyama (Plecoptera, Scopuridae) . Proceedings of Arthropodan Embryological Society of Japan , 32 : 35 – 37 . (in Japanese)
- Kjer , K. M. , Carle , F. L. , Litman , J. and Ware , J. 2006 . ‘A molecular phylogeny of Hexapoda’ . Arthropod Systematics & Phylogeny , 64 : 35 – 44 .
- Klapálek , F. 1896 . ‘Über die Geschlechtstheile der Plecopteren, mit besonderer Rücksicht auf die Morphologie der Genitalanhänge’ . Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften Wien, Mathematisch-Naturwissenschaftliche Klasse , 105 : 683 – 738 . pls. I–V
- Klass , K. D. (ed.) . 2003 . ‘Proceedings of the 1st Dresden Meeting on Insect Phylogeny: “Phylogenetic Relationships within the Insect Orders” (Dresden, September 19–21, 2003)’ . Entomologische Anhandlungen , 61 : 110 – 172 .
- Klausnitzer , B. 1996 . “ ‘Insecta (Hexapoda), Insekten’ ” . In Spezielle Zoologie, Teil 1: Einzeller und Wirbellose Tiere , Edited by: Westheide , W. and Rieger , R. 601 – 681 . Stuttgart : Gustav Fischer Verlag .
- Knight , A. W. , Nebeker , A. V. and Gaufin , A. R. 1965a . ‘Description of the eggs of common Plecoptera of Western United States’ . Entomological News , 76 : 105 – 111 .
- Knight , A. W. , Nebeker , A. V. and Gaufin , A. R. 1965b . ‘Further descriptions of the eggs of Plecoptera of Western United States’ . Entomological News , 76 : 233 – 239 .
- Kristensen , N. P. 1975 . ‘The phylogeny of hexapod “orders”. A critical review of recent accounts’ . Zeitschrift für Zoologische Systematik und Evolutionsforschung , 13 : 1 – 44 .
- Kristensen , N. P. 1991 . “ ‘Phylogeny of extant hexapods’ ” . In The insects of Australia. A Textbook for Students and Research Workers , Edited by: CSIRO, Division of Entomology . 125 – 140 . Carlton : Melbourne University Press .
- Kukalová-Peck , J. 1978 . ‘Origin and evolution of insect wings and their relation to metamorphosis, as documented by the fossil record’ . Journal of Morphology , 156 : 53 – 126 .
- Kukalová-Peck , J. 1991 . “ ‘Fossil history and the evolution of hexapod structures’ ” . In The Insects of Australia. A Textbook for Students and Research Workers , Edited by: CSIRO, Division of Entomology . 141 – 179 . Carlton : Melbourne University Press .
- Kukalová-Peck , J. 2008 . ‘Phylogeny of Higher Taxa in Insecta: Finding Synapomorphies in the Extant Fauna and Separating them from Homoplasies’ . Evolutionary Biology , 35 : 4 – 51 .
- Matsuda , R. 1970 . ‘Morphology and evolution of the insect thorax’ . Memoirs of the Entomological Society of Canada , 76 : 1 – 431 .
- Mills , H. B. 1932 . ‘The life history and thoracic development of Oligotoma texana(Mel.) (Embidiina)’ . Annals of the Entomological Society of America , 25 : 648 – 652 .
- Misof , B. , Niehuis , O. , Bischoff , I. , Rickert , A. , Erpenbeck , D. and Staniczek , A. 2007 . ‘Towards an 18S phylogeny of hexapods: Accounting for group-specific character covariance in optimized mixed nucleotide/doublet models’ . Zoology , 110 : 409 – 429 .
- Nelson , C. H. 1991 . “ ‘Preliminary note on the comparative morphology of the stonefly pretarsus (Plecoptera)’ ” . In Overview and Strategies of Ephemeroptera and Plecoptera , Edited by: Alba-Tercr , J. and Sanchez-Ortega , A. 135 – 156 . Gainesville , Florida: : The Sandhill Crane Press Inc. .
- Nelson , C. H. ‘Surface ultrastructure and evolution of tarsal attachment structures in Plecoptera (Hexapoda: Arthropoda)’ . International Perspectives in Mayfly and Stonefly Research. Proceedings of the 12th International Conference on Ephemeroptera and the 16th International Symposium on Plecoptera, Stuttgart 2008 . Edited by: Staniczek , A. H. Aquatic Insects, 31, (Suppl. 1), 523–545
- Pass , G. , Gereben-Krenn , B.-A. , Merl , M. , Plant , J. , Szucsich , N. U. and Tögel , M. 2006 . ‘Phylogenetic relationships of the orders of Hexapoda: Contributions from the Circulatory Organs for a Morphological Data Matrix’ . Arthropod Systematics & Phylogeny , 64 : 165 – 203 .
- Pictet , F. J. 1841 . Histoire naturelle générale et particulière des insectes Névroptères. Famille des Perlides , Genève : Kessmann . Paris: J.-B- Baillière
- Rosciszewska , E. 1996 . ‘Egg capsule structure of the stonefly Protonemura intricata (Ris 1902) (Plecoptera: Nemouridae)’ . Acta Biologica Cracoviensa, Series Zoologica , 38 : 41 – 48 . plate 7
- Sattler , C. 1968 . ‘Untersuchungen zur Lebensweise, insbesondere zur Ethologie, neotropischer Embiopteren (Insecta)’ unpublished doctoral thesis, University of Giessen, Dept. of Zoology
- Sinichenkova , N. D. 1997 . “ ‘Paleontology of stoneflies’ ” . In Ephemeroptera & Plecoptera: Biology – Ecology-Systematics , Edited by: Landolt , P. and Sartori , M. 561 – 565 . Fribourg : Mauron + Tinguely & Lachat .
- Terry , M. D. 2003 . ‘Phylogeny of the Polyneopterous Insects With Emphasis on Plecoptera: Molecular and Morphological Evidence’ unpublished Ph.D. dissertation, Brigham Young University, Provo, Utah. http://contentdm.lib.byu.edu/ETD/image/etd376.pdf
- Terry , M. D. and Whiting , M. F. 2005 . ‘Mantophasmatodea and phylogeny of the lower neopterous insects’ . Cladistics , 21 : 240 – 257 .
- Townsend , G. D. and Pritchard , G. 1998 . ‘Larval growth and development of the stonefly Pteronarcys californica (Insecta: Plecoptera) in the Crowsnest River, Alberta’ . Canadian Journal of Zoology , 76 : 2274 – 2280 .
- Wheeler , W. C. , Whiting , M. , Wheeler , Q. D. and Carpenter , J. C. 2001 . ‘The phylogeny of the extant hexapod orders’ . Cladistics , 17 : 113 – 169 .
- Wichard , W. 1997 . ‘Insekten erobern den aquatischen Lebensraum’ . Verhandlungen des Westdeutschen Entomologentags , 1996 : 19 – 28 .
- Wichard , W. and Eisenbeis , B. 1979 . ‘Fine structural and histochemical evidence of chloride cells in stonefly larvae 3. The floriform chloride cells’ . Aquatic Insects , 1 : 185 – 191 .
- Wichard , W. and Komnick , H. 1971 . ‘Electron microscopical and histochemical evidence of chloride cells in tracheal gills of mayfly larvae’ . Cytobiologie , 3 : 215 – 228 .
- Wichard , W. and Komnick , H. 1973a . ‘Feinstruktur der Chloridzellen von Eintagsfliegenlarven’ . Verhandlungen der Deutschen Zoologischen Gesellschaft , 66 Jahresversammlung, 80–83
- Wichard , W. and Komnick , H. 1973b . ‘Feinstruktureller und histochemischer Nachweis von Chloridzellen bei Steinfliegenlarven. 1. Die coniformen Chloridzellen’ . Cytobiologie , 7 : 297 – 314 .
- Wichard , W. and Komnick , H. 1974 . ‘Feinstruktureller und histochemischer Nachweis von Chloridzellen bei Steinfliegenlarven. 2. Die caviformen und bulbiformen Chloridzellen’ . Cytobiologie , 8 : 297 – 311 .
- Wichard , W. , Komnick , H. and Abel , J. J. Jr . 1972 . ‘Typology of ephemerid chloride cells’ . Zeitschrift für Zellforschung , 132 : 533 – 551 .
- Willmann , R. 2003 . ‘Die phylogenetischen Beziehungen der Insecta: Offene Fragen und Probleme’ . Verhandlungen des Westdeutschen Entomologentags , 2001 : 1 – 64 .
- Willmann , R. 2005 . “ ‘Phylogenese und das System der Insekten’ ” . In Lehrbuch der Speziellen Zoologie, Band I: Wirbellose Tiere, 5. Teil Insecta , 2nd ed. , Edited by: Dathe , H. H. Heidelberg , , Berlin : Spektrum Akademischer Verlag . corrected new printing, pp. 1–65
- Zompro , O. 2004 . ‘Revision of the genera of the Areolatae, including the status of Timema and Agathemera (Insecta Phasmatodea)’ . Abhandlungen Naturwissenschaftlicher Verein in Hamburg (NF) , 37 : 1 – 327 .
- Zompro , O. 2005 . ‘Inter- and intra-ordinal relationships of the Mantophasmatodea, with comments on the phylogeny of the polyneopteran orders (Insecta: Polyneoptera)’ . Mitteilungen des Geologisch-Paläontologischen Instituts der Universität Hamburg , 89 : 85 – 116 .
- Zwick , P. 1973 . ‘Insecta Plecoptera. Phylogenetisches System und Katalog’ . Das Tierreich , 94 : I – XXXII . 1–465
- Zwick , P. 1980 . ‘Plecoptera (Steinfliegen)’ . Handbuch der Zoologie , 4 ( 22/7 ) : 1 – 115 .
- Zwick , P. 1983 . ‘The Neoperla of Sumatra and Java (Indonesia) (Plecoptera: Perlidae)’ . Spixiana , 6 : 167 – 204 .
- Zwick , P. 1991 . “ ‘Biometric studies of the growth and development of two species of Leuctra and of Nemurella pictetii (Plecoptera: Leuctridae and Nemouridae)’ ” . In Overview and Strategies of Ephemeroptera and Plecoptera , Edited by: Alba-Tercr , J. and Sanchez-Ortega , A. 515 – 526 . Gainesville , Florida : The Sandhill Crane Press Inc. .
- Zwick , P. 2000 . ‘Phylogenetic system and zoogeography of the Plecoptera’ . Annual Review of Entomology , 45 : 709 – 746 .
- Zwick , P. and Hohmann , M. 2003 . ‘Direct development, no diapause, in Taeniopteryx nebulosa (Plecoptera, Taeniopterygidae)’ . Lauterbornia , 47 : 141 – 151 .
- Zwick , P. and Teslenko , V. A. 2002 . ‘Development and life history of Far Eastern Russian Pteronarcys spp. (Plecoptera, Pteronarcyidae)’ . Archiv für Hydrobiologie , 153 : 503 – 528 .