Abstract
The Ephemeroptera are usually regarded as the sister group of the remaining Pterygota. Their wing base sclerites and pterothoracic musculature are compared with that of other basal pterygote lineages. It is shown that most elements of the neopteran wing base are also present in Ephemeroptera and Odonata. The wing base in the ground plan of Pterygota is presumably composed of three axillaries and a proximal median plate. The first axillary is provided with two muscles. The third axillary is equipped with one short muscle in the ground plan of Pterygota. A second muscle, which inserts at the third axillary and originates from the episternum, is most likely an autapomorphic character of Neoptera. The results imply that the wing base of Plecoptera is close to the pterygote ground plan. It is assumed that the wing bases of Ephemeroptera and Odonata are secondarily stiffened. The so-called basalare and its associated muscles in Ephemeroptera and Odonata are probably not homologous to the basalare and respective muscles in Neoptera. Though the wing bases of both Ephemeroptera and Odonata show similar modifications their specialisations may have evolved independently from each other.
Introduction
The development of wings in insects was one key character for the evolutionary success of this diverse group. It opened up new ecological niches and increased the efficiency of dispersal. Other advantages of the ability to fly were certainly better chances to escape from predators and to detect new food resources.
To date there is no general agreement on the derivation of wings and the evolution of wing base sclerites. It is often assumed that insect wings are leg derivates (Kukalová-Peck Citation1983), or derived from lateral tergal expansions, the paranota (Hamilton Citation1971, Citation1972a,Citationb,Citationc), or rather originate from both tergites and pleurites (Snodgrass Citation1935).
The number and homology of axillary plates in basal Pterygota has always been controversial. The hypothesis that assumes only one axillary plate in Ephemeroptera, two axillary plates in Odonata, and three axillary plates in Neoptera, representing the apomorphic character state in the latter group (Hamilton Citation1971) is only rarely maintained nowadays (Gullan and Cranston Citation2005). Grandi (Citation1947) even assumed that the wing base sclerites of Ephemeroptera are ‘pseudopteralia’ without homology to the wing base sclerites of other Pterygota. Most authors today agree on a homology between the axillaries of at least Ephemeroptera and Neoptera. However, it is not clear which element of the mayfly wing base is the homologue to which of the wing base elements of Neoptera (e.g. Brodsky Citation1970, Citation1974, Citation1994; Matsuda Citation1956; Tsui and Peters Citation1972). According to Kukalová-Peck (Citation1983, Citation1987, Citation1991), the wing base in the pterygote ground plan consists of 32 sclerites, which are secondarily fused in different ways in extant Pterygota. Boudreaux (Citation1979) also suggested that the sclerites of the wing base in Ephemeroptera are probably a result of a secondary fusion correlated with weak flight abilities. The paleopterous wing resting position was assumed to be a primitive character by Boudreaux (Citation1979). In contrast, Brodsky (Citation1994), Kukalová-Peck (Citation1991), Rasnitsyn (Citation2002) and Willmann (Citation1998) assumed that Odonata and Ephemeroptera secondarily lost the ability to fold their wings horizontally over the abdomen.
The basalare and subalare are assumed to be of pleural origin. Most authors have no doubt that the basalare and the subalare of Ephemeroptera are the homologue of the respective sclerites in Neoptera (Matsuda Citation1970; Brodsky Citation1974; Hasenfuß Citation2008).
According to these different opinions on the derivation of wings and wing base morphology, the phylogenetic relationships among the three basal clades of Pterygota are likewise controversially discussed (e.g. Börner Citation1909; Martynov Citation1925; Lemche Citation1940; Schwanwitsch Citation1943; Hennig Citation1953; Kristensen Citation1975, Citation1981, Citation1991; Fürst von Lieven Citation2000; Gorb et al. Citation2000; Staniczek Citation2000, Citation2001; Bechly et al. Citation2001; Hovmöller et al. Citation2002; Ogden and Whiting Citation2003, Citation2005). Recently, Willkommen (Citation2008) presented a homology of wing base sclerites and flight muscles based on morphological investigations.
Materials, methods and abbreviations
Material examined
Ephemeroptera (imagines): Siphlonurus aestivalis (Eaton, 1903); Baetis fuscatus (Linnaeus, 1761), Centroptilum luteolum (Müller, 1776); Cloeon dipterum (Linnaeus, 1761); Habroleptoides confusa Sartori & Jacob, 1986; Habrophlebia lauta Eaton, 1884; Paraleptophlebia submarginata (Stevens, 1836); Ecdyonurus submontanus Landa, 1969; Ecdyonurus venosus (Fabricius, 1775); Epeorus assimilis Eaton, 1885; Heptagenia coerulans (Rostock, 1878); Heptagenia sulphurea (Müller, 1776); Rhithrogena semicolorata (Curtis, 1834); Serratella ignita (Poda, 1761); Ephemera danica Müller, 1764; Caenis horaria (Linnaeus, 1758); Caenis rivulorum Eaton, 1884; Ephoron virgo (Olivier, 1791); Exeuthyplocia minima (Ulmer, 1916).
Ephemeroptera (larvae): Baetis sp.
Plecoptera (imagines): Pteronarcys reticulata Burmeister, 1839; Isoperla grammatica Poda, 1761; Isoperla goertzi Illies, 1952; Perlodes microcephalus Pictet, 1833; Capnia vidua Klapálek, 1904; Brachyptera seticornis (Klapálek, 1902).
Plecoptera (larvae): Brachyptera seticornis (Klapálek, 1902).
Odonata (imagines): Sympetrum cf. striolatum Müller, 1764.
Most specimens were preserved in 70–80% ethanol and subsequently dissected under a Leica MZ16 stereomicroscope. Drawings were made by using a drawing tube on a Leica MZ16 stereomicroscope. Photographs were made with a Nikon Coolpix camera.
The terminology of muscles primarily follows Matsuda (Citation1970). The terminology of sclerites of Ephemeroptera generally follows Kluge (Citation1994, Citation2004) except for the wing base sclerites that are termed axillaria (1Ax, 2Ax, 3Ax) herein. Roman numbers indicate mesothoracic (II) or metathoracic (III) elements. Unless otherwise noted, the head is directed to the top in specimens figure in dorsal view, and to the left in specimens figure in lateral view.
Abbreviations
| ANP | = |
anterior notal wing process |
| ASA | = |
anterior subalar apodeme |
| Ax | = |
axillary sclerite (1Ax, 2Ax, 3Ax = first, second, third axillary sclerite) |
| A1 | = |
anterior anal vein |
| BA | = |
basalare |
| BA.Cm | = |
basalar-coxal muscle (sensu Kluge Citation1994, Citation2004) |
| BSc | = |
basisubcostale |
| BA.SmI | = |
basalar-sternal muscle inferior (sensu Kluge Citation1994, Citation2004) |
| BA.SmS | = |
basalar-sternal muscle superior (sensu Kluge Citation1994, Citation2004) |
| BA.Trm | = |
basalar-trochanteral muscle (sensu Kluge Citation1994, Citation2004) |
| C | = |
costal vein |
| Cx | = |
coxa |
| fwp | = |
forewing pad |
| hwp | = |
hind wing pad |
| LPs | = |
lateroparapsidal suture |
| MA | = |
anterior medial vein |
| p | = |
pleural muscle |
| PMP | = |
proximal median plate |
| PNP | = |
posterior notal wing process |
| PSA | = |
posterior subalar apodeme |
| PWP | = |
pleural wing process |
| p-s | = |
pleurosternal muscle |
| p-ti (cx) | = |
pleurotrochantinal (coxal) muscle |
| p-tr | = |
trochanteral muscle |
| R | = |
(anterior) radial vein |
| Rs | = |
radial sector |
| SA | = |
subalare |
| SA.Cm | = |
subalar-coxal muscle |
| Sc | = |
subcostal vein |
| t-cx | = |
tergocoxal muscle |
| t-p | = |
tergopleural muscle |
| t-s | = |
tergosternal muscle |
Results and discussion
Axillary sclerites
The wing base of Ephemeroptera () is composed of elements which are homologous with the first, second and third axillary (1Ax, 2Ax and 3Ax; ) of Neoptera (Willkommen Citation2008). The basal plate of the wing base of Ephemeroptera is most likely the homologue of the neopteran proximal median plate (PMP; ) (Willkommen Citation2008).
Figure 1. Homology of the axillary sclerites of Ephemeroptera and Neoptera (modified after Willkommen Citation2008). (a) Habroleptoides confusa (Ephemeroptera), right forewing. (b) Perlodes microcephalus (Plecoptera), right hind wing.
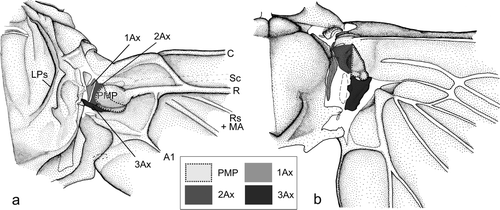
The 1Ax of Neoptera () is sclerotised only in the dorsal layer of the wing membrane. It articulates with 2Ax distally and is connected to the subcostal vein (Snodgrass Citation1935). In the examined Plecoptera there is a muscle attached to the 1Ax, which runs to the pleural ridge (t-p 11; ). This muscle is even present in Capnia vidua, a brachypterous stonefly. A muscle running from the 1Ax (homology after Willkommen Citation2008) to the pleural ridge is also present in Siphlonurus aestivalis (t-p 11 in ), Siphlonurus columbianus McDunnough, 1925 (see Matsuda Citation1956), and other basal Ephemeroptera (Parameletus chelifer Bengtsson, 1908; Ametropus fragilis Albarda, 1878; Metretopus norvegicus (Eaton, 1871)) (see Brodsky Citation1974). In most Ephemeroptera there is a second muscle, which extends between 1Ax and the furca. According to Matsuda (Citation1970), this muscle is a plesiomorphic character in Pterygota and homologous with muscle 45 of Lepisma sp. (Zygentoma). Among adult Pterygota it has been retained only in Ephemeroptera (Matsuda Citation1970).
Figure 2a–f. Direct flight muscles of the mesothorax of Ephemeroptera (Siphlonurus aestivalis). Muscle homology after Willkommen Citation2008 (modified after Willkommen Citation2008).
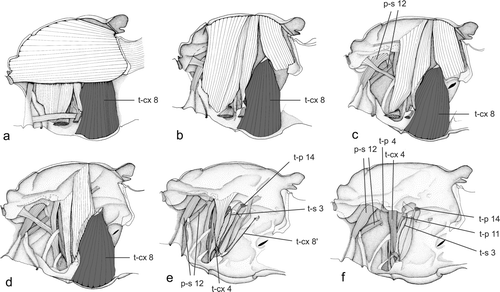
Figure 3a–e. Direct flight muscles of the synthorax of Odonata (Sympetrum cf. striolatum). Muscle homology after Willkommen Citation2008 (modified after Willkommen Citation2008).
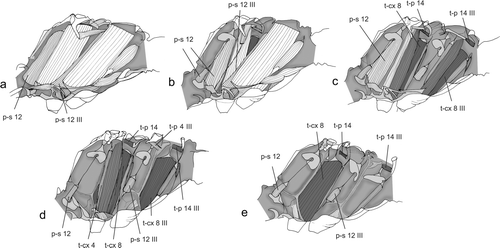
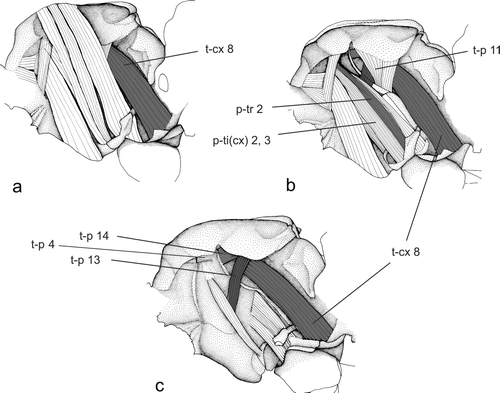
Figure 4a–c. Direct flight muscles of the mesothorax of Plecoptera (Brachyptera seticornis). (modified after Willkommen Citation2008).
The 2Ax of Neoptera is sclerotised in both the dorsal and ventral layer of the wing membrane. It is connected to the 3Ax, the PMP, and the anterior radial vein. On its ventral side the 2Ax articulates with the pleural wing process (PWP) (Snodgrass Citation1935). A convex axillary flexion line is located proximally to the 2Ax (Wootton Citation1979). In the ground plan of Neoptera there is no muscle is attached to the 2Ax. The 2Ax of Ephemeroptera shows all the above mentioned characters, but it is additionally fused with PMP.
The 3Ax is sclerotised in both the dorsal and ventral layer of the wing membrane. It is connected to the 2Ax, PMP, anal veins, and to the posterior notal wing process (PNP) (Snodgrass Citation1935). At least one muscle is attached to the 3Ax. In Plecoptera there are two muscles attached to the 3Ax. The first muscle (t-p 14; ) is ventrally attached to the pleuron posterior to the PWP. The second muscle (t-p 13; ) is attached to the episternum anterior to the pleural ridge.
The 3Ax of nearly all examined Ephemeroptera is tightly connected to the PMP. In E. danica for example there is a suture between those elements. There is one muscle (t-p 14, , ) between the 3Ax and the pleuron posterior to the PWP in all examined Ephemeroptera.
Most elements of the wing base of Neoptera are present in the ground plan of Pterygota, namely 1Ax, 2Ax, 3Ax, and PMP. Contrary to previous hypotheses (Snodgrass Citation1935), two muscles are attached to 1Ax in the ground plan of Pterygota – the axillar-pleural muscle (t-p 11; , ) and the axillar-furcal muscle (t-s 3; , ). Among adult Pterygota, the axillar-furcal muscle has been retained only in Ephemeroptera. It is possible that the reduction of the axillar-furcal muscle is an autapomorphic character of Metapterygota (Odonata + Neoptera). It cannot be entirely ruled out that the reduction of the axillar-furcal muscle has happened convergently in Odonata and Neoptera; the Odonata are adapted to a manoeuvrable hunting flight.
Most likely only a single muscle between the 3Ax and the upper part of the pleuron is present in the pterygote ground plan (t-p 14).
On the basis of their different flight adaptation it is more likely that the wing bases of Ephemeroptera (2Ax and PMP fused; ) and Odonata (2Ax, PMP and 3Ax fused) are secondarily fused and do not represent the ancestral condition.
The enigmatic question whether the ability to fold the wings horizontally over the abdomen is a ground plan character of Pterygota or rather represents an apomorphic character of Neoptera is still unresolved. The differently specialised flight apparatuses of the two basal lineages of Pterygota (Ephemeroptera and Odonata) together with their different flight behaviour (Ephemeroptera: specialised mating flight; Odonata: adapted to a manoeuvrable hunting flight) suggest that these taxa are more advanced from the groundplan of Pterygota than previously assumed. The inability to fold the wings horizontally over the abdomen could be a result of secondary stiffening in consequence of a partial fusion of the wing base sclerites. The Plecoptera, a basal lineage within the Neoptera, are most likely very close to the pterygote ground plan regarding many aspects of their flight system (Willkommen and Hörnschemeyer Citation2007; Willkommen Citation2008). Other than in Ephemeroptera and Odonata, two muscles are attached to the 3Ax in Plecoptera (t-p 13, t-p 14; ). The first muscle (t-p 14) extends between the 3Ax and the pleuron next to the PWP. This muscle is also present in Ephemeroptera and Odonata (t-p 14; , , ). The second muscle, however, extends between the 3Ax and the episternum (t-p13). This muscle is not present in Ephemeroptera and Odonata. It is either present in the groundplan of Pterygota and reduced in Ephemeroptera and Odonata, which may be associated with the flight adaptations in these groups, or this muscle is a new character of the Neoptera. In the outgroup Zygentoma there are several tergo-pleural muscles present (Barlet Citation1953, Citation1954). According to Matsuda (Citation1970), t-p 13 is a new formation of the Pterygota, but Matsuda gives no reasoning for this assumption. It would need a thorough re-examination of the thoracic muscles in primitive Zygentoma to assess this character.
In any case there is not only one muscle attached to the 3Ax in the ground plan of Neoptera as previously assumed, but there is a second muscle (t-p 13) present, extending between the 3Ax and the episternum. The presence of t-p 13 in Neoptera could be the key-character for the ability to fold the wings horizontally over the abdomen.
Pleural wing base sclerites
There are two pleural wing base sclerites present in Pterygota. The basalare (BA) is located anteriorly to the PWP. It articulates with the basisubcostale (BSc) (Brodsky Citation1994). The basalare is assumed to be a pleural element (Snodgrass Citation1935; Matsuda Citation1970; Hasenfuß Citation2008). The subalare is located posterior to the pleural wing process. Usually it is also supposed that the subalare is derived from the pleuron (Snodgrass Citation1935; Hasenfuß Citation2008), but according to Matsuda (Citation1970), the subalar-coxal muscle is termed t-cx 8 (tergo-coxal muscle), which would imply a tergal origin of the subalare. This terminology was used by Matsuda (Citation1970), because Maki (Citation1938) has shown that this muscle is attached to the lateral part of the tergum in larvae of Leucophaea sp. (Blattaria).
Basalare
The basalare is not detached from the episternum in Plecoptera. It articulates anteriorly with the basicostale and posteriorly with the basisubcostale. There are two muscles attached to the basalare. The first muscle is ventrally attached to the coxa, and the second one to the trochanter (Willkommen Citation2008). There is no generally accepted interpretation of the basalare and its associated muscles in the Ephemeroptera ().
Table 1. Different hypotheses on the presence (+), absence (−), or different interpretation (1Ax) of basalar muscles in Ephemeroptera. For explanations of “*” and “**” see text.
According to Knox (Citation1935) there are five muscles attached to the basalare in Ephemeroptera: Pm1 and Pm2, which is the paired muscle p-s 12 sensu Matsuda (Citation1970); Pm3, a basalar-trochanteral muscle; Pm4, which is t-cx 4 sensu Matsuda (Citation1970) and Pm5, which is p 5 sensu Matsuda (Citation1970).
Matsuda (Citation1956) mentions only three muscles attached to the basalare of Ephemeroptera (p 5 and the paired p-s 12), but Matsuda (Citation1970) also suspects that p 5 could be t-p 4 (a muscle extending between the laterophragma or the subtegula and the pleural ridge). Matsuda (Citation1970) also mentions a basalar-trochanteral muscle (p-tr 2), which is also listed as an axillar-trochanteral muscle (t-tr 2, see above and ).
Kluge (Citation1994) lists five muscles that are attached to the basalare: BA.Cm – t-cx 4 sensu Matsuda Citation1970; BA.Pm – p 5 sensu Matsuda Citation1970; BA.SmI and BA.SmS – the paired p-s 12 sensu Matsuda Citation1970; BA.Trm.
According to Tsui and Peters (Citation1972) four muscles are attached to the basalare: II-8, II-9, which are the paired p-s12 sensu Matsuda Citation1970; II10, which is p 5 sensu Matsuda Citation1970; II-12a.
Brodsky (Citation1974) specifies five muscles attached to the basalare: TTrm2; TCxm5, which is t-cx 4 sensu Matsuda Citation1970; TSm1 and TSm2, which is the paired p-s 12 sensu Matsuda Citation1970; TPm3, which is p 5 sensu Matsuda Citation1970. However, in a compilation (Brodsky Citation1974, , 2) Brodsky lists TTrm2 as absent in all examined Ephemeroptera. Matsuda (Citation1970) also contradicted himself in stating that ‘t-cx 4 is attached to the 1Ax in Ephemeroptera’ (Matsuda Citation1970, p. 120, 125), but also mentioning that ‘t-cx 4 is attached to 2Ax in Ephemeroptera, but to 1Ax in Ecdyonurus’ (Matsuda Citation1970, p. 66) (see * in ). Matsuda also interpreted the basalar-trochanteral muscle differently in Ephemeroptera. It is either attached to the basalare and named p-tr 2 (Matsuda Citation1970: 76, 124), or attached to the 1Ax and named t-tr 2 (Matsuda Citation1970, p. 75, 120) (see ** in ).
All previous authors agree that the muscles p-s 12 and p 5 are attached to the basalare in Ephemeroptera (Knox Citation1935; Matsuda Citation1956, Citation1970; Tsui and Peters Citation1972; Brodsky Citation1974; Kluge Citation1994). Some authors assume that t-cx 4 is an axillary muscle (Matsuda Citation1956, Citation1970; Tsui and Peters Citation1972) or a basalar-coxal muscle (Kluge Citation2004). This leads to the question how the basalare is really defined and how many muscles are attached to it in Ephemeroptera.
Most authors have regarded the crescent-shaped sclerite at the anterior wing base of Ephemeroptera as basalare (BA; ). The morphological study by Willkommen (Citation2008) however revealed that only two of the above mentioned five muscles are attached to this sclerite. In Siphlonurus aestivalis, the first muscle is dorsally attached to the upper part of the crescent-shaped sclerite and runs to the profurca. The second muscle is stretched between the ventral part of the crescent-shaped sclerite and the presternite (Willkommen Citation2008). Both muscles correspond to p-s 12 sensu Matsuda (Citation1970) who regarded these two muscles as a unit. The third muscle (p 5), which is generally interpreted as a basalar muscle in Ephemeroptera (Knox Citation1935; Matsuda Citation1956, Citation1970; Tsui and Peters Citation1972; Brodsky Citation1974; Kluge Citation1994), is attached to a separate sclerite. Matsuda (Citation1970) mentioned that p 5 could be t-p 4. The muscle p5 is attached to a sclerite located at the anterior base of the wing, in between tegula and tergum, and posterior to the posterior arc of the prealar bridge. This short muscle is also present in Odonata, where it is assumed to be attached to the 1Ax (Tannert Citation1958, t-p 11 sensu Matsuda Citation1970). I assume muscle t-p11 of Odonata (sensu Matsuda Citation1970) and p 5 of Ephemeroptera to be homologues. These muscles are not basalar or axillar muscles, but rather one and the same muscle (t-p 4 sensu Matsuda Citation1970) running from the subtegula (or the prescutum in Odonata) to the upper part of the pleuron (Willkommen Citation2008).
Figure 5. Mesopleuron of a male imago of Siphlonurus aestivalis. BA – basalare of Ephemeroptera sensu Kluge Citation2004 (grey areas sclerotised; modified after Willkommen Citation2008).
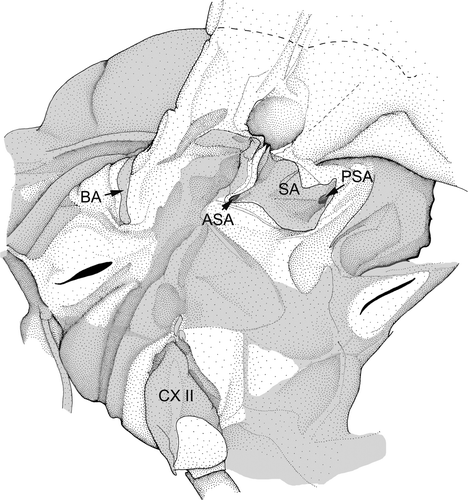
The muscle t-cx 4 is either assumed to be a basalar muscle (Brodsky Citation1974; Knox Citation1935; Kluge Citation1994, Citation2004) or an axillar muscle (Matsuda Citation1956, Citation1970; Tsui and Peters Citation1972). This muscle is attached to a sclerite lying anteriorly to ANP. It is located posterior to t-p 4, which is not a genuine basalar muscle (see above).
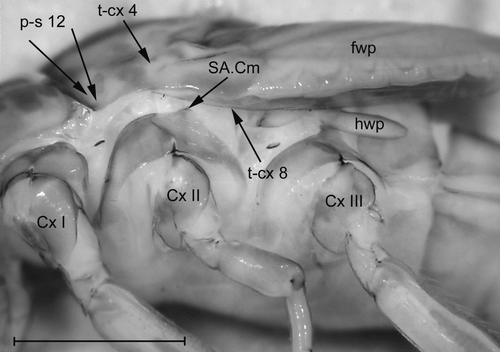
In mayfly larvae, both p-s 12 and t-cx 4 are attached to the tergum (), whereas in larvae of Plecoptera the basalar-coxal muscle is attached to the anterior part of the pleural sclerite. The muscles p-s 12 of Ephemeroptera are most likely homologous with two muscles present in Odonata (21, 22 sensu Asahina Citation1954; p-s 12; ), which are stretched between the anterolateral part of the humeral plate and the pre-episternal apodeme (Willkommen Citation2008).
Figure 6. Mesopleuron and metapleuron of a late larva of Baetis sp., the arrows show the attachments of the basalar and subalar muscles. SA.Cm – subalar-coxal muscle of Ephemeroptera (terminology sensu Kluge Citation2004); fwp – forewing pad; hwp – hind wing pad. Scale: 1 mm (modified after Willkommen Citation2008).
Consequently, the crescent-shaped sclerite at the anterior wing base of Ephemeroptera is most likely homologous to a part of the humeral plate of Odonata. In contrast to Hasenfuß (Citation2008) I assume that these sclerites of Ephemeroptera and Odonata are most likely not homologous with the basalare of Neoptera (Willkommen Citation2008). Likewise, Kukalová-Peck et al. (Citation2009) assume that the dorsal part of the basalar sclerite of Ephemeroptera (sensu Knox Citation1935) is homologous to the anterior plate of the wing base of Odonata. The basalare sensu Knox (Citation1935) is however composed of different sclerites, namely the crescent-shaped sclerite, and two tergal sclerites. The morphological studies by Willkommen (Citation2008) reveal that only the crescent-shaped sclerite is the homologue to a part of the anterior plate of the wing base of Odonata. I assume the anterior tergal sclerite to be the homologue of the subtegula of Neoptera. In the three basal pterygote lineages there is a tergopleural muscle attached to it (t-p 4) (see above). The posterior tergal sclerite is located anteriorly to ANP and posteriorly to the last mentioned sclerite. The muscle t-cx 4 is attached to this sclerite.
Hasenfuß (Citation2008) homologised the basalare of Neoptera with a detached part of the anteriormost of three subcoxal sclerites of Lepisma saccharina Linnaeus, 1758 (Iv sensu Hasenfuß Citation2008). He assumes the homology of trochantinus and pleuron (including basalare) in Lepismatidae (Zygentoma) and Neoptera. Hasenfuß consequently assumes the presence of the trochantinus and basalare in the groundplan of Dicondylia. However, in my point of view the status of the trochantinus and also the presence of the basalare (and the basalar-trochanteral muscle) in Ephemeroptera and Odonata is not confirmed. Furthermore, it is not known if the sclerite Iv is also present in Tricholepidion gertschi Wygodzinsky, 1961, which is generally regarded as the basal clade within Zygentoma or even Dicondylia (Bitsch and Bitsch Citation2000; Staniczek Citation2000). Barlet (Citation1980) in his investigation of the thorax of T. gertschi does not recognise a detached part of the anteriormost of three subcoxal sclerites in this species. To clarify the origin and homology of the trochantinus and the basalare (with the associated muscles) it is necessary to re-examine the plesiomorphic T. gertschi in this respect.
Subalare
According to Knox (Citation1935), Kluge (Citation1994) and Matsuda (Citation1956, Citation1970), three muscles are attached to the subalare of Ephemeroptera – a subalar-coxal muscle (t-cx 8 sensu Matsuda Citation1970), a subalar-sternal muscle (t-s 5 sensu Matsuda Citation1970), and a subalar-furcal muscle (t-s 4 sensu Matsuda Citation1970). Matsuda mentioned that t-s 5 could be a derivative of t-cx 8, but without reasoning. According to Brodsky (Citation1974) four muscles are attached to the subalare of Ephemeroptera because he classified the bipartite subalar-coxal muscle as two separate muscles. Tsui and Peters (Citation1972) also classified the subalar-coxal muscle as two separate muscles, but according to Tsui and Peters (Citation1972) another muscle which is attached to the posterior part of the median plate originates from the subalare in Ephemeroptera. The last mentioned muscle is in fact t-p 14. It is stretched between the 3Ax and the upper part of the pleuron in Ephemeroptera, Odonata and Neoptera. Most authors have no doubt that the subalar-coxal muscle of Ephemeroptera is homologous to the subalar-coxal muscle of Neoptera (Matsuda Citation1970, Brodsky Citation1994). The homology of the subalar-sternal muscle and the subalar-furcal muscle is, however, controversial.
In larvae of Plecoptera the future subalare (SA) develops from the membrane above the pleural sclerite, and it is also located there in the imago. A large subalar-coxal muscle (t-cx 8; ) is attached to the subalare of Plecoptera. In the larvae of Ephemeroptera, the subalar-coxal muscle is attached to the upper part of the pleural sclerite (SA.Cm; ). The subalar-sternal muscle of Ephemeroptera (t-cx 8; ) shows the same development as the subalar-coxal muscle in Plecoptera. In the winged stages of Ephemeroptera the subalar muscles are attached to a large sclerite, previously termed subalare, which is located in the membrane above the pleural sclerite (SA; ). Most likely this sclerite in alate mayflies is a product of a fusion of two sclerites. The subalar-sternal muscle is attached to one of these sclerites. The other sclerite represents the anterior part of the larval pleuron to which the subalar-coxal muscle is attached. So only the posterior part of the subalare of the winged stages of Ephemeroptera is the homologue of the subalare of Plecoptera. The subalar-sternal muscle of Ephemeroptera (t-cx 8; ) is homologous with the subalar-coxal muscle of Neoptera, but the subalar-coxal muscle of Ephemeroptera (SA.Cm) is most likely not homologous to the subalar-coxal muscle of Neoptera. The subalar-furcal muscle of Ephemeroptera (t-cx8´; ) is attached to the posterior subalar apodeme (PSA; Figure. 5). Its origin is the same as the origin of the subalar-sternal muscle in mayfly larvae. This indicates that the subalar-furcal muscle could be a derivate of the subalar-sternal muscle of Ephemeroptera, and both muscles together are most likely homologous with the subalar-coxal muscle of Neoptera.
The position of the ventral attachment of the subalar-sternal muscle in the groundplan of Pterygota is not clear. In Ephemeroptera, this muscle is attached to the furcasternum, while in Odonata and Neoptera it is attached to the posterior coxal rim. According to Matsuda t-cx 8 is probably homologous to a tergo-coxal muscle of Lepisma saccharina. Brodsky (Citation1974) assumed that the sternal attachment of the subalar-sternal muscle of Ephemeroptera represented a specialisation of the mayfly wing apparatus. Indeed, the subalar-sternal muscle, the subalare, and the furcasternite of Ephemeroptera are obviously enlarged. Correlated with these specialisations it is likely that the ventral attachment of the pterygote subalar-coxal muscle has shifted to the furcasternum in Ephemeroptera. From this, it is concluded that the loss of the subalar-sternal muscle can no longer be considered an autapomorphy of Metapterygota as a homologue of this muscle is present in both Odonata and Neoptera (Willkommen Citation2008).
Acknowledgements
I would like to thank PD Dr T. Hörnschemeyer, Prof. Dr R. Willmann (Göttingen) and Dr A. Staniczek (Stuttgart) for their general support, fruitful discussions, and encouragement during the time of my doctoral thesis. I am very indebted to A. Staniczek for his thorough editing of the first version of this paper. Thanks also to Dr G. Bechly (Stuttgart) for valuable comments and discussions. Sincere thanks to Prof. Dr P. Zwick (Schlitz), Dr A. Staniczek (Stuttgart), Dr J.-L. Gattolliat (Lausanne), Dipl. Geol. J. Brinkmann (Tübingen), Dipl. Biol. F. Wieland, Dr C. Richter, PD Dr T. Hörnschemeyer (all Göttingen), and Dr M. Ohl (Berlin) for the loan and donation of specimens. This work was supported by a grant to PD Dr T. Hörnschemeyer from the Deutsche Forschungsgemeinschaft (DFG HO 2306/2–1, 2–3).
References
- Asahina , S. 1954 . “ ‘A morphological study of a relic dragonfly Epiophlebia superstes Selys (Odonata, Anisozygoptera)’ ” . 1 – 153 . Tokyo : Japan Society for the Promotion of Science .
- Barlet , J. 1953 . ‘Morphologie du thorax de Lepisma saccharina L. (Apterygote Thysanoure). II. Musculature 1’ . Bulletin et Annales de la Société Entomologique de Belgique , 89 : 214 – 236 .
- Barlet , J. 1954 . ‘Morphologie du thorax de Lepisma saccharina L. (Aptérygote Thysanoure). II. Musculature 2’ . Bulletin et Annales de la Société Entomologique de Belgique , 90 : 299 – 321 .
- Barlet , J. 1980 . ‘Remarques concernant le thorax de Tricholepidion gertschi Wyg. (Aptérygotes Thysanoures)’ . Bulletin et Annales de la Société Royale d' Entomologie de Belgique , 116 : 215 – 232 .
- Bechly , G. , Brauckmann , C. , Zessin , W. and Gröning , E. 2001 . ‘New results concerning the morphology of the most ancient dragonflies (Insecta: Odonatoptera) from the Namurian of Hagen-Vorhalle (Germany)’ . Journal of Zoological Systematics and Evolutionary Research , 39 : 209 – 226 .
- Bitsch , C. and Bitsch , J. 2000 . ‘The phylogenetic interrelationships of the higher taxa of apterygote hexapods’ . Zoologica Scripta , 29 : 131 – 156 .
- Börner , C. 1909 . ‘Neue Homologien zwischen Crustaceen und Hexapoden. Die Beißmandibel der Insekten und ihre phylogenetische Bedeutung. Archi- und Metapterygota’ . Zoologischer Anzeiger , 34 : 100 – 125 .
- Boudreaux , H. B. 1979 . Arthropod Phylogeny, with Special Reference to Insects , New York : John Wiley & Sons .
- Brodsky , A. K. 1970 . ‘Organization of the flight system of the mayfly Ephemera vulgata L. (Ephemeroptera)’ . Entomological Review , 49 ( 2 ) : 184 – 188 .
- Brodsky , A. K. 1974 . ‘Evolution of the wing apparatus in the Ephemeroptera’ . Entomological Review , 53 ( 2 ) : 35 – 43 .
- Brodsky , A. K. 1994 . The Evolution of the Insect Flight , Oxford : Oxford University Press .
- Fürst von Lieven , A. 2000 . ‘The transformation from monocondylous to dicondylous mandibles in the Insecta’ . Zoologischer Anzeiger , 239 : 139 – 146 .
- Gorb , S. N. , Kesel , A. and Berger , J. 2000 . ‘Microsculpture of the wing surface in Odonata: evidence for cuticular wax covering’ . Arthropod Structure & Development , 29 : 129 – 135 .
- Grandi , M. 1947 . ‘Contributi allo studio degli “Efemeroidei” italiani. VIII. Gli scleriti ascellari (pseudopteralia) degli Efemeroidei, loro morfologia e miologia comparate’ . Bollettino dell’Istituto di Entomologia della Università degli Studi di Bologna , 16 : 85 – 114 .
- Gullan , P. J. and Cranston , P. S. 2005 . The Insects. An Outline of Entomology , Davis , CA : Blackwell Publishing .
- Hamilton , K. G.A. 1971 . ‘The insect wing. Part I. Origin and development of wings from notal lobes’ . Journal of the Kansas Entomological Society , 44 : 421 – 433 .
- Hamilton , K. G.A. 1972a . ‘The insect wing. Part II. Vein homology and the archetypal wing’ . Journal of the Kansas Entomological Society , 45 : 54 – 58 .
- Hamilton , K. G.A. 1972b . ‘The insect wing. Part III. Venation of the orders’ . Journal of the Kansas Entomological Society , 45 : 145 – 162 .
- Hamilton , K. G.A. 1972c . ‘The insect wing. Part IV. Venational trends and the phylogeny of the winged orders’ . Journal of the Kansas Entomological Society , 45 : 295 – 308 .
- Hasenfuß , I. 2008 . ‘The Evolutionary Pathway to Insect Flight – a Tentative Reconstruction’ . Arthropod Systematics & Phylogeny , 66 : 19 – 35 .
- Hennig , W. 1953 . ‘Kritische Bemerkungen zum phylogenetischen System der Insekten’ . Beiträge zur Entomologie , 3 : 1 – 85 .
- Hovmöller , R. , Pape , T. and Källersjö , M. 2002 . ‘The Palaeoptera problem: basal pterygote phylogeny inferred from 18S and 28S rDNA sequences’ . Cladistics , 18 : 313 – 323 .
- Kluge , N. J. 1994 . ‘Pterothorax structure of mayflies (Ephemeroptera) and its use in systematics’ . Bulletin de la Société Entomologique de France , 99 : 41 – 61 .
- Kluge , N. J. 2004 . The Phylogenetic System of Ephemeroptera , Dordrecht : Kluwer Academic Publishers .
- Knox , V. 1935 . “ ‘The body-wall and musculature of the thorax’ ” . In The Biology of Mayflies , Edited by: Needham , J. G. , Traver , J. R. and Hsu , Y.-C. 135 – 178 . New York : Comstock Publishing Co .
- Kristensen , N. P. 1975 . ‘The phylogeny of hexapod “orders”. A critical review of recent accounts’ . Zeitschrift für zoologische Systematik und Evolutionsforschung , 13 : 1 – 44 .
- Kristensen , N. P. 1981 . ‘Phylogeny of insect orders’ . Annual Review of Entomology , 26 : 135 – 157 .
- Kristensen , N. P. 1991 . “ ‘Phylogeny of extant hexapods’ ” . In The Insects of Australia , 125 – 140 . CSIRO , , Melbourne : Melbourne University Press .
- Kukalová-Peck , J. 1983 . ‘Origin of the insect wing and wing articulation from the arthropodan leg’ . Canadian Journal of Zoology , 61 : 1618 – 1669 .
- Kukalová-Peck , J. 1987 . ‘New Carboniferous Diplura, Monura, and Thysanura, the hexapod ground plan, and the role of thoracic side lobes in the origin of wings (Insecta)’ . Canadian Journal of Zoology , 65 : 2327 – 2345 .
- Kukalová-Peck , J. 1991 . “ ‘Fossil history and the evolution of hexapod structures’ ” . In The Insects of Australia , 141 – 179 . CSIRO , , Melbourne : Melbourne University Press .
- Kukalová-Peck , J. , Peters , J. G. and Soldán , T. ‘Homologisation of the anterior articular plate in the wing base of Ephemeroptera and Odonatoptera’ . International Perspectives in Mayfly and Stonefly Research. Proceedings of the 12th International Conference on Ephemeroptera and the 16th International Symposium on Plecoptera, Stuttgart 2008 . Edited by: Staniczek , A. H. Aquatic Insects, 31 (Suppl. 1), 459–470
- Lemche , H. 1940 . ‘The origin of winged insects’ . Videnskabelige Meddelelser fra dansk naturhistorisk Forening i København , 104 : 127 – 168 .
- Maki , T. 1938 . Studies on the thoracic musculature of insects, Memoirs of the Faculty of Science and Agriculture, Taihoku Imperial University . 24 : 1 – 343 .
- Martynov , A. V. 1925 . ‘Über zwei Grundtypen der Flügel bei den Insecten und ihre Evolution’ . Zeitschrift für Morphologie und Ökologie der Tiere , 4 : 465 – 501 .
- Matsuda , R. 1956 . ‘Morphology of the thoracic exoskeleton and musculature of a mayfly Siphlonurus columbianus McDunnough (Siphlonuridae, Ephemeroptera). A contribution to the subcoxal therory of the insect thorax’ . Journal of the Kansas Entomological Society , 29 : 92 – 113 .
- Matsuda , R. 1970 . ‘Morphology and evolution of the insect thorax’, Memoirs of the Entomological Society of Canada . 76 : 1 – 431 .
- Ogden , T. H. and Whiting , M. F. 2003 . ‘The problem with “the Paleoptera problem:” sense and sensitivity’ . Cladistics , 19 : 432 – 442 .
- Ogden , T. H. and Whiting , M. F. 2005 . ‘Phylogeny of Ephemeroptera (mayflies) based on molecular evidence’ . Molecular Phylogenetics and Evolution , 37 : 625 – 643 .
- Rasnitsyn , A. P. 2002 . “ ‘Subclass Scarabaeona Laicharting, 1781. The winged insects (=Pterygota Lang, 1888)’ ” . In History of Insects , Edited by: Rasnitsyn , A. P. and Quicke , D. L.J . 75 – 82 . Dordrecht : Kluwer Academic Publishers .
- Schwanwitsch , B. N. 1943 . ‘Subdivision of Insecta Pterygota into subordinate groups’ . Nature , 527 : 727 – 728 .
- Snodgrass , R. E. 1935 . Principles of Insect Morphology , New York and London : McGraw-Hill Book Co .
- Staniczek , A. H. 2000 . ‘The mandible of silverfish (Insecta: Zygentoma) and mayflies (Ephemeroptera): its morphology and phylogenetic significance’ . Zoologischer Anzeiger , 239 : 147 – 178 .
- Staniczek , A. H. 2001 . “ ‘Der Larvenkopf von Oniscigaster wakefieldi McLachlan, 1873 (Insecta: Ephemeroptera: Oniscigastridae)’ ” . Ph.D. thesis, Eberhard-Karls-Universität Tübingen, Germany
- Tannert , W. 1958 . ‘Die Flügelgelenkung bei Odonaten’ . Deutsche Entomologische Zeitschrift (Neue Folge) , 5 : 394 – 455 .
- Tsui , P. T.P. and Peters , W. L. 1972 . ‘The comparative morphology of the thorax of selected genera of the Leptophlebiidae (Ephemeroptera)’ . Journal of Zoology , 168 : 309 – 367 .
- Willkommen , J. 2008 . ‘The morphology of the pterothorax of Ephemeroptera, Odonata and Plecoptera (Insecta) and the homology of wing base sclerites and flight muscles’ . Stuttgarter Beiträge zur Naturkunde A, Neue Serie , 1 : 203 – 300 .
- Willkommen , J. and Hörnschemeyer , T. 2007 . ‘The homology of wing base sclerites and flight muscles in Ephemeroptera and Neoptera and the morphology of the pterothorax of Habroleptoides confusa (Insecta: Ephemeroptera: Leptophlebiidae)’ . Arthropod Structure & Development , 36 : 253 – 269 .
- Willmann , R. 1998 . “ ‘Advances and problems in insect phylogeny’ ” . In Arthropod relationships (systematics association, special volume series) , Edited by: Fortey , R. A. and Thomas , R. H. 269 – 279 . London : Chapman & Hall .
- Wootton , J. R. 1979 . ‘Function, homology and terminology in insect wings’ . Systematic Entomology , 4 : 81 – 93 .