ABSTRACT
Pseudoscorpions can be found in different microhabitats, one of which is moss. In this paper, we report the first records of pseudoscorpions associated with mosses in Mexico. We found three species: Albiorix edentatus, Paratemnoides nidificator and a new species of the genus Cocinachernes which is described below as C. akalchesis sp. nov. The specimens were collected from moss of the genus Bryum. This new species has globose spermathecae with four tubes: two short and two long, with ocular spots in the carapace; trichobothrium distance between t and st is less than the distance of t from the fingertip, Femur L/B ranges from 2.4 to 2.9, tibia from 1.68 to 1.9, chela is 2.9 (without pedicel), and hand (without pedicel) ranges from 2.53 to 2.88; galea short and slightly robust, without divisions in males; anterior operculum with 36 setae long and acuminated, posterior operculum with 20 acuminated setae. This new species represents the second known species of Cocinacheres from Mexico.
http://zoobank.org/urn:lsid:zoobank.org:pub:E907BC57-49AB-430D-8F4D-0B1C19EE830
Introduction
The floodplain forest represents an important habitat for invertebrates that have developed different strategies: 1. Stay near the water line and move in advance of floods. 2. Move to non-flooded trunk and canopy areas in the inundation forest. 3. Move to an adjacent dryland biotope during inundation. 4. Evolve adaptations for remaining in flooded terrestrial areas. 5. Combine one or more of these features (Adis et al. Citation1988; Adis Citation1997; Adis & Junk Citation2002).
Epiphytic plants, such as mosses, lichens, bromeliads, and tillandsias, represent a refuge for different groups of arthropods. Mites, springtails, tardigrades, pseudoscorpions, and other invertebrates can be found in mosses (Gerson Citation1969). Moss provides both food and optimal conditions for the development of pseudoscorpions. Moreover, in the inundated rainforest, the presence of epiphytic plants is crucial because water levels in these areas remain high (from one to several meters) for 4–7 months of the year (Adis et al. Citation1988; Adis & Junk Citation2002), and pseudoscorpions make a vertical migration (up and down the vegetation) using moss as a refuge to survive during flooding (Adis Citation1997).
Pseudoscorpions are small arachnids that inhabit diverse microhabitats, such as caves, under rocks, logs, bark, soil, ground litter, and nests of rodents and social insects (Muchmore Citation1990a; Martínez et al. Citation2021). Additionally, they can be found associated with various animals, including rodents (on hair), dragonflies, coleopterans (under elytra), and other arachnids (Muchmore Citation1971; Hoff & Jennings Citation1974; Aguiar & Bührnheim Citation1992, Citation1998; Villegas-Guzmán et al. Citation2022). Neobisium sp. Chamberlin, Citation1930 (Neobisiidae), Apochthonius moestus (Banks, 1891), and Kleptochthonius sp. Chamberlin, Citation1949 (Chthoniidae) are three species known to be associated with moss (Graves & Graves Citation1969).
Table 1. Reported pseudoscorpions associated with mosss.
In Mexico, there are currently 185 species in 71 genera and 18 families, recorded in 29 states. In Quintana Roo, there are records of ten species: Garypus decolor Muchmore, Citation1991 (Garypidae), Olpiolum amplum Hoff, Citation1964, and Aphelolpium cayanum Muchmore, Citation1979 (Olpiidae), Tyrannochthonius innominatus Muchmore, Citation1996 and Aphrastochthonius sp. (Chthoniidae), Coprochernes quintanarooensis Muchmore, Citation1990, Epactiochernes tumidus (Banks, 1895), Epichernes navarroi Muchmore, Citation1990, Parachernes virginicus (Banks, 1895), and Pachychernes attenuatus Muchmore, Citation1990 (Cherrnetidae) (Muchmore Citation1990b; Villegas-Guzmán Citation2013). Muchmore (Citation1990b) mentioned that in Quintana Roo can be found species that have been registered in neighboring states and countries such as Yucatan, Campeche, Chiapas, Belize, and Guatemala. For this reason, the number of species in this state could be as large as 35 species. The aim of this paper is to report a new species of Cocinachernes and the pseudoscorpions associated with mosses in Quintana Roo. The genus Cocinachernes was described by Hentschel and Muchmore (Citation1989) from material collected in dry litter at Cocinas island in Jalisco, Mexico. Before this paper only one species, C. foliosus was known, which is characterized by presenting a globose spermatheca with four short and terminally expanded tubules; in addition to having the entire body covered by setae pinnately feathered and leaflike vestitural setae.
Materials and methods
Pseudoscorpions were collected in a floodplain forest in ‘Ejido Nicoláas Bravo’ at the municipality of Othón P. Blanco, Quintana Roo () (18°28’73’’N. 89°03’50.2W), in samples of moss 15 × 15 cm gathered on trees during three periods of 2011: April-May (droughts), July-August (rains), and October-November (northerly winds). Samples were placed in plastic bags and carried to the laboratory, where they were processed using Berlese-Tullgren funnels to separate arthropods. Pseudoscorpions were processed using Hoff’s (Citation1949) technique, modified following Wirth and Marston (Citation1968). Specimens were measured in millimeters using Chamberlin’s (Citation1931) method and the modifications of Benedict and Malcolm (Citation1977) concerning the measures of the chela. Terminology follows Chamberlin (Citation1931), Harvey (Citation1992) and Judson (Citation2007). Specimens are deposited in the Colección Nacional de Arácnidos of the Instituto de Biología, UNAM, and others in the Colección de Acarología de la Escuela Nacional de Ciencias Biológicas, IPN.
Abbreviations
The following abbreviations are used in the text for the chelal trichobothria: b – basal; sb – sub-basal; st – subterminal; t – terminal; ib – interior basal; isb – interior sub-basal; ist – interior sub-terminal; it – interior terminal; eb – exterior basal; esb – exterior sub-basal; est – exterior sub-terminal; et – exterior terminal. As well as in the text L – long; B – breadth.
Results
We found 17 pseudoscorpions in three samples of mosses of the genus Bryum, collected during the rainy season. They belong to three species: Albiorix edentatus Chamberlin Citation1930, 1♀ and 3 protonymphs (Ideoroncidae) (), Paratemnoides nidificator (Balzan, 1888) 2 ♀♀ (Atemnidae) and a new species of the genus Cocinachernes 6♀♀, 3 ♂♂, 1 deutonymph, and 1 protonymph (Chernetidae) ().
Taxon treatment
Family Chernetidae Menge 1885
Subfamily Chernetinae
Genus Cocinachernes Hentschel and Muchmore Citation1989
Cocinachernes foliosus Hentschel and Muchmore : 345–349
Type species
Cocinachernes foliosus Hentschel and Muchmore Citation1989 by original designation.
Diagnosis
Cocinachernes differs from other chernetid genera by the following combination of character states: carapace with two transverse furrows without eyes or ocular spot; four setae in the rallum; absence of the tactile seta from tarsus IV; cheliceral hand with 7 setae; b and sb terminally denticulated; leaflike setae on body and appendages; female galea moderate in size, with six small branches; male galea smaller and without branches; spermathecae spherical with four short and broad tubes; each finger with 29–32 marginal teeth and several external and 1 or no internal accessory teeth. male anterior genital operculum with a group of about 35 setae, four of which are larger and located medially, posterior operculum with 20 setae on the face and along the posterior margin (Hentschel & Muchmore Citation1989).
Distribution
Cocinachernes is only known from the type species (male) and two paratypes (females) from Cocinas Island, Jalisco, Mexico, collected in dry litter.
Cocinachernes akalchesis Villegas-Guzmán & Córdova-Tabares sp. nov.
Zoobank: E907BC57-49AB-430D-8F4D-0B1C19EE830
Material examined
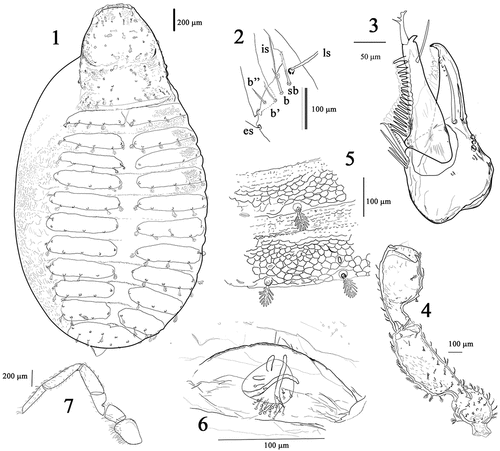
Figures 1–7. Cocinachernes akalchesis, female from Mexico. 1 body. Left hand chelicera setae. 3. Right chelicera, 4 left pedipalp. 5. Setae and ornament of tergites. Female genitalia, spermathecal. 7 Leg IV right. Scale bar 3 = 50 μm; 5 = 100 μm; 7 = 200 μm.
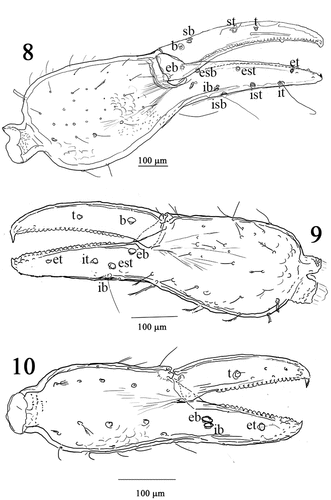
Figures 8–10. Cocinachernes akalchesis, 8 Chela female. 9 Chela deutonymph. 10 Chela protonymph. Scale bar 8 and 9 = 200 μm; 10 = 100 μm.
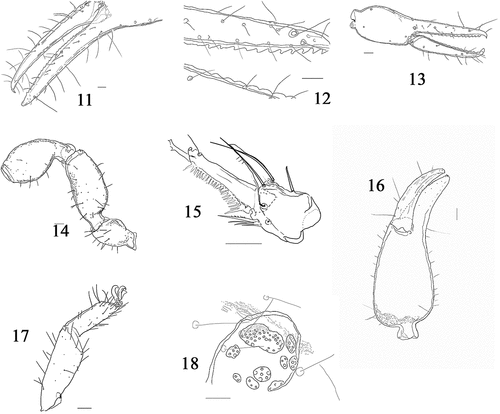
Figures 11–18. Albiorix edentates, 11 chela female. 12 tooth of fixed and movable finger chela female. 13 chela protonymph. Scale bar 11 and 12 = 200 μm; 13 = 100 μm. (14–18) Paratemnoides nidificator, 14 right pedipalp. 15 left chelicera. 16 chela female. 17. Leg IV. 18. Spermathecae. Scale bar 14, 15 and 16 = 200 μm; 17 = 100 μm; 18 = 50 μm.
Holotype
Male: México, Quintana Roo, Othón P. Municipality of Blanco in Ejido Nicolás Bravo (18°28’73’’ N, 89°03’50.2’’W, at 103 m) in samples of moss of the genus Bryum collected in trees in July–August 2011 (CNAN-T 01386).
Paratypes
Mexico: Quintana Roo 2♂, 6♀, 1 deutonymph, 1 protonymph, same data as holotype.
Diagnosis
This new species has globose spermathecae with 4 tubes: 2 short and 2 long, with ocular spots in the carapace; the trichobothrium distance between t and st is less than the distance of t from the fingertip, femur L/B 2.4–2.9, tibia 1.68–1.9, chela 2.9 (without pedicel); hand (without pedicel) 2.53–2.88; galea short and slightly robust, without divisions in males; the anterior operculum has 36 setae long and acuminated, and the posterior operculum has 20 acuminated setae.
Description (adults)
Pedipalps, tergites, and carapace moderately sclerotized; legs, sternites, and pleural membranes light yellowish brown. Setae on the carapace, palp, and tergites pinnately feathered (), whereas setae on the sternites are acuminate.
Chelicerae: hand with 7 setae, sb, b, and b’’ denticulated (). Movable finger with acuminate seta (gs). Galea is short and slightly robust, without divisions in males (0.035–0.049), and has 2–5 rami (0.049–0.058) in females. Rallum with four blades (): two small smooth and two long dentate, each three quarters of an inch long. Serrula has an exterior with 16–18 lamellae.
Description (adults)
Pedipalps, tergites and carapace reddish-brown color, moderately sclerotized; legs, sternites and pleural membranes light yellowish brown. Setae of carapace, palp, tergites with pinnately feathered (), whereas in sternites setae are acuminate.
Chelicerae: hand with 7 setae, sb, b’, and b’’ denticulated (). Movable finger with acuminate seta (gs). Galea short and slightly robust, without divisions in males (0.035–0.049), females with 2–5 rami (0.049–0.058). Rallum with 4 blades (): two small smooth and two longest dentate, three quarters long. Serrula exterior with 16–18 lamellae.
Pedipalp (): surface reticulated, extended, slightly robust, trochanter 1.28–1.0 (♂), 1.06–1.57 (♀), femur 2.4–2.9 (♂), 2.6–2.71 (♀), patella 1.68–1.9 (♂), 1.95–2.03 (♀) times longer than wide, hand (without pedicel) 2-53-2.88 (♂), 2.7–2.76 (♀) x longer than wide; movable finger 1.12–1.14 × as long as hand (without pedicel). Trochanter with a rounded dorsal protuberance 1–1.5 (♂), 1.1–1.6 (♀) x longer than wide. Fixed finger with 30–37 (♂), 31–36 (♀) marginal teeth with the end of the dental row at esb level, plus 1–3 (♂), 0–3 (♀) external accessory teeth and 4 (♂), 0–4 (♀) internal accessory teeth; movable finger with 31–36 (♂), 35–38 (♀) marginal teeth, with the end of the dental row at sb level, plus 1–5 (♂), 0–3 (♀) external accessory teeth and 1–4 (♂), 0–3 (♀) internal accessory teeth. Fixed finger with 8 trichobothria (): distance between fingertip and it on fixed finger slightly longer than the distance between ist and isb; it much closer to et than to est., distance from est to isb more than 3× the distance between esb and eb. Movable finger with 4 trichobothria: st on movable finger much closer to t to than sb, distance between t and st is less than the distance of t from the fingertip (). Venom apparatus well developed in the movable finger with nodus ramosus very close to trichobothria t.
Carapace: reticulated throughout and granulated in the perimeter zone, with ocular spots, medial zone with 2 transverse furrows well developed, and other furrows close to posterior margin, 0.73–0.76 (♂), 1.05–1.09 (♀) x long as wide; with two ocular spots, with 8–11 (♂), 8–9 (♀) setae on anterior margin, 10–11 (♂), 9–12 (♀) setae on posterior margin, and 52–57 setae (♂, ♀) in the rest of the carapace ().
Coxal region: coxae of pedipalps with 18–20 setae (♂), 20–23 (♀); chaetotaxy of coxae I–IV: ♂, 12: 14: 16: 28–30; ♀, 22–24, 12–13, 17–19, 35–36. Coxae I and IV are approximately of the same width.
Legs: cuticle granulated. Setae strongly clavated and pinnately feathered. Leg I with trochanter 1 (♂), 1.27–1.3 (♀), femur 0.50–079 (♂), 0.71–0.77 (♀), patella 1.91–2.31 (♂), 2.5–2.4 (♀), tibia 2.6–3.2 (♂), 2.4–2.5 (♀), tarsus 2.84–4.7 (♂), 4.7–5.1 (♀) x longer than wide. Leg IV () with trochanter 1.76–1.31 (♂), 1-43-1.48 (♀), femur + patella 3.52–3.59 (♂), 3.34–3.18 (♀), tibia 4.31–4.5 (♂), 3.95–4.34 (♀), tarsus 4.4–5 (♂), 4.7–5 (♀) x longer than wide. Tibiae and tarsi of legs III and IV without tactile setae. Tarsal claws simple; arolium complete, distinctly shorter than claws.
Abdomen: reticulated tergites with pinnately feathered setae (). Tergites I-X and sternites IV-X divided (♂, ♀). Tergal chaetotaxy: ♂ 10: 10:10: 12: 12: 12: 12: 12: 12: 12: 4; ♀ 9: 10: 9 12: 13: 11: 11: 12: 12: 12: 10: Sternal chaetotaxy ♂ 10:17:13:12:10:10:8:6; ♀ 8: 15: 13: 12: 10: 11: 8: 3. Four tactile seta in both sexes.
Genitalia: Male anterior operculum with 36 setae long and acuminated and posterior operculum with 20 acuminated setae. Female anterior operculum with 32 short and acuminated setae, posterior with 10 short and acuminated setae. Male genitalia is the typical chernetid form (Vachon Citation1938). Spermatheca of female with four tubules, two long and two shorts, not terminally expanded ().
Dimensions: Male holotype and two paratypes in parentheses: Body length 2.11 (2.38–2.40). Pedipalps: trochanter 0.23/0.23 (0.36–0.31/0.24–0.239), femur 0.50/0.21 (0.71–0.67/0.25), patella 0.44/0.26 (0.60–0.56/0.30–0.28), chela (without pedicel) 1.04 (0.97–1.04), movable finger length 0.58 (0.54-0-0.57). Chelicera 0.22/0.09 (0.25–0.0.28/0.12–0.16). Carapace 0.54/0.71 (0.78–0.81/0.74–0.85). Leg I: trochanter 0.11/0.11 (0.13/0.11–0.13), femur 0.06/0.12 (0.11–0.09/0.13–0.15), patella 0.18/0.09 (0.13–0.15/0.27–0.28), tibia 0.2/0.076 (0.27–0.084), tarsus 0.15/0.06 (0.26–0.29/0.054–0.062). Leg IV: trochanter ¿/? (0.19–0.27/0.14–0.15), femur+patella 0.38/0.11 (0.54/0.15), tibia 0.35/0.08 (0.42–0.46/0.1–0.11), tarsus 0.27/0.06 (0.1–0.11/0.33–0.35).
Female (n = 6)
Paratype body length 2.57–2.95. Pedipalps: trochanter 0.27–0.43/0.25–0.27, femur 0.64–0.73/0.25–0.27, patella 0.57–0.65/0.29–0.32, chela (without pedicel) 1.04–1.1/0.38–0.41, movable finger length 0.54–0.58. Chelicera 0.30–0.27/0.2–0.12. Carapace: 0.93–0.81/0.88–0.74. Leg I: trochanter 0.15–0.13/0.15–0.13, femur 0.12–0.1/0.16–0.13, patella 0.31–0.29/0.13–0.18, tibia 0.33–0.27/0.1–0.08, tarsus 0.33–0.27/0.07–0.05. Leg IV: trochanter 0.28–0.23/0.19–0.16, femur+patella 0.62–0.54/0.18–0.17, tibia 0.5–0.42/0.12–0.11, tarsus 0.38–0.32/0.08–0.07.
Deutonymph
Morphology generally as in adults. Pedipalps, carapace and tergites pale brown, legs and sternites light yellow.
Chelicera: hand with 5 setae sb and b finely denticulate and the remainder acuminate; movable finger with 1 subdistal setae (gs). Galea long and slender with 3 rami. Rallum composed of 4 blades. Serrula exterior with 13 blades.
Pedipalp: trochanter 1, femur 2.4, patella 1.5, chela (without pedicelo) 2.8 × longer that wide. Fixed chelal finger with 6 trichobothria: eb, est and et; isb below of it ib, movable chelal finger with 2 trichobothria: b and t.
Chelal teeth (): fixed finger with 22 marginal teeth, movable finger with 25 marginal teeth, both fingers without accessory teeth. Venom apparatus present in movable finger with nodus ramosus near t and tooth 14.
Carapace: 1.02 × longer than wide; with small ocular spots; with 4 setae in anterior margin and 6 on posterior margin, the rest of carapace with 27 setae, with 2 transverse furrows.
Legs: mostly as in adult.
Abdomen: Tergites I-X and sternites I-X divided. Tergal chaetotaxy: 6: 7: 8: 8: 8: 8: 8: 8: 8:7:4 (including 2 tactile setae). Sternal chaetotaxy 4: 4: 4: 4: 6: 8: 6: 7: 6: 4: 4 (including 2 tactile setae). Pleural membrane rugose.
Dimensions (n = 1) Body length 1.5. Pedipalp: trochanter 0.16/0.16, femur 0.35/0.15, patella 0.25/0.16, chela (without pedicel) 0.58/0.21, movable finger length 0.35. Carapace 0.43/0.45. Leg I: trochanter 0.08/0.08, femur 0.04/0.09, patella 0.13/0.08, tibia 0.17/0.05, tarsus 0.17/0.03. Leg IV: trochanter 0.08/0.08/, femur+patella 0.27/0.12, tibia 0.23/0.1, tarsus 0.19/0.05.
Protonymph
Morphology generally as in adults. Pedipalps, carapace and tergites pale brown, legs and sternites pale brown.
Chelicerae: missing
Pedipalp: trochanter 1, femur 2.2, patella 1.6, chela (without pedicel) 2.92 × longer that wide. Fixed chelal finger with 3 trichobothria: eb, ib and et, movable chelal finger with 1 trichobothrium t located in middle of the finger.
Chelal teeth (10): fixed finger with 20 marginal teeth, movable finger with 22 marginal teeth, both fingers without accessory teeth. Venom apparatus present in a movable finger with nodus ramosus near teeth 13.
Carapace: 1.02 × longer than wide; with small ocular spots; with 4 setae in anterior margin and 6 on posterior margin, the rest of carapace with 24 setae, with 2 transverse furrows.
Legs: mostly as in adult.
Abdomen: Tergites I-X and sternites I-X divided. Tergal chaetotaxy: 6: 6: 6: 6: 6: 6: 6: 6: 6: 6: 6 (including 2 tactile setae). Sternal chaetotaxy 6: 6: 6: 6: 6: 6: 5: 5: 5: 2: 4 (including 2 tactile setae). Pleural membrane rugose.
Dimensions (n = 1): Body length 1.07. Pedipalp: trochanter 0.12/0.12, femur 0.25/0.12, patella 0.19/0.12, chela (without pedicel) 0.45/0.15, movable finger length 0.25. Carapace 0.41/0.40. Leg I: trochanter 0.05/0.07, femur 0.04/0.08, patella 0.12/0.06, tibia 0.12/0.05, tarsus 0.12/0.04. Leg IV: trochanter 0.08/0.08/, femur + patella 0.19/0.08, tibia 0.16/0.07, tarsus 0.17/0.04.
Etymology
The species name, akalchesis, was derived from the Latinized Mayan word for ‘flooded.’ Ak’alche refers to the place where the moss samples were collected in lowland, inundable rainforest.
Discussion
Mosses are key drivers of nutrient and carbon dynamics in many terrestrial ecosystems, as they regulate leaf litter decomposition. However, litter decomposition responds to changes in bryosphere cover, moss species, and bryosphere-associated biota. Microarthropods play a critical role in composition. Specifically, the contribution of microarthropods to litter decomposition in the bryosphere is unclear (Grau-Andres et al. Citation2021). The results described here - two families and two genera for Quintana Roo – have now been added to the state’s list of 13 species in six families. The organisms were found in the rainy season, when the study area was flooded, and we considered that the pseudoscorpions found here were taking refuge in the mosses, experiencing a vertical migration to avoid drowning conditions (Gerson Citation1969; Adis Citation1997; Adis & Junk Citation2002). Our observations of the two of the three species of pseudoscorpions here studied showed coexisting adults and nymphs (A. edentatus and C. akalchensis), which suggests that in the mosses they are carrying out their entire life cycle because this micro habitat provides them with stable humidity and temperature for their survival, as well as mites and springtails on which they prey (Weygoldt Citation1969).
Paratemnoides nidificator, known to inhabit under tree bark (Ribeiro et al. Citation2018), has been found in mosses, possibly due to the favorable environmental conditions they provide. In the case of A. edentatus and C. akalchesis, this marks the first instance of their registration on moss. A. edentatus had previously been recorded under rocks (Chamberlin Citation1930), making this the first documented instance of the species in Mexico. On the other hand, the genus Cocinachernes, which includes C. akalchesis, has been typically found in dry litter (Hentschel & Muchmore Citation1989). The observed pseudoscorpions of these species are likely engaging in a vertical migration within trees to distance themselves from the water line. Conducting collections of litter and soil during the dry season would be intriguing to determine whether the pseudoscorpion species recorded in this study are also present in these microhabitats. Mosses have been identified as a much stronger driver of the microarthropod community compared to vascular plants (Bokhorst et al. Citation2014).
Acknowledgments
The authors thank Wendy Varguez, Yuritza Cruz, Yareli Dzul, and Juan Ek for collecting the mosses from which we extracted the pseudoscorpions. Sergio Cahuich for allowing the collection of mosses on his property. Funding for this study was provided by Instituto Tecnológico Federal 3381.10P to LQC-P. RJM thanks the Vicerrectoria de Investigación y Postgrado, University of Panama, for providing the financial means to complete this project through CUFI-2022-CNET-EP-008 grants. We thank Margarita Ojeda, Oscar Francke, and Dora Isabel Quirós for their comments on the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Adis J. 1997. Estratégias de sobrevivencia de invertebrados terrestres em florestas inundáveisda Amazónia central: uma resposta à inundação de longo período. Acta Amaz. 27:43–54. https://doi.org/10.1590/1809-43921997271054.
- Adis J, Junk WJ. 2002. Terrestrial invertebrates inhabiting lowland river floodplains of Central Amazonia and Central Europe: a review. Freshw Biol. 47(4):711–731.
- Adis J, Mahnert V, De Morais JW, Gomes-Rodrigues JM. 1988. Adaptation of an Amazonian pseudoscorpion (Arachnida) from dryland forests to inundation forests. Ecology. 69:287–291.
- Aguiar NO, Bührnheim PF. 1992. Pseudoscorpiones (Arachnida) em associação forética com Passalidae (Insecta, Coleoptera) no Amazonas, Brasil. Amazonia. 12:187–205.
- Aguiar NO, Bührnheim PF. 1998. Phoretic pseudoscorpions associated with flying insects in Brazilian Amazonia. J Arachnol. 26:452–459.
- Benedict EM, Malcolm DR. 1977. Some garypoid false scorpions from western North America (Pseudoscorpionida: Garypidae and Olpiidae). J Arachnol. 5:113–132.
- Bokhorst S, Wardle DA, Nilsson MC, Gundale MJ. 2014. Impact of understory mosses and dwarf shrubs on soil micro-arthropods in a boreal forest chronosequence. Plant Soil. 379(1):121–133.
- Chamberlin JC. 1931. The arachnid order Chelonethida. Stanford Univ Publ Biol. 7:1–284.
- Gerson U. 1969. Moss-arthropod associations. Bryologist. 72(4):495–500.
- Grau-Andres R, Wardle DA, Kardol P. 2021. Bryosphere loss impairs litter decomposition consistently across moss species, litter types, and micro-arthropod abundance. Ecosystems. 25:1–13.
- Graves RC, Graves ACF. 1969. Pseudoscorpions and spider from moss, fungi, rhododendron leaf litter and other microcommunities in the Highlands area of Western North Carolina. Ann Entomol Soc Am. 62:267–269.
- Harvey MS. 1992. The phylogeny and classification of the Pseudoscorpionida (Chelicera: Arachnida). Invertebr Biol. 6:1373–1435.
- Hentschel E, Muchmore WB. 1989. Cocinachernes foliosus, a new genus and species of pseudoscorpion (Chernetidae) from Mexico. J Arachnol. 17:345–349.
- Hoff CC. 1949. The pseudoscorpions of Illinois. Bull Ill Nat Hist Surv. 24:409–498.
- Hoff CC, Jennings DT. 1974. Pseudoscorpions on a spider. Entomol News. 85:21–22.
- Judson MLI. 2007. A new endangered pseudoscorpion of the genus Lagynochthonius (Arachnida, Chelonethi, Chthoniidae) from Vietnam, with notes on chelal morphology and the composition of the Tyrannochthoniini. Zootaxa. 1627:53–68.
- Kolesnikov VB, Christophoryová J, Przhiboro AA, Turbanov IS. 2022. The pseudoscorpions of the Caucasian Sphagnum bogs: part I. Description of Neobisium (Neobisium) adjaricum sp. nov. and redescription of the holotype of N.(N.) vilcekii Krumpál, 1983 (Arachnida, Pseudoscorpiones, Neobisiidae). ZooKeys. 1100:165–190.
- Krajčovičová K, Ivinskis P, Rimšaitė J, Christophoryová J. 2022. Pseudoscorpions (Arachnida: Pseudoscorpiones) from the Curonian Spit National Park with Dinocheirus panzeri as a newly recorded genus and species from Lithuania. Arachnol Mitt. 64:52–56.
- Martínez RJ, Guzmán GAV, Quirós DI, Emmen D. 2021. Associated pseudoscorpions (Arachnida: Pseudoscorpiones) with waste heaps of Atta colombica (Guérin-Méneville, 1844) (Hymenoptera: Formicidae) in Panama. Rev Chil Entomol. 47:67–74.
- Muchmore WB. 1990a. Pseudoscorpionida. In: Navarro L and Robinson J, editors. Diversidad biológica de la reserva de Sian Ka’an, Quintana Roo. México: Centro de Investigaciones de Quintana Roo. p. 155–173.
- Muchmore WB. 1990b. Pseudoscorpionida. In: Dindal DL, editor. Soil biology guide. New York: John Wiley and Sons. p. 503–527.
- Muchmore WR. 1971. Phoresy by north and Central American pseudoscorpions. Proc Rochester Acad Sci. 12:77–97.
- Ribeiro RF, Gomes FC, Tizo AFS, Tizo-Pedroso E, Del-Claro K. 2018. Cooperative foraging in neotropical pseudoscorpions: effects of prey changes on behavioral adjustments of colonies. Acta Ethol. 21:153–161.
- Vachon M. 1938. Recherches anatomiques et biologiques sur la reproduction et le developpement des Pseudoscorpions. Annls Sci Nat (Zool). 1: 1–207.
- Villegas-Guzmán GA. 2013. Pseudoescorpiones (Arachnida: Pseudoscorpiones) de la Laguna Colombia, Isla Cozumel, Quintana Roo, México. Folia Entomol Mex. 12:117–120.
- Villegas-Guzmán GA, Chinchilla-Romero FA, Martínez RJ. 2022. A new species of Epichernes (Pseudoscorpiones: Chernetidae) associated with rodents in Costa Rica. Stud Neotrop Fauna Environ. 58:1–8.
- Weygoldt P. 1969. The biology of pseudoescorpions. Cambridge, Massachusetts: Harvard University Press. p. 145.
- Wirth WW, Marston M. 1968. A method for mounting small insects on microscope slides in Canada balsam. Ann Entomol Soc Am. 68:783–784.