Abstract
Chronic inflammatory liver disease regardless of aetiology leads to failing regeneration and fibrosis, ending in cirrhosis. Both in man and in animals this worldwide health problem has no definitive cure. Chronic liver injury causes hepatic stellate cells to proliferate and differentiate into matrix-producing cells. New therapeutic options will be developed upon detailed understanding of the molecular mechanisms driving liver fibrosis. This may lead to new anti-fibrotic therapies which need to be tested in suitable models before application in the veterinary and human clinic. On the other side, to restore the failing regenerative capacity of the diseased liver cells, adult progenitor cells are of interest, as an alternative to whole organ transplantation. In order to find the most suitable large animal model it is important to recognise that the typical histopathological reaction pattern of the liver can differ between mammalian species. It is therefore imperative that specialists in veterinary internal medicine and pathology, being familiar with the diseases and pathologies of the liver in different animal species, are teaming-up in finding the best models for veterinary and human liver diseases. Several large animal models have been mentioned, like pigs, sheep, and dogs. Based on the observations that man and dog share the same hepatopathies and have identical clinical, pathological and pathogenetic reaction patterns during the development of liver disease, the dog seems to be a properly suited species to test new therapeutic strategies for pets and their best friends.
1. Introduction
The liver, one of the largest organs in the body, has an enormous reserve capacity and plays a key role in many metabolic processes and homeostasis of the body. It consists of epithelial cell types (hepatocytes, cholangiocytes and progenitor cells), mesenchymal cell types (hepatic stellate cells) and sinusoidal cells (Kuppfer cells, endothelial cells). There is a continuous interplay between these different cell types in the liver and their surrounding stroma. Adult liver parenchyma is composed of hundreds of thousands of lobules that can be considered as the smallest structural and functional units. From the portal areas blood is supplied from the smallest portal vein and hepatic artery branches to the sinusoids, which are oriented towards the central veins of the lobules. Going from periportal to pericentral, hepatocytes have different functions, which is called ‘metabolic zonation’ (Burke and Tosh Citation2006). The huge adaptive capacity of the liver implies that signs of disease and concomitant dysfunction of the liver often become clinically apparent in the chronic stage when the repair mechanisms are hampered.
Chronic hepatic injury activates a general aetiology-independent process characterised by fibrogenesis and liver regeneration. The sequence of events starts with the primary insult (e.g. toxins, viruses), followed by regenerating hepatocytes, activation of non-hepatic cells and fibrosis. When mature hepatocytes are damaged or hampered in their replication, the liver progenitor cell (LPC) compartment may be activated (Shibata et al. Citation2006). Hepatic fibrosis, a common manifestation of chronic liver diseases, is the result of accumulation of extracellular matrix (ECM) and can progressively lead to cirrhosis (Friedman Citation2003). Cirrhosis is defined as a diffuse process characterised by fibrosis of the liver and the conversion of normal liver architecture into structurally abnormal nodules, micro- or macronodular (van den Ingh et al. Citation2006). Liver cirrhosis represents a worldwide health problem and no definitive cure other than transplantation is available (Salgado et al. Citation2000). In human medicine, a short supply of donor organs is a serious problem, and for people in need of a new liver and are on the waiting list, a suitable donor liver often comes too late. A strong pressure has been put to evaluate alternatives for whole organ transplantation since the last two decades. One of these alternatives is liver cell transplantation. This technique has been studied for more than four decades, mainly in mice and rats. Although liver cell transplantation has also been used in over 200 human beings with different kind of liver diseases (Fisher and Strom Citation2006), the step from principle to practice still remains full of technical and theoretical hurdles. The availability of a suitable large animal model in which the pathophysiology of chronic liver disease closely resembles that in man is lacking (Weber et al. Citation2009). A dog model with an inducible, controllable and reproducible form of chronic hepatitis (CH) with a well understood cause might be a valuable tool for evaluating new therapeutic strategies. Spee et al., IJzer et al. and Schotanus et al. demonstrated that the molecular pathophysiology of canine fibrotic liver diseases is highly comparable to the pathophysiology of their human counterparts (Spee et al. Citation2006a; Schotanus et al. Citation2009; IJzer et al. Citation2010).
In human medicine, the molecular pathogenesis of diseases associated with copper excess (Wilson's disease, WD) is of great interest (De Bie et al. 2007a). Several animal models resembling WD have been described (Vonk et al. Citation2008). The Long Evans Cinnamon (LEC) rat and (Jackson) toxic milk mouse are naturally occurring models for Wilson disease. LEC rats have a deletion in the ATP7B gene resulting in the total loss of the Wilson's disease protein (Wu et al. Citation1994) and toxic milk and Jackson toxic milk mice have missense mutations in ATP7B that affects ATP7B function leading to an incomplete activation (Biempica et al. Citation1988; Coronado et al. Citation2001; Roberts et al. Citation2008). Another interesting animal model for studying the molecular pathophysiology of copper accumulation, is the Bedlington terrier with copper toxicosis (CT). CT in the Bedlington terrier is caused by a deletion of exon-2 of the COMMD1 gene which results in the absence of a functional COMMD1 protein (van de Sluis et al. Citation2002). The result is a progressive accumulation of copper in the hepatocellular lysosomes in the centrolobular region around the central vein in the liver, histologically distinguishable from the normal situation at one year of age, which leads to progressive hepatitis and cirrhosis. At the department of Clinical Sciences of Companion Animals of the University of Utrecht, a COMMD1 deficient dog population has been created for longitudinal follow-up studies on the development of copper-associated CH. This unique animal model allows an in-depth characterisation of the fibrosis, regeneration, copper and oxidative stress pathways.
2. Liver regeneration
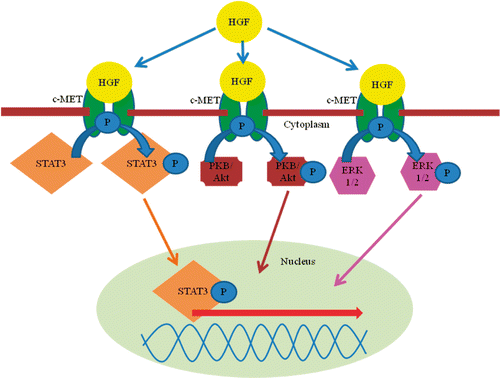
Many growth factors and cytokines have been implicated in regulating liver regeneration. Hepatocyte growth factor (HGF), a ligand for the c-Met proto-oncogene product (MET), is one of the most potent stimulators of hepatocyte proliferation (Michalopoulos and DeFrances Citation1997; Huh et al. Citation2004). In liver, HGF is mainly produced by hepatic stellate cells, sinusoidal endothelial cells and Kupffer cells (Schirmacher et al. Citation1993; LeCouter et al. Citation2003). For the regulation of the release and the activation of HGF during regeneration metalloproteinases, tissue inhibitor of metalloproteinases (TIMP) levels, urokinase-type plasminogen activator (uPA) and HGF-activator are important (Naldini et al. Citation1992; Mohammed et al. Citation2005). HGF is secreted as a single-chain, 83-kDa precursor without biological activity (pro-HGF) and proteolytic cleavage into HGF is necessary for activation. Maturation of pro-HGF into the bioactive dimer takes place in the extracellular environment. Naldini et al. showed that uPA activates pro-HGF in vitro, although at a 1000-fold lower activity as HGF-activator. Thus, a crucial limiting step occurs in the HGF signaling pathway after its secretion (Naldini et al. Citation1992, Citation1995). After binding of HGF to c-Met, signalling to multiple transducers is mediated, including the Akt/PKB, ERK1/2 and STAT3 (signal transducer and activator of transcription 3; ; Darnell Citation1997; Borowiak et al. Citation2004). Other cytokines important in activation of the STAT pathway include transforming growth factor (TGF) alpha and interleukin (IL)-6 (Zimmers et al. Citation2003; Santoni-Rugiu et al. Citation2005). Recently, the idea came up that the activation of multiple pathways is required for liver regeneration instead of one single humoral agent such as HGF (Taub Citation2004). Taub proposed an essential circuitry required for liver regeneration compassed of three types of pathways: cytokine, growth factor and metabolic networks that link liver function with cell growth and proliferation (Fausto and Campbell Citation2003; Taub Citation2004; Fausto et al. Citation2006).
Figure 1. HGF signalling. Notes: After activation, HGF binds to its receptor c-MET. Autophosphorylation of c-MET induces multiple intra-cellular pathways (STAT3, PKB/Akt, and ERK1/2). HGF: hepatocyte growth factor; c-MET, c-MET tyrosine kinase receptor; STAT3, signal transducer and activator of transcription 3; PKB, protein kinase B; ERK, one of the MAP (mitogen activated protein) kinases.
When mature hepatocytes are damaged or inhibited in their replication, a reserve compartment, in humans called the LPC compartment and in rodents the oval cell compartment, is activated. This compartment resides in the smallest and most peripheral branches of the biliary tree, the ductules and canals of Hering. Keratin (K)7 and K19 are generally accepted as immunohistochemical markers for the identification for LPCs (Libbrecht and Roskams Citation2002; Roskams et al. Citation2003, Citation2004; Roskams Citation2006), but cholangiocytes also stain positive. Unfortunately, no universal LPC marker specific to this compartment has been identified to date (Bird et al. Citation2008). Recently, two interesting potential candidates were described; TWEAK through its receptor Fn14 (Jakubowski et al. Citation2005) and FoxoL1 (Sackett et al. Citation2009). Progenitor cell activation is seen in the majority of subacute to chronic human liver diseases and the degree of activation increases with the severity of the disease (Roskams Citation2006; Bird et al. Citation2008). A general trigger for progenitor cell activation is a lack of the mature cell compartments ability to proliferate (Shibata Citation2006). Progenitor cells are able to survive when hepatocytes are lost due to toxic damage or viral infections (Roskams Citation2006) and, in view of their bipotent differentiation potential towards hepatocytes or cholangiocytes, progenitor cells would be interesting cells to use for cell therapy (Weiss et al. Citation2008b). The mechanisms controlling LPC activation and differentiation are under intense investigation (Kung and Forbes Citation2009) and these processes could be driven by different signals acting onto the LPC niche as result of the stage (acute or chronic) and type of disease (parenchymal or biliary; Scadden Citation2006; Jones and Wagers Citation2008; Spee et al. Citation2010). Interestingly, Wnt signalling is involved in the LPC response in mice (Hu et al. Citation2007), rats (Apte et al. Citation2008) and humans (Yang et al. Citation2008; Spee et al. Citation2010) and activation of the Wnt pathway plays a significant role in LPC expansion.
3. Liver fibrosis
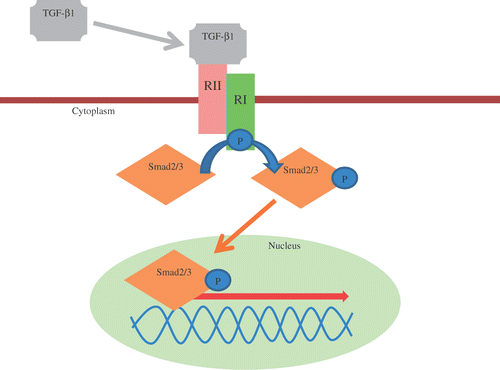
Hepatic fibrosis results of an accumulation of extracellular matrix (ECM) and when it progresses it can lead to cirrhosis (Friedman Citation2003). ECM deposition is the result of increased synthesis and/or decreased breakdown. The bulk of ECM in the fibrotic liver is produced by myofibroblast (MF)-like cells. Three different MF-like cells have been described in rat and man based on location and immunohistochemical profile (Cassiman et al. Citation2002; IJzer et al. Citation2010). These three types comprise portal or septal MF, interface MF and perisinusoidally located hepatic stellate cells (HSCs). HSCs are non-parenchymal, quiescent cells that, when activated, show a cytoplasmic alpha-smooth muscle actin (α-SMA) immunoreactivity. It has already been established that a transient increase of transforming growth factor β1 (TGF-β1) in the liver, mainly produced by the HSC, promotes fibrosis with the formation of extracellular matrix (ECM) components and suppresses hepatocyte proliferation (Michalopoulos Citation2007). The signalling responses to TGF-β1 are mediated by two receptors, TGF-β receptor type I (TGF-β RI) and TGF-β receptor type II (TGF-β RII) at the cell surface and their intracellular substrates, the Smad proteins (). Recently, is has been shown that β-catenin dependent Wnt signalling is involved in keeping HSCs quiescent (Kordes et al. Citation2008). Important regulators of proteolytic activity which determine ECM turnover are plasminogen activator-plasmin system components. Plasmin, generated from circulating plasminogen by proteolytic cleavage by uPA, is capable of degrading ECM components directly by proteolysis, and indirectly by inhibiting deposition of ECM by the activation of matrix metalloproteinases (MMPs). In this way, an upregulation of uPA in the liver might inhibit the deposition of ECM and reverse hepatic fibrosis (Murphy et al. Citation1992). Matrix degrading proteases are inhibited in their activity by the tissue inhibitor of metalloproteinase (TIMP). Overall, matrix remodelling is an important component of liver regeneration and the point at which cirrhosis or extensive fibrosis becomes irreversible has not been well defined (Bonis et al. Citation2001; Iredale Citation2007).
Figure 2. TGF-β/Smad pathway. Notes: The signalling response to TGF-β1 is mediated by its two receptors TGF-β RII for binding and TGF-β RI for phosphorylation at the cell surface and the intracellular substrates, the Smad proteins. TGF-β: transforming growth factor β; RI: transforming growth factor β receptor 1; RII transforming growth factor β receptor 2.
4. Apoptosis and necrosis
Several modes of cell death have been classified, apoptosis and necrosis being the most important ones (Kroemer et al. Citation2009). Apoptosis, a regulated form of cell death, can be initiated through two fundamental pathways: the intracellular organelle-based intrinsic pathway and the death receptor or extrinsic pathway. Both pathways usually interact with each other (Green and Reed Citation1998; Kim et al. Citation2006). The mechanisms involved in apoptosis connect cell death to inflammation and fibrosis (Kim et al. Citation2006). Regulation of the apoptotic cascades in liver cells is complex, but appears to be commonly triggered via the extrinsic pathway through activation of death receptors (Fas, TNF receptor 1, TRAIL-R1 and R2; Los et al. 1995). Mitochondrial dysfunction plays an important role in augmenting the apoptotic processes and integrating death receptor initiated and stress signals into a common final pathway. Mitochondrial release of cytochrome C is a common event in apoptosis and triggers a final caspase-dependent apoptosis cascade resulting in cellular fragmentation (Hengartner Citation2000; Canbay et al. Citation2004). Depending on the balance between the production and removal by antioxidant systems, reactive oxygen species (ROS) may function as signalling molecules to induce damage to mitochondria and disrupt cellular function and integrity, finally resulting in apoptosis (Koopman et al. Citation2010). ROS includes the superoxide anion (), hydrogen peroxide (H2O2) and the highly reactive hydroxyl radical (
) (Valko et al. Citation2007). Necrosis represents a form of cell death with simultaneous disruption of multiple pathways and is accompanied by complete release of cellular constituents into the extracellular space, which can initiate an inflammatory response. The mode of cell death by toxic stimuli, e.g. ROS, seems to be concentration dependent, with low concentrations inducing apoptosis and high concentrations necrosis (Kroemer et al. Citation2009).
5. Regulation of copper homeostasis
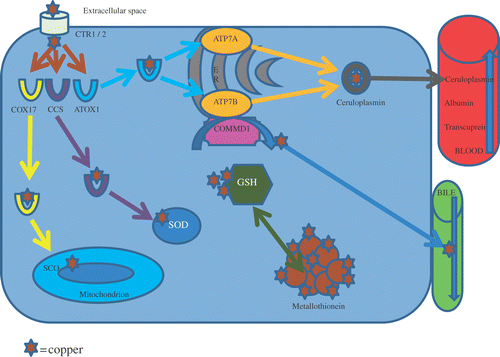
Copper, an essential micronutrient, plays an important function as a cofactor for a number of cellular processes (Culotta and Gitlin Citation2001). Copper homeostasis is regulated by copper uptake in the gastro-intestinal tract, distribution through the body, and excretion mainly into the bile (). At the cellular level, the copper transporters 1 and 2 (CTR1/2) regulate the intake, after which the distribution in the cell is mediated via the so called copper chaperones (COX17, CCS, ATOX1). ATOX1 delivers copper to the copper transporting ATPases (ATP7A (Menkes disease gene) and ATP7B (Wilson's disease gene)) in the secretory pathway, CCS distributes copper to Cu/Zn superoxide dismutase (SOD1), and COX17 delivers copper to cytochrome c oxidase in the mitochondria. Copper efflux occurs via the copper ATPase pumps encoded by ATP7B and ATP7A. Both these proteins exhibit copper-induced trafficking and redistribution in response to changes in copper abundance (Lutsenko and Petris Citation2003; Lutsenko Citation2010). In intestinal epithelial cells, copper is transported across the basolateral membrane by ATP7A, where it is transported via the portal circulation to the liver. Excess liver copper is removed by biliary excretion via the ATP7B copper pump and ATP7B deficiency results in a loss of biliary excretion of copper as well as a lack of copper incorporation into secreted proteins produced in the liver, with ceruloplasmin (CP) being the most notable example (Mercer Citation2001). CP is a metalloprotein that binds copper during synthesis and is secreted into the blood. In plasma, 90% of copper is bound to CP. Free intracellular copper is sequestered by metallothioneins. Metallothionein (MT1A) is known to be regulated by intracellular copper levels (Coyle et al. Citation2002). MT1A mRNA as well as protein concentrations are known to increase after acute copper administration (Packman et al. Citation1987). Spee et al. found MT1A mRNA concentrations to be significantly decreased in dogs with primary copper toxicosis (Spee et al. Citation2006). Free copper is highly toxic due to its ability to generate hydroxyl radicals and high hepatic levels of copper induce oxidative stress (Koopman et al. Citation2010). Recent data suggest that both hepatocellular necrosis and apoptosis may be triggered by copper-induced cell damage (Ferenci et al. Citation1996; Strand et al. Citation1998).
Figure 3. Copper trafficking within the cell. Notes: Copper enters the cell via copper transporters, after which the distribution in the cell is mediated via the copper chaperones to the endoplasmatic reticulum, mitochondrion or metallothionein. Copper efflux to bile or blood occurs via the copper ATPase pumps. CRT: copper transporter; COX17 (cytochrome c oxidase assembly protein), CCS (copper transporter for superoxide dismutase), ATOX1 (anti-oxidant protein 1); SCO, target-specific copper transporters; SOD, superoxide dismutase; COMMD1, copper metabolism murr1 domain containing protein 1; GSH, glutathione; ATP7A, Menkes disease protein; ATP7B, Wilson's disease protein.
6. Oxidative stress
Free copper ions are capable of forming hydroxyl radicals via the Haber–Weiss reaction (Bremner Citation1998). The final outcome of this reaction is a toxic hydroxyl radical which can damage DNA, proteins and lipids and can create other ROS [60].
To be protected against these toxic radicals, cells possess several important proteins and molecules involved in the defence against oxidative stress. Most of the anti-oxidants can be grouped into either enzymatic defences or non-enzymatic defences (Gaetke and Chow Citation2003).
6.1. The non-enzymatic defences
Glutathione (GSH) is part of the cellular non-enzymatic antioxidant system, which also includes vitamins C and E, caretenoids and flavonoids. GSH is present in high concentrations in the cytosol, nucleus and mitochondria and is very important in the protection against oxidative damage, both by direct reaction with ROS and as an electron donor for peroxidases (Koopman et al. Citation2010). GSH is oxidised to GSSG (oxidised glutathione) by ROS and glutathione peroxidise (GPX). Glutathione Reductase (GR) continually recycles GSSG back to GSH (Costa et al. Citation2003), keeping the GSH/GSSG ratio high and under normal circumstance 99% of GSH is reduced (Reed Citation1990).
6.2. The enzymatic defences: Cu-Zn superoxide dismutase (SOD), catalase and glutathione peroxidise (GPX)
The superoxide dismutase family, SOD-1 (Cu/ZnSOD), -2 (MnSOD) and 3 (ECSOD), is specialised in eliminating from external sources and those produced within the mitochondrial matrix as byproducts of oxygen metabolism through the electron transport chain (McCord and Fridovich Citation1969). SOD is a metalloenzyme, essential for the dismutation of
to H2O2 in the cytosol:
Regulation of SOD genes plays an important role in balancing the concentration of ROS. Diverse transcriptional factors like Nuclear Factor-KappaB (NK-κB), Activator Protein 1 and 2 (AP-1 and AP-2) and Specificity Protein 1 (Sp1) have been shown to play important roles in regulating expression levels of SOD (Miao and St Clair Citation2009).
In most mammalian cell types H2O2 is broken down by catalase and GPX:
7. Copper-associated liver diseases
Hepatic copper accumulation can result from increased copper intake, inherited metabolic defects in the hepatic copper metabolism or from a reduced biliary excretion of copper. In dogs, regarding inherited metabolic copper storage diseases, copper always starts to accumulate centrolobularly, which differs from secondary copper loading due to cholestasis or increased intake which is localised to the periportal area (Fuentealba et al. Citation1989; Cisternas et al. Citation2005; van den Ingh et al. Citation2006).
7.1. Wilson's disease in man
Wilson's disease (WD) is an autosomal recessive inherited copper storage disorder which was first described in 1912 (Wilson Citation1912; Bearn and Kunkel Citation1953). The diseased appeared to be caused by mutations or reduced expression of the ATP7B gene. An impaired function of ATP7B reduces the excretion of copper into the bile or incorporation into ceruloplasmin (CP) (Tanzi et al. Citation1993). In the blood, CP concentration is reduced and the non-CP bound copper is increased (Bearn and Kunkel Citation1953). The therapeutic options of patients with WD include medical treatment, dietary restrictions and orthotopic liver transplantation (OLT). Pharmacological treatments for WD include chelating agents (penicillamine, trientine) and zinc salts (Brewer et al. Citation1987; Scheinberg et al. Citation1987). OLT is reserved for patients with end stage liver failure. After successful OLT, patients require no further therapy specific to WD. The prognosis for patients who comply with pharmacotherapy is excellent, even if CH or cirrhosis is present at the time of diagnosis (Schilsky et al. Citation1991). Although the affected gene for WD is known, and progress has been made into the molecular events leading to WD (De Bie et al. 2007) further molecular characterisation is feasible and necessary. Several inbred rodent strains are available to study the consequences and to dissect the involved disease mechanisms of copper overload in the liver (Biempica et al. Citation1988; Wu et al. Citation1994; Coronado et al. Citation2001; Roberts et al. Citation2008).
7.2. The toxic milk (tx) mouse and the Jackson toxic (tx j) mouse
There are two versions of tx mouse (toxic milk and the Jackson toxic milk mouse), both having missense mutations in ATP7B that do not disrupt ATP7B synthesis but affect the function of the Wilson's disease protein (Biempica et al. Citation1988; Coronado et al. Citation2001; Roberts et al. Citation2008). The tx mouse, although an accepted animal model for WD, shows differences with WD besides clear similarities: liver morphology of adult tx mice show significant differences form WD livers and neurological defects have not been observed (Biempica et al. Citation1988; Buiakova et al. Citation1999; Fuentealba and Aburto Citation2003).
7.3. The Long-Evans Cinnamon (LEC) rat
LEC rats have a naturally occurring deletion in the ATP7B gene, resulting in the loss of the protein and they share many clinical and biochemical features with WD, but neurological defects have not been observed (Wu et al. Citation1994; Buiakova et al. Citation1999; Fuentealba and Aburto Citation2003). Hepatic copper accumulation occurs prior to the development of hepatitis and hepatitis can be prevented by treatment with penicillamine. When left untreated, they develop CH and often progress into hepatocellular carcinoma (Masuda et al. Citation1988; Sawaki et al. Citation1990).
7.4. ATP7B-/- mice
Huster et al. have evaluated ATP7B-/- mice, genetically engineered by targeted inactivation of the WD gene (spliced mRNA is present but the ATP7B protein is not produced), as a model for analysis for copper toxicity in the liver (Buiakova et al. Citation1999; Huster et al. Citation2006, Citation2007). They also compared these mice with tx mice and LEC rats and found besides clear similarities also differences: LEC rats developed carcinomas which was not observed in the ATP7B-/- mice and tx mice had milder liver pathology compared with the ATP7B-/- (Huster et al. Citation2006). A remarkable finding was the time-dependent decrease in hepatic copper content, most likely due to age-dependent upregulation of copper-handling mechanisms independent of ATP7B (Huster et al. Citation2006). Overall, it was concluded that ATP7B -/- mice represent a valuable model to study hepatic WD (Lutsenko Citation2008).
7.5. Canine copper associated CH
Canine CH is a complex disease which occurs frequently in dogs (Poldervaart et al. Citation2009). Clinical signs can develop at any age, and the history of a CH patient indicates illness present for several weeks to months. It is morphologically characterised by fibrosis, hepatocellular necrosis and apoptosis, a mononuclear or mixed inflammatory cell infiltrate, ductular proliferation and eventually nodular regeneration and cirrhosis. Copper storage is a well-known cause for the development of CH in dogs. For the diagnosis of copper associated CH (CACH), a histochemical rubeanic acid copper stain (graded from 0–5) demonstrating semi-quantitative copper grades of three or more in liver, is sufficient (Spee et al. Citation2006b; van den Ingh et al. Citation2006). The treatment of CACH patients exists of either reduced intake of copper via the diet or by oral zinc administration, or increased copper excretion from the body using a copper chelating agent, e.g. penicillamine, or a combination of both. For only one dog breed, the Bedlington Terrier (BT), the genetic defect has been found (van de Sluis et al. Citation2002). In this breed, a lack of exon-2 of the COMMD1 gene is correlated with copper toxicosis. The precise role of this protein is not yet clear, but increased evidence indicates the involvement of COMMD1 in cellular trafficking and ubiquitination processes (Burkhead et al. Citation2009). An increased number of breeds have been discovered with primary copper toxicosis. Hoffmann et al. demonstrated a form of CACH in the Labrador Retriever (Hoffmann et al. Citation2006) and a retrospective review of CH cases referred to our clinic revealed that one third of the CH cases was copper related (Poldervaart et al. Citation2009). In addition, primary copper toxicosis was overrepresented in English and American cocker spaniels, Cavalier King Charles spaniels, Labrador and Golden retrievers, West Highland white terriers and German pointers (Poldervaart et al. Citation2009).
8. Functional aspects of COMMD1
COMMD1 is the founding member of a recently discovered family of COMMD. This protein family consists of ten members which are widely conserved throughout evolution and share certain functional properties (Maine and Burstein Citation2007). COMMD1 stands for Copper Metabolism MURR1 Domain protein 1 and plays an important role in the regulation of ATP7B, NF-κB, and delta epithelial sodium channels (Biasio et al. Citation2004; de Bie et al. 2007b; Burkhead et al. Citation2009). In addition, COMMD1 restricts HIV-1 replication in resting T lymphocytes by inhibiting basal and cytokine-stimulated NF-κB activity (Ganesh et al. Citation2003). COMMD1 was shown to bind copper in vitro, but whether this binding represents an in vivo property is uncertain (Narindrasorasak et al. Citation2007). A role of COMMD1 in copper export was demonstrated by an increased retention of copper in different cell cultures in which COMMD1 was down regulated with small interfering RNA (Burstein et al. Citation2004; Spee et al. Citation2007). However, the precise function of COMMD and the mechanism through which COMMD1 performs its multiple roles are not yet understood. COMMD1 appears to be involved in the proteolysis of ATP7B (Maine and Burstein Citation2007) and Maine et al. demonstrated that XIAP (X-linked IAP (inhibitor of apoptosis)) functions as the ubiquitin ligase of COMMD1 (Maine et al. Citation2009). Van den Berghe et al. demonstrated that ATP7B protein misfolding leads to the induction of an increased COMMD1 binding, consistent with a proposed general role of COMMD1 in protein folding, maturation and degradation (van den Berghe et al. Citation2009). Burkhead et al. proposed COMMD1 as a scaffold protein in a distinct sub-compartment of the endocytic pathway and that it functions as a regulator of structurally unrelated membrane transporters (Burkhead et al. Citation2009). Weiss et al. concluded that the COMMD1 protein plays a role in the copper excretion pathway but is not involved in the copper mediated translocation of ATP7B (Weiss et al. Citation2008a). Vonk et al. showed COMMD1 to be a regulator of SOD maturation and activity and might be involved in the defense against toxic superoxide anions (Vonk et al. Citation2010). To understand the in vivo role of COMMD1 van der Sluis et al. created COMMD1 knockout mice, but the mutation was embryonically lethal (van de Sluis et al. Citation2007). Gene expression analysis revealed that the HIF (hypoxia inducible factor) pathway was upregulated when COMMD1 was deficient. It was unclear, however, whether this was a direct effect of lack of COMMD1 or due to changes in the placental development. In another paper, van der Sluis et al. demonstrated that COMMD1 promotes the proteolysis of HIF-1α (van de Sluis et al. Citation2009). Overall, the COMMD1 protein seems to be a kind of traffic agent in escorting target proteins to the proteasome for further breakdown or incorporation in (apical) membranes.
9. Conclusions
Pathways of regeneration and fibrosis can be studied in vitro in cell culture or tissue slices, and in vivo in zebrafish, mouse or rat models. Proof of principle for new strategies of intervention can be obtained in rodent models. However, it is still a giant step from these fundamental animal model studies to the clinical application. The availability of a suitable large animal model for such studies will greatly enhance the selection and validation of effective new strategies before they enter the expensive and time consuming clinical phase.
In order to find the most suitable large animal model it is important to recognise that the typical histopathological reaction pattern of the liver is different between mammalian species.
Based on our observations that man and dog share the same hepatopathies and have identical clinical, pathological and pathogenetic reaction patterns during the development of liver disease, the dog seems to be a properly suited species to test new therapeutic strategies.
With this recognition, the need for a well controlled and reproducible form of canine hepatitis became apparent. We have established a dog model with copper-induced hepatitis due to a COMMD1 deficiency with excellent properties for the evaluation of anti-fibrotic and anti-oxidant therapies and liver cell transplantation experiments. Different pathways (fibrosis, regeneration, copper metabolism and oxidative stress) have been characterised. These dogs combined with state-of-art clinical treatment options, close collaboration with owners and breeding organisations, detailed histological knowledge and almost unlimited molecular tools allow to investigate the feasibility of hepatocyte, or (adult) stem cell- and/or gene-therapy to cure CH in man (‘s best friend) and to evaluate new therapeutic strategies for WD.
References
- Apte , U , Thompson , MD , Cui , S , Liu , B , Cieply , B and Monga , SP . 2008 . Wnt/β-catenin signaling mediates oval cell response in rodents . Hepatology , 47 ( 1 ) : 288 – 295 .
- Bearn , AG and Kunkel , HG . 1953 . Genetic and biochemical aspects of Wilson's disease . Am J Med , 15 ( 4 ) : 442 – 446 .
- Biasio , W , Chang , T , McIntosh , CJ and McDonald , FJ . 2004 . Identification of Murr1 as a regulator of the human delta epithelial sodium channel . J Biol Chem , 279 ( 7 ) : 5429 – 5434 .
- Biempica , L , Rauch , H , Quintana , N and Sternlieb , I . 1988 . Morphologic and chemical studies on a murine mutation (toxic milk mice) resulting in hepatic copper toxicosis . Lab Invest , 59 ( 4 ) : 500 – 508 .
- Bird , TG , Lorenzini , S and Forbes , SJ . 2008 . Activation of stem cells in hepatic diseases . Cell Tissue Res , 331 ( 1 ) : 283 – 300 .
- Bonis , PA , Friedman , SL and Kaplan , MM . 2001 . Is liver fibrosis reversible? . N Eng J Med , 344 ( 6 ) : 452 – 454 .
- Borowiak , M , Garratt , AN , Wüstefeld , T , Strehle , M , Trautwein , C and Birchmeier , C . 2004 . Met provides essential signals for liver regeneration . Proc Natl Acad Sci USA , 101 ( 29 ) : 10608 – 10613 .
- Bremner , I . 1998 . Manifestations of copper excess . Am J Clin Nutr , 67 ( 5 Suppl ) : 1069S – 1073S .
- Brewer , GJ , Terry , CA , Aisen , AM and Hill , GM . 1987 . Worsening of neurologic syndrome in patients with Wilson's disease with metal penicillamine therapy . Arch Neurol , 44 ( 5 ) : 490 – 493 .
- Buiakova , OI , Xu , J , Lutsenko , S , Zeitlin , S , Das , K , Das , S , Ross , BM , Mekios , C , Scheinberg , IH and Gilliam , TC . 1999 . Null mutation of the murine ATP7B (Wilson disease) gene results in intracellular copper accumulation and late-onset hepatic nodular transformation . Hum Mol Genet , 8 ( 9 ) : 1665 – 1671 .
- Burke , ZD and Tosh , D . 2006 . The Wnt/beta-catenin pathway: master regulator of liver zonation? . Bioessays , 28 ( 11 ) : 1072 – 1077 .
- Burkhead , JL , Organ , CT , Shinde , U , Haddock , G and Lutsenko , S . 2009 . COMMD1 forms oligomeric complexes targeted to the endocytic membranes via specific interactions with phosphatidylinositol 4,5-biphosphate . J Biol Chem , 284 ( 1 ) : 696 – 707 .
- Burstein , E , Ganesh , L , Dick , RD , van De Sluis , B , Wilkinson , JC , Klomp , LW , Wijmenga , C , Brewer , GJ , Nabel , GJ and Duckett , CS . 2004 . A novel role for XIAP in copper homeostasis through regulation of MURR1 . EMBO J , 23 ( 1 ) : 244 – 254 .
- Canbay , A , Friedman , S and Gores , GJ . 2004 . Apoptosis: the nexus of liver injury and fibrosis . Hepatology , 39 ( 2 ) : 273 – 278 .
- Cassiman , D , Libbrecht , L , Desmet , V , Denef , C and Roskams , T . 2002 . Hepatic stellate cell/myofibroblast subpopulations in fibrotic human and rat livers . J Hepatol , 36 ( 2 ) : 200 – 209 .
- Cisternas , FA , Tapia , G , Arredondo , M , Cartier-Ugarte , D , Romanque , P , Sierralta , WD , Vial , MT , Videla , LA and Araya , M . 2005 . Early histological and functional effects of chronic copper exposure in rat liver . Biometals , 18 ( 5 ) : 541 – 551 .
- Coronado , V , Nanji , M and Cox , DW . 2001 . The Jackson toxic milk mouse as a model for copper loading . Mamm Genome , 12 ( 10 ) : 793 – 795 .
- Costa , NJ , Dahm , CC , Hurrell , F , Taylor , ER and Murphy , MP . 2003 . Interactions of mitochondrial thiols with nitric oxide . Antioxid Redox Signal , 5 ( 3 ) : 291 – 305 .
- Coyle , P , Philcox , JC , Carey , LC and Rofe , AM . 2002 . Metallothionein: the multipurpose protein . Cellular and Molecular Life Sciences , 59 : 627 – 47 .
- Culotta , VC and Gitlin , JD . 2001 . Disorders of copper transport , Scriver CR, Beauclet AL, Sly W, Valle D, editors. . Vol. II. The metabolic and molecular bases of inherited disease. New York (NY): McGraw-Hill. p. 3105–3126
- Darnell Jr , JE . 1997 . STATs and gene regulation . Science , 277 ( 5332 ) : 1630 – 1635 .
- de Bie , P , Muller , P , Wijmenga , C and Klomp , LW . 2007a . Molecular pathogenesis of Wilson and Menkes disease; correlation of mutations with molecular defects and disease phenotypes . J Med Gen , 44 ( 11 ) : 673 – 88 .
- de Bie , P , van de Sluis , B , Burstein , E , van de Berghe , PV , Muller , P , Berger , R , Gitlin , JD , Wijmenga , C and Klomp , LW . 2007b . Distinct Wilson's disease mutations in ATP7B are associated with enhanced binding to COMMD1 and reduced stability of ATP7B . Gastroenterology , 133 ( 4 ) : 1316 – 1326 .
- Fausto , N and Campbell , JS . 2003 . The role of hepatocytes and oval cells in liver regeneration and repopulation . Mech Dev , 120 ( 1 ) : 117 – 130 .
- Fausto , N , Campbell , JS and Riehle , KJ . 2006 . Liver regeneration . Hepatology , 43 ( 2 suppl 1 ) : S45 – S53 .
- Ferenci , P , Gilliam , TC , Gitlin , JD , Packman , S , Schilsky , ML , Sokol , RJ and Sternlieb , I . 1996 . An international symposium on Wilson's and Menkes’diseases . Hepatology , 24 ( 4 ) : 952 – 958 .
- Fisher , RA and Strom , SC . 2006 . Human hepatocyte transplantation: worldwide results . Transplantation , 82 ( 4 ) : 441 – 449 .
- Friedman , SL . 2003 . Liver fibrosis – from bench to bedside . J Hepatol , 38 ( Suppl 1 ) : S38 – S53 .
- Fuentealba , I , Haywood , S and Trafford , J . 1989 . Variations in the intralobular distribution of copper in the livers of copper-loaded rats in relation to the pathogenesis of copper storage disease . J Comp Path , 100 ( 1 ) : 1 – 11 .
- Fuentealba , IC and Aburto , EM . 2003 . Animal models of copper-associated liver disease . Comp Hepatol , 3;2 ( 1 ) : 5
- Gaetke , LM and Chow , CK . 2003 . Copper toxicity, oxidative stress, and antioxidant nutrients . Toxicology , 189 ( 1–2 ) : 147 – 163 .
- Ganesh , L , Burstein , E , Guha-Niyogi , A , Louder , MK , Mascola , JR , Klomp , LW , Wijmenga , C , Duckett , CS and Nabel , GJ . 2003 . The gene product Murr1 restricts HIV-1 replication in resting CD4+ lymphocytes . Nature , 426 ( 6968 ) : 853 – 857 .
- Green , DR and Reed , JC . 1998 . Mitochondria and apoptosis . Science , 281 ( 5381 ) : 1309 – 1312 .
- Hengartner , MO . 2000 . The biochemistry of apoptosis . Nature , 407 ( 6805 ) : 770 – 776 .
- Hoffmann , G , van den Ingh , TSGAM , Bode , P and Rothuizen , J . 2006 . Copper-associated chronic hepatitis in Labrador Retrievers . J Vet Intern Med , 20 ( 4 ) : 856 – 861 .
- Hu , M , Kurobe , M , Jeong , YJ , Fuerer , C , Ghole , S , Nusse , R and Sylvester , KG . 2007 . Wnt/β- catenin signaling in murine hepatic transit amplifying progenitor cells . Gastroenterology , 133 ( 5 ) : 1579 – 1591 .
- Huh , CG , Factor , VM , Sánchez , A , Uchida , K , Conner , EA and Thorgeirsson , SS . 2004 . Hepatocyte growth factor/c-met signalling pathway is required for efficient liver regeneration and repair . Proc Natl Acad Sci USA , 101 ( 13 ) : 4477 – 4482 .
- Huster , D , Finegold , MJ , Morgan , CT , Burkhead , JL , Nixon , R , Vanderwerf , SM , Gilliam , CT and Lutsenko , S . 2006 . Consequences of copper accumulation in the livers of the Atp7b-/- (Wilson disease gene) knockout mice . Am J Pathol , 168 ( 2 ) : 423 – 434 .
- Huster , D , Purnat , TD , Burkhead , JL , Ralle , M , Fiehn , O , Stuckert , F , Olson , NE , Teupser , D and Lutsenko , S . 2007 . High copper selectively alters lipid metabolism and cell cycle machinery in the mouse model of Wilson disease . J Biol Chem , 282 ( 11 ) : 8343 – 8355 .
- IJzer , J , Schotanus , BA , Vander Borght , S , Roskams , TA , Kisjes , R , Penning , LC , Rothuizen , J and van den Ingh , TSGAM . 2010 . Characterisation of the hepatic progenitor cell compartment in normal liver and in hepatitis: an immunohistochemical comparison between dog and man . Vet J , 184 ( 3 ) : 308 – 314 .
- Iredale , JP . 2007 . Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ . J Clin Invest , 117 ( 3 ) : 539 – 548 .
- Jakubowski , A , Ambrose , C , Parr , M , Lincecum , JM , Wang , MZ , Zheng , TS , Browning , B , Michaelson , JS , Baetscher , M Wang , B . 2005 . TWEAK induces liver progenitor cell proliferation . J Clin Invest , 115 ( 9 ) : 2330 – 2340 .
- Jones , DL and Wagers , AJ . 2008 . No place like home: anatomy and function of the stem cell niche . Nat Rev Mol Cell Biol , 9 ( 1 ) : 11 – 12 .
- Kim , R , Emi , M , Tanabe , K , Murakami , S , Uchida , Y and Arihiro , K . 2006 . Regulation and interplay of apoptotic and non-apopototic cell death . J Pathol , 208 ( 3 ) : 319 – 326 .
- Koopman , WJ , Nijtmans , LG , Dieteren , CE , Roestenberg , P , Valsecchi , F , Smeitink , JA and Willems , PH . 2010 . Mammalian mitochondrial complex I: biogenesis, regulation and reactive oxygen species generation . Antioxid Redox Signal , 12 ( 12 ) : 1431 – 1470 .
- Kordes , C , Sawitza , I and Häussinger , D . 2008 . Canonical Wnt signaling maintains the quiescent stage of hepatic stellate cells . Biochem Biophys Res Commun , 367 ( 1 ) : 116 – 123 .
- Kroemer , G , Galluzzi , L , Vandenabeele , P , Abrams , J , Alnemri , ES , Baehrecke , EH , Blagosklonny , MV , El-Deiry , WS , Golstein , P Green , DR . 2009 . Classification of cell death: recommendations of the Nomenclature Committee on Cell Death (2009) . Cell Death Differ. (2009) , 16 ( 8 ) : 3 – 11 .
- Kung , JW and Forbes , SJ . 2009 . Stem cells and liver repair . Curr Opin Biotechnol , 20 ( 5 ) : 1 – 7 .
- LeCouter , J , Moritz , DR , Li , B , Phillips , GL , Liang , XH , Gerber , HP , Hillan , KJ and Ferrara , N . 2003 . Angiogenesis-independent endothelial protection of liver: role of VEGFR-1 . Science , 22 ( 5608 ) : 309 – 325 .
- Libbrecht , L and Roskams , T . 2002 . Hepatic progenitor cells in human liver diseases . Semin Cell Dev Biol , 13 ( 6 ) : 389 – 396 .
- Los , M , Van de Craen , M , Penning , LC , Schenk , H , Westendorp , M , Baeuerle , PA , Dröge , W , Krammer , PH , Fiers , W and Schulze-Osthoff , K . 1995 . Requirement of an ICE/CED-3 protease for Fas/APO-1 mediated apoptosis . Nature , 375 ( 6526 ) : 81 – 83 .
- Lutsenko , S . 2008 . Atp7b-/- mice as a model for studies of Wilson's disease . Biochem Soc Trans , 36 ( Pt6 ) : 1233 – 1238 .
- Lutsenko , S . 2010 . Human copper homeostasis: a network of interconnected pathways . Curr Opinion Chem Biol , 14 ( 2 ) : 211 – 217 .
- Lutsenko , S and Petris , MJ . 2003 . Function and regulation of the mammalian copper-transporting atpases: insights from biochemical and cell biological approaches . J Membr Biol , 191 ( 1 ) : 1 – 12 .
- Maine , GN and Burstein , E . 2007 . COMMD proteins: COMMing to the scene . Cell Mol Life Sci , 64 ( 15 ) : 1997 – 2005 .
- Maine , GN , Mao , X , Muller , PA , Komarck , CM , Klomp , LW and Burstein , E . 2009 . COMMD1 expression is controlled by critical residues that determine XIAP binding . Biochem J , 417 ( 2 ) : 601 – 609 .
- Masuda , R , Yoshida , MC , Sasaki , M , Dempo , K and Mori , M . 1988 . High susceptibility to hepatocellular carcinoma development in LEC rats with hereditary hepatitis . Jpn J Cancer Res , 79 ( 7 ) : 828 – 835 .
- McCord , JM and Fridovich , I . 1969 . Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) . J Biol Chem , 244 ( 22 ) : 6049 – 6055 .
- Mercer , JF . 2001 . The molecular basis of copper-transport diseases . Trends Mol Med , 7 ( 2 ) : 64 – 69 .
- Miao , L and St Clair , DK . 2009 . Regulation of superoxide dismutase genes: implications in disease . Free Radic Biol Med , 47 ( 4 ) : 344 – 356 .
- Michalopoulos , GK . 2007 . Liver regeneration . J Cell Physiol , 213 ( 2 ) : 286 – 300 .
- Michalopoulos , GK and DeFrances , MC . 1997 . Liver regeneration . Science , 276 ( 5309 ) : 60 – 66 .
- Mohammed , FF , Pennington , CJ , Kassiri , Z , Rubin , Js , Soloway , PD , Ruther , U , Edwards , DR and Khokha , R . 2005 . Metalloproteinase inhibitor TIMP-1 affects hepatocyte cell cycle via HGF activation in murine liver regeneration . Hepatology , 41 ( 4 ) : 857 – 867 .
- Murphy , G , Atkinson , S , Ward , R , Gavrilovic , J and Reynolds , JJ . 1992 . The role of plasminogen activators in the regulation of connective tissue metalloproteinases . Ann N Y Acad Sci , 4 ( 667 ) : 1 – 12 .
- Naldini , L , Tamagnone , L , Vigna , E , Sachs , M , Hartmann , G , Birchmeier , W , Daikura , Y , Tsubouchi , H , Blasi , F and Comoglio , PM . 1992 . Extracellular proteolytic cleavage by urokinase is required for activation of hepatocyte growth factor/scatter factor . EMBO J , 11 ( 13 ) : 4825 – 4833 .
- Naldini , L , Vigna , E , Bardelli , A , Follenzi , A , Galimi , F and Comoglio , PM . 1995 . Biological activation of pro-HGF (hepatocyte growth factor) by urokinase is controlled by a stoichiometric reaction . J Biol Chem , 270 ( 2 ) : 603 – 611 .
- Narindrasorasak , S , Kulkarni , P , Deschamps , P , She , YM and Sarkar , B . 2007 . Characterization and copper binding properties of human COMMD1 (MURR1) . Biochemistry , 46 ( 11 ) : 3116 – 3128 .
- Packman , S , Palmiter , RD , Karin , M and O’Toole , C . 1987 . Metallothionein messenger RNA regulation in the mottled mouse and Menkes kinky hair syndrome . J Clin Invest , 79 ( 5 ) : 1338 – 1342 .
- Poldervaart , JH , Favier , RP , van den Ingh , TSGAM , Penning , LC and Rothuizen , J . 2009 . Canine hepatitis in a referral population: a retrospective overview (2002–2006) . J Vet Intern Med , 23 ( 1 ) : 72 – 80 .
- Reed , D . 1990 . Glutathione: toxicological implications . Annu Rev Pharmacol toxicol , 30 : 603 – 631 .
- Roberts , EA , Robinson , BH and Yang , S . 2008 . Mitochondrial structure and function in the untreated Jackson toxic milk (tx-j) mouse, a model for Wilson disease . Mol Genet Metab , 93 ( 1 ) : 54 – 65 .
- Roskams , T . 2006 . Different types of liver progenitor cells and their niches . J Hepatol , 45 ( 1 ) : 1 – 4 .
- Roskams , TA , Libbrecht , L and Desmet , VJ . 2003 . Progenitor cells in diseased human liver . Semin Liver Dis , 23 ( 4 ) : 385 – 396 .
- Roskams , TA , Theise , ND , Balabaud , C , Bhagat , G , Bhathal , PS , Bioulac-Sage , P , Brunt , EM , Crawford , JM , Crosby , HA Desmet , V . 2004 . Nomenclature of the finer branches of the biliary tree: canals, ductules, and ductular reactions in human livers . Hepatology , 39 ( 6 ) : 1739 – 1745 .
- Santoni-Rugiu , E , Jelnes , P , Thorgeirsson , SS and Bisgaard , HC . 2005 . Progenitor cells in liver regeneration: molecular responses controlling their activation and expansion . APMIS , 113 ( 11–12 ) : 876 – 902 .
- Sackett , SD , Li , Z , Hurtt , R , Gao , Y , Wells , RG , Brondell , K , Kaestner , KH and Greenbaum , LE . 2009 . Foxl1 is a marker of bipotential hepatic progenitor cells in mice . Hepatology , 49 ( 3 ) : 920 – 929 .
- Salgado , S , Garcia , J , Vera , J , Siller , F , Bueno , M , Miranda , A , Segura , A , Grijalva , G , Segura , J Orozco , H . 2000 . Liver cirrhosis is reverted by urokinase-type plasminogen activator gene therapy . Mol Ther , 2 ( 6 ) : 545 – 515 .
- Sawaki , M , Enomoto , K , Takahashi , H , Nakajima , Y and Mori , M . 1990 . Phenotype of preneoplastic and neoplastic liver lesions during spontaneous liver carcinogenesis of LEC rats . Carcinogenesis , 11 ( 10 ) : 1857 – 1861 .
- Scadden , DT . 2006 . The stem-cell niche as an entity of action . Nature , 441 ( 7097 ) : 1075 – 1079 .
- Scheinberg , IH , Jaffe , ME and Sternlieb , I . 1987 . The use of trientine in preventing the effects of interrupting penicillamine therapy in Wilson's disease . N Eng J Med , 317 ( 4 ) : 209 – 213 .
- Schilsky , ML , Scheinberg , IH and Sternlieb , I . 1991 . Prognosis of wilsonian chronic active hepatitis . Gastroenterology , 100 ( 3 ) : 762 – 767 .
- Schirmacher , P , Geerts , A , Jung , W , Pietrangelo , A , Rogler , CE and Diens , HP . 1993 . The role of Ito cells in the biosynthesis of HGF-SF in the liver . Exs , 65 : 285 – 299 .
- Schotanus , BA , van den Ingh , TSGAM , Penning , LC , Rothuizen , J , Roskams , TA and Spee , B . 2009 . Cross-species immunohistochemical investigation of the activation of the liver progenitor cell niche in different types of liver disease . Liver Int , 29 ( 8 ) : 1241 – 1252 .
- Shibata , C , Mizuguchi , T , Kikkawa , Y , Nobuoka , T , Oshima , H , Kawasaki , H , Kawamoto , M , Katsuramaki , T , Mitaka , T and Hirata , K . 2006 . Liver repopulation and long-term function of rat small hepatocyte transplantation as an alternative cell source for hepatocyte transplantation . Liver Transpl , 12 ( 1 ) : 78 – 87 .
- Spee , B , Arends , B , van den Ingh , TSGAM , Brinkhof , B , Nederbragt , H , IJzer , J , Roskams , T , Penning , LC and Rothuizen , J . 2006a . Transforming growth factor B-1 signalling in canine hepatic diseases: new models for human fibrotic liver pathologies . Liver Int , 26 ( 6 ) : 716 – 725 .
- Spee , B , Arends , B , van den Ingh , TSGAM , Penning , LC and Rothuizen , J . 2006b . Copper metabolism and oxidative stress in chronic inflammatory and cholestatic liver diseases in dogs . J Vet Intern Med , 20 ( 5 ) : 1085 – 1092 .
- Spee , B , Arends , B , van Wees , AM , Bode , P , Penning , LC and Rothuizen , J . 2007 . Functional consequences of RNA interference targeting COMMD1 in a canine hepatic cell line in relation to copper toxicosis . Anim Genet , 38 ( 2 ) : 168 – 170 .
- Spee , B , Carpino , G , Schotanus , BA , Katoonizadeh , A , Vander Borght , S , Gaudio , E and Roskams , T . 2010 . Characterisation of the activated liver progenitor cell niche, potential involvement of Wnt and Notch signaling . Gut , 59 ( 2 ) : 247 – 257 .
- Strand , S , Hofmann , WJ , Grambihler , A , Hug , H , Volkmann , M , Otto , G , Wesch , H , Mariani , SM , Hack , V Stremmel , W . 1998 . Hepatic failure and liver cell damage in acute Wilson's disease involve CD95 (APO-1/Fas) – mediated apoptosis . Nat Med , 4 ( 5 ) : 588 – 593 .
- Tanzi , RE , Petrukhin , K , Chernov , I , Pellequer , JL , Wasco , W , Ross , B , Romano , DM , Parano , E , Pavone , L Brzustowicz , LM . 1993 . The Wilson's disease gene is a copper-transporting ATPase with homology to the Menkes's disease gene . Nat Genet , 5 ( 4 ) : 344 – 350 .
- Taub , R . 2004 . Liver regeneration: from myth to mechanism . Nat Rev Mol Cell Biol , 5 ( 10 ) : 836 – 847 .
- Valko , M , Leibfritz , D , Moncol , J , Cronin , MT , Mazur , M and Telser , J . 2007 . Free radicals and antioxidants in normal physiological functions and human disease . Int J Biochem Cell Biol , 39 ( 1 ) : 44 – 84 .
- van den Berghe , PV , Stapelbroek , JM , Krieger , E , de Bie , P , van de Graaf , SF , de Groot , RE , van Beurden , E , Spijker , E , Houwen , RH Berger , R . 2009 . Reduced expression of ATP7B affected by Wilson disease-causing mutations is rescued by pharmalogical folding chaperones 4-phenylbutyrate and curcumin . Hepatology , 50 ( 6 ) : 1783 – 1795 .
- van den Ingh TSGAM, Van Winkle TJ, Cullen JM, Charles JA, Desmet VJ. 2006. WSAVA liver standardization group. Standards for clinical and histological diagnosis of canine and feline liver diseases, Chapter 7. Philadelphia (PA): Saunders Elsevier. Chapter 7, Morphological classification of parenchymal disorders of the canine and feline liver; p. 85–101.
- van de Sluis , B , Groot , AJ , Vermeulen , J , van der Wall , E , van Diest , PJ , Wijmenga , C , Klomp , LW and Vooijs , M . 2009 . COMMD1 promotes pVHL and O2-independent proteolysis of HIF-1alpha via HSP90/70 . PLoS One , 4 ( 10 ) : e7332
- van de Sluis , B , Muller , P , Duran , K , Chen , A , Groot , AJ , Klomp , LW , Liu , PP and Wijmenga , C . 2007 . Increased activity of hypoxia-inducible factor 1 is associated with early embryonic lethality in commd1 null mice . Mol Cell Biol , 27 ( 11 ) : 4142 – 4156 .
- van de Sluis , B , Rothuizen , J , Pearson , PL , van Oost , BA and Wijmenga , C . 2002 . Identification of a new copper metabolism gene by positional cloning in a purebred dog population . Hum Mol Genet , 11 ( 2 ) : 165 – 173 .
- Vonk , WI , Wijmenga , C , Berger , R , van de Sluis , B and Klomp , LW . 2010 . Cu/Zn Superoxide dismutase maturation and activity are regulated by COMMD1 . J Biol Chem , 285 ( 37 ) : 28991 – 289000 .
- Vonk , WIM , Wijmenga , C and van de Sluis , B . 2008 . Relevance of understanding mammalian copper homeostasis . Am J Clin Nutr , 88 ( 3 ) : 840S – 845S .
- Weber , A , Groyer-Picard , MT , Franco , D and Dagher , I . 2009 . Hepatocyte transplantation in animal models . Liver Transpl , 15 ( 1 ) : 7 – 14 .
- Weiss , KH , Lozoya , JC , Tuma , S , Gotthardt , D , Reichert , J , Ehehalt , R , Stremmel , W and Füllekrug , J . 2008a . Copper-induced translocation of the Wilson disease protein ATP7B independent of Murr1/COMMD1 and Rab7 . Am J Pathol , 173 ( 6 ) : 1783 – 1794 .
- Weiss , TS , Lichtenauer , M , Kirchner , S , Stock , P , Aurich , H , Christ , B , Brockhoff , G , Kunz-Schughart , LA , Jauch , KW Schlitt , HJ . 2008b . Hepatic progenitor cells from adult human livers for cell transplantation . Gut , 57 ( 8 ) : 1129 – 1138 .
- Wilson , SAK . 1912 . Progressive lenticular degeneration: a familial nervous disease associated with cirrhosis of the liver . Brain , 34 : 20 – 509 .
- Wu , J , Forbes , JR , Chen , HS and Cox , DW . 1994 . The LEC rat has a deletion in the copper transporting ATPase gene homologous to the Wilson disease gene . Nat Genet , 7 ( 4 ) : 541 – 545 .
- Yang , W , Yan , HX , Chen , L , Liu , Q , He , YQ , Yu , LX , Zhang , SH , Huang , DD , Tang , L Kong , XN . 2008 . Wnt/β-catenin signaling contributes to activation of normal and tumorigenic liver progenitor cells . Cancer Res , 68 ( 11 ) : 4287 – 4295 .
- Zimmers , TA , McKillop , IH , Pierce , RH , Yoo , JY and Koniaris , LG . 2003 . Massive liver growth in mice induced by systemic interleukin 6 administration . Hepatology , 38 ( 2 ) : 326 – 334 .