Abstract
Prompted by FAO/WHO's and the European Commission's recognition that documents on Good Farming Practices (GFPs) and Good Veterinary Practices (GVPs) in apicultural production are hardly available, part 1 of this contribution provides an update of current apicultural production and associated best practices to ensure animal and public health. Major bee health and disease prevention issues and risk management options at the primary production level are summarised with particular reference to the role of the veterinary practitioner/consultant and the official veterinarian in a control function in the safe production of honey.
1. Introduction
Apiculture (beekeeping) is the practice of the management of honey bees in hives, principally for the honey and pollination (ecosystem services). Hives of bees also produce other products such as wax, royal jelly, propolis and pollen. In addition, an important aspect of beekeeping is the production of bees, queens, package bees, etc. The assurance of bee health has recently become of ecological concern with the advent of phenomena such as Colony Collapse Disorder (Berenbaum Citation2007; EFSA Citation2008; Hendrikx et al. Citation2009; Higes et al. Citation2009; Vanengelsdorp et al. Citation2009; Neumann and Carreck Citation2010). As a result, the European Parliament has called upon the Commission ‘to incorporate into its veterinary policy, research into, and actions to tackle bee disease’ (European Parliament Citation2008). Each Member State funds bee health research to differing degrees, various international research projects are currently being conducted and research priorities from the apicultural production sector have been formulated, both at EU level and in each Member State (e.g. COLOSS (Neumann Citation2010a), BEE DOC (Bilikova and Simuth Citation2010; De Graaf Citation2010; Forsgren Citation2010; Moritz Citation2010; Paxton Citation2010) and STEP (Neumann Citation2010b) Bee SHOP (Jaffé et al. Citation2008), ALARM (Grabaum et al. Citation2006), APENET (Mutinelli et al. Citation2009)).
The European Rapid Alert System for Food and Feed (RASFF) has in the past few years also substantiated claims that contaminated and hence potentially unsafe honey is still being produced in the European Union and beyond (RASFF Citation2004, Citation2005, Citation2006, Citation2007, Citation2008). It is now timely that the veterinary profession is updated on how it can help to improve apicultural performance.
The role of the veterinarian in apiculture has to date been a modest one, because in some countries the responsibility for bee health does not lie with the veterinary authorities. Hence, in many veterinary curricula, bee health and disease prevention (if at all considered important enough to be specifically addressed at the undergraduate level) is usually restricted to basic bee pathology and sometimes covered only as part of the elective study programme. However, in some countries commercial apiculture is significant and beekeepers are increasingly seeking the support of knowledgeable scientists to advise them on disease prevention and treatment, safe honey production and the related legislative aspects. Also, for veterinarians in a control function (‘official veterinarians’ e.g. those affiliated with the competent authorities) a more solid knowledge of beekeeping practices and associated animal and public health issues is necessary to ensure the performance of responsible inspection and auditing exercises. Consequently, postgraduate Diploma courses for veterinarians who professionally have to deal with bee health and honey safety (Apimondia.org Citation2009) as well as a blog for veterinary experts in honey bee pathologies (Apivet.eu Citation2009) are now being offered.
Probably the most important role for veterinarians in apiculture is education, because education of beekeepers is crucial in the correct identification of bee diseases, and the correct application of veterinary medicines. In this context, it should be acknowledged that expert beekeepers will play an important role in training and bee health surveillance and here again veterinarians could be part of education programmes.
In this article, an overview of bee health and disease is presented from a preventive veterinary medicine point of view. A further paper (Formato et al. Citation2011) will deal more specifically with the elaboration of a risk management system for assuring the safety of unprocessed honey, based on Hazard Analysis Critical Control Point (HACCP) principles. The purpose of these companion papers is to update the readership on apicultural essentials and to indicate options for risk management in which they are or might become involved, in their capacity as veterinary practitioner/consultant (e.g. as teacher in courses for beekeepers) or in their function as official veterinarians in the context of European regulations (European Commission Citation2004a, Citation2004b; Smulders et al. Citation2005), so as to contribute to ongoing international efforts to improve bee health and honey safety.
2. The background of beekeeping
In 2008, the European Food Safety Authority (EFSA) published data from a survey which included amongst other elements, the current level of honey production and its geographical distribution across Europe (). Although not completely complying with FAOSTAT and EUROSTAT data, said survey identified Spain, Hungary, Germany, Romania and Poland as the top-five largest producers of honey (EFSA Citation2008). A more recent document [Regulation (EU) No 726/2010] has identified Spain as the country in Europe with the largest bee population (2,459,373 beehives) and estimates the total European bee population at 13,985,091 million beehives (European Commission Citation2010).
Figure 1. Distribution of average honey production in Europe combining FAOSTAT and EUROSTAT data (reprinted figure with permission from EFSA (2008)).
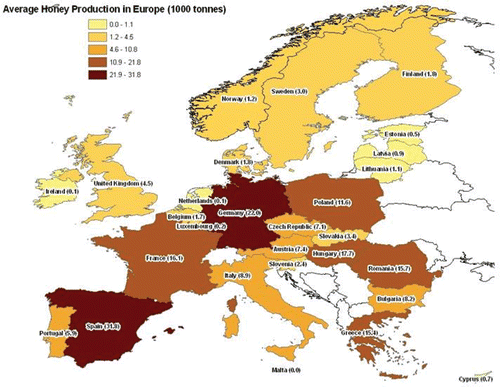
Annually an approximate 1.2 million tonnes of honey are produced globally, about 400,000 tonnes of which are traded internationally, main exporters being China, Argentina and Mexico. The degree of self sufficiency of the EU is 40–50% (Michaud Citation2005). Consequently, products marketed in Europe are sometimes mixes of indigenously produced and imported honey (Ortelli et al. Citation2004).
A more comprehensive overview of apiculture is provided in the following literature: International Bee Research Association (IBRA; www.ibra.org.uk), the beekeeping portal (www.apiculture.com), Danant (Citation1975) or Crane (Citation1990). All these sources include detailed background information and the various technical terms generally used in literature on beekeeping. This first section aims at introducing some essentials.
A population of honeybees (Apis mellifera spp) in a colony includes one queen (with a life span of 3–4 years) which exclusively lays eggs in the cells of the comb. Unfertilised eggs (containing a unique combination of 50% of the queen's genes) develop into haploid ‘drones’ (i.e. male bees which die upon mating or are expelled from the beehive before overwintering). Following one or more nuptial flights the queen establishes herself in the hive and begins laying eggs in the comb, consisting of thousands of hexagonal cells made by worker bees from wax produced in glands on the underside of their abdomen. Hatching of the eggs takes place in 3–4 days, worker bees feed hatched larvae for 5–7 days whereupon they ‘cap’ the cells with a semi-permeable wax membrane allowing the larvae to pupate rendering either virgin queens, worker bees or drones (i.e. after ca. 16, 21 and 24 days after the egg has been laid, respectively). Worker bees have a life cycle of a few weeks in summer and up to several months in areas with extended winter. Approximately, 20 young worker bees, between 6 and 12 days old, feed the queen with ‘royal jelly’ (secreted from the hypopharyngeal glands) allowing her to continually produce eggs (up to 1500 in mid-summer). Cells are used to develop larvae from the eggs the queen has laid (‘brood cells’) whilst other cells serve the purpose of storing honey and pollen.
A colony typically will vary in size from summer (50,000 or more) to winter (5–15,000 individuals). Apiaries (areas containing one or more man-made beehives in which colonies are kept and cared for) may consist of one to (in commercial beekeeping) hundreds of hives.
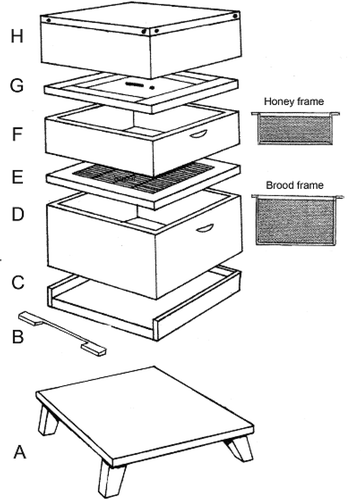
A diagrammatic presentation of the typical modern beehive is included in .
Figure 2. Essential features of the typical modern beehive (reprinted figure with permission from P. Gordon (2004)) (A) Hive stand: Providing clearance from ground level thus avoiding damp, reducing accessibility for intruders (e.g. mice). (B) Entrance block: Denying or reducing bees’ access to hive, in Wintertime equipped with a mouse guard. (C) Floor board: Rest for brood box with entrance allowing access for bees. (D) Brood box: Series of vertically hanging brood frames on which queen can lay eggs and worker bees perform their various tasks (feeding larvae, caring for drones and queen). (E) Queen excluder: Wire construction preventing queen from laying eggs in super; equally impassable for drones. (F) Super: Containing vertically oriented shallower frames for honey storage; in productive years more than one super necessary. (G) Crown board: Insulation board preventing the escape of too much warmth from super. It contains apertures (1) to insert feeder for supplementary feeding, (2) for bee escape (one-way exit: return through this aperture impossible). (H) Roof: Water-proof cover with metal gauze ventilation holes (inaccessible to insects, robber bees and wasps).
In terms of veterinary public health, apiculture is significant as it involves such fields as food safety, environmental hygiene, human nutrition and disease control [(as bees are involved in the transfer of some communicable diseases listed by the World Organisation for Animal Health (OIE; )].
Bees play a significant role in public health for the following reasons:
| 1. | because the incorrect use of some veterinary treatments (e.g. acaricides, antibiotics) may lead to residues of these substances being concentrated in honey, which may also be the case for environmental contaminants such as phytotherapeutics, polycyclic aromatic compounds (e.g. benzene and dioxin), heavy metals and radionuclides, | ||||
| 2. | as bees function as ‘monitors’ for the environmental contamination with the above or other substances used in agriculture (Devillers and Delègue Citation2002; Yazgan et al. Citation2006), | ||||
| 3. | as honey has been incriminated in cases of infant botulism caused by spores of clostridial toxin producers [(e.g. Clostridium botulinum); See: Formato et al. Citation2011)], | ||||
| 4. | as after swarming of colonies these may invade houses or buildings, | ||||
| 5. | because at times honeybees may be highly defensive, the more so after hybridisation with imported bee breeds, after which they can develop particular aggressiveness (‘africanized bees’). | ||||
3. Bee health and disease prevention
3.1. Good farming practices
lists the various hazards encountered in apicultural practice and the associated Good Farming Practices (GFPs, ‘best beekeeping practices’) preventing these. It characterises the hazards in terms of adverse effects on bee health and honey safety, honey quality and production efficiency and qualitatively indicates the severity of these effects. specifically summarises the various agents that jeopardise the honeybee's health and the measures to prevent or treat the associated illnesses. Extended reviews on the various bee diseases have recently become available (Journal of Invertebrate Pathology Citation2010).
Table 1. Analysis of hazards to bee health (BH), honey safety (HS), honey quality (HQ), production efficiency (PE) and the associated Good Farming Practices (GFPs) preventing these.
Table 2. Bee health (BH) and disease prevention and possible ramifications for unprocessed honey safety and quality – summarised.
Both tables substantiate the validity of taking risk management measures. These have been formulated in the current European legislation (EU Regulation 852/2004) which stipulates that the primary food producer is responsible for ensuring that any food put on the market is safe, which includes: (1) ensuring that primary products are protected against contaminations, having regard to any processing that primary products will subsequently undergo, (2) complying with control of hazards in primary production and associated operations, (3) ensuring that staff handling foodstuffs are in good health and undergo training on health risks, (4) taking adequate measures to keep clean any facilities, equipment, containers, crates, vehicles and vessels used, (5) taking account of the results of any relevant analyses carried out on samples taken from animals or other samples that have importance to human health, (6) using veterinary medicinal products correctly, as required by the relevant legislation, (7) taking appropriate remedial action when informed of problems identified during official controls, (8) keeping and retaining records related to measures put in place to control hazards in an appropriate manner and for an appropriate period, commensurate with the nature and size of the food business and to make relevant information contained in these records available to the competent authority and receiving food business operators on request, and, finally, (9) keeping records on veterinary medicinal products or other treatments administered to the animals, dates of administration and withdrawal periods (European Commission Citation2004c).
GFPs play an important role in controlling potential health hazards (Sperber et al. Citation1998) and constitute the foundations upon which a further HACCP plan is to be based (NACMCF Citation1997). They consist of the described series of practical operations and managerial procedures which are indispensable for the healthy functioning of apiaries. Moreover, the adherence to GFPs in beekeeping activities is highly advantageous to apiary performance, as it improves the health of the hives, increases hive production, decreases medicinal costs and yields a healthier and higher quality honey for the consumer market.
3.2. The veterinarian's role in apiculture – good veterinary practices
Both veterinary practitioners and veterinarians in a control function may play a role in apiculture. In some countries the former are involved in such tasks as controlling the health status of bee hives, advising beekeepers on proper treatment options of the various pathologies (possibly involving laboratory analysis), prescribing veterinary medicines, assisting in the introduction of and adherence to bee health and honey safety assurance systems, providing instructions on how to keep records in log books as stipulated in European legislation and in the training of beekeepers in general.
In its recently issued Terrestrial Animal Health Code, the World Organisation for Animal Health (OIE) has indicated that ‘the permanent official sanitary surveillance of apiaries should be under the authority of the Veterinary Authority’ and should be performed by either representatives of this authority or by those of approved organisations [possibly assisted by specially trained ‘health inspectors or advisers’ (e.g. experienced beekeepers)] (OIE Citation2009). Among the tasks of the official veterinarians possibly involved are, as specifically proposed by OIE and already partly included in European legislation (European Commission Citation2004b): (1) annual official visits to apiaries during the most appropriate period of detection of diseases and additional (unexpected) visits to apiaries where breeding or transport operations are carried out for trade or transfer to other regions or any other purpose whereby diseases could be spread, as well as to apiaries located in the vicinity, and, finally, special visits for sanitary surveillance to sectors where breeding apiaries have been approved for export purposes, (2) collecting sampling for purposes of diagnosis of contagious diseases and despatching them to an official laboratory for analysis, the results of which are to be communicated to the Veterinary Authority with the shortest delay [notably, the EFSA (http://www.efsa.europa.eu/en/scdocs/doc/27e.pdf, page 29) has identified the absence of specific laboratory facilities as a critical point regarding the efficiency of the systems of monitoring the causes of bee health problems], and, finally, (3) applying hygiene measures comprising in particular the treatment of colonies of bees, as well as disinfection of the equipment and possibly the destruction of affected or suspect colonies and of the contaminated equipment so as to ensure the rapid eradication of any outbreak of a contagious disease. Additional tasks, as laid down in European and national legislation, include official control of records contained in log books (European Commission Citation2004b), residue control – as per national residue plans – of all hive products (honey, royal jelly, propolis, pollen), examination of imported bees for exotic pests (e.g. Aethina and Tropilaelaps), of transport documents (e.g. those on hive or family movements, new purchases) and possibly the official veterinarian's involvement in the training of beekeeping personnel.
Although the drug resistance to antibiotics is not widely reported (e.g. in Europe) the development of resistance against pharmaceuticals used in apiculture is a major concern. Examples include drug resistance of major bee pathogens such as Paenibacillus larvae spp [notably against tetracyclines in the USA and Canada where – as opposed to Europe – these substances are registered for use in bees (Flores Citation2007)] or Varroa [e.g. against Amitraz, fluvalinate and coumaphos (Mutinelli and Baggio Citation2004]. The fact that, consequentially, some pharmaceuticals for treating bee diseases are showing reduced or no therapeutical efficiency has prompted action plans by local authorities to permit the import of pharmacologically active substances not yet registered in the country where they are to be administered. Pending the registration of some of these pharmaceuticals, and/or legislative changes that would allow beekeepers to reach a suitably qualified person (SQP) status permitting them administration of such drugs, veterinary practitioners are called upon to be available to guide this transitional situation, which obviously is restricted to veterinary ‘Prescription Only Medicines’ (POM-V). This ‘cascade principle’ is widely used across Europe (RCVS Citation2009a, Citation2009b; VMD Citation2009)]. In Part 2 of this article a list of drugs currently authorised in the various EU Member States has been included (Formato et al. Citation2011).
Of particular significance is the prescription and proper administration of suitable veterinary medicines in apicultural practice. Whereas more general definitions of GVPs (i.e. standards specifying both veterinary ethics, principles of conduct and requirements relating to the quality management of veterinary operations) are available, such as those formulated by FVE (Citation2002), their utility has been challenged as they are too unspecific for purposes of ensuring the responsible use of veterinary drugs (FAO Citation2001). The use of more topical ‘Good Practices in the use of Veterinary Drugs’ (GPVDs as defined in FAO's procedural manual), has in the past already been suggested, e.g. by Van Miert (Citation1990) who defined GFPs as a more drug-use focused concept, namely: ‘the selective use (in accordance with directions of instructions for users) of veterinary drugs registered by the authorities in those indications in which they are permitted when the diagnosis has been established and in which the problem of residues in foods of animal origin on using these agents has been taken into account’.
Residues of veterinary drugs and other (environmental) contaminants in apicultural products have in Europe over the past few years been systematically monitored and reported through the RASFF. It appears from these reports that residues are still an issue in honey (and in many other food products for that matter), particularly, when imported into the EU from Asia (notably China). Also indigenously produced honey still contains these, albeit in markedly fewer samples (European Commission Citation2009b). Annually an approximate 50 notifications of antibiotics residues are reported for honey, and significantly more of pesticides (e.g. 180 notifications in 2007) (RASFF Citation2004, Citation2005, Citation2006, Citation2007). The most recent report (RASFF Citation2008) indicates that nitrofurane (metabolites) still represent the most notified hazard, that sulphonamide residues are found less than before, and that other substances (notably erythromycin) are on the rise. In the 2008 Commission Staff Working Document on the implementation of national residue monitoring plans in the Member States, it is reported that the noncompliant results for antibacterials dominated in honey (DG SANCO Citation2009).
The legislation on the use of antimicrobial substances in apiculture varies considerably among countries (Mutinelli and Rademacher Citation2002; Mutinelli Citation2006). For instance, in the US and Canada antibiotic use is authorised in beekeeping. However – whereas in the EU drugs such as streptomycin, tetracyclines and sulphonamides are regarded as safe for use in farm animals and Maximum Residue Levels (MRLs, i.e. the maximum concentrations of residue considered as not presenting a hazard for the consumer or the manufacturing process) have been set for their occurrence in beef, lamb and pork (often in several hundred ppb) – the EU prohibits the use of antibiotics in apiculture. This is largely explained by the fact that the pharmaceutical industry considers apiculture to be a niche market and consequently has not invested in collecting and submitting data to the European Medicines Evaluation Agency (EMEA) with a view to obtain marketing authorisation (Martin Citation2003; Mutinelli Citation2006). Because of their bacteriostatic nature [antibiotics merely temporarily mask the severity of bee diseases caused by microbial pests (American foulbrood, AFB; European Foulbrood, EFB; and nosemosis) rather than eliminate the responsible agents], it is also often argued that it would be wiser to refrain from – instead of trying to regulate (and therefore justify) the use of antibiotics in apiculture. Incidentally, some European Member States apply the cascade principle to permit the use of antibiotics by authorised officers of the competent authorities.
In Directive 96/23/EC there are no MRLs set for honey (European Commission Citation1996), so the mandatory monitoring programmes for residues in honey as required in this Directive focuses on residues of veterinary medicinal products and environmental contaminants. However, it should be noted that MRLs have now been set for certain plant protection agents in various products (including honey), a summary of which has been listed by DG SANCO (Citation2010). Non compliant levels in honey of the following substances (that have also been used for plant protection) have been reported: streptomycin, pyrethroids, organochlorine compounds and organophosphates (EFSA Citation2008). In the absence of MRLs and because many third countries use antibiotics in apiculture, the EU has recently (as per (EC) 470/2009) opened the possibility of extrapolating MRLs from one species/commodity to others (including honey) and will hence allow Member States to import honey from third countries, provided so-called ‘Minimum Required Performance Limits’ (MRPLs) that serve as ‘Reference Points for Action’ (RPA, i.e. concentrations technically detectable in a food control laboratory based on decision limits as defined in Commission Decision 2002/657/EC) are not exceeded, certain administrative procedures are followed (possibly including informing Commission services) and defined sampling procedures are complied with (European Commission Citation2009c). Currently, the only MRPL established is that for Chloramphenicol (0.3 µg/kg) (European Commission Citation2009c). Several analytical procedures for assessing antimicrobial residue levels in honey have been described in the past few years (e.g. Edder et al. Citation2002; Ortelli et al. Citation2004; Martinez Vidal et al. Citation2009).
However, the prevalence of residues of antimicrobial agents is not an exclusive problem of third countries. For instance, whereas analyses of antimicrobial residues in honey of different origin by Edder et al. (Citation2002) and Reybroeck (Citation2003) indicated that the residue problem is not restricted to a certain geographical area of origin of the honey (except for chloramphenicol exclusively found in honey produced in China) their data also prove that in many, including EU, countries different veterinary products (streptomycins, tetracyclines and sulphonamides) are used and sometimes simultaneously. Incidentally, antimicrobial residues in honey do not appear to be necessarily the result of illegal application in apiculture. For instance, Brasse (Citation2001) reports low concentrations (<20 µg/kg) in fruit honey from nectar collected in pear orchards since the blossoms are sometimes sprayed with streptomycin preparations like Fructosin® or Plantomycin® for the treatment of fire blight (Erwinia amylovora). It has also been suggested that such low concentrations found in honey of African origin (notably from Zambia) are ‘natural’ and result from the natural occurrence of Streptomyces in the environment (Bradbear Citation2005).
Such and other observations have prompted FAO/WHO's Codex Alimentarius Commission to issue a circular letter requesting information ‘on veterinary drugs registered across the world, to provide data on honey consumption, considering both direct and indirect honey intake, and to also provide information regarding the existence of Good Veterinary Practice (GVP) in honey production’ (FAO/WHO Citation2009). The European Commission has recently acknowledged that there are indeed hardly if any publications on GVPs applied to beekeeping activities (European Commission Citation2009a). By this article, the authors hope to have contributed to remedying this situation.
4. Conclusions
Adhering to GFPs in beekeeping represents the foundation upon which bee health assurance and disease prevention should be based and it is a prerequisite for introducing additional risk management measures. European legislation (European Commission Citation2004a, Citation2004b) holds the beekeeper primarily accountable for the assurance of the safety of honey and other apicultural products. In their efforts they are supported by consultants, in most European countries including veterinary practitioners, for assisting them in keeping their bee colonies healthy, production efficiency up to standards and for assuring the safety and quality of the raw products supplied to the processing industry. To this end, beekeepers should also be able to rely on veterinarians adhering to more clearly formulated GVPs. It is timely that the veterinary profession recognises the role it should and could play in assuring bee health and honey safety. Consequently, establishments for veterinary education (at least those in countries where apiculture is significant) are well-advised to pay more attention to including in their curricula all relevant elements, so as to prevent their graduates from being rightfully criticised for their relative incompetence in this area of animal production.
Only provided both GFPs and GVPs are respected, are further risk management measures according to a systematically elaborated and implemented HACCP system for primary apicultural production meaningful and effective. The latter will be dealt with in Part 2 of this article (Formato et al. Citation2011).
Acknowledgements
Dr Jane Richardson (EFSA, Parma, Italy) and Dr Ivor Davis (past President of the British Beekeeping Association) are gratefully acknowledged for their useful comments on this study.
References
- Apimondia.org. [Internet]. 2009. Veterinarians and honey bee industry in France; [accessed 2010 Oct 14]. Available from: http://www.apimondia.org/2009/bee-health/Veterinarians%20and%20honey%20bees%20industry%20in%20France%20-%20L%20HOSTIS%20Monique.pdf
- Apivet.eu. [Internet]. 2009. Veterinarians and bee health involvement taking France as an example; [accessed 2010 Oct 14]. Available from: http://www.apivet.eu/2009/12/veterinarians-and-bee-health-involvement-taking-france-as-an-example.html
- Berenbaum , M . [Internet]. 2007. Colony Collapse Disorder and Pollinator Decline, Statement before the Subcommittee on Horticulture and Organic Agriculture of the US House of Representatives, 29 March 2007; [accessed 2009 Feb 2]. Available from: www.7nationalacademies.org/ocga/testimony/Colony_Collapse_Disorder-and-Pollinator_Decline.asp
- Bilikova , K and Simuth , J . 2010 . The structure and function of antimicrobial peptides in defence of honeybee against microbial pathogens . Proceedings of the 4th European Conference of Apidology; 2010 Sep 7–9; Ankara . 2010 , Turkey. pp. 104 – 105 .
- Bradbear , N . [Internet]. 2005. Foreword to the proceedings of the Bees for Development Honey Trade Workshop; [accessed 2009 Nov 20]. Available from: www.beesfordevelopment.org/info/proceedings_HTW1/
- Brasse , D . 2001 . Stellungnahme der BBA zum streptomycin-problem. Teil 1 Ursachen und Bedingungen für die Zulassung von Plantomycin . ADIZ , 35 ( 6 ) : 24 – 25 . Teil 2 Bewertung der Rückstandswerte in Honig, ADIZ. 35:24–25
- Crane , E . 1990 . Bees and beekeeping; science, practice and world resources , 624 New York : William Heinemann .
- Danant. 1975. The hive and the honeybee. Ed. Danant & Sons, ISBN.13-146741
- De Graaf DC. 2010. Report of the activities of the BEEDOC Diagnostic Department. Proceedings of the 4th European Conference of Apidology; 2010 Sep 7–9; Ankara, Turkey, p. 103
- Devillers , J and Delègue , MH . 2002 . Honey bees: estimating the environmental impact of chemicals , 332 London : Taylor & Francis .
- SANCO. , DG . [Internet]. 2009. SANCO/11030/2009 (POOL/E5/2009/11030/11030-EN.doc). Commission Staff Working Document on the implementation of national residue monitoring plans in the member states in 2008 (Council Directive 96/23/EC); [accessed 2010 Oct 14]. Available from: http://ec.europa.eu/food/food/chemical-safety/residues/workdoc_2008_en.pdf
- SANCO , DG . [Internet]. 2010. Eu Pesticides database (Directive 91/414/EEC); [accessed 2011 March 24]. Available from: http://ec.europa.eu/sanco_pesticides/public/
- Edder , P , Ortelli , D and Corvi , C . [Internet]. 2002. Survey of antibiotics residues in honey on the Swiss market. Poster presented at: 4th International Symposium on Veterinary Drugs and Hormones Residues Analysis; June 2002; Antwerp, Belgium; [accessed 2009 Nov 20]. Available from: http://etat.geneve.ch/des/SilverpeasWebFileServices/reference15pdf?component=kmelia704&SourceFile=1166702
- European Commission. 1996. Council Directive 96/23/EC of 29 April 1996 on measures to monitor certain substances and residues thereof in live animals and animal products, O.J. L125, 23-5-1996, p. 10–32
- European Commission. 2004a. Regulation No. 854/2004 of the European Parliament and the Council of 29 April 2004, containing specific rules concerning specific rules concerning official controls on products of animal origin, Official Journal of the European Union, Brussels, O.J. L155/206
- European Commission. 2004b. Regulation No. 882/2004 of the European Parliament and the Council of 29 April 2004, on official controls performed to ensure the verification of compliance with feed and food law, animal health and welfare rules. Official Journal of the European Union, Brussels, O.J. L165
- European Commission. 2004c. Regulation (EC) No. 852/2004 of the European Parliament and the Council of 29 April 2004, on the hygiene of foodstuffs. Official Journal of the European Union, O.J. L138/1
- European Commission. [Internet]. 2009a. European Commission Comments of 17/07/2009 on Codex Letter CL/2009/21-RDVF: request for comments/information on veterinary drugs registered for honey production and bee health, honey consumption and Good Veterinary Practices in honey production; [accessed 2009 Dec 7]. Available from: http://ec.europa.eu/food/fs/ifsi/eupositions/ccrdvf_CL2009-21_en.pdf
- European Commission [Internet]. 2009b. Food safety – from farm to the fork; residues of veterinary medicinal products – control and monitoring; [accessed 2009 Dec 10]. Available from: http://ec.europa.eu/food/food/chemicalsafety/residues/control_en.htm
- European Commission [Internet]. 2009c. DG SANCO. Food safety – from the farm to the fork; [accessed 2009 Dec 2]. Available from: ec,europa.eu/food/food/chemical safety/residues/third_countries_en.htm
- European Commission. 2010. Regulation No. 726/2010 of the European Parliament and the Council of 12 August 2010, on amending Regulation (EC) No. 9178/2004 on detailed rules to implement Council Regulation (EC) No. 797/2004 on measures improving general conditions for the production and marketing of apiculture products, Official Journal of the European Union, Brussels, O.J. L213/29
- [EFSA] European Food Safety Authority . 2008 . Bee mortality and bee surveillance in Europe (EFSA-Q-2008-428) . EFSA J , 154 : 1 – 28 .
- European Parliament [Internet]. 2008. Resolution of November 2008 on the situation in the beekeeping sector, P6_TA(2008)0567; [accessed 2009 Dec 3]. Available from: www.europarl.europa.eu/sides
- [FAO] Food and Agriculture Organization of the United Nations [Internet]. 2001. Matters referred to the committee by the Codex Alimentarius Commission and other commissions; [accessed 2009 Dec 7]. Available from: http://www.fao.org/docrep/meeting/005/x7102e/x7102e09.htm
- [FAO/WHO] Food and Agriculture Organization of the United Nations/World Health Organization Codex Alimentarius Commission. 2009. Request for recommendation on veterinary drugs registered for honey production and bee health, honey consumption and Good Veterinary Practices in honey production, Circular Letter CL 2009/21-21-RDVF
- Flores , A . 2007. Helping beekeepers to beat American Foulbrood, Agricultural Research Magazine, July, 55, No. 6
- Formato , G , Zilli , R , Condoleo , R , Marrozzi , S , Davis , I and Smulders , FJM . 2011 . Risk management in primary apicultural production , a Hazard Analysis Critical Control Point approach to assuring the safety of unprocessed honey, Vet Quart (in press). Part 2 . Doi: 10.1080/01652176.2011.567755
- Forsgren , E . 2010 . BEEDOC Prevention Department . Proceedings of the 4th European Conference of Apidology; 2010 7–9 Sep; Ankara . 2010 , Turkey. pp. 104
- [FVE] Federation of Veterinarians of Europe [Internet]. 2002. Code of good veterinary practice; [accessed 2009 Dec 7]. Available from: http://www.fve.org/news/publications/pdf/gvp.pdf
- Gordon , P . 2004 . Getting started with bees , 95 Saffron Walden, Essex : Broad Leys .
- Grabaum , R , Hammen , V , Kühn , I , Klotz , S , Settele , J , Biesmeijer , JC and Wolf , T . [Internet]. 2006. The European field site network of the ALARM project – cross border GIS solutions for research. Proceedings of International Symposium on Geoinformatics in European Nature Protection Regions; 2006 Nov 13–14; Dresden, pp. 149–156; [accessed 2010 Oct 14]. Available from: http://www.ufz.de/data/Grabaum-et-al_ALARM-FSN_NatureProtection_GIS5072.pdf
- Hendrikx , P , Chauzat , MP , Debin , M , Neuman , P , Fries , I , Ritter , W , Brown , M and Mutinelli , F . Le Conte Y, Gregorc A. [Internet]. 2009. Bee mortality and bee surveillance in Europe. [accessed 2011 March 24]. Available from: http://www.efsa.europa.eu/en/supporting/pub/27e.htm
- Higes , M , Martín-Hernandez , R , Martínez-Salvador , A , Garrido-Ballón , E , González-Port , AV , Meana , A and Bernal , JL . del Nozal MJ, Bernal J. 2009. A preliminary study of the epidemiological factors related to honeybee colony loss in Spain. Environmental Microbiology Report, Blackwell Publishing Ltd
- Jaffé , R , Wolf , S and Moritz , RFA . 2008. Bees in Europe and Sustainable Honey Production (BEESHOP): a European research network. Bee Improvement and Conservation Magazine, 29:28,29,32
- Journal of Invertebrate Pathology [Internet]. 2010. Special issue on bee diseases, various authors; [accessed 2010 Jan 18]. Available from: http://www.sciencedirect.com/science?_ob=PublicationsURL&_tockey=%23TOC%236888%232010%23998969999.8998%231594152%23FLA%23&_cdi=68888&_pubType=J&auth=y&_acct=acct=C000050221&_version=1&urlVersion==&userid=
- Martin , P . 2003 . Veterinary drug residues in honey . Apiacta , 38 : 21 – 23 .
- Martinez Vidal , JL , Del Mar Aguilera-Luiz , M , Romero-Gonzalez , R and GarridoFrenich , A . 2009 . Multiclass analysis of antibiotic residues in honey by ultraperformance liquid chromatography-tandem mass spectrometry . J Agric Food Chem , 57 : 1760 – 1767 .
- Michaud , V . 2005 . Antibiotic residues in honey – the FEEDM view . Apiacta , 40 : 52 – 54 .
- Moritz , RFA . 2010 . Bees in Europe and the decline of honeybee colonies (BEEDOC) . Proceedings of the 4th European Conference of Apidology; 2010 Sep 7–9; Ankara . 2010 , Turkey. pp. 103
- Mutinelli , F . 2006 . Controlling Diseases of Bees in the EU . Regul Aff J Pharma , 17 : 207 – 210 .
- Mutinelli , F and Baggio , A . 2004 . Use of medicinal drugs against Varoosis . Apiacta , 39 : 53 – 62 .
- Mutinelli , F and Rademacher , E . 2002 . European legislation governing the use of drugs in bee colonies to control varroosis: a case study . Regul Aff J , 13 : 401 – 406 .
- Mutinelli , F , Sabatini , AG , Gallina , A , Medrzycki , P , Sgolastra , F , Bortolotti , L and Porrini , C . [Internet]. 2009. APENET: network for monitoring honeybee mortality and colony losses in Italy. 41st Apimondia Congress; 2009 Sep 16; Montpellier, France; [accessed 2010 Oct 14]. Available from: http://www.apimondia.org/2009/bee-health/symposia/Apenet,%20network%20for%20monitoring%20honeybee%20mortality%20and%20colony%20losses%20in%20Italy%20-%20MUTINELLI%20Franco.pdf
- [NACMCF] National Advisory Committee on Microbiological Criteria for Foods. 1997. Hazard analysis and critical control point principles and application guidelines; 1997 Aug 14; FSIS, Washington, DC
- Neumann , P . 2010a . The COLOSS network . Proceedings of the 4th European Conference of Apidology; 2010 Sep 7–9; Ankara . 2010a , Turkey. pp. 105
- Neumann , P . [Internet]. 2010b. EU projects/networks. Proceedings of Workshop: Diagnostics in honeybees: from sampling to data analyses; 2010 Aug 30–Sep 1; Ghent, Belgium, p. 3; [accessed 2010 Oct 14]. Available from: http://www.coloss.org/documents/BEEDOC_Proceedings_Workshop_Ghent_2010.pdf
- Neumann , P and Carreck , NL . 2010 . Honey bee colony losses. Special Issue on Colony Losses . J Apicult Res , 49 ( 1 ) : 1 – 6 .
- [OIE] Office International des Épizooties [Internet]. 2007. Listed diseases; [accessed 2009 Dec 6]. Available from: http://www.oie.int/eng/maladies/old_en_classification2007.htm
- [OIE] Office International des Épizooties [Internet]. 2009. Terrestrial animal health code, Chapter 4.14. Hygiene and disease security procedures in apiaries; [accessed 2009 Dec 6]. Available from: http://www.oie.int/eng/normes/MCODE/en_chapitre_1.4.14.pdf
- Ortelli , D , Edder , P and Corvi , C . 2004 . Analysis of chloramphenicol residues in honey by liquid chromatography-tandem mass spectrometry . Chromatographia , 59 : 61 – 64 .
- Paxton , RJ . 2010 . BEEDOC: novel treatments for the control of honey bee diseases . Proceedings of the 4th European Conference of Apidology; 2010 Sep 7–9; Ankara . 2010 , Turkey. pp. 104
- [RASFF] Rapid Alert Systems for Food and Feed [Internet]. 2004. Annual report on the functioning of the RASFF 2004. European Commission Health and Consumer Protection Directorate General, Annual Report 2004; [accessed 2009 Dec 5]. Available from: http://ec.europa.eu/food/food/rapidalert/report2004_en.pdf
- [RASFF] Rapid Alert Systems for Food and Feed [Internet]. 2005. Annual report 2005. European Commission Health and Consumer Protection Directorate General, Annual Report, 2005; [accessed 2009 Dec 5]. Available from: http://ec.europa.eu/food/food/rapidalert/report2005_en.pdf
- [RASFF] Rapid Alert Systems for Food and Feed [Internet]. 2006. Annual report 2006. European Commission Health and Consumer Protection Directorate General, Annual Report, 2006; [accessed 2009 Dec 5]. Available from: http://www.efet.gr/docs/rasff/report%202006.pdf
- [RASFF] Rapid Alert Systems for Food and Feed [Internet]. 2007. Annual report 2007. European Commission Health and Consumer Protection Directorate General, Annual Report, 2007; [accessed 2009 Dec 5]. Available from: http://www.efet.gr/docs/rasff/report2007.pdf
- [RASFF] Rapid Alert Systems for Food and Feed [Internet]. 2008. Annual Report 2008. European Commission Health and Consumer Protection Directorate General, Annual Report, 2008; [accessed 2009 Dec 5]. Available from: http://www.efet.gr/docs/rasff/report2008_en.pdf
- Reybroeck , W . 2003 . Residues of antibiotics and sulphonamides in honey on the Belgian market . Apiacta , 38 : 23 – 30 .
- [RCVS] Royal College of Veterinary Surgeons. 2009a. Bees under your care; Interim advice on prescribing medicines for bees, RCVS News, June, 2009-12-05
- [RCVS] Royal College of Veterinary Surgeons [Internet]. 2009b. RVCS Advice Note 28; [accessed 2009 Dec 4]. Available from: www.rcvs.org.uk/advicenotes
- Smulders , FJM , Kainz , R and Weijtens , MJM . 2005 . “ The new EU legislation on food control and how veterinarians fit in ” . In Veterinary public health and food safety assurance Vol. 4. Towards a risk-based chain control , Edited by: Smulders , FJM . 201 – 224 . The Netherlands : Wageningen Academic .
- Sperber , WH , Stevenson , KE , Bernard , DT , Deibel , KE , Moberg , LJ , Hontz , LR and Scott , VN . 1998 . The role of prerequisite programs in managing a HACCP system . Dairy Food Environ Sanit , 18 ( 7 ) : 418 – 423 .
- Vanengelsdorp , D , Evans , JD , Saegerman , C , Mullin , C , Haubruge , E , Nguyen , BK , Frazier , M , Frazier , J , Cox-Foster , D Chen , Y . 2009 . Colony collapse disorder: a descriptive study . PLoS One , 4 ( 8 ) : e6481
- Van Miert ASJPAM . 1990 . Veterinary drugs and good veterinary practice . Tijdschr Diergeneesk , 115 ( 3 ) : 125 – 131 .
- [VMD] Veterinary Medicines Directorate [Internet]. 2009. Action plan on the availability of medicines for bees; [accessed 2009 Dec 3]. Available from: www.vmd.gov.uk/VetSQP/Beeactionplan.pdf
- Yazgan , S , Horn , H and Isengard , HD . 2006 . Honey as bioindicator by screening the heavy metal content of the environment . Deutsche Lebensmittel-Rundschau , 102 ( 5 ) : 192 – 194 .