Abstract
In managing risks associated with the human consumption of honey, all sectors of the production chain must be considered, including the primary production phase. Although the introduction of the Hazard Analysis Critical Control Point (HACCP) system has not been made compulsory for purposes of quality and safety control in farming operations, European legislation makes many references to the key role of primary production in food safety management and the HACCP system has been indicated as the preferred tool to ensure that consumers are provided with safe foods. This article describes a systematic HACCP-based approach to identifying, preventing and controlling food safety hazards occurring in primary apicultural production. This approach serves as a useful tool for beekeepers, food business operators, veterinary advisors, and for Food and Veterinary Official Control Bodies in their planning and conducting of audits and for establishing priorities for the evaluation of training programmes in the apicultural sector.
1. Introduction
For safeguarding food safety ‘from farm-to-fork’, farmers and food processors need to base their efforts on risk analysis. Risk analysis includes risk assessment (the result of hazard analysis, exposure assessment and risk estimation), risk management (taking the appropriate measures to control risks in a particular link of the production or marketing chain) and risk communication (informing actors further down this chain about residual risks that remain at delivery). Various options for the management of risks exist, notably the Hazard Analysis Critical Control Point (HACCP) system (for an explanation see below) as explicitly indicated in European legislation for achieving food safety (e.g. EC 1999, 2002a, 2004a, 2004b, 2004c). Even if – at the present time – it is not mandatory in the European Union (EU) to apply HACCP principles to primary production, Regulation (EC) No. 852/2004 states that ‘Member States shall encourage the development of national guides to good practice for hygiene’ and that ‘Food hazards present at the level of primary production should be identified and adequately controlled’ (EC Citation2004a). In suggesting the adoption of HACCP as a useful risk management approach, it is essential to realize that this is only meaningful provided basic measures of safety and quality assurance are adhered to first. These ‘Good Farming and Good Veterinary Practices’ (GFPs; GVPs) are universally applicable, based on scientific and well-documented proof and are absolute prerequisites. They represent the ‘foundations’ of risk management, in that they principally prevent external hazards being introduced in a production environment. Additionally, relying on a HACCP system essentially serves to significantly reduce or even eliminate those hazards that, despite strict adherence to best practices, may still prevail. Untermann (Citation1998) has compared such a system with a house, where good practices and structural facilities represent its foundations and walls, without which the ‘HACCP roof’ would collapse.
Hazard and risk analysis can address various concerns. For instance, whereas the main focus of the Office International des Épizooties is on risks for both animal and human health, the Codex Alimentarius Commission (CAC) concentrates on domestic risks and exclusively addresses those related to biological, chemical and physical agents of serious public health concern (CAC Citation2002). In addition, approaches which are primarily concerned with identifying animal welfare risks have recently been described (Smulders Citation2009). Depending on the main focus of scientific analysis, a choice has to be made. Considering the scope of the present contribution, CAC's approach is the obvious one. Consequently, although animal health and disease prevention issues indeed need to be considered (Formato and Smulders Citation2011), in this article, people who eat honey are defined as the population possibly at risk.
The HACCP system has primarily been designed for purposes of safety assurance in food processing (International Committee on the Microbiological Specification for Foods Citation1988). Over the past decades several documents have become available for the honey processing area (Instituto Nacional de Tecnologia Industrial Citation2005; Food and Agriculture Organization of the United Nations and World Health Organization Citation2006; WHO Citation2008; Canadian Food Inspection Agency Citation2010). However, although the relevance of measures to be taken by beekeepers is stressed, none of these documents specifically address risk management options at the primary apiary level.
In some parts of the world (notably China, Argentina, Mexico and Central and Southern European countries), a sizeable number of large-scale commercial outfits, honey packers and bottlers of food products exist, which are well advised to consider an ‘online’ safety and quality assurance approach (thus minimizing economic losses) and to document that they have lived up to their contractual obligations. It can be argued that for small-scale operations – and this applies to most apiaries in areas with a cold and humid climate – the formal application of the HACCP system is less suitable and overly complicated, and that beekeepers should rely on common sense and the advice by their beekeepers associations or bee health advisors. Clearly, in these situations intervention options are restricted to end-product control (e.g. in the UK by Trading Standard Officers, who ensure the quality and safety of food products, as well as by the Food Standards Agency that can request any jar of honey for analysis and withdraw the entire batch if necessary). However, it can equally be argued that any beekeeper who aims to commercialize his product could benefit from adhering to a safety and quality management system based on HACCP principles, obviously applying the flexibility principle. Ultimately, it will be up to the legislator to decide if and to what extent the introduction of HACCP at the apiary level can be justified.
In the first part of this contribution (Formato and Smulders Citation2011), hazards occurring in apicultural practice in general were analysed and associated best practices (GFPs and GVPs) relevant for bee health and disease prevention were identified. The purpose of this companion article is to formulate appropriate ‘on-farm’ risk management measures, which specifically address the safety of unprocessed honey and have been identified by following a systematic HACCP approach.
2. HACCP applied to primary apiary production
The HACCP methodology described by the CAC (Citation1999) defines seven principles of HACCP, namely: conduct hazard analysis, assess CCPs, define critical limits, develop a monitoring system, establish corrective actions, develop a verification procedure and ensure that a good documentation system is in place. To avoid overlooking anything, CAC has identified 12 associated tasks to be performed: (1) assemble an HACCP team, (2) describe the product, (3) identify the intended use, (4) set up the flow diagram, (5) on-site confirmation of the flow diagram, (6) list hazards associated with each step and control measures, (7) determine CCPs, (8) establish critical limits for each CCP, (9) establish a monitoring system for each CCP, (10) establish corrective actions, (11) establish verification procedures, and, finally, (12) establish documentation and record keeping. In the following sections, these tasks are systematically addressed.
2.1. Assembly of an HACCP team
An expert HACCP team develops the HACCP plan according to the specific needs. As the team has to be authoritative, it should include at least the beekeeper and the veterinarian consultant (or an equivalent expert on HACCP at farm level).
2.2. Description of the product (unprocessed honey)
The final product of primary apicultural production is honey in combs intended to be further processed to obtain honey for human consumption.
Honey is the natural sweet substance produced – from the nectar of plants, from secretions of living parts of plants or from insects sucking these from the plants – by Apis mellifera bees. Upon collection, the bees transform nectar or secretions by adding specific substances of their own, whereafter the product is deposited, dehydrated and stored in honeycombs for further ripening (EC Citation2001). Honey is composed primarily of the sugars glucose and fructose; its third greatest component is water. Honey also contains numerous other types of sugars, as well as acids, proteins and minerals (National Honey Board Citation2005). The chemical composition and quality of honey is highly dependent on its floral origin (Crane Citation1980). According to Bogdanov et al. (Citation1999), it is mainly constituted of carbohydrates (ranging from 73% to 83%) and water (generally ranging from 14.5% to 18.5%). Minor components are organic acids (0.6%), proteins (0.3%), amino acids (0.05%) and minerals (0.1%). Honey is not a good source for vitamins, lipids and aromatic substances.
Generally, after extraction from the comb, honey is bottled in jars. Honey can be subjected to a variety of processing methods yielding several variants (). Because of its unique composition and the processing of nectar by the bees through the introduction of enzymes which change its chemical composition, honey is suitable for long-term storage and can remain stable for many years, provided stored at temperatures <12°C and in airtight containers.
Table 1. Honey – description of the product variants (EC 2001).
2.3. Identification of the intended use
Honey in combs must be further processed (e.g. through extraction, filtration, decantation and packaging) to obtain honey for human consumption that could be delivered to industry in food-safe drums or buckets, or to retail shops in jars or other packages.
2.4. Construction of the flow diagram
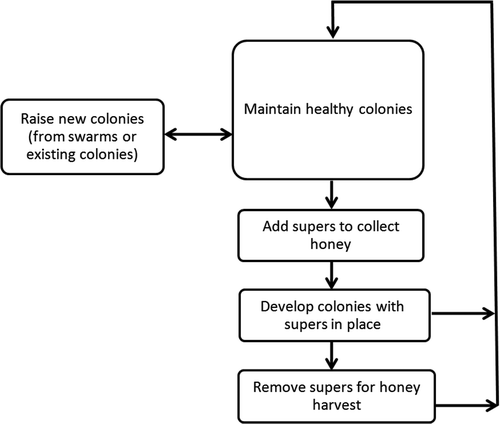
The operative phases of beekeeping are to be systematically listed to better identify the various stages where hazards, that could compromise honey safety, can occur (CAC Citation1999). Breaking up beekeeping activities into operative phases () generates a list of major production stages which are described in the following sections.
2.4.1. Maintain healthy colonies
This phase includes different stages of colony management, some of which are associated with the specific beekeeping calendar (Hopper Citation1983):
| 1. | ‘Summer keeping of hives after super removal’: colonies are usually at their maximum level of strength and, at the same time, the infestation with the Varroa destructor mite is at such a high level that death of the bee colonies may ensue. In this phase, it is essential to protect beehives against this pest by proper treatments (Rice et al. Citation2004; Delaplane et al. Citation2005; Formato and Smulders Citation2011, their );
Table 2. Analysis of hazards to public health, possibly associated with honey ingestion – summarized.
Table 3. Examples of insecticide preparations used in plants or against ecoparasites. | ||||
| 2. | ‘Autumn keeping of the colonies’: in this phase, an inspection of the hives is recommendable to verify: (1) the presence of the queen, (2) the absence of pathologies (e.g. varroosis) and symptoms of viruses (e.g. honeybees smaller, black and with deformed wings) and (3) that an adequate amount of honey and pollen supply is stored in the brood box to last through the next winter phase. If the stocks are poor, the beekeeper must provide supplementary feed to the hives (see of Formato and Smulders (Citation2011,). Treatments against Varroa mites in autumn are important, because particularly in this season treatment is less harmful as the bee-brood is hardly, if at all, present in the hive, so the parasites can be maximally exposed to the drugs. | ||||
| 3. | ‘Winter keeping of the colonies’: bees, especially at low ambient temperatures, remain in the hives and consume honey so as to produce heat and keep the cluster warm (Flottum Citation2005). Since bees are dormant during winter, it is better to limit (or preferably refrain from) inspection as soon as possible to avoid cold stress and the possible breakage of the winter cluster. Regular feeding of the hives with hard candy is recommended for the weaker colonies during very cold and rainy seasons. In this context it should be stressed that – in cooler climates – enough feed must be supplied no later than September, as the next opportunity will not present itself until February or March. Finally, it is important before the winter season to inspect the beekeeping equipment and to take specific precautionary measures following GFP instructions ( of Formato and Smulders (Citation2011)). | ||||
| 4. | ‘Keeping of colonies before supering’: in this phase, the beekeeper should visit each hive to verify the presence of the queen and to monitor the presence of signs of diseases (especially American/European foulbrood and nosemosis). It is also important to replace the old frames with new ones (so as to take advantage of the bee's natural tendency to produce wax, which is typical of this time of the year), to take preventive measures against swarming, in unusually wet springs to provide weaker colonies with extra food thus preventing starvation, and to treat the colonies against Varroa infestation ( and of Formato and Smulders (Citation2011)). Incidentally, beekeepers should (and many do) monitor for pests and diseases at each apiary visit. | ||||
2.4.2. Raise new colonies
The beekeepers can produce their own stocks of bees and nuclei, and they may also do their own queen rearing (Pellet Citation1918; Laidlaw, Jr. and Page Jr. 1997) or they can purchase nuclei, swarms, colonies or queens. Before purchasing or harvesting these from the environment, beekeepers must: (1) critically select suppliers and verify that the sanitary condition of the bee population is satisfactory, (2) take quarantine measures and (3) treat bees against mites ( and of Formato and Smulders (Citation2011)).
2.4.3. Add supers to collect honey
When the colonies are big enough to expand and fill the honey supers (usually in spring/summer), the beekeeper can super the hives to obtain the honey harvest.
2.4.4. Develop colonies with supers in position
While bees are collecting honey, the beekeeper must monitor their performance and:
| - | add empty supers when the ones originally placed have been filled; | ||||
| - | check the status of less productive [e.g. ‘orphan’ (dequeened)] hives with empty or less-filled supers. | ||||
2.4.5. Remove supers for honey harvest
After the combs are filled with ripened honey, the beekeeper must remove the supers as per GFPs ( of Formato and Smulders (Citation2011)). The final output of this phase will be the harvested honey.
2.5. On-site confirmation of the flow diagram
Considering that HACCP plans should be tailor-made for each individual production unit (CAC Citation1999), the HACCP team must confirm the accuracy of the flow diagram on site. Any observed deviation is to result in an amendment of the original flow diagram. The flow diagram has to be confirmed on the apiary for each stage.
2.6. Listing of all potential hazards associated with each step, conducting hazard analysis, and considering any measures to control identified hazards
Principle 1 of the CAC approach to HACCP (CAC Citation1999) requires a systematic consideration of hazards to public health, i.e. the listing of all possible biological, physical and chemical agents associated with the consumption of honey. To achieve this, one relies on scientific data and surveillance systems. The hazards to be considered in the finished or unfinished honey are those possibly introduced during the various production stages and that are reported to cause illness in humans. In these are summarized, and reference is made to scientific sources providing further information on the (severity of) adverse effects.
2.6.1. Biological hazards
In honey, moulds and yeasts are the only microorganisms able to proliferate. Whereas some bacteria like Bacillus and Clostridium can survive in honey, their growth is unlikely. In practice, spores of Bacillus and Clostridium, moulds and yeasts prevail in honey with certain regularity (Snowdon and Cliver Citation1996; EC Citation2002b). Ripened honey is a rather shelf-stable product, as it is composed of elements that avoid bacterial proliferation and disable its main pathogens from 10 days up to 2 months. Therefore, it generally does not represent a product associated with microbiological hazards with the possible exception of clostridial toxin producers (Clostridium botulinum, Clostridium baratii, Clostridium butyricum).Although these pathogens cannot multiply or produce toxins due to the inhibitory properties of honey, they can survive in the product as spores. C. botulinum, C. baratii, C. butyricum are reported to be responsible for cases of infant botulism (occurring in children of <1 year of age, usually as a result of pacifiers having been dipped in honey). Of these, C. botulinum is the most common agent of disease. Although infant botulism is a serious illness, mortality is very low and the level and frequency of contamination of honey with spores of clostridia is generally low (EC Citation2002b).
In some parts of the world – notably in New Zealand where the native and widely distributed tutu plants (Coriaria arborea) co-exist with the vine hopper (Scolypopa sp.) – bees may produce ‘toxic honey’. When in dry periods nectar from more attractive flowers is not available, bees (rather than collecting nectar and pollen from tutu plants) feed on the ‘honeydew’ left on the plant by the sap sucking vine hopper. Such honeydew contains the potent and very stable tutin toxin. Particularly hazardous is the ingestion of comb honey, which (as opposed to extracted honey, as this is often blended with other honeys) can contain high concentrations of tutin. In Europe, tutu plants are not indigenous. In New Zealand this risk is managed by removing hives and supers before dry periods and by monitoring for these conditions within a 3-km radius around the apiary (New Zealand Food Safety Authority Citation2008).
Similarly, intoxications can be caused by the consumption of honey contaminated with the grayanotoxin prevalent in nectar of some plants belonging to the family Ericaceae (Rhododendron albiflorum, Rhododendron macrophyllum, Rhododendron occidentale, Rhododendron ponticum, Azalea pontica, Kalmia latifolia and Kalmia angustifolia). Clinical symptoms () generally occur when unprocessed honey from a single affected apiary is consumed, but – as these usually disappear within 24 h – intervention is rarely necessary (Center for Food Safety and Applied Nutrition Citation2005). Although found in some ornamental gardens, these plants are not indigenous in Europe and consequently toxin concentrations hardly ever reach dangerous levels in honeys produced in Europe.
Although there is some evidence that the pyrrolizidine alkaloids present in ragwort (Senecio jacobaea) and in other plants (e.g. Echium, Senecio, Borago, Tussilago and Cynoglossum) might be carcinogenic and it is known that ragwort alkaloids may occur in milk of cows and goats grazing infested areas and in the honey of bees working on those (Edgar et al. Citation2002), no fully validated or standardized methods exist to measure the pyrrolizidine alkaloids content in honey (Kempf et al. Citation2010), no incidents of pyrrolizidine alkaloids poisoning have been attributed to consumption of honey (WHO Citation1988) and no data are available on which to query the safety of these products for human consumption. Although the bitter taste prevents people from consuming large doses, it is reported that ragwort imparts a taint to honey making it unfit for sale (Parsons and Cuthbertson Citation2001). However, as the nectar from various sources are mixed, generally this does not represent a problem.
2.6.2. Physical hazards
Whereas foreign objects accidentally placed in honey do represent a hazard in (and therefore must be considered in HACCP plans for) honey processing, they do not compromise the safety of unprocessed honey produced at the primary apiary level.
2.6.3. Chemical hazards
2.6.3.1. Pharmaceuticals with antimicrobial action
Most beekeepers in Asia, the USA, South America and Canada rely on the use of substances with antimicrobial activity, such as tetracycline antibiotics, sulpha drugs, chloramphenicol and tylosine (Edder et al. Citation2002, Reybroeck Citation2003; Ortelli et al. Citation2004; Lopez et al. Citation2008, Formato and Smulders Citation2011). The European apicultural sector [as represented by the COPA-COGECA Honey Working Party and the European Federation of Honey Packers and Distributors (FEEDM)] is against the registration of any antimicrobial substance for bees so as to protect the consumers’ image of honey as being a ‘natural’ and ‘healthy’ product, and proposes establishing reference points of action for imported honey, that should only take into account environmental contamination, and decidedly not residues of antibiotics used to combat bee diseases (Bruneau et al. Citation2009; FEEDM/COPA-COGECA Citation2009). As combating bacterial (American or European foulbrood) or fungal diseases (nosemosis) without being able to rely on antibiotics is more difficult, less responsible European beekeepers still rely on their illicit use. Implicitly, the HACCP system fails to prevent such practices, particularly since these will obviously not be documented. Therefore, the only remaining risk management option is to rely on end-product control by the competent authority, i.e. monitoring for the proper use of veterinary drugs on apiaries and by examining hive products.
2.6.3.2. Insecticides
One must distinguish between the permissiveness of the use of pesticides against insects pathogenic for plants, those allowed for use in mammalian animal species or those approved for use in bees ( and ). Although many proprietary products are marketed to beekeepers, there are many ‘home-made concoctions’ applied to hives using the same ingredients which are included in agricultural pesticides. This can potentially give rise to residues in the product. For instance, whilst fluvalinate-based preparations such as Apistan® are authorized for use in apiculture, the plant preparations based on the same active ingredient (e.g. Maverik®) are not. The inherent danger of relying on such unsuitable drugs (e.g. because some plant preparations are cheaper) is that GVPs regarding a proper dosage, manner of administration and withdrawal times are likely to be abused with inherent risks for residues in honey (i.e. at too high dosages) or development of resistance of the pathogen against the drug (at too low dosages). The consequences can even become particularly hazardous for public health when plant preparations such as Birlane® or Supona®, known for their carcinogenic properties, are used to combat mites in the apiary.
Table 4. Drugs authorized in EU Member States for their use in apiculture (European Medicine Evaluation Agency 2009).
2.6.3.3. Other environmental contaminants
Other chemical hazards of environmental origin – like heavy metals, PCBs, polycyclic aromatic hydrocarbons and pesticides – are also relevant for honey safety. Indeed, those hazards could equally be prevented by adhering to GFPs [i.e. ensuring proper positioning of the apiary as indicated by Formato and Smulders (Citation2011) in their ].
2.7. Determine CCPs
A CCP is defined as ‘a step at which control can be applied and is essential to prevent or eliminate a food safety hazard or reduce it to an acceptable level’. Determination of CCPs is carried out with a decisional tree, as included in the Codex HACCP guideline (CAC Citation1999). The following need to apply for a step to be a CCP:
| 1. | The step needs to be listed as representing a hazard. | ||||
| 2. | Validated measures that allow keeping the hazard in check need to exist. | ||||
| 3. | The step needs to represent an intervention option (if answer is yes: CCP; if not: further consider 4 and 5). | ||||
| 4. | The step should involve a level of contamination beyond critical limits. | ||||
| 5. | A subsequent step eliminating or significantly reducing the hazard does not exist (if this the case: CCP, if such a step does exist: address the hazard through adherence to GFPs) | ||||
The outcome of such a procedure is the identification of those production steps that can be monitored, corrected, verified and documented. According to these criteria and procedures, only the following two steps need to be further considered.
2.7.1. Treatment against Varroa mites
Treatments against V. destructor can be realized with low environmental impact products (i.e. relatively harmless chemical compounds, normally present in nature and authorized for organic beekeeping), such as organic acids (e.g. oxalic acid, lactic acid, formic acid) or essential oils (e.g. thymol, menthol, eucalyptol) or with products that have a high environmental impact (i.e. for which specific MRLs have been established, which are produced by synthesis in a laboratory and are not authorized for organic beekeeping), such as organophosphates and pyrethroids. lists the drugs authorized for use in apiculture in the various EU Member States (European Medicine Evaluation Agency Citation2009).
Only EU authorized medicines are allowed for hive treatments, provided these are used with supers in such a position that contamination of honey can be excluded. Legislation (EC Citation2004a) holds beekeepers to record in a log book the name of the drug administrator, the nature of the drug used, the dates of its administration and the withdrawal period (if any). In general, those treatments that have a low environmental impact are strongly influenced in their varroacide activity by the quantity of the sealed brood that is in the brood box. Normally, the higher their efficacy is, the lower the quantity of the sealed brood, as the Varroa mite cannot ‘escape’ from the treatment within the capped brood cells (because products like oxalic acid cannot permeate into capped cells). Efficacy is also dependent of the environmental conditions (e.g. temperature and humidity). However, at the same time, they have the great advantage of not presenting problems for honey contamination and the consumer's health. Compounds with a higher environmental impact, though usually more efficient to eliminate mites, could, on the other hand, result in dangerous levels of residues in honey products, if not properly administered.
As chemical hazards associated with treatments against V. destructor in the phase of ‘Add supers to collect honey’ satisfy the first three criteria of the decisional tree, treatment against V. destructor represents a CCP.
2.7.2. Production steps allowing contamination with clostridial spores
Contamination of honey with clostridial spores may occur during the following phases: (1) ‘Breeding hives with supers in position’ and (2) ‘Remove supers for honey harvest’. Primary sources of spore contamination of honey may include pollen, sweeteners destined as food for bees, the digestive tracts of honey bees, dust, air, earth and nectar (Nakano et al. Citation1992). These sources are impossible to control. Secondary sources of spore contamination of honey are contamination through air, food handlers, cross-contamination, equipment, etc. As regards these sources, the mentioned production phases would appear to be CCPs. However, due to the impracticability of microbiological monitoring of botulinum-producing spores in apicultural practice – which is essentially related to the high costs involved in the analysis and the sporadic occurrence of the pathogen (EC Citation2002b) – only the adherence to GFPs and targeted operatives’ training programmes are realistic options for the prevention of this hazard. Hence, records on the training programme as well as those on the actual procedures followed during harvesting of the supers must be kept by the beekeeper or his assistants, to assess whether or not best practices have indeed been adhered to (Formato and Smulders Citation2011; their ). Field inspections are to be conducted by the beekeeper or his assistants in the application of HACCP principles, at least once a year to verify this. In addition, honey is generally labelled ‘not to be consumed by infants less than 1 year old’, to account for any residual uncertainty.
2.8. Establish critical limits for each CCP
Critical limits for chemical hazards of the only CCP identified include:
| - | presence of supers on top of brood boxes before expiration of the withdrawal period (if applicable) of the administered drug; | ||||
| - | absence, within the brood box, of any hive treatment device. | ||||
2.9. Establish a monitoring system for each CCP
To monitor hazards associated with pharmaceutical/chemical treatments, immediately before hive supering, the beekeeper will verify by documentation the following:
| - | Are treatments still ongoing? | ||||
| - | Has a particular withdrawal time of an agent in question been reported in the medicines record book, and – if so – has it been adhered to? | ||||
| - | Are any devices from previous hive treatments (e.g. sponges, strips) still present in the brood box? | ||||
2.10. Establish corrective actions
The corrective actions associated with the CCP include:
| - | delaying supering until the end of the treatments; | ||||
| - | no supers to be placed on the hives during treatments; and | ||||
| - | removal of all application tools. | ||||
2.11. Establish verification procedures
The verification procedure includes:
| - | during harvesting of every super, verification in the log book of the information about the nature and termination of treatments, and the dates of positioning of the supers; | ||||
| - | drawing samples (by the official controlling bodies) from at least one super per apiary for purposes of analysing the level of contamination with residues of authorized drugs. | ||||
2.12. Establish documentation and record keeping
Accurate and efficient record keeping is essential to application of a HACCP system and for substantiating that legal and contractual obligations have been fulfilled. To be documented are, for instance, the hazard analysis, all the reference documents substantiating the risk assessment, CCP determination and critical limit determination. The record-keeping system should include information drawn from a specific log book in which have been recorded:
| - | all monitoring activities; | ||||
| - | other relevant information about the treatment (following GFP); | ||||
| - | the date of supers’ positioning (for purposes of comparing with the dates of previous treatments) | ||||
| - | the corrective actions eventually adopted; and | ||||
| - | the verification procedures adopted. | ||||
3. Conclusions
Provided a set of basic GFPs and GVPs is adhered to, the establishment of a supplementary HACCP system for the management of risks associated with the production of unprocessed honey is meaningful.
Whereas the presence of botulinum toxin producing spores may indeed represent a hazard during the harvesting of supers, its prevention is preferably addressed through relying on the adherence to GFPs and through training of the operatives, as in apicultural practice monitoring of this hazard is impracticable. Thus the ‘feasibility’ principle, as formulated in Regulation (EC) No. 852/2004 is taken into account, which states that: ‘The HACCP requirements … should provide sufficient flexibility to be applicable in all situations, including in small businesses’.
The HACCP system also allows for significantly reducing the risks of hazards associated with chemical or pharmaceutical treatments which could lead to residues being present in unprocessed honey. In this context, the only CCP identified is the phase of ‘Develop colonies with supers in position’.
The application of the HACCP system in apiculture is an achievable goal, albeit it will inevitably increase the level of obligations to be fulfilled by the individuals responsible. By the same token, it will offer beekeepers the opportunity to clearly identify hazards, to focus prevention strategies following a priority ranking approach and it will allow fulfilment of contractual obligations in a longitudinally integrated honey production and marketing chain. Finally, the suggested approach promises to be useful for veterinary advisors supporting Food Business Operators (FBOs) and for the competent authorities in planning and conducting their audits and establishing priorities for the evaluation of FBO training programmes.
Acknowledgements
Dr Jane Richardson (European Food Safety Authority, Parma, Italy) is gratefully acknowledged for her useful comments on the manuscript.
References
- Bogdanov , S , Lüllmann , C , Martin , P , Von der Ohe , W , Russmann , H , Vorwohl , G , Persano Oddo , L , Sabatini , AG , Marcazzan , GL Piro , R . 1999 . Honey quality and international regulatory standards: review by the International Honey Commission . Bee World , 80 : 61 – 69 .
- Bruneau , E , Delso , NS and Besana , A . 2009. Securing bee health and honey quality [MI(09)8583]. Personal presentation at the workshop on ‘Medicines for bees and what the EMEA can do to increase the availability’, Dec 14 and 15
- CAC 1999. Recommended international code of practice: general principles of food hygiene. Rome, Italy: FAO/WHO. Document CAC/RCP 1-1996, Rev. 4-2003
- CAC 2002. Principles and guidelines for the conduct of microbiological risk assessment. Rome, Italy: FAO/WHO. Document CAC/GL30
- Canadian Food Inspection Agency. 2010. Food safety enhancement program (FSEP) manual [Internet]. Available from: www. inspection.gc.ca/english/fssa/polstrat/haccp/haccpe.shtml; accessed 2001 Feb 14
- Center for Food Safety and Applied Nutrition. 2005. Grayanotoxin. In: The “Bad Bug Book” – Foodborne pathogenic microorganisms and natural toxins handbook. USA: Handheldmed Inc. Available from: http://www.fda.gov/Food/FoodSafety/FoodborneIllness/FoodborneIllnessFoodbornePathogensNaturalToxins/BadBugBook/ucm071128.htm; accessed 2009 Dec 17
- Costa , LG . 2006 . Current issues in organophosphate toxicology . Clin. Chim. Acta , 366 ( 1–2 ) : 1 – 13 .
- Crane , EE . 1980 . A book of honey , Oxford : Oxford University Press .
- Delaplane , KS , Berry , JA , Skinner , JA , Parkman , JP and Hood , WM . 2005 . Integrated pest management against Varroa destructor reduces colony mite levels and delays treatment threshold . J. Apic. Res , 44 ( 4 ) : 157 – 162 .
- Duffus , JH . 2002 . ‘Heavy metals’, a meaningless term? (IUPAC Technical Report) . Pure Appl. Chem , 74 : 793 – 807 .
- Edder , P , Ortelli , D and Corvi , C . 2002. Survey of antibiotics residues in honey on the Swiss market. Poster presented at the 4th International Symposium on Veterinary Hormones and Drug Residue Analysis; Jun 4–7; Antwerp, Belgium. Available from: http://etat.geneve.ch/des/SilverpeasWebServices/reference-15pdf?component=kmelia704&SourceFile=1166702; accessed 2009 Nov 20
- Edgar , JA , Roeder , E and Molyneux , RJ . 2002 . Honey from plants containing pyrrolizidine alkaloids: a potential threat to health . J Agric. Food Chem , 50 ( 10 ) : 2719 – 2730 .
- Edwards , M . 2004 . Detecting foreign bodies in rood, Woodhead Food Series, No. 92 , Cambridge : Woodhead Publishing Ltd .
- European Commission [EC]. 1999. White Paper on food safety, EU Commission, COM 719, Final, 2000 Jan 12
- EC 2001. Council Directive 2001/110/EC of 20 December 2001 relating to honey. Official Journal of the European Communities, L 10, 2002 Jan 12, p. 47
- EC 2002a. Regulation 178/2002 of the European Parliament and the Council of 28 January 2002, laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. Official Journal of the European Union, Brussels, O.J. L 31/1
- EC 2002b. Opinion of the scientific committee on veterinary measures relating to public health on honey and microbiological hazards. Adopted on 19–20 June 2002
- EC 2004a. Regulation (EC) No. 852/2004 of the European Parliament and of the Council of 29 April 2004, containing specific rules for the Hygiene of foodstuffs. Brussels: Official Journal of the European Union. O.J. L138/1
- EC 2004b. Regulation (EC) No. 853/2004 of the European Parliament and the Council of 29 April 2004, containing specific rules for the hygiene of products of animal origin. Brussels: Official Journal of the European Union. O.J. L139/55
- EC 2004c. Regulation (EC) No. 854/2004 of the European Parliament and the Council of 29 April 2004, containing specific rules concerning official controls on products of animal origin. Brussels: Official Journal of the European Union. O.J. L155/206
- European Food Safety Authority. 2009. Reasoned opinion of EFSA prepared by the Pesticides Unit (PRAPeR) on the 2007 Annual Report on Pesticides Residues according to Article 32 of Regulation (EC) No 396/2005. EFSA Scientific Report 305, 1–106
- European Medicine Evaluation Agency. 2009. Co-ordination group for mutual recognition and decentralised procedures – veterinary. London: EMEA. Report No. EMEA/497311/2009, rev. 1., Aug 24
- Extoxnet. 2009. Pesticides information profiles [Internet]. Available from: http://extoxnet.orst.edu/pips/pyrethri.htm; accessed 2010 Dec 18
- FEEDM/COPA-COGECA. 2009. Letter to Mr. Eric Poudelet, European Commission, DG SANCO [MI (09)5244:1]
- Flottum , K . 2005 . The backyard beekeeper: an absolute beginner's guide to keeping bees in your yard and garden , Beverley, , USA : Rockport Publishers .
- Food and Agriculture Organization of the United Nations and World Health Organization. 2006. FAO/WHO guidance to governments on the application of HACCP in small and/or less-developed food business. Rome: FAO Food and Nutrition. Paper No. 86
- Formato , G and Smulders , FJM . Forthcoming 2011 Risk management in primary apicultural production. Part 1: bee health and disease prevention and associated best practices. Vet. Quart. 31(1):29–47
- Fredes , C and Montenegro , G . 2006 . Heavy metals and other trace element contents in Chilean honey . Cien. Inv.Agr , 33 ( 1 ) : 50 – 58 .
- Hilbert , F and Smulders , FJM . 2004 . “ Antibiotics; resistance in food-borne pathogens ” . In Encyclopedia of meat sciences , Edited by: Jensen , WK , Devine , C and Dikeman , M . Vol. 1 , 38 – 43 . Amsterdam : Elsevier .
- Hopper , T . 1983 . Guide to bees and honey , USA : Sterling Pub. Co. Inc .
- Instituto Nacional de Tecnologia Industrial. 2005. HACCP & hygiene in honey production. Personal presentation, Argentina, October/November 2005. Available from: www.apiculturaonline.com/pdf/HACCP.pdf; accessed 2011 Feb 14
- International Committee on the Microbiological Specification for Foods . 1988 . Microorganisms in foods 4: Application of the Hazard Analysis Critical Control Point (HACCP) system to ensure microbiological safety and quality , Oxford : Blackwell Science .
- Kempf , M , Wittig , M , Reinhard , A , von der Ohe , K , Blacquiere , T , Raezke , KP , Michel , R , Schreier , P and Beuerle , T . 2010 . Pyrrolizidine alkaloids in honey: comparison of analytical methods . Food Addit. Contam. Part A: Chem. Anal. Control Exposure Risk Assess , 12 : 1 – 16 .
- Laidlaw Jr , HH and Page Jr , RE . 1997 . Queen rearing and bee breeding , 224 Cheshire : Wicwas Press .
- Lopez , MI , Pettis , JS , Smith , IB and Chu , PS . 2008 . Multiclass determination and confirmation of antibiotic residues in honey using LC-MS/MS . J. Agric. Food Chem , 56 ( 5 ) : 1553 – 1559 .
- Nakano , H , Yoshikuni , Y , Hashimoto , H and Sakaguchi , G . 1992 . Detection of Clostridium botulinum in natural sweetening . Int. J. Food Microbiol , 16 : 117 – 121 .
- National Honey Board. 2005. Honey. A reference guide to nature's sweeteners. [Internet]. Available from: http://www.honey.com/images/downloads/refguide.pdf; accessed 2010 Oct 16
- New Zealand Food Safety Authority. 2008. Background on toxic honey. [Internet]. Available from: www.nzfsa.govt.nz/animalproducts/publications/info-pamphlet/bee-products; accessed 2009 Dec 1
- Ortelli , D , Edder , P and Corvi , C . 2004 . Analysis of chloramphenicol residues in honey by liquid chromatography-tandem mass spectrometry . Chromatographia , 59 : 61 – 64 .
- Parsons , WT and Cuthbertson , EG . 2001 . Noxious weeds of Australia , Collingwood : CSIRO Publishing .
- Pellet , FC . 1918 . Practical queen rearing , Hamilton, Illinois : American Bee Journal. . Available from: http://www.archive.org/details/practicalqueenre00pellrich; accessed 2011 April 1
- Reybroeck , W . 2003 . Residues of antibiotics and sulphonamides in honey on the Belgian market . APIACTA , 38 : 23 – 30 .
- Rice , ND , Winston , ML and Higo , HA . 2004 . Integrated pest management for the parasitic mite Varroa destructor (Anderson and Trueman) in colonies of honey bees (Apis mellifera) . Am. Bee J , 144 ( 10 ) : 791 – 795 .
- Safe , S , Bandiera , S , Sawyer , T , Robertson , L , Safe , L , Parkinson , A , Thomas , PE , Ryan , DE , Reik , LM and Levin , W . 1985 . PCB's: structure-function relationships and mechanisms of action . Environ. Health Perspect , 60 : 47 – 56 .
- Smulders , FJM . 2009 . “ A practicable approach to assessing risks for animal welfare – methodological considerations ” . In Food safety assurance and veterinary public health. Vol. 5, Welfare of production animals: assessment and management of risks. Wageningen , Edited by: Smulders , FJM and Algers , B . 239 – 274 . The Netherlands : Academic Publishers .
- Snowdon , JA and Cliver , DO . 1996 . Microorganisms in honey . Int. J. Food Microbiol , 31 ( 1 ) : 1 – 26 .
- Untermann , F . 1998 . Microbial hazards in foods . Food Control , 9 : 119 – 126 .
- Wiedenfeld , H and Edgar , J . 2010 . Toxicity of pyrrolizidine alkaloids to humans and ruminants . Phytochem. Rev , 10 ( 1 ) : 137 – 151 .
- [WHO] World Health Organization. 1988. Pyrrolizidine alkaloids. Geneva: Environmental Health Criteria International Programme on Chemical Safety (IPCS). Report No. 80
- WHO 2001WHO global strategy for containment of antimicrobial resistance. Geneva: WHO. Report No. WHO/CDS/DRS/2001.2
- WHO 2008. Hazard Analysis and Critical Control Point generic models for some traditional foods: a manual for the Eastern Mediterranean Region. Amman: ERMO. ISBN 978-92-9021-5905