Abstract
Background: Enterococcus hirae-associated endocarditis, characterized by a peak in mortality during the second week of the grow-out, and occasionally lameness, was diagnosed at Dutch broiler farms.
Objectives: Field cases were studied to increase knowledge on clinical and pathological characteristics, pathogenesis and epidemiology of these infections.
Animals and methods. In total, 1266 birds of 25 flocks from 12 farms were examined. Post-mortem examinations, bacteriology, histopathology, PCR and DNA fingerprinting was carried out. Six flocks were followed longitudinally (n = 1017 birds).
Results: Average mortality was 4.1% for the entire grow-out, of which 36% was attributed to endocarditis. Fibrinous thromboendocarditis of the right atrioventricular (AV) valve was found in 24% of hearts, compared to 7% and 4% with lesions of left and both AV valves, respectively. Thrombotic lesions were found in 24% (n = 432) of lungs, but only in larger branches of the Arteria pulmonalis. Occasionally, thrombi were found in the Arteria ischiadica externa and in liver and brain vessels. Enterococcus was cultured from 54% (n = 176) of heart and in 75% (n = 28), 62% (n = 106) and 31% (n = 16) of liver, bone marrow and lung samples, respectively. Further identification, using the Rapid ID Strep 32 API system and a PCR targeting mur-2 and mur-2ed genes was carried out on a subset of Enterococcus positive isolates (n = 65): both techniques identified the isolates as Enterococcus hirae. Pulsed-field gel electrophoresis did not indicate evidence of clonality between farms and flocks.
Conclusions: The relevance of these findings for pathogenesis and epidemiology of E. hirae infections is discussed.
Clinical importance. This study may facilitate diagnosis of field cases and may contribute to the design of further research and development of control measures.
1. Introduction
Endocarditis is an inflammation of the inner lining of the heart muscle, the endocardium and usually involves the heart valves, which is often caused by bacteria. In humans, bacterial endocarditis is associated with congenital heart disease, degenerative valvular disease, prosthetic valves, haemodialysis, intravenous drug abuse and nosocomial bacteriaemia due to surgical procedures (Hill et al. Citation2006). Staphylococcus aureus, streptococci and enterococci are responsible for more than 80% of human cases of endocarditis (Fitzgerald et al. Citation2006). In contrast to endocarditis in humans, lesions can develop in companion and farm animals without detectable evidence of valve abnormalities and are usually caused by Streptococcus spp. , Enterococcus spp. , S. aureus, Escherichia coli, Actinobacillus equuli, Arcanobacterium pyogenes, Erysipelothrix spp., Pseudomonas aeruginosa, Pasteurella multocida and Bartonella spp. (Maxie Citation2007).
Bacterial endocarditis in poultry is usually caused by streptococci and enterococci. Enterococcus faecalis has been the most common isolate in naturally occurring infections (Domermuth and Gross Citation1969; Jortner and Helmboldt Citation1971; Chadfield et al. Citation2004). Other bacteria associated with naturally occurring and experimentally induced endocarditis include Enterococcus faecium (Sandhu Citation1988), Streptococcus zooepidemicus (Peckham Citation1966), S. aureus, P. multocida (Gross and Domermuth Citation1962) and Enterococcus hirae (McNamee and King Citation1996; Chadfield et al. Citation2005a). Enterococcus durans has been described as causative agent of endocarditis, but only under experimental conditions (Domermuth and Gross Citation1969).
In addition to endocarditis, enterococci are frequently associated with many other disease conditions in poultry. In day-old chicks enterococci are often associated with yolk sac infection (Landman Citation2002). E. faecalis has been related to hepatic granulomas in turkeys (Moore and Gross Citation1968; Hernandez et al. Citation1972), ascites in hens (Huan Citation1997) and pulmonary hypertension in broilers (Tankson et al. Citation2001). Spontaneous cases of AA amyloid arthropathy and concomitant systemic amyloidosis caused by arthropathic and amyloidogenic E. faecalis strains have been described in brown layers (Landman et al. Citation1994) and broiler breeders (Steentjes et al. Citation2002). In domestic ducks E. faecalis has been isolated from arthritic joints (Bisgaard Citation1981), whereas E. faecium has been associated with acute septicaemia in white pekin ducklings (Sandhu Citation1988). Enterococcus cecorum is increasingly found in broiler and broiler breeder flocks with signs of lameness and is mostly associated with arthritis, spondylitis, femoral head necrosis and osteomyelitis (Devriese et al. Citation2002; De Herdt et al. Citation2009; Kense and Landman Citation2010; Stalker et al. Citation2010). E. durans in young chickens has been found in birds with bacteraemia and encephalomalacia (Cardona et al. Citation1993; Abe et al. Citation2006), while E. hirae has been reported in cases of focal necrosis of the brain of young chicks (Devriese et al. Citation1991a; Randall et al. Citation1993), in cases of diarrhoea in first week layer chicks (Kondo et al. Citation1997) and in broilers with osteomyelitis (Kolbjørnsen et al. Citation2011).
The present report describes naturally occurring E. hirae-associated endocarditis in broilers. Birds, organs and cultures of 25 affected flocks were subjected to further examination and for six of these flocks the progression of lesions and related mortality was studied longitudinally from two weeks of age onwards Results of post-mortem examinations, bacteriology, histopathology, PCR and DNA fingerprinting of isolated bacteria are described.
2. Materials and methods
2.1. Case history
Spontaneous E. hirae-associated endocarditis was first noticed in the Netherlands in 2004, when it was diagnosed in 15 flocks on 10 broiler farms. In the following year endocarditis was diagnosed again, predominantly affecting the same broiler farms as in 2004. In total, 21 flocks of 12 different broiler farms were diagnosed with endocarditis by the Animal Health Service (GD), Deventer, the Netherlands in 2004 and 2005 and were included in the study. In 2008 and 2009, four different flocks with signs of endocarditis and lameness and originating from three of the previously studied farms were studied.
Broilers of affected flocks originated from different hatcheries, parent stocks and three different breeds. All flocks consisted of male and female broilers, housed on litter floors. Flock sizes ranged from approximately 8000 to 49,000 and biosecurity measures varied greatly between the farms included in this study. The farms were located in the southwestern part of the Netherlands and on average at a distance of 44 km from each other (range 3 km to 86 km). Exception was one farm that was located in the northeastern part of our country at an average distance of 172 km (range 134 km to 223 km) from the other flocks.
During the second week of the grow-out period a mortality peak was observed in affected flocks, while the overall average mortality was 4.1%. This is not significantly higher than the average mortality in Dutch broiler flocks in 2004 and 2005, being 4.0%, which was calculated based on an average fattening period of 43 days (Animal Sciences Group – Wageningen UR Citation2006). However, if the mortality of diseased flocks was compared to unaffected flocks at the same farm, a significant increase was observed. In order to quantify the impact of endocarditis on flock health, the percentage of total mortality ascribed to endocarditis, was estimated per flock. These estimations, were based on the increase in mortality compared to normal mortality for flocks at the same farm (n = 11 flocks), on post-mortem examinations of a random selection of perished birds throughout the entire grow-out period (n = 5 flocks) and on an estimation of the farmer (n = 1 flock). In Flock A4 all perished birds were examined for endocarditis lesions from two weeks of age onwards (when endocarditis pathology was first observed). This enabled an exact calculation of mortality attributed to endocarditis. Flock data of the 2004 and 2005 outbreaks are summarized in .
Table 1. Summary of the flock data, post-mortem analysis and bacteriology of the 2004 and 2005E. hirae-associated endocarditis outbreaks.
2.2. Post-mortem examination and further analysis
During the 2004 outbreak, 145 birds of the affected flocks were predominantly collected during the second week of the grow-out period and sent for further examination to Animal Health Service (GD), Deventer, the Netherlands. In order to study the course of infection and progression of lesions, all carcasses from Flock A4 of the 2005 outbreak were examined for endocarditis pathology from the second week onwards (n = 579). Furthermore, during that same year a random selection of carcasses (n = 438) from another five flocks, collected at various time points during the grow-out period, were also examined to assess the occurrence of endocarditis (). In addition, 104 carcasses from four flocks of three farms (farms A, C and E, according to the notation in ) were examined in 2008 and 2009.
During the post-mortem examination special attention was paid to the occurrence of lesions of endocarditis, bacteraemia and thrombotic processes. Additionally, the occurrence of joint pathology was assessed. Inspection of the endocardium was performed after opening of the right and the left ventricle to expose the right and left atrioventricular (AV) valves. Both atria were removed and the Vena cava superior was cut towards the apex of the heart, separating the right from the left ventricle. The left ventricle wall was cut towards the apex, after entering one of the pulmonary vessels. The blind pouch underneath the triangular muscle plate of the right AV valve and the surface of the left AV valve were inspected carefully for endocarditis lesions. Liver, lungs, spleen and kidneys were inspected in detail for the occurrence of lesions. For this purpose, the lungs had to be sliced transversally into 2–4 mm pieces, as thrombotic lesions were not visible externally. Arteries and veins located in the lumbar and sacral area were also inspected for presence of thrombosis.
After post-mortem examination, samples of heart (n = 72), liver (n = 9), lungs (n = 19), kidneys (n = 16), spleen (n = 15) and brain (n = 16) were collected and stored in 10% buffered formalin, embedded in paraffin and sectioned for haematoxylin and eosin staining.
In addition, presence of endocarditis pathology and liver- and lung lesions in birds of Flock A4, at the end of the grow-out period, was assessed by examining 300 randomly selected hearts, livers and lungs from the slaughterhouse.
2.3. Bacteriological examination
From each flock a number of hearts (n = 176), livers (n = 28), lungs (n = 16) and bone marrow (n = 106) were left intact during post-mortem examination, until sampling for bacteriology (). These organs were selected, based on post-mortem findings suggesting the presence of endocarditis or bacteraemia, i.e. thrombotic processes in one of the lungs, foci in the liver or a swollen appearance of the heart at the AV valve region. Furthermore, in order to assess the occurrence of bacteraemia, blood samples were collected from 30 live birds from Flock A4 at day 28 for bacteriology. Blood samples were collected aseptically (after cleansing the skin with 70% ethanol) by Vena ulnaris aspiration using a 2 cc syringe and needle. One mL of blood was incubated with 10 mL of brain-heart infusion broth (Oxoid B.V., Haarlem, the Netherlands) at 37°C overnight. Flasks showing bacterial growth were subsequently plated out on sheep blood agar.
Bacteriological cultures were made from heart, liver, lungs, bone marrow or diluted blood on sheep blood agar. After aerobic incubation of the agar plates for 24 h at 37°C small grey transparent to white colonies were identified as Lancefield Group D isolates, using Streptex® (bioTRADING Benelux B.V., Mijdrecht, the Netherlands). Preliminary identification of the Enterococcus species involved was carried out by carbohydrate fermentation tests and by determining growth in 6.5% saline, according to Quin et al. (Citation1994).
A subset of Enterococcus positive isolates was chosen for further phenotypic examination to determine the Enterococcus species. These isolates originated from the 2004 and 2005 outbreaks (n = 54, from 21 flocks) and from the cases in 2008 and 2009 (n = 11 from farms A and E), and contained samples from heart (n = 30), lung (n = 3), liver (n = 4), bone marrow (n = 22) and blood (n = 6). Further phenotypic characterization of the Enterococcus species was carried out using the API rapid ID 32 STREP system (bioMérieux, Marcy-l’Etoile, France), pyrrolidonyl aminopeptidase tests (PYR diatabsT, Rosco diagnostica A/S Taastrup, Denmark) according to Manero and Blanch (Citation1999) and by testing the utilization of pyruvate. The latter test was done to discriminate between asaccharolytic variants of E. faecalis and E. durans or E. hirae according to Facklam and Collins (Citation1989).
2.4. Genotypic characterization: PCR and Pulsed-field gel electrophoresis
The same subset of 54 isolates from the 2004 and 2005 outbreaks used for further phenotypic characterization of the Enterococcus species, and five additional isolates were subjected to a PCR assay, targeting the species specific primer pairs mur-2 ed (E. durans) and mur-2 (E. hirae), to differentiate between these species according to the procedures described by Arias et al. (Citation2006). These isolates originated from heart (n = 34), lung (n = 3), liver (n = 4), bone marrow (n = 12) and blood (n = 6) from 21 different flocks. In addition, isolates identified as E. durans (n = 9) and E. cecorum (n = 2) were used as controls.
Genetic relatedness of these isolates was studied using pulsed-field gel electrophoresis (PFGE) analysis, according to modification of the procedure described by Kuzucu et al. (Citation2005). Briefly, preparation of intact genomic DNA in agarose blocks was performed by a slight modification of the method described by Jacobsen et al. (Citation1999). Agarose blocks were incubated overnight at 35°C in lysis buffer (6 mM Tris-HCl, 1.0 M NaCl, 100 mM Na-EDTA, 0.5% Brij 58, 0.2% sodium deoxycholate, 0.5% sodium lauroyl sarcosine, 1 mg/mL lysozyme, 5 U/mL mutanolysin, pH 7.6) on a rocker platform. The plugs were subsequently incubated at 50°C for 24 h in a waterbath in EDTA-sarcosine-proteinase solution (0.5 EDTA, 1% sodium lauroyl sarcosine, 0.1 mg/mL proteinase K, pH 8). The plugs were washed four times for 1 h in TE buffer (10 mM Tris-HCl, 0.1 M EDTA, pH 7.6). Restriction enzyme digestion was performed with 20 U of SmaI (New England Biolabs Inc. Hitchin, Hertfordshire, UK) according to the supplier's instructions for 24 h at 25°C. DNA fragments were resolved in 1% (w/v) PFGE certified agarose (Bio-Rad) in 0.5× TBE buffer by PFGE using the CHEF DR-III apparatus (Bio-Rad, Hercules, USA). Digested fragments were separated using ramp times 5–40 s for 24 h at a 120° angle with a voltage gradient of 6 V/cm at 14°C. The agarose gels were stained with ethidium bromide (0.5 µg/mL) and visualized under UV light. The Low Range PFGE Marker (2.03–194.0 kbp; Bio-Rad) was included as size marker and normalization reference. PFGE patterns were assessed for genetic relatedness according to Tenover et al. (Citation1995).
2.5. Statistical analysis
Statistical analyses were applied on post-mortem examination data of the 2005 outbreak (Flocks A4, K, L1, L2, I2 and E2) using SPSS 12.0.1 for Windows (SPSS Inc. Citation2003). The level of statistical significance was set at p < 0.05.
Logistic models were used to determine whether macroscopic lesions other than heart lesions, i.e. lesions in lungs, liver, kidneys, femur or Arteria ischiadica externa were associated with the presence of endocarditis and localization of the heart lesions. The macroscopic lesions served as dependent binary variables for the subsequent analyses. All variables were given score one when lesions or abnormalities were present and zero when considered normal. ‘Week of grow-out’ (WEEK) and farm (FARM) were entered into the model to correct for age and farm effects. The categorical covariate for presence of endocarditis and localization of heart lesions (ENDO_LOC) consisted of four categories (0 = absence of heart lesions, 1 = left-sided endocarditis, 2 = right-sided endocarditis and 3 = both left- and right-sided endocarditis). The first category was chosen as reference category for WEEK (second week for all variables, except for lung lesions which were not assessed until the third week of the grow-out period), FARM (Farm A) and ENDO_LOC (absence of heart lesions). For each of the macroscopic lesions odds ratio (OR) and 95% confidence interval (95% CI) were determined. A similar model was run with category 1 of ENDO_LOC as reference category to determine whether significant differences between right- and left-sided endocarditis were present for the different macroscopic lesions.
A logistic model was constructed to determine whether presence and localization of the heart lesions was associated with ‘Week of grow-out’. Presence of left-sided endocarditis (EC_L), right-sided endocarditis (EC_R), both left- and right-sided endocarditis (EC_LR) and presence of endocarditis without specification of localization (EC) served as dependent binary variables for the subsequent analyses. WEEK was added as categorical covariate with the second week of the grow-out period as reference category and FARM was entered into the model to correct for farm effects with Farm A as reference category. OR and 95% CI were determined for EC_L, EC_R, EC_LR and EC.
Model fit was evaluated using the Hosmer and Lemeshow test for goodness of fit, –2 log likelihood values and normality of the deviance residuals.
3. Results
3.1. Clinical observations
At inspection of the affected flocks, perished birds were usually found on their back, similar to flipovers. In some flocks birds with unilateral or bilateral posterior paralysis were found, coinciding with the peak in mortality. Farmers did not complain about detrimental effects of the disease on technical results of the flock, i.e. body weight at slaughter and feed conversion ratio.
In , data from the different flocks from the 2004 and 2005 outbreaks with endocarditis pathology are summarized. On average 36% of the total mortality was attributed to the E. hirae-associated endocarditis. The estimated percentages of total mortality, related to endocarditis, varied widely between flocks. Estimations based on comparing mortality of affected flocks with normal mortality at the farm ranged between 11–67%. The most reliable and accurate estimations were obtained by means of post-mortem analysis of various random samples taken during the grow-out period of five flocks and after analysing all perished birds of one flock (Flock A4) during the 2005 outbreak. In the first case the percentage mortality attributed to endocarditis varied between 12–41%, while in the latter case it was 46% ().
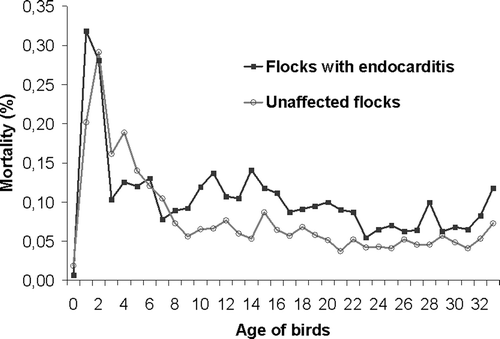
In , the course of mortality per day is shown for four of the six studied flocks (Flocks A4, K, I2 and E2), diagnosed with E. hirae-associated endocarditis during 2005. Mortality in the affected flocks was slightly higher throughout the entire grow-out period, compared to the mortality of unaffected flocks of the same farms. Between day 9 and 22 a small peak in mortality seemed to be present, which was consistent with the observations reported by the farmers.
3.2. Post-mortem examinations
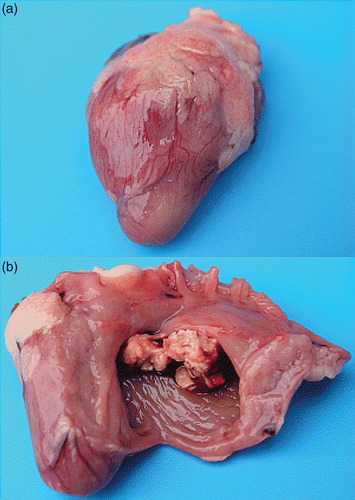
Affected hearts showed a more pronounced rounded shape compared to normal hearts (). In severe cases, fibrinous vegetative lesions of the endocardium were seen shining through the myocardium. At dissection of the myocardium, cauliflower-like vegetative lesions were found lining the endocardium at the height of the AV valves. In most cases vegetations of the right AV valve were seen (), however in some instances, the left AV valve was affected, with or without concomitant right-sided lesions.
Figure 2. Gross lesions in birds with E. hirae-associated endocarditis with (a) an intact heart with a more pronounced rounded shape, due to vegetative lesions of the right AV valve and (b) inner aspect of the same heart with vegetative lesions of the right AV valve.
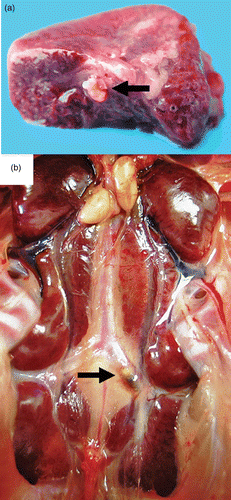
In birds of the 2005 outbreak, pale and swollen kidneys were observed in 6%, liver lesions (liver foci, hemorrhages or signs of necrosis) in 8% and femur abnormalities (necrosis of the femoral neck or cartilage damage of the femoral head) in 60% of all birds (n = 1017). Thrombotic lesions were found in 24% of the investigated lungs (n = 432) (), and a thrombus in the A. ischiadica externa, directly after branching off from the descending aorta, was only found in 0.8% of the investigated cases (n = 833) ().
Figure 3. Thrombotic lesions in birds with E. hirae-associated endocarditis with (a) a thrombus in a large branch of the A. pulmonalis of the lung (arrow) and (b) a thrombus in the left A. ischiadica externa (arrow).
From Flock A4 (n = 27,069 broilers at the end of the grow-out period) 300 randomly selected hearts, livers and lungs were obtained from the slaughterhouse. Abnormalities were observed in none of these organs. This is in agreement with data on mortality. After a peak around the second week, mortality decreases gradually towards the end of the grow-out period ( and ) suggesting that the prevalence of endocarditis decreases with increasing age of the broilers.
3.3. Detailed distribution of endocarditis and thrombotic lesions
OR and 95% confidence intervals that were calculated to measure the association between macroscopic lesions in lungs, liver, kidneys, femur or A. ischiadica externa and the localization of endocarditis lesions in the heart are presented in . Compared to birds without endocarditis, the presence of right-sided endocarditis increased the risk of having thrombotic lesions in the lungs by 300 times (OR = 299.7) and for both left- and right-sided endocarditis by 69 times (OR = 68.9). The risk for liver lesions was significantly increased when left-sided endocarditis (OR = 14.0), right-sided endocarditis (OR = 6.1) and both left- and right-sided endocarditis (OR = 6.1) were observed. The risk for kidney lesions was only significantly increased by left-sided endocarditis (OR = 9.6).
Table 2. Odds Ratios and 95% confidence intervals for the associations between localization of endocarditis lesions in the heart and presence of other lesions in birds of Flocks A4, K, L1/L2, and E2 of the 2005 outbreak.
Table 3. Distribution of lesions in the hearts of succumbed broilers of flocks A4, K, L1/L2, I2 and E2 per week of the grow-out period from day 15 onwards.
When left-sided endocarditis was chosen as reference category (data not shown) significant differences with right-sided endocarditis were observed for liver (OR = 0.43, 95% CI = 0.22–0.84) and kidney lesions (OR = 0.17, 95% CI = 0.06–0.48). Thrombotic lesions in lungs were only present in birds with right-sided or both left- and right-sided endocarditis, therefore ORs for lung lesions could not be reliably estimated using left-sided endocarditis as reference category.
In , distribution of endocarditis lesions with time is presented for the 2005 outbreak (n = 1017). The number of birds with endocarditis lesions decreases with increasing age, except for lesions of the right AV valve, which are more prevalent in the third week of the grow-out period compared to the second week. Hearts affected with endocarditis showed right AV lesions in 70% of the cases, compared to 19% for left-sided endocarditis pathology. In 12% of the cases, both heart ventricles were affected simultaneously. Associations between WEEK and endocarditis (data not shown) were only seen for left-sided endocarditis in week 4 (OR = 0.21, 95% CI = 0.10–0.46) and week 5 (OR = 0.27, 95% CI = 0.08–0.96), compared to week 2 of the grow-out period. The risk for right-sided endocarditis was significantly higher in week 3 (OR = 1.98, 95% CI = 1.30–3.02) when compared to week 2. For endocarditis on both sides of the heart no significant association with WEEK was found.
3.4. Histopathology
Histopathological interpretation was complicated by autolysis, since most organs were harvested from birds that were found dead at the farm.
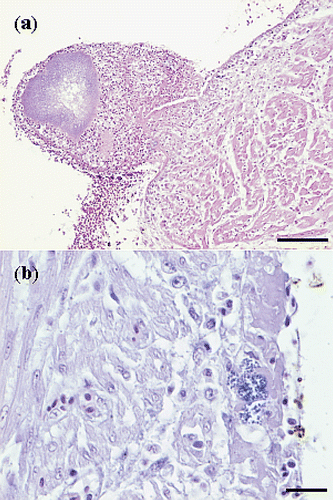
Tissue sections of affected hearts revealed fibrinous thromboendocarditis, localized on or beneath the surface of the left AV valve or localized at the blind pouch underneath the triangular muscle plate of the right AV valve (). The valvular lesions consisted of fibrin and cocci, frequently accompanied by heterophils and macrophages (). Occasionally, a distinct pedunculus was present between the fibrinous process and the endocardium. Most lesions were covered by a layered thrombus, composed of erythrocytes, fibrin and accumulations of small cocci. The stroma of the AV valves was unaffected in most cases, except for one affected heart where a nest of small cocci was found in the stroma of the left AV valve. Furthermore, suppurative inflammation of the stroma of the valve of the Arteria pulmonalis, characterized by the presence of cocci and intact and degenerated heterophils, was found in one bird. Necrosis of myocardial fibres and infiltration with mononuclear cells and heterophils, just below the surface of the endocardium, was observed in a few cases. However, cocci were not found in the myocardium and branches of the coronary circulation in the myocardium did not contain thrombi.
Figure 4. Haematoxylin and eosin sections of hearts with E. hirae-associated endocarditis showing (a) fibrinous thromboendocarditis of the right atrioventricular valve: the vegetative lesion is covered by a thrombus with erythrocytes, fibrin and accumulations of cocci, mural attachment of the vegetative process is present and the myocardium seems unaffected (bar = 100 µm), and (b) a subendocardial lesion, with numerous cocci, embedded in fibrillar eosinophilic proteinaceous material (fibrin): some inflammatory cells are present and the myocardium seems unaffected (bar = 20 µm).
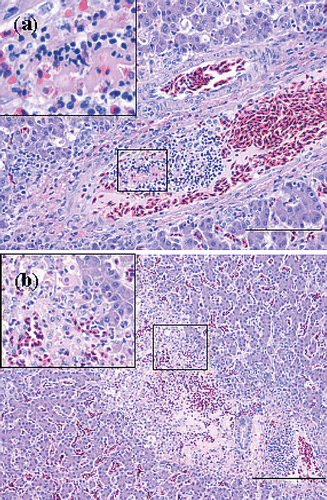
In the lungs, thrombi containing many large accumulations of small cocci, were observed in several large branches of the A. pulmonalis. In some sections endarteritis of the A. pulmonalis was present with mural attachment of thrombi, containing cocci ( and ). The smaller branches of this vessel following the subdivisions of the bronchi and the capillary network in the parabronchi were unaffected (). Signs of hypoxia due to the occlusion of these arteries, i.e. infarcts and necrosis, were not observed in most sections. One section showed necrosis and signs of ischaemia, extending to the whole diameter of a small number of parabronchi, with large numbers of erythrocytes in the adjacent vessels. In one section of the lungs hyperplasia of muscle fibres of the parabronchi was present.
Figure 5. Haematoxylin and eosin sections of thrombotic lesions in the A. pulmonalis of the lungs from birds with E. hirae-associated endocarditis, with (a) thrombosis and endarteritis of a large branch of the A. pulmonalis, with mural attachment, containing accumulations of small cocci (bar = 500 µm), and (b) thrombosis and endarteritis in a large branch of the A. pulmonalis: the smaller branches of the A. pulmonalis following the subdivisions of the bronchi and the capillary network in the parabronchi are unaffected (bar = 500 µm).
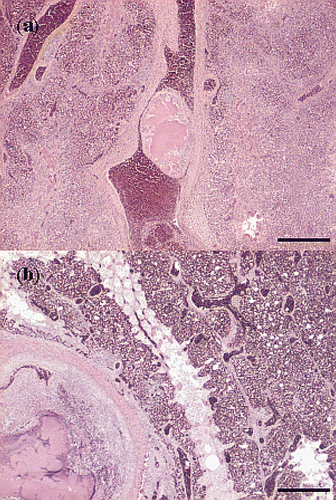
In the liver, numerous foci were present that appeared to be consecutive stages of thrombosis, necrosis and inflammation, i.e. necrotic foci, small abscesses and granulomas without accumulations of cocci and without an apparent zonal distribution. In some hepatic vessels, most likely portal veins, (partial) occlusive thrombi containing macrophages and heterophils were present (). Accumulations of small cocci were present in several veins and sinusoids without surrounding reactive processes. Some foci were characterized by coagulative necrosis, containing heterophil infiltrations with large mesenchymal cells, possibly activated Kupffer cells, and infarction with erythrocytes (). The necrotic foci in most sections were not surrounded by macrophages. Ischaemic necrosis was also found, covering several periportal areas. Reactive changes were hardly present, but some debris of erythrocytes was found in several vessels. The affected areas were surrounded by normal liver tissue.
Figure 6. Haematoxylin and eosin sections of thrombotic lesions in the liver from birds with E. hirae-associated endocarditis. with (a) thrombosis of a periportal vein: insert showing a thrombus being composed of fibrin and debris and disintegrating heterophils present in the thrombus (bar = 100 µm), and (b) subacute process consisting of focal coagulative necrosis with heterophil infiltration and large mesenchymal cells, possibly activated Kupffer cells, and infarction with erythrocytes: insert showing necrosis, mesenchymal cells and heterophils (bar = 200 µm).
Leptomengititis, with mononuclear or mixed cellular infiltrates, was present and showed a random multifocal distribution in the brain. Mononuclear perivascular cuffing in the hemispheres was found and surrounding astrocytes were occasionally vacuolized. Mural thrombosis of some smaller vessels was found, without the presence of cocci. Few reactive changes were found adjacent to the lesions. In one bird the hemisphere ventricle contained a purulent mass in which small cocci were abundantly present.
Most sections of the spleen showed depletion of lymphoid cells in the ellipsoids and reticular phagocytic activity and in some sections eosinophilis surrounding the penicillar arteries were found. Some of the sections showed the presence of many lymphoid follicles. All these changes resembled the appearance of the general reaction pattern of the spleen. On some occasions, small accumulations of small cocci were seen.
Kidney sections showed hyperplasia of renal cells in the mesangium and Bowman's capsule in only a few birds. Accumulations of cocci, signs of thromboembolic processes, vascular lesions or glomerulonephritis were not found in any of the sections. In a few other kidneys, necrosis of the tubular epithelium of reptile-type nephrons and purulent material within the tubular lumen existed, without major changes in the interstitium.
3.5. Bacteriology
Detailed information on the results of the bacteriological analysis per flock for the 2004 and 2005 outbreaks can be found in . Bacteriology of the affected hearts revealed that Enterococcus was the major pathogen associated with endocarditis lesions. In 54% of the examined hearts that were investigated during the 2004, 2005, 2008 and 2009 outbreaks (n = 176) Enterococcus was found, whereas less frequently E. coli (16%) and a mixture of bacteria (23%) were cultured. From livers (n = 28), bone marrow (n = 106), and lungs (n = 16), Enterococcus was cultured in 75%, 60% and 31% of the submitted samples, respectively. Other pathogens that were cultured from livers and bone marrow were E. coli (4% of cases) and a mixture of opportunistic bacteria (10%). A large percentage of lungs (69%) contained mixtures of bacteria, whereas E. coli (31%) was present in a smaller number of lung samples. In blood (n = 30) taken from live birds of Flock A4 Enterococcus was cultured in 20% of the samples.
According to the API rapid ID 32 STREP system, 53 isolates were classified as E. hirae (n = 65), in 11 cases differentiation between E. hirae and E. durans could not be made and in a single case E. faecalis was identified. Other isolates of the same farm were characterized as E. hirae. The pyrrolidonyl aminopeptidase test was positive in all cases and the pyruvate test was negative for all samples, except for the E. faecalis isolate.
3.6. Genotypic characterization: PCR and PFGE
The PCR identified 58 of the isolates from the outbreaks (n = 59) as E. hirae, based on amplification with mur-2 primers and the absence of amplification with mur-2ed primers. In one isolate, amplification did not occur for both the mur-2 and the mur-2ed primers, which was the same strain that was classified as E. faecalis according to phenotypic characterization. Amplification with mur-2 primers did not occur in control samples with E. durans isolates, while the E. cecorum isolates tested negative for both primers.
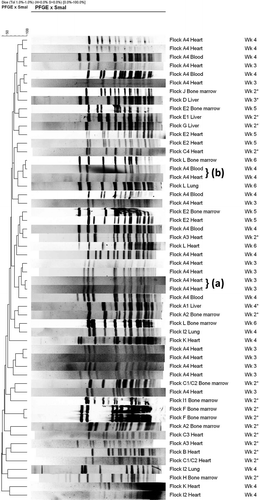
PFGE banding patterns for isolates of heart, liver, bone marrow, lungs and blood collected during the 2004 and 2005 outbreak are shown in . PFGE showed a genetically highly diverse group of isolates. Both between and within flocks (e.g. A4), isolates were different. Genetically closely related isolates were present within a flock, but not between flocks, and mostly originated from the same source (e.g. heart, Flock A4, Wk 3). In banding patterns from different sources (e.g. heart and blood, Flock A4, Wk 4) some genetic relatedness was observed. Isolates of E. hirae bacteria from the same source (e.g. heart), but from different flocks, did not show homology. The isolate originating from a lung from flock I2 that was identified as E. faecalis showed a different banding pattern compared to isolates originating from the heart and lung of the same flock, that were identified as E. hirae according to phenotypic and PCR identification.
Figure 7. PFGE banding patterns for isolates of E. hirae from heart, liver, bone marrow, blood and lungs from affected flocks from the 2004 (denoted with *) and 2005 outbreak at different times during the grow-out period. Genetically closely related isolates are present within some flocks, but not between flocks, and mostly originated from the same source, i.e. heart (a). Some genetic relatedness was present in isolates from the same flock but of different sources, i.e. heart and blood (b).
4. Discussion
E. hirae-associated endocarditis was studied in 25 Dutch broiler flocks, with increased mortality, peaking between day 9 and 22 of the grow-out period. Although the mortality reported in affected flocks did not seem to significantly exceed the reference mortality of Dutch broiler farms in 2004 and 2005, it had clearly increased if compared to the mortality of previous unaffected flocks of the same farm. The average percentage of the total mortality in these flocks, attributed to endocarditis, was 36%.
In addition to vegetative processes of the endocardium, thrombotic lesions were found in lungs, liver and occasionally in the A. ischiadica externa. The latter finding has not been described previously. Occlusion of the A. ischiadica externa might have caused the lameness, which was reported in some of the affected flocks. Lameness may also have been caused by bone and joint lesions, i.e. osteomyelitis, femoral head fractures and epiphysiolysis of the coxofemoral joints, which have been associated with E. cecorum septicemia (Devriese et al. Citation2002; De Herdt et al. Citation2009; Kense and Landman Citation2010; Stalker et al. Citation2010) and recently with endocarditis-associated E. hirae infections (Kolbjørnsen et al. Citation2011).
Endocarditis-associated pathology of liver, spleen, kidney, central nervous system and myocardium, as a result of septic or thromboembolic processes, have been reported previously (Gross and Domermuth Citation1962; Jortner and Helmboldt Citation1971; Randall and Pearson Citation1991; Cardona et al. Citation1993; Randall et al. Citation1993; Wages Citation2003). However, histopathological evidence for thromboembolism, i.e. infarcts or occlusion of blood vessels, was not found in the myocardium, spleen or kidney in this study. In some brain and liver vessels, thrombi were found, but without the presence of cocci. Possibly, parts of the vegetations of the heart may have detached, forming aseptic thrombi, as described for domestic animals (Maxie Citation2007). Alternatively, the cocci, which may only be present in a more acute stage, had vanished, as only birds that died spontaneously (most likely representing a more subacute or chronic stage of the disease) were examined. However, evidence of rather acutely occurring mortality in affected birds was also observed in this study. Accumulations of small cocci in several veins and sinusoids in the liver, without surrounding reactive processes, were found in some cases. This may have been a result of intravital dissemination of bacteria prior to death and subsequent multiplication after death. Furthermore, the aspect of the lesions in the liver, i.e. coagulation necrosis and infarction with heterophil infiltrate in the absence of typical granuloma morphology, indicates that these lesions developed only shortly prior to death. As the occurrence of left-sided endocarditis decreased more rapidly with time compared to right-sided endocarditis, broilers with left-sided endocarditis may have died more quickly, which may have been a result of the increased risk for development of liver and kidney lesions in these birds. Furthermore, the absence of lesions in organs from an affected flock at slaughter suggests that most birds with endocarditis-associated pathology died before the age of 35 days.
Thrombotic lesions of the lungs were reported previously by Gross and Domermuth (Citation1962) in one bird with experimentally induced endocarditis and by Randall and Pearson (Citation1991) in field cases of this disease, whereas the present study describes thrombotic lung lesions in 24% of the field cases examined during the 2005 outbreak. The lung lesions consisted of thrombi containing small cocci, in some cases accompanied by endarteritis, and were only found in the larger branches of the A. pulmonalis. It is unclear, however, whether these lung lesions were caused by emboli, originating from valvular vegetations or by locally multiplying enterococci in the endothelium, preceded or followed by formation of thrombi. In the case of thromboembolism, however, thrombotic lesions would have been expected in the smaller branches of the A. pulmonalis. However, the risk for lung lesions in birds with endocarditis of the right AV valve was 300 times higher compared to birds without endocarditis, suggesting that instead of emboli, enterococci might have travelled from the valve vegetations to the lungs. The question remains as to why the enterococci specifically invaded the larger branches of the A. pulmonalis. It can be hypothesized that certain factors, i.e. local damage of the surface of the endothelium (Leask et al. Citation2003) or blood flow kinetics (Isberg and Barnes Citation2002) facilitate adhesion of enterococci. In addition, selective adherence of enterococci, due to the production of biofilm, the presence of specific surface components on the bacteria, i.e. pili, or receptors on the endothelium might determine whether adhesion of bacteria to the endothelium can take place (Rozdzinski et al. Citation2001; Nallapareddy et al. Citation2006). Furthermore, it has been shown that enterococci, such as E. faecalis, E. faecium and Enterococcus avium, can induce platelet aggregation activity (Johnson Citation1994), which may play a role in the formation of thrombi and on the pathogenesis.
Bacteriological analysis of hearts, livers, bone marrow and lungs showed that E. hirae was the major pathogen involved. Phenotypic characterization, with the API rapid ID 32 STREP system and genotypic characterization with a PCR targeting mur-2 and mur-2ed genes showed similar results but, in contrast to the PCR, the API system could not always differentiate between E. durans and E. hirae. The only isolate that was examined with both techniques and that was not an E. hirae or E. durans isolate according to the PCR and API system, was identified as E. faecalis by the API system. Another molecular technique, i.e. full-length 16S rRNA sequencing (Patel et al. Citation1998; Manero et al. Citation2002) was used to verify the correct classification of the isolates by the PCR and this yielded the same results, but was only carried out on five of the E. hirae isolates (results not shown). In a few cases, E. coli, E. faecalis and mixtures of several opportunistic bacteria were isolated from the affected organs as well. The significance of these findings is currently unknown, but considering the small number of isolations, these infections were considered as opportunistic or possibly resulting from autolysis. In a study by Tankson et al. (Citation2002) with young chicks, several species of bacteria including E. coli, E. faecalis and E. durans were transiently present in hearts and lungs, with an incidence of bacterial isolation of 15%. In the Netherlands, however, E. hirae is rarely cultured from organs of flocks without signs of endocarditis. Since E. hirae was isolated from a majority of the selected organs and (histo)pathological lesions in these organs were similar to endocarditis-associated pathology as previously described for E. hirae field and experimental infections (Chadfield et al. Citation2005a; Chadfield et al. Citation2005b), it is likely that E. hirae was the cause of endocarditis in these flocks.
Evidence of clonality between E. hirae isolates originating from endocarditis lesions, affected livers, lungs, bone marrow and blood was not found, as deduced from the PFGE banding patterns. The diversity of isolates identified by PFGE indicates that these infections were polyclonal in nature, which is in contrast with the clonality of arthropathic and amyloidogenic E. faecalis strains, a bacterial species which has also been associated with endocarditis lesions (Landman et al. Citation1999) and with E. hirae outbreaks in Danish broiler flocks, where clonality was found for some, but not for all of the outbreaks (Chadfield et al. Citation2005a).
Based on the severity of the lesions found at post-mortem examination and histopathology and the fact that similar vegetative proliferations of mitral valves were present from 5 to 7 days after intravenous inoculation with both Streptococcus gallinaceus and E. hirae (Chadfield et al. Citation2005b), it can be deduced that infection with E. hirae probably started at one week of age or earlier. In birds that were experimentally inoculated with another Enterococcus species, i.e. E. faecalis, highest mortality occurred between the fifth and sixteenth day post-inoculation (Gross and Domermuth Citation1962). In another study (Domermuth and Gross Citation1969), even after intravenous inoculation of E. faecalis, the average time of death remained similar, i.e. 14 days. However for E. durans and E. faecium it was on average 28 days. Based on these studies, and considering that the peak of mortality due to endocarditis lesions occurred between the ninth and the twenty-second day of the grow-out period in this study, it seems reasonable to assume that most E. hirae affected flocks, were infected at hatch or shortly after.
Considering the assumption made before, that infection most probably occurred at hatch or shortly after, it must have started at the hatchery, during transport or at arrival at the farm; alternatively, a combination of the previous possibilities may have occurred. Regarding the hatchery as a source of contamination it is well known that during hatch explosive growth of E. faecalis occurs (Landman et al. Citation2000), as this bacterium and E. faecium dominate the intestinal flora in the day-old chick. In contrast, the intestinal flora of 3 to 5 week old broilers is dominated by E. faecium and Streptococcus alactolyticus, followed by E. hirae and E. durans (Devriese et al. Citation1991b). Nevertheless, since E. hirae can be part of the intestinal chicken flora, it may have reached the hatchery through soiled eggs which could then have been a contamination source for day-old chicks, albeit in low numbers, in view of what has been stated earlier. Subsequently, transport vehicles departing from the hatchery might have also been contaminated with E. hirae. Other sources of contamination could be from the environment through visitors and/or fomites. Also, the possibility of persisting infections on the farm should be considered. The latter case was suggested by the fact that E. hirae-associated endocarditis sometimes reoccurred on the same farm. However, this is not supported by the PFGE findings, which demonstrated that clonality of E. hirae isolates was absent within farms, both between subsequent flocks, and within the same flock. The PFGE data rather support another hypothesis, which is that different types of E. hirae bacteria, that form part of the intestinal flora in chickens, could under certain circumstances (e.g. dysbacteriosis, enteric infections or other conditions that compromise the intestinal villous epithelial integrity, co-infection with immune-system compromising agents, etc.) translocate from the intestinal tract (Rozdzinski et al. Citation2001; Krueger et al. Citation2004) and induce bacteraemia with subsequent endocarditis.
Further research on the occurrence of E. hirae in the parent flocks, hatcheries, transport vehicles and environmental sampling at affected broiler farms may elucidate the source of E. hirae disease outbreaks. Also, research on the pathogenesis of these infections and associated risk factors may contribute to knowledge of the disease and may facilitate the development of control measures to reduce E. hirae-associated mortality and lameness at broiler farms.
Acknowledgements
The authors thank the participating broiler farms for supplying flock data and chickens, and Hans Vernooij for his advice on the statistical analyses.
References
- Abe , Y , Nakamura , K , Yamada , M and Yamamoto , Y . 2006 . Encephalomalacia with Enterococcus durans infection in the brain stem and cerebral hemisphere in chicks in Japan . Avian Dis , 50 ( 1 ) : 139 – 141 .
- Animal Sciences Group – Wageningen UR. 2006. Kwantitatieve informatie veehouderij 2006–2007. Lelystad: Animal Sciences Group of Wageningen UR
- Arias , CA , Robredo , B , Singh , KV , Torres , C , Panesso , D and Murray , BE . 2006 . Rapid identification of Enterococcus hirae and Enterococcus durans by PCR and detection of a homologue of the E. hirae mur-2 gene in E. durans . J Clin Microbiol , 44 ( 4 ) : 1567 – 1570 .
- Biomerieux technical library. Rapid ID 32 strep [Internet]. Marcy-l’Etoile, France: BioMerieux. Available from: http://industry.biomerieux-usa.com/support/techlibrary/api/index.asp
- Bisgaard , M . 1981 . Arthritis in ducks. Aetiology and public health aspects . Avian Pathol , 10 : 11 – 21 .
- Cardona , CJ , Bickford , AA , Charlton , BR and Cooper , GL . 1993 . Enterococcus durans infection in young chickens associated with bacteremia and encephalomalacia . Avian Dis , 37 ( 1 ) : 234 – 239 .
- Chadfield , MS , Bojesen , AM , Christensen , JP , Juul-Hansen , J , Saxmose Nielsen , S and Bisgaard , M . 2005b . Reproduction of sepsis and endocarditis by experimental infection of chickens with Streptococcus gallinaceus and Enterococcus hirae . Avian Pathol , 34 ( 3 ) : 238 – 247 .
- Chadfield , MS , Christensen , JP , Christensen , H and Bisgaard , M . 2004 . Characterization of streptococci and enterococci associated with septicaemia in broiler parents with a high prevalence of endocarditis . Avian Pathol , 33 ( 6 ) : 610 – 617 .
- Chadfield , MS , Christensen , JP , Juhl-Hansen , J , Christensen , H and Bisgaard , M . 2005a . Characterization of Enterococcus hirae outbreaks in broiler flocks demonstrating increased mortality because of septicemia and endocarditis and/or altered production parameters . Avian Dis , 49 ( 1 ) : 16 – 23 .
- De Herdt , P , Defoort , P , Van Steelant , J , Swam , H , Tanghe , L , Van Goethem , S and Vanrobaeys , M . 2009 . Enterococcus cecorum osteomyelitis and arthritis in broiler chickens . Vlaams Diergeneeskd Tijdschr , 78 ( 1 ) : 44 – 48 .
- Devriese , LA , Cauwerts , K , Hermans , K and Wood , AM . 2002 . Enterococcus cecorum septicemia as a cause of bone and joint lesions resulting in lameness in broiler chickens . Vlaams Diergeneeskd Tijdschr , 71 : 219 – 221 .
- Devriese , LA , Ducatelle , R , Uyttebroek , E and Haesebrouck , F . 1991a . Enterococcus hirae infection and focal necrosis of the brain of chicks . Vet Rec , 129 ( 14 ) : 316
- Devriese , LA , Hommez , J , Wijfels , R and Haesebrouck , F . 1991b . Composition of the enterococcal and streptococcal intestinal flora of poultry . J Appl Bacteriol , 71 ( 1 ) : 46 – 50 .
- Domermuth , CH and Gross , WB . 1969 . A medium for isolation and tentative identification of fecal streptococci, and their role as avian pathogens . Avian Dis , 13 ( 2 ) : 394 – 399 .
- Facklam , RR and Collins , MD . 1989 . Identification of Enterococcus species isolated from human infections by a conventional test scheme . J Clin Microbiol , 27 ( 4 ) : 731 – 734 .
- Fitzgerald , JR , Foster , TJ and Cox , D . 2006 . The interaction of bacterial pathogens with platelets . Nat Rev Microbiol , 4 ( 6 ) : 445 – 457 .
- Gross , WB and Domermuth , CH . 1962 . Bacterial endocarditis of poultry . Am J Vet Res , 23 : 320 – 329 .
- Hernandez , J , Roberts , ED , Adams , LG and Vera , T . 1972 . Pathogenesis of hepatic granulomas in turkeys infected with Streptococcus faecalis var. liquefaciens . Avian Dis , 16 ( 2 ) : 201 – 216 .
- Hill , EE , Herijgers , P , Herregods , MC and Peetermans , WE . 2006 . Evolving trends in infective endocarditis . Clin Microbiol Infect , 12 ( 1 ) : 5 – 12 .
- Huan , S . 1997 . Study on ascites in hens caused by Streptococcus faecalis infection . Chin J Vet Med , 23 ( 5 ) : 9 – 10 .
- Isberg , RR and Barnes , P . 2002 . Dancing with the host; flow-dependent bacterial adhesion . Cell , 110 ( 1 ) : 1 – 4 .
- Jacobsen , CN , Rosenfeldt Nielsen , V , Hayford , AE , Moller , PL , Michaelsen , KF , Paerregaard , A , Sandstrom , B , Tvede , M and Jakobsen , M . 1999 . Screening of probiotic activities of forty-seven strains of Lactobacillus spp. by in vitro techniques and evaluation of the colonization ability of five selected strains in humans . Appl Environ Microbiol , 65 ( 11 ) : 4949 – 4956 .
- Johnson , AP . 1994 . The pathogenicity of enterococci . J Antimicrob Chemother , 33 ( 6 ) : 1083 – 1089 .
- Jortner , BS and Helmboldt , CF . 1971 . Streptococcal bacterial endocarditis in chickens. Associated lesions of the central nervous system . Vet Pathol , 8 ( 1 ) : 54 – 62 .
- Kense , MJ and Landman , WJM . 2010 . Enterococcus cecorum , Fachgespräch über geflügelkrankheiten : infections in broiler breeders and their offspring: molecular epidemiology. Paper presented at 79 . DVG Fachgruppe Geflügel und Deutsche Gruppe der WVPA; Hannover
- Kolbjørnsen Ø, David B, Gilhuus M. Bacterial osteomyelitis in a 3-week-old broiler chicken associated with Enterococcus hirae. Vet Pathol. published online [Internet]. [cited 2011 Feb 28]. Available from: http://vet.sagepub.com/content/early/2011/01/26/0300985810396513 doi: 10.1177/0300985810396513
- Kondo , H , Abe , N , Tsukuda , K and Wada , Y . 1997 . Adherence of Enterococcus hirae to the duodenal epithelium of chicks with diarrhoea . Avian Pathol , 26 ( 1 ) : 189 – 194 .
- Krueger , WA , Krueger-Rameck , S , Koch , S , Carey , V , Pier , GB and Huebner , J . 2004 . Assessment of the role of antibiotics and enterococcal virulence factors in a mouse model of extraintestinal translocation . Crit Care Med , 32 ( 2 ) : 467 – 471 .
- Kuzucu , C , Cizmeci , Z , Durmaz , R , Durmaz , B and Ozerol , IH . 2005 . The prevalence of fecal colonization of enterococci, the resistance of the isolates to ampicillin, vancomycin, and high-level aminoglycosides, and the clonal relationship among isolates . Microb Drug Resist , 11 ( 2 ) : 159 – 164 .
- Landman , WJM . 2002 . Animal health and production compendium , Wallingford : CAB International. . Streptococcus and enterococcus infections in poultry
- Landman , WJM , Gruys , E and Dwars , RM . 1994 . A syndrome-associated with growth depression and amyloid arthropathy in layers – a preliminary-report . Avian Pathol , 23 ( 3 ) : 461 – 470 .
- Landman , WJM , Mekkes , DR , Chamanza , R , Doornenbal , P and Gruys , E . 1999 . Arthropathic and amyloidogenic Enterococcus faecalis infections in brown layers: a study on infection routes . Avian Pathol , 28 ( 6 ) : 545 – 557 .
- Landman , WJM , Veldman , KT , Mevius , DJ and Doornenbal , P . 2000 . Contamination of Marek's disease vaccine suspensions with Enterococcus faecalis and its possible role in amyloid arthropathy . Avian Pathol , 29 ( 1 ) : 21 – 25 .
- Leask , RL , Jain , N and Butany , J . 2003 . Endothelium and valvular diseases of the heart . Microsc Res Tech , 60 ( 2 ) : 129 – 137 .
- Manero , A and Blanch , AR . 1999 . Identification of Enterococcus spp. with a biochemical key . Appl Environ Microbiol , 65 ( 10 ) : 4425 – 4430 .
- Manero , A , Vilanova , X , Cerda-Cuellar , M and Blanch , AR . 2002 . Characterization of sewage waters by biochemical fingerprinting of enterococci . Water Res , 36 ( 11 ) : 2831 – 2835 .
- Maxie , MG . 2007 . Jubb, Kennedy & Palmer's Pathology of Domestic Animals , Edinburgh : Saunders Ltd. . Endocardial Disease; p. 27–30
- McNamee , PT and King , DC . 1996 . Endocarditis in broiler breeder rearers due to Enterococcus hirae . Vet Rec , 138 ( 10 ) : 240
- Moore , WE and Gross , WB . 1968 . Liver granulomas of turkeys – causative agents and mechanism of infection . Avian Dis , 12 ( 3 ) : 417 – 422 .
- Nallapareddy , SR , Singh , KV , Sillanpaa , J , Garsin , DA , Hook , M , Erlandsen , SL and Murray , BE . 2006 . Endocarditis and biofilm-associated pili of Enterococcus faecalis . J Clin Invest , 116 ( 10 ) : 2799 – 2807 .
- Patel , R , Piper , KE , Rouse , MS , Steckelberg , JM , Uhl , JR , Kohner , P , Hopkins , MK , Cockerill , FR 3rd and Kline , BC . 1998 . Determination of 16S rRNA sequences of enterococci and application to species identification of nonmotile Enterococcus gallinarum isolates . J Clin Microbiol , 36 ( 11 ) : 3399 – 3407 .
- Peckham , MC . 1966 . An outbreak of streptococcosis (apoplectiform septicemia) in white rock chickens . Avian Dis , 10 ( 4 ) : 413 – 421 .
- Quin , PJ . Carter, ME, Markey, BK, Carter, GR. 1994. Clinical veterinary microbiology. Spain: Wolfe Publishing. Chapter 9, The streptococci and related cocci; p. 127–136
- Randall , CJ and Pearson , DB . 1991 . Enterococcal endocarditis causing heart failure in broilers . Vet Rec , 129 ( 24 ) : 535
- Randall , CJ , Wood , AM and MacKenzie , G . 1993 . Encephalomalacia in first-week chicks . Vet Rec , 132 ( 16 ) : 419
- Rozdzinski , E , Marre , R , Susa , M , Wirth , R and Muscholl-Silberhorn , A . 2001 . Aggregation substance-mediated adherence of Enterococcus faecalis to immobilized extracellular matrix proteins . Microb Pathog , 30 ( 4 ) : 211 – 220 .
- Sandhu , TS . 1988 . Fecal streptococcal infection of commercial white pekin ducklings . Avian Dis , 32 ( 3 ) : 570 – 573 .
- Inc. , SPSS . 2003. SPSS 12.0.1 for Windows [computer program]. Version 12.0.1. Chicago, Illinois, USA: SPSS Inc. Chicago, Illinois, US
- Stalker , MJ , Brash , ML , Weisz , A , Ouckama , RM and Slavic , D . 2010 . Arthritis and osteomyelitis associated with Enterococcus cecorum infection in broiler and broiler breeder chickens in Ontario, Canada . J Vet Diagn Invest , 22 ( 4 ) : 643 – 645 .
- Steentjes , A , Veldman , KT , Mevius , DJ and Landman , WJM . 2002 . Molecular epidemiology of unilateral amyloid arthropathy in broiler breeders associated with Enterococcus faecalis . Avian Pathol , 31 ( 1 ) : 31 – 39 .
- Tankson , JD , Thaxton , JP and Vizzier-Thaxton , Y . 2001 . Pulmonary hypertension syndrome in broilers caused by Enterococcus faecalis . Infect Immun , 69 ( 10 ) : 6318 – 6322 .
- Tankson , JD , Thaxton , JP and Vizzier-Thaxton , Y . 2002 . Bacteria in heart and lungs of young chicks . J Appl Microbiol , 92 ( 3 ) : 443 – 450 .
- Tenover , FC , Arbeit , RD , Goering , RV , Mickelsen , PA , Murray , BE , Persing , DH and Swaminathan , B . 1995 . Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: Criteria for bacterial strain typing . J Clin Microbiol , 33 ( 9 ) : 2233 – 2239 .
- Wages , DP . 2003 . Diseases of poultry , Ames, Iowa : Iowa State Press. . Chapter 24, Enterococcosis; p. 809–812