Abstract
Background: Glucocorticoids are suggested to precipitate laminitis and induce insulin resistance in horses.
Hypothesis/Objectives: To assess insulin sensitivity and the basal amount of glucose metabolized in equine pituitary pars intermedia dysfunction (PPID).
Animals and methods: The euglycaemic hyperinsulinaemic clamp (EHC) technique was performed in seven horses with a diagnosis of PPID based on the presence of hypertrichosis and positive dexamethasone suppression-test results comprising one gelding and six mares with a mean age of 21.1 ± 5.8 (SD; range 15–34) years. Results were compared with those from five negative (healthy) controls comprising two geldings and two mares with a mean age of 10.0 ± 2.5 (range 7–13) years and six positive (diseased) controls comprising two geldings and four mares with a mean age of 12.5 ± 4.5 (range 8–21) years examined during the same period. Differences were assessed by means of the Mann–Whitney U test.
Results: Mean basal rate of glucose metabolism (9.0 ± 4.2 versus 16.0 ± 5.2 µmol/kg BW/min; p = 0.030) and mean glucose metabolism rate-to-plasma insulin concentration ratio (2.9 ± 1.6 versus 6.2 ± 2.7 × 10−6; p = 0.048) were significantly lower in PPID horses than in negative controls, respectively. No differences were found between both control groups.
Conclusions and clinical importance: In horses suffering from PPID it seems important to reduce the insulin resistance, thereby potentially decreasing the risk of laminitis as being a major complication of equine PPID. Plasma glucose concentration following fasting might be considered in the screening of horses for PPID.
1. Introduction
Insulin resistance is defined as a condition in which normal concentrations of insulin produce a subnormal physiologic response (Kahn Citation1978), whereas insulin sensitivity is the reciprocal of insulin resistance. Glucose usage and production are regulated in part by insulin, which is synthesized and secreted by the beta cells of the islets of Langerhans in the pancreas (Kahn Citation1978). Direct and indirect methods of varying complexity are currently employed for assessing insulin resistance. The direct methods comprise the glucose clamp techniques and the insulin suppression test (using somatostatin or a somatostatin analogue to suppress endogenous secretion of insulin) and the indirect methods include the classical glucose tolerance tests. The so-called minimal model is a physiologic compartmental representation of the classical glucose tolerance tests. Besides, there are simple surrogate indexes for insulin resistance derived from fasting steady-state conditions (Muniyappa et al. Citation2008). To study glucose metabolism and insulin sensitivity, a glucose clamp technique was developed by Andres et al. in 1966. Especially the euglycaemic hyperinsulinaemic clamp (EHC) technique is regarded as gold standard with reference to assessment of peripheral insulin sensitivity (DeFronzo et al. Citation1979; Wallace and Matthews Citation2002). In typical glucose tolerance tests, the dose of glucose is fixed, and the measure of tolerance is the plasma glucose concentration. In the clamp techniques, the plasma glucose concentration is fixed and the quantity of glucose administered becomes the measure of tolerance (DeFronzo et al. Citation1979). There are two types of glucose clamp techniques reported for use in horses, the hyperglycaemic clamp technique (Rijnen and van der Kolk Citation2003) and the EHC technique (Powell et al. Citation2002; Rijnen and van der Kolk Citation2003; Annandale et al. Citation2004; Pratt et al. Citation2005, Citation2006; de Graaf-Roelfsema et al. Citation2006). The principle of both clamp techniques is that the rate of glucose infusion required to maintain a steady-state is an index of glucose metabolism (DeFronzo et al. Citation1979). With reference to the EHC, the rate of glucose infusion required to maintain euglycaemia equals the quantity of glucose taken up by all tissues in response to exogenous insulin administration, provided that endogenous glucose entry rate remains constant. The amount of glucose metabolized divided by the plasma insulin concentration during the steady-state of the EHC reflects the quantity of glucose metabolized per unit of insulin in plasma and, as a result, is a reasonable index of the sensitivity of tissues to exogenous insulin (DeFronzo et al. Citation1979).
Increased insulin sensitivity as assessed by use of the EHC as the gold standard is associated with equine disorders, such as polysaccharide storage myopathy (Annandale et al. Citation2004) and equine motor neuron disease (van der Kolk et al. Citation2005). Furthermore, minimal model of insulin and glucose dynamics has documented insulin resistance in metabolic syndrome in ponies (Kronfeld et al. Citation2006; Treiber et al. Citation2006b) and in laminitis prone ponies without pituitary pars intermedia dysfunction (PPID) in June (Bailey et al. Citation2008). A decrease in the insulin-to-glucose ratio in ponies with PPID has been associated with reduced insulin secretion (Garcia and Beech Citation1986; Kronfeld et al. Citation2005).
Hyperadrenocorticism in the equine species almost invariably originates from an adenoma of the pars intermedia of the pituitary gland (pituitary-dependent; Heinrichs et al. Citation1990; van der Kolk et al. Citation2004) as pituitary-independent hyperadrenocorticism had been described in the equine species only once (van der Kolk et al. Citation2001). As a consequence, equine hyperadrenocorticism can be classified as Cushing's disease regarded by definition as pituitary-dependent. However, there is general consensus that histo-pathologically unconfirmed cases should be referred to as PPID. Clinically, however, the diagnosis of PPID is challenging although many diagnostic possibilities are available like the dexamethasone suppression test (Dybdal et al. Citation1994), the TRH stimulation test (Beech et al. Citation2007) and basal plasma ACTH (corticotrophin) measurement (van der Kolk et al. Citation1995; Couëtil et al. Citation1996).
PPID was first described in horses in 1932 (Pallaske Citation1932) and is the most frequently confirmed endocrinopathy in aged horses and ponies (van der Kolk et al. Citation1993, Citation2004; Dybdal et al. Citation1994; Schott 2002). Initially, these animals have hypertrichosis, which is a remarkable clinical sign. The two major complications of equine PPID are diabetes mellitus with concurrent weight loss, and laminitis (van der Kolk et al. Citation1993; Schott et al. Citation2001).
It has been demonstrated that decreased glucose availability causes separation of hoof lamellae in vitro suggesting insulin resistance may be involved in the pathogenesis of laminitis by decreasing laminar glucose metabolism (Pass et al. Citation1998; Treiber et al. Citation2006a). Glucocorticoids are suggested to precipitate laminitis (Eustace and Redden Citation1990) and in agreement recent studies have shown that dexamethasone treatment can induce insulin resistance in horses (Firshman et al. Citation2005; Tiley et al. Citation2007, Citation2008).
The objective of the study reported here was to determine glucose metabolism and insulin sensitivity by use of the EHC as the gold standard of assessment of insulin sensitivity in horses suffering from spontaneous PPID.
2. Materials and methods
2.1. Horses
Among the equine patients admitted to the Equine Clinic of Utrecht University, cases with a tentative clinical diagnosis of PPID prospectively as based on the presence of hypertrichosis were selected. Inclusion criteria were (besides hypertrichosis) abnormal overnight dexamethasone suppression-test results as reflected by a 17 h post-dexamethasone plasma cortisol concentration above 27.6 nmol/L following 0.04 mg/kg BW intramuscularly (Dybdal et al. Citation1994) and absence of any treatment including corticosteroids the previous 60 days. As a result, two Warmblood horses and five mixed-breed (non-Shetland) ponies with a diagnosis of PPID were included for the study during October through December. These animals comprised one gelding and six mares with a mean age of 21.1 ± 5.8 (SD; range 15–34) years and weighing 365 ± 185 (range 160–610) kg. Mean body condition scores (Henneke et al. Citation1983) were 4.3 ± 1.7 (range 2–7) for horses with PPID. Clinical signs in these horses included hypertrichosis (n = 7), hyperhidrosis (n = 3), bulging of supraorbital fat (n = 4), laminitis (n = 4) and seizures (n = 1). Because of severe laminitis (Obel grade 4) one of the seven PPID animals (an 18-year-old pony mare) was euthanased at the owner's request and the clinico-pathologic diagnosis PPID was confirmed post-mortem.
Two clinically healthy Warmblood horses and three Shetland ponies from the herd of research horses from Utrecht University were used as negative controls also during October through December comprising three geldings and two mares with a mean age of 10.0 ± 2.5 (range 7–13) years and weighing 350 ± 175 (range 160–592) kg. None of the control animals had abnormal dexamethasone suppression-test results with post-dexamethasone plasma cortisol concentrations always below 27.6 nmol/L 17 h following administration associated with basal ACTH concentrations always below the cutoff value of 12.1 pmol/L (van der Kolk et al. Citation1995). Mean body condition scores were 5.6 ± 0.5 (range 5–6) for negative controls.
In addition, among the equine patients admitted to the Equine Clinic of Utrecht University 2 Warmblood horses, one Friesian Horse, one Quarter Horse, one Fjord Horse and a Shetland pony were used as positive control horses also during October through December comprising two geldings and four mares with a mean age of 12.5 ± 4.5 (range 8–21) years and weighing 447 ± 171 (range 155–690) kg. Mean body condition scores were 4.3 ± 2.2 (range 2–9) for these positive controls. The diseased control horses suffered from chronic laminitis, lower motor neuron disease, sand colitis, pododermatitis, melanomas and obesitas. The overnight dexamethasone suppression test was not performed in the positive control horses due to the absence of consent by the owners.
The experiment was carried out during October through December in order to standardize diet with the animals housed individually in boxes. The diet consisted of grass silage supplemented with concentrate feed and met nutrient requirements for maintenance and performance. The total diet contained 10% ash, 14.5% crude protein, 1.3% crude fat, 20% crude fibre and 56.2% other carbohydrates.
The study was approved by the Committee for Animal Welfare of Utrecht University (protocol number 0609.0601).
2.2. The EHC technique
The EHC technique was performed as described previously (Rijnen and van der Kolk Citation2003; van der Kolk et al. Citation2005) and based on former studies (DeFronzo et al. Citation1979; Elmahdi Citation1998). A catheter was placed in each jugular vein after food was withheld for 12 h. One of the catheters was used for infusion of glucose as a 50% solution and insulin (Actrapid recombinant human insulin (100 U/mL); Novo Nordisk A/S, Bagsvaerd, Denmark), whereas the other catheter was used for obtaining blood samples. During the experiment, every 10 min, a blood sample (heparinized blood syringe) was taken for measurement of the concentration of glucose. Within 2 minutes, the glucose concentration of the 10-min heparinized blood sample was determined by an automated analyzer. The plasma insulin concentration was determined in three samples (lithium heparin tube) taken during the steady-state of the EHC as well as in a basal sample. Immediately after intravenous administration of the priming dose of 45 mU/kg BW insulin (dissolved in 50 mL sodium chloride as a 0.9% solution) within 10 min to induce hyperinsulinaemia (plasma insulin concentration >1435 pmol/L), insulin infusion was started with a constant infusion rate of 6 mU/kg BW/minute. Glucose infusion was started simultaneously with an infusion rate of 8.6 µmol/kg BW/min as previously described (Elmahdi Citation1998). During the insulin and glucose infusions, blood samples were taken every 10 min for measurement of the concentration of glucose according to the protocol by Bergman et al. (Citation1985). Glucose infusion rate was adjusted when the preceding blood glucose value differed from the euglycaemic concentration (range 3.9–5.6 mmol/L). Plasma for measurement of the concentration of insulin was separated and stored at –20°C until insulin concentrations were measured. A steady-state condition was presumed to exist when the plasma glucose concentration and the glucose infusion rate were concurrently held constant for at least 30 min following an equilibrium period and the plasma glucose concentration was within the range for euglycaemic values and did not change more than 0.1 mmol/L. An equilibrium period of at least 120 min after the start of the experimental procedure was used in each clamp before glucose infusion rates during a steady-state were used to quantitate the actions of insulin on glucose uptake by peripheral tissues. After maintaining a steady-state for 30 min, the insulin and glucose infusions were stopped. To prevent hypoglycaemia following the experimental procedure, horses and ponies were first given 200–300 mL of glucose as a 50% solution intravenously. After removal of the catheters, horses and ponies also received carbohydrates in the form of 1 kg of concentrate feed along with roughage.
During steady-state of the blood glucose concentration, the glucose infusion rate must equal the glucose metabolism rate, provided that endogenous glucose production is completely suppressed by hyperinsulinaemia or hyperglycaemia. The glucose metabolism rate was computed as: M (mmol/kg/min) = INF (mmol/kg/min) – UC (mmol/kg/min) – SC (mmol/kg/min), where M is the glucose metabolism rate, INF is the glucose infusion rate, UC is the rate of urinary glucose loss and SC the is the so-called space correction factor (DeFronzo et al. Citation1979). The rate of urinary glucose loss (mmol/kg BW/min) was calculated as urinary glucose concentration (mmol/L): (BW × 120) as previously published (Rijnen and van der Kolk Citation2003). The urine glucose concentration was determined in urine collected via catheterization within 15 min after ending the clamp test. As the plasma glucose concentration is not maintained constant perfectly during the glucose clamp procedure a correction must be made. The space correction factor adjusts for glucose that has been added or removed from the glucose space (i.e. extracellular volume). The plasma glucose concentrations at the beginning (G 1) and end (G 2) of each 10-min period are considered. The space correction is calculated as
The plasma insulin concentration was determined during the steady-state of the blood glucose concentration. The mean glucose metabolism rates and the mean glucose metabolism rate-to-plasma insulin concentration ratios were calculated from three measurements/horse based on three different intervals, whereas the mean plasma glucose and insulin concentrations were calculated from the four and three measurements/horse, respectively.
2.3. Assays
The heparinized blood glucose concentration was assayed within 2 min following collection by use of an automated analyzer (ABL-605 radiometer Copenhagen, Westlake, OH, USA). Plasma insulin, cortisol and ACTH concentrations were measured by means of a radioimmunoassay kit (Diagnostic Products Corporation, Los Angeles, CA, USA) validated for use in horses (van der Kolk et al. Citation1995).
2.4. Statistical analyses
The significance of differences between the groups was assessed by use of the Mann–Whitney U test (2-tailed). Values of p < 0.05 were considered significant. The results are presented as mean ± SD. SPSS version 12.0 (SPSS, Chicago, IL, USA) was used as statistical program.
3. Results
The mean (n = 7) plasma cortisol concentration in PPID horses remained above the cutoff value of 27.6 nmol/L (98.6 ± 87.1 nmol/L) 17 h following the administration of dexamethasone associated with an average basal ACTH concentration of 56.3 ± 51.2 (n = 5) pmol/L. The mean basal plasma glucose concentration after food was withheld for 12 h in PPID horses was significantly higher than in negative control horses (5.9 ± 1.4 versus 4.9 ± 0.3 mmol/L, respectively; p = 0.028) in contrast to basal plasma insulin concentrations (405 ± 682 versus 10.8 ± 6.5 pmol/L, respectively; p = 0.060). Two out of the seven animals with PPID had a basal plasma glucose concentration above the upper limit of the reference rate (5.6 mmol/L), namely 9.1 mmol/L in an 18-year-old pony mare and 6.1 mmol/L in a 23-year-old pony mare. Mean rate of glucose metabolism of horses with PPID was significantly lower than in negative control animals (9.0 ± 4.2 versus 16.0 ± 5.2 µmol/kg BW/min, respectively; p = 0.030) () associated with a significantly lower mean glucose metabolism rate-to-plasma insulin concentration ratio of horses with PPID than in negative control animals (2.9 ± 1.6 versus 6.2 ± 2.7 × 10−6, respectively; p = 0.048) (). The greatest reduction in rate of glucose metabolism and glucose metabolism rate-to-plasma insulin concentration ratio was observed in an 18-year-old pony mare with PPID (confirmed post-mortem), weight loss and associated laminitis. In this animal, a reduction in glucose metabolism to 5% of normal (basal rate of glucose metabolism 0.8 µmol/kg BW/min) and a 60 times increase in insulin resistance (basal glucose metabolism rate-to-plasma insulin concentration ratio 0.097 × 10−6) were measured associated with a basal plasma insulin concentration of 287 pmol/L and a basal plasma glucose concentration of 9.1 mmol/L.
Figure 1. Box plots of rate of glucose metabolism (M in µmol/kg BW/min) in five negative control horses (control), six positive control horses (diseased) and seven horses suffering from PPID. The box represents the interquartile range (i.e. 25–75% range) and the horizontal bar in the box represents the median value. For each box plot, the T-bar represents the median value. Differences between negative control horses and horses suffering from PPID were statistically significant (p=0.030).
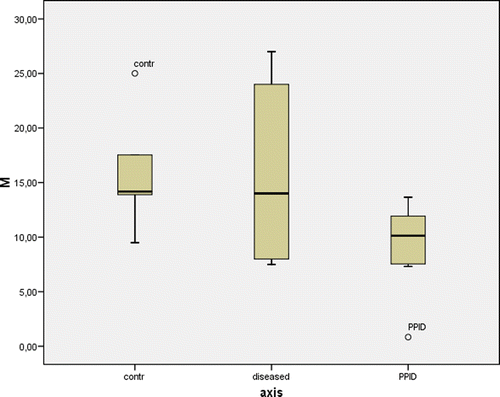
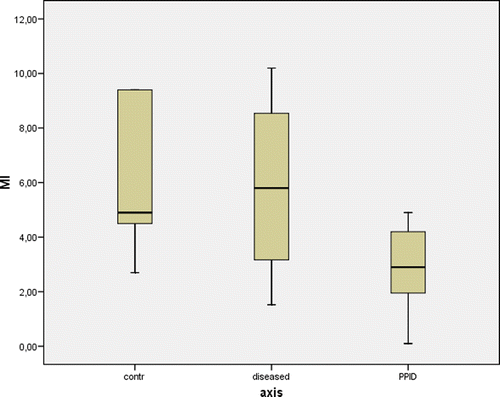
Figure 2. Box plots of rate of glucose metabolism rate-to-plasma insulin concentration ratio (M/I) in five negative control horses (control), six positive control horses (diseased) and seven horses suffering from PPID. The box represents the interquartile range (i.e. 25–75% range) and the horizontal bar in the box represents the median value. For each box plot, the T-bar represents the median value. Differences between negative control horses and horses suffering from PPID were statistically significant (p = 0.048).
The mean age of the negative control animals was significantly (p = 0.003) lower than that of those with PPID. Neither mean rate of glucose metabolism nor mean glucose metabolism rate-to-plasma insulin concentration ratio did differ significantly between animals with laminitis (n = 4) and animals without laminitis (n = 3) within the group of horses with PPID.
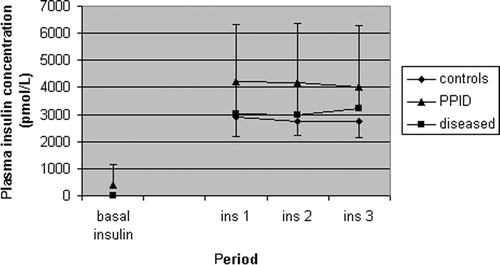
Mean space correction (indicating the quality of the steady-state) did not differ significantly between horses with PPID and negative controls (−0.0050 ± 0.084 versus 0 ± 0.0016 mmol/kg BW/min, respectively; p = 0.584). In addition, the mean plasma glucose concentration during the steady-state of the clamp test in the groups is also shown () in order to visualize the quality of the steady-state in various groups. Furthermore, mean time to reach steady-state also did not significantly differ between horses with PPID and negative controls (200 ± 76 versus 166 ± 58 min, respectively; p = 0.445). The mean plasma insulin concentration during the steady-state of the clamp test in PPID horses was significantly higher than in negative control horses (4145 ± 1979 versus 2800 ± 563 pmol/L, respectively; p = 0.045) ().
Figure 3. Mean (±SD) heparinized blood glucose concentration in five negative control horses (controls), six positive control horses (diseased) and seven horses suffering from PPID at the start and during the steady-state of the EHC test.
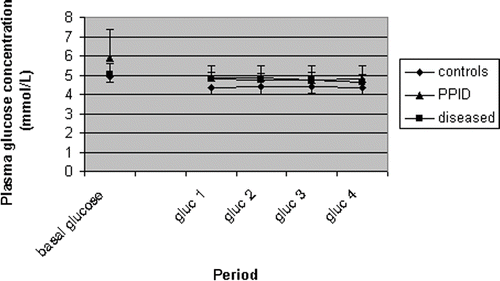
Figure 4. Mean (±SD) plasma insulin concentration in five negative control horses (controls), six positive control horses (diseased) and seven horses suffering from PPID at the start and during the steady-state of the EHC test. Values during the steady-state differed significantly between negative control horses and horses suffering from PPID (p = 0.045).
Neither mean rate of glucose metabolism (15.8 ± 7.49 µmol/kg BW/min; P = 0.181) nor mean glucose metabolism rate-to-plasma insulin concentration ratio (5.8 ± 3.1 × 10−6; p = 0.101) in positive controls did differ significantly from negative control horses. The mean basal plasma insulin concentration after food was withheld for 12 h in positive control horses was significantly lower than in PPID horses (16.5 ± 17.3 versus 404.7 ± 736.9; p = 0.035).
4. Discussion
The diagnosis of equine PPID remains a challenge and the confirmation of such a diagnosis in general mainly depends on dynamic endocrinological function tests. However, there are only few published studies available concerning a considerable number of histo-pathologically confirmed cases from which the sensitivity (and preferably the specificity) with regard to the disorder could be determined. There is general consensus that basal plasma ACTH concentration (van der Kolk et al. Citation1995; Couëtil et al. Citation1996; Perkins et al. Citation2002), the dexamethasone suppression test (Dybdal et al. Citation1994) and ACTH concentration following administration of thyrotropin-releasing hormone (Beech et al. Citation2007) are the most sensitive and specific diagnostic tests for PPID. It is not clear whether (Couëtil et al. Citation1996) or not (Donaldson et al. Citation2005) ponies have lower basal plasma ACTH concentrations than horses. However, seasonal changes in plasma ACTH concentration and dexamethasone suppression-test results should be considered when interpreting endocrine test results (Donaldson et al. Citation2005; Beech et al. Citation2009; Frank et al. Citation2010; Lee et al. Citation2010; Place et al. Citation2010). As a consequence, basal plasma ACTH concentration and dexamethasone suppression-test results in equids suspected of PPID should be compared with matched controls. All negative control animals used in this study had dexamethasone suppression-test results within the reference range in contrast to PPID animals. As the mean basal plasma glucose concentration in PPID horses was significantly higher than that in negative control horses after food was withheld for 12 h this fact might be considered in the screening of horses for PPID similar to the urinary corticoid:creatinine ratio (van der Kolk et al. Citation1994).
A study of the repeatability of both the minimal model of insulin and glucose dynamics and the EHC found that the EHC provided more closely repeatable results in the equine species (Pratt et al. Citation2005). Although the minimal model approach is less labour-intensive than the EHC technique as there is no need for the continuous adjustment of glucose infusion rates and so bedside glucose readings are not necessary (Wallace and Matthews Citation2002), this method may be affected by endogenous insulin secretion and as a consequence is much less reliable in individuals with impaired insulin secretion and/or significant insulin resistance (Muniyappa et al. Citation2008).
By using the EHC, horses with PPID showed a 40% reduction in mean rate of glucose metabolism associated with a 2.1 times higher insulin resistance. This fact was corroborated by the significantly higher mean plasma insulin concentration during the steady-state of the EHC in PPID animals similar to the situation following dexamethasone administration (Firshman et al. Citation2005). These findings support the hypothesis that decreased glucose metabolism in equine PPID might be due to insulin resistance and focuses attention on the functioning of insulin clearance in these patients. In addition, in this study the maximal reduction in rate of glucose metabolism (observed in an 18-year-old pony with PPID) was 95% of the negative control horses, which is similar to the glucose infusion rate required to maintain euglycaemia in a 29-year-old mare with suspected insulin resistance as previously reported (Firshman and Valberg Citation2007).
As mentioned before, minimal model of insulin and glucose dynamics has documented insulin resistance in metabolic syndrome in ponies (Kronfeld et al. Citation2006; Treiber et al. Citation2006b). Although it is clear that obese horses are at increased risk of developing insulin resistance, the mechanisms for this are not well-understood. It is not currently known whether the mechanisms associated with obesity and insulin resistance in man are similar to those that cause insulin resistance in horses and care should be taken when comparing the two conditions (Firshman and Valberg Citation2007). Remarkably, neither altered mean insulin sensitivity nor altered mean rate of glucose metabolism were found in the group of horses suffering from various diseases in this study.
As has been mentioned before, a decrease in the insulin-to-glucose ratio in equines with PPID had been associated with reduced insulin secretion (Garcia and Beech Citation1986; Kronfeld et al. Citation2005; Durham et al. 2009). The hyperglycaemic clamp technique is regarded as gold standard for quantification of the sensitivity of beta cells to glucose (DeFronzo et al. Citation1979; Wallace and Matthews Citation2002). In this study, we were unable to assess insulin secretion, given the technique used, namely the EHC technique.
As the mean age of the control animals was significantly lower than that of those with PPID the effect of age on EHC results should be considered. To the authors’ knowledge, no studies have been reported yet in which insulin sensitivity and glucose metabolism were investigated by means of the EHC in relationship with ageing in horses. Based on unpublished observations from our group no such relationship could be demonstrated in 17 healthy Warmbloods (mean age 9.3 ± 3.0 ranging from 3 to 16 years) between age and either rate of glucose metabolism (r = 0.231; p = 0.373) or glucose metabolism rate-to-plasma insulin concentration ratio (r = 0.094; p = 0.719) during EHC (Pearson product moment correlation test (2-tailed) used).
Cushing's disease in human beings commonly occurs in women of childbearing age, but may occur at any age. In the equine species, females are also affected more frequently (Heinrichs et al. Citation1990; van der Kolk et al. Citation2004). However, in human Cushing's disease the most frequent finding is a microadenoma in the anterior lobe of the pituitary gland (Wilson et al. Citation1998) in contrast to an adenoma in the pars intermedia in the equine species (Heinrichs et al. Citation1990; van der Kolk et al. Citation2004). In comparison, the disorder in women was associated with a similar (49%) overall reduction in mean rate of glucose metabolism (Nosadini et al. Citation1983).
PPID or hyperadrenocorticism is characterized by glucocorticoid excess. Recent equine studies have shown that dexamethasone treatment can induce insulin resistance. In adult Standardbreds, 21 days of intravenous dexamethasone treatment (0.08 mg/kg BW, q 48 h) resulted in a 4.1 times increase in insulin resistance associated with a 70% reduction in the glucose infusion rate required to maintain euglycaemia (Tiley et al. Citation2008). Compared to the data obtained in this study, the ability of dexamethasone to induce insulin resistance seems greater than the magnitude of insulin resistance observed in horses with PPID. It is well-established that cortisol excess causes insulin resistance in man, but the mechanisms responsible for this insulin resistance are poorly understood although possibly due to an impairment of peripheral insulin action located beyond the hormone-receptor binding step (Nosadini et al. Citation1983) regarding the phosphatidylinositol 3-kinase (PI 3-K)-dependent pathway (Muntoni and Muntoni Citation2011).
It should be realized that there may be breed differences in insulin sensitivity as it has been reported that ponies were less insulin sensitive than Standardbred horses (Jeffcott et al. Citation1986). This finding was corroborated by an EHC study, in which Shetland ponies were found to have a decreased rate of glucose metabolism as compared with Warmblood horses (Rijnen and van der Kolk Citation2003). The data presented here may be influenced by the imbalance in horse to pony ratios in the negative control versus PPID group. In this study, data obtained from two horses and five mixed-breed (non-Shetland) ponies with a clinical diagnosis of PPID were compared with those from two clinically healthy horses and three Shetland ponies. However, statistical differences might be expected to be even more prominent if non-Shetland ponies were used in the negative control group.
It has been demonstrated that decreased glucose metabolism causes separation of hoof lamellae in vitro and has been suggested to be involved in the pathogenesis of laminitis due to insulin resistance (Pass et al. Citation1998; Treiber et al. Citation2006a). However, no significant differences were found between laminitic and non-laminitic horses with PPID in this study likely due to the low numbers of animals used although the 18-year-old pony mare with PPID and most severe laminitis had a rate of glucose metabolism of about nil. The latter finding is in accord with the suggestion that insulin has a rapid, detrimental effect on the lamellar tissues. It has been hypothesized that decreased glucose metabolism due to insulin resistance might be the most important stage in the pathogenesis of laminitis (French et al. Citation2000) perhaps mediated via disturbances in vascular function (de Laat et al. Citation2010). As a consequence, in horses suffering from PPID it seems important to reduce insulin resistance, thereby potentially decreasing the risk of laminitis as being a major complication of the disorder.
Acknowledgements
This work was supported by the Committee for the Furtherance of Veterinary and Comparative Pathological Research, Houten, the Netherlands.
References
- Andres , R , Swerdloff , R , Pozefsky , T and Coleman , D . 1966 . Manual of feedback technique for the control of glucose concentration , 486 – 491 . White Plains, NY : Mediad .
- Annandale , EJ , Valberg , SJ , Mickelson , JR and Seaquist , ER . 2004 . Insulin sensitivity and skeletal muscle glucose transport in horses with equine polysaccharide storage myopathy . Neuromuscul Disord , 14 : 666 – 674 .
- Bailey , SR , Habershon-Butcher , JL , Ransom , KJ , Elliott , J and Menzies-Gow , NJ . 2008 . Hypertension and insulin resistance in a mixed-breed population of ponies predisposed to laminitis . Am J Vet Res , 69 : 122 – 129 .
- Beech , J , Boston , R , Lindborg , S and Russell , GE . 2007 . Adrenocorticotropin concentration following administration of thyrotropin-releasing hormone in healthy horses and those with pituitary pars intermedia dysfunction and pituitary gland hyperplasia . J Am Vet Med Assoc , 231 : 417 – 426 .
- Beech , J , Boston , RC , McFarlane , D and Lindborg , S . 2009 . Evaluation of plasma ACTH, alpha-melanocyte-stimulating hormone, and insulin concentrations during various photoperiods in clinically normal horses and ponies and those with pituitary pars intermedia dysfunction . J Am Vet Med Assoc , 235 : 715 – 722 .
- Bergman , RN , Finegood , DT and Ader , M . 1985 . Assessment of insulin sensitivity in vivo . Endocr Rev , 6 : 45 – 86 .
- Couetil , L , Paradis , MR and Knoll , J . 1996 . Plasma adrenocorticotropin concentration in healthy horses and in horses with clinical signs of hyperadrenocorticism . J Vet Intern Med , 10 : 1 – 6 .
- DeFronzo , RA , Tobin , JD and Andres , R . 1979 . Glucose clamp technique: a method for quantifying insulin secretion and resistance . Am J Physiol , 237 : E214 – E223 .
- de Graaf-Roelfsema , E , van Ginneken , MME , van Breda , E , Wijnberg , ID , Keizer , HA and van der Kolk , JH . 2006 . The effect of long-term exercise on glucose metabolism and peripheral insulin sensitivity in standardbred horses . Equine Vet J. Suppl.: , : 221 – 225 .
- de Laat , MA , McGowan , CM , Sillence , MN and Pollitt , CC . 2010 . Equine laminitis: induced by 48 h hyperinsulinaemia in Standardbred horses . Equine Vet J , 42 : 129 – 135 .
- Donaldson , MT , McDonnell , SM , Schanbacher , BJ , Lamb , SV , McFarlane , D and Beech , J . 2005 . Variation in plasma adrenocorticotropic hormone concentration and dexamethasone suppression test results with season, age, and sex in healthy ponies and horses . J Vet Intern Med , 19 : 217 – 222 .
- Durham AE, Hughes KJ, Cottle HJ, Rendle DI, Boston RC. 2009. Type 2 diabetes mellitus with pancreatice beta cell dysfunction in 3 horses confirmed with minimal model analysis. Equine Vet J. 41:924–929
- Dybdal , NO , Hargreaves , KM , Madigan , JE , Gribble , DH , Kennedy , PC and Stabenfeldt , GH . 1994 . Diagnostic testing for pituitary pars intermedia dysfunction in horses . J Am Vet Med Assoc , 204 : 627 – 632 .
- Elmahdi , BEM . 1998. Comparative aspects of glucose metabolism in camels, sheep, horses and ponies [PhD Dissertation]. [Hannover, Germany]: Bovine Clinic, Veterinary School, University of Hannover
- Eustace , RA and Redden , RR . 1990 . Iatrogenic laminitis . Vet Rec , 126 : 586
- Firshman , AM and Valberg , SJ . 2007 . Factors affecting clinical assessment of insulin sensitivity in horses . Equine Vet J , 39 : 567 – 575 .
- Firshman , AM , Valberg , SJ , Karges , TL , Benedict , LE , Annandale , EJ and Seaquist , ER . 2005 . Serum creatine kinase response to exercise during dexamethasone-induced insulin resistance in Quarter Horses with polysaccharide storage myopathy . Am J Vet Res , 66 : 1718 – 1723 .
- Frank , N , Elliott , SB , Chameroy , KA , Tóth , F , Chumbler , NS and McClamroch , R . 2010 . Association of season and pasture grazing with blood hormone and metabolite concentrations in horses with presumed pituitary pars intermedia dysfunction . J Vet Intern Med , 24 : 1167 – 1175 .
- French , K , Pollitt , CC and Pass , MA . 2000 . Pharmacokinetics and metabolic effects of triamcinolone acetonide and their possible relationships to glucocorticoid-induced laminitis in horses . J Vet Pharmacol Ther , 23 : 287 – 292 .
- Garcia , MC and Beech , J . 1986 . Equine intravenous glucose tolerance test: glucose and insulin responses of healthy horses fed grain or hay and of horses with pituitary adenoma . Am J Vet Res , 47 : 570 – 572 .
- Heinrichs , M , Baumgartner , W and Capen , CC . 1990 . Immunocytochemical demonstration of proopiomelanocortin-derived peptides in pituitary adenomas of the pars intermedia in horses . Vet Pathol , 27 : 419 – 425 .
- Henneke , DR , Potter , GD , Kreider , JL and Yeates , BF . 1983 . Relationship between condition score, physical measurements and body fat percentage in mares . Equine Vet J , 15 : 371 – 372 .
- Jeffcott , LB , Field , JR , McLean , JG and O'Dea , K . 1986 . Glucose tolerance and insulin sensitivity in ponies and Standardbred horses . Equine Vet J , 18 : 97 – 101 .
- Kahn , CR . 1978 . Insulin resistance, insulin insensitivity, and insulin unresponsiveness: a necessary distinction . Metabolism , 27 : 1893 – 1902 .
- Kronfeld , DS , Treiber , KH and Geor , RJ . 2005 . Comparison of nonspecific indications and quantitative methods for the assessment of insulin resistance in horses and ponies . J Am Vet Med Assoc , 226 : 712 – 719 .
- Kronfeld , DS , Treiber , KH , Hess , TM , Splan , RK , Byrd , BM , Staniar , WB and White , NW . 2006 . Metabolic syndrome in healthy ponies facilitates nutritional countermeasures against pasture laminitis . J Nutr , 136 : 2090S – 2093S .
- Lee , ZY , Zylstra , R and Haritou , SJ . 2010 . The use of adrenocorticotrophic hormone as a potential biomarker of pituitary pars intermedia dysfunction in horses . Vet J , 185 : 58 – 61 .
- Muniyappa , R , Lee , S , Chen , H and Quon , MJ . 2008 . Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage . Am J Physiol Endocrinol Metab , 294 : E15 – E26 .
- Muntoni , S and Muntoni , S . 2011 . Insulin Resistance: Pathophysiology and Rationale for Treatment . Ann Nutr Metab , 58 : 25 – 36 .
- Nosadini , R , Del Prato , S , Tiengo , A , Valerio , A , Muggeo , M , Opocher , G , Mantero , F , Duner , E , Marescotti , C Mollo , F . 1983 . Insulin resistance in Cushing's syndrome . J Clin Endocrinol Metab , 57 : 529 – 536 .
- Pallaske , G . 1932 . Zur Kasuistik seltener Geschwuelste bei den Haustieren . Z Krebsfor , 36 : 342 – 353 .
- Pass , MA , Pollitt , S and Pollitt , CC . 1998 . Decreased glucose metabolism causes separation of hoof lamellae in vitro: a trigger for laminitis? . Equine Vet J. Suppl.: , : 133 – 138 .
- Place , NJ , McGowan , CM , Lamb , SV , Schanbacher , BJ , McGowan , T and Walsh , DM . 2010 . Seasonal variation in serum concentrations of selected metabolic hormones in horses . J Vet Intern Med , 24 : 650 – 654 .
- Perkins , GA , Lamb , S , Erb , HN , Schanbacher , B , Nydam , DV and Divers , TJ . 2002 . Plasma adrenocorticotropin (ACTH) concentrations and clinical response in horses treated for equine Cushing's disease with cyproheptadine or pergolide . Equine Vet J , 34 : 679 – 685 .
- Powell , DM , Reedy , SE , Sessions , DR , Schanbacher , B , Nydam , DV and Divers , TJ . 2002 . Effect of short-term exercise training on insulin sensitivity in obese and lean mares . Equine Vet J. Suppl.: , : 81 – 84 .
- Pratt , SE , Geor , RJ and McCutcheon , LJ . 2005 . Repeatability of 2 methods for assessment of insulin sensitivity and glucose dynamics in horses . J Vet Intern Med , 19 : 883 – 888 .
- Pratt , SE , Geor , RJ and McCutcheon , LJ . 2006 . Effects of dietary energy source and physical conditioning on insulin sensitivity and glucose tolerance in standardbred horses . Equine Vet J Suppl , 2006 : 579 – 584 .
- Rijnen , KEPM and van der Kolk , JH . 2003 . Determination of reference range values indicative of glucose metabolism and insulin resistance by use of glucose clamp techniques in horses and ponies . Am J Vet Res , 64 : 1260 – 1264 .
- Schott , HC . 2002 . Pituitary pars intermedia dysfunction: equine Cushing's disease . Vet Clin Equine , 18 : 237 – 270 .
- Schott , HC , Coursen , CL , Eberhart , SW , Nachreiner , RJ , Refsal , KR , Ewart , SL and Marteniuk , JV . 2001. The Michigan Cushing's project. Proceedings of the 47th Annual American Association of Equine Practitioners Convention. p. 22–24
- Tiley , HA , Geor , RJ and McCutcheon , LJ . 2007 . Effects of dexamethasone on glucose dynamics and insulin sensitivity in healthy horses . Am J Vet Res , 68 : 753 – 759 .
- Tiley , HA , Geor , RJ and McCutcheon , LJ . 2008 . Effects of dexamethasone administration on insulin resistance and components of insulin signaling and glucose metabolism in equine skeletal muscle . Am J Vet Res , 69 : 51 – 58 .
- Treiber , KH , Kronfeld , DS and Geor , RJ . 2006b . Insulin resistance in equids: possible role in laminitis . J Nutr , 136 : 2094S – 2098S .
- Treiber , KH , Kronfeld , DS , Hess , TM , Byrd , BM , Splan , RK and Staniar , WB . 2006a . Evaluation of genetic and metabolic predispositions and nutritional risk factors for pasture-associated laminitis in ponies . J Am Vet Med Assoc , 228 : 1538 – 1545 .
- van der Kolk , JH , Heinrichs , M , van Amerongen , JD , Stooker , RC , in de Wal , LJ and van den Ingh , TSGAM . 2004 . Evaluation of pituitary gland anatomy and histopathologic findings in clinically normal horses and horses and ponies with pituitary pars intermedia adenoma . Am J Vet Res , 65 : 1701 – 1707 .
- van der Kolk , JH , Ijzer , J , Overgaauw , PA and van der Linde-Sipman , JS . 2001 . Pituitary-independent Cushing's syndrome in a horse . Equine Vet J , 33 : 110 – 112 .
- van der Kolk , JH , Kalsbeek , HC , van Garderen , E , Wensing , Th and Breukink , HJ . 1993 . Equine pituitary neoplasia: a clinical report of 21 cases (1990–1992) . Vet Rec , 133 : 594 – 597 .
- van der Kolk , JH , Kalsbeek , HC , Wensing , Th and Breukink , HJ . 1994 . Urinary concentration of corticoids in normal horses and horses with hyperadrenocorticism . Res Vet Sci , 56 : 126 – 128 .
- van der Kolk , JH , Rijnen , KEPM , Rey , F , de Graaf-Roelfsema , E , Grinwis , GCM and Wijnberg , ID . 2005 . Evaluation of glucose metabolism in three horses with lower motor neuron degeneration . Am J Vet Res , 66 : 271 – 276 .
- van der Kolk , JH , Wensing , Th , Kalsbeek , HC and Breukink , HJ . 1995 . Laboratory diagnosis of equine pituitary pars intermedia adenoma . Domest Anim Endocrinol , 1995;12 : 35 – 39 .
- Wallace , TM and Matthews , DR . 2002 . The assessment of insulin resistance in man . Diabet Med , 19 : 527 – 534 .
- Wilson , JD , Foster , DW , Kronenberg , HM and Larsen , PR . 1998 . Williams textbook of endocrinology , 9th , Philadelphia : WB Saunders Company .