Abstract
Coccidiosis in chickens is a parasitic disease with great economic significance, which has been controlled successfully for decades using mainly anticoccidial products. However, large-scale and long-term use of anticoccidial drugs has led to the worldwide development of resistance against all these drugs. In order to minimize the occurrence of resistance, the rotation of various anticoccidial drugs in single and/or shuttle programmes is used. Unfortunately, this has not solved the anticoccidial resistance problem. Recently, live anticoccidial vaccines have been incorporated into rotation programmes, resulting in an increasing incidence of anticoccidial drug-sensitive Eimeria spp. field isolates, which may ameliorate the efficacy of anticoccidial drugs. Nevertheless, possible upcoming bans restricting the use of anticoccidials as feed additives, consumer concerns on residues and increasing regulations have prompted the quest for alternative coccidiosis control strategies. Although management and biosecurity measures could halt the introduction of Eimeria spp. to a farm, in practice they do not suffice to prevent coccidiosis outbreaks. Phytotherapy, aromatherapy and pre- and probiotics either show conflicting, non-consistent or non-convincing results, and have therefore not been applied at a large scale in the field. So far, live attenuated and non-attenuated anticoccidial vaccines have proved to be the most solid and successful coccidiosis prevention and control strategy. Despite the drawbacks associated with their production and use, their popularity is increasing. If with time, the immunogenicity of subunit vaccines can be improved, they could represent the next generation of highly efficient and low-cost anticoccidial strategies.
1. Introduction
Coccidiosis is a disease caused by parasites of the genus Eimeria and Isospora belonging to the phylum Apicomplexa with a complex life cycle, affecting mainly the intestinal tract of many species of mammals and birds. It is of great economic significance in farm animals, especially chickens. Most knowledge on coccidiosis has been obtained from chickens, where the disease has been studied most intensively as it is in commercial poultry that this parasite causes the most damage due to the fact that birds are reared together in large numbers and high densities. The economic significance of coccidiosis is attributed to decreased animal production (higher feed conversion, growth depression and increased mortality) and the costs involved in treatment and prevention. Worldwide, the annual costs inflicted by coccidiosis to commercial poultry have been estimated at 2 billion €, stressing the urgent need for more efficient strategies to control this parasite.
2. Anticoccidial products
Numerous anticoccidial drugs have been introduced since 1948, when sulphaquinoxaline and nitrofurazone were first approved by the American Food and Drug Administration (Chapman Citation1997; McDougald Citation2003; Conway and McKenzie 2007). Most of the anticoccidial products currently approved in different regions of the world for the prevention of coccidiosis in chickens are listed in . Moreover, national regulatory limitations may apply to some products. A number of anticoccidials have been withdrawn overtime because of safety or efficacy issues; nevertheless, many of these drugs are still available and used.
Table 1. Contemporary anticoccidial products and recommended doses for prophylactic treatment of coccidiosis in chickens (modified from Conway and McKenzie 2007).
2.1. Categories
The anticoccidial products can be classified in three categories according to their origin (Chapman 1999a, 1999b; Allen and Fetterer Citation2002).
| 1. | Synthetic compounds. These compounds are produced by chemical synthesis and often referred to as ‘chemicals’. Synthetic drugs have a specific mode of action against the parasite metabolism. For example, amprolium competes for the absorption of thiamine (vitamin B1) by the parasite. | ||||
| 2. | Polyether antibiotics or ionophores. These products are produced by the fermentation of Streptomyces spp. or Actinomadura spp. and destroy coccidia by interfering with the balance of important ions like sodium and potassium. The following groups of ionophores exist: | ||||
| • | Monovalent ionophores (monensin, narasin and salinomycin). | ||||
| • | Monovalent glycosidic ionophores (maduramicin and semduramycin). | ||||
| • | Divalent ionophores (lasalocid). | ||||
| 1. | Mixed products. A few drug mixtures, consisting of either a synthetic compound and ionophore (nicarbazin/narasin (Maxiban®)) or two synthetic compounds (meticlorpindol/methylbenzoquate (Lerbek®)), are also used against coccidiosis. | ||||
2.2. Mode of action
Although detailed knowledge on the selective action of anticoccidial compounds against specific stages of the parasite is often lacking (Wang Citation1982), a broad categorization of the mode of action of anticoccidials on the parasite metabolism has been undertaken (Chapman Citation1997).
2.2.1. Products affecting cofactor synthesis
Several anticoccidial products influence essential biochemical pathways of the parasitic cell by affecting an important cofactor (Greif et al. Citation2001).
Ethopabate, which is often used in combination with amprolium to improve the spectrum of efficacy, is a folate antagonist and blocks a step in the synthesis of para-aminobenzoic acid (PABA) and prevents the formation of nucleic acids of vitamins (Rogers et al. Citation1964). It is most active against Eimeria maxima and Eimeria brunetti.
Similar to ethopabate, sulphonamides prevent the synthesis of dihydrofolate by interfering with the dihydropteroate synthetase reaction, blocking the conjugation of pteridine and PABA. Dihydropteroate synthetase is only present in the parasite. They are very effective against E. brunetti, E. maxima and Eimeria acervulina and to a much lower degree against Eimeria tenella and Eimeria necatrix (Ryley and Betts Citation1973). A major drawback of sulphonamides is their small safety margin, which easily leads to intoxications especially if they are used as treatment for coccidiosis outbreaks.
A third product affecting the folate pathway of coccidia is pyrimethamine. It prevents the reduction of dihydrofolate to tetrahydrofolate by inhibiting the enzyme dihydrofolate reductase. Pyrimethamine has a clear synergistic effect with sulphonamides (Kendall and Joyner Citation1956). Ethopabate, sulphonamides and pyrimethamine affect the second generation of schizonts (Reid Citation1973, 1975).
Thiamine analogues, like amprolium, block the absorption of thiamine completely and have probably an antagonistic effect on the vitamin B1 supply. Amprolium seems especially efficacious during schizogony as then the demand of thiamine is at its highest (James Citation1980). There is a difference in sensitivity of the thiamine transport system of host and parasite (more sensitive) to amprolium. It affects the first generation of schizonts and to a lesser extent the gametogony (Reid Citation1973) allowing immune response to develop.
2.2.2. Products affecting mitochondrial function
Quinolone drugs, amongst which buquinolate, decoquinate and nequinate (methyl benzoquate) are listed, show anticoccidial activity at very low concentrations. These products inhibit the respiration of coccidia by blocking the electron transport in their mitochondria (Wang Citation1975). Quinolones arrest the development of sporozoites (Yvoré Citation1968; Reid Citation1973).
Meticlorpindol is the most important compound of the pyridone group. Similar to the quinolones, it inhibits electron transport in mitochondria, but possibly at another level as cross-resistance with quinolones does not occur. A synergistic effect between meticlorpindol and 4-hydroxiquinolones has been described (Challey and Jeffers Citation1973). A widely used pyridone–quinolone combination drug is Lerbek®, consisting of meticlorpindol and methyl benzoquate.
The true mode of action of nicarbazin (4,4′-dinitrocarbanilide) is unknown. The product has been shown to inhibit both the succinate-linked NAD reduction in mitochondria of beef hearts and the energy dependent transhydrogenase and accumulation of Ca2+ ions by rat liver mitochondria (Dougherty Citation1974). It is not suitable for layers as it negatively affects the egg production and quality.
The exact anticoccidial mechanism of robenidine (a guanidine derivative) is still unknown. However, from studies in mammals, it is assumed that it inhibits the oxidative phosphorylation of mitochondria (Wong et al. Citation1972).
Another anticoccidial drug possibly affecting mitochondrial function is the triazinetrione compound toltrazuril, which is applied in drinking water for preventive and therapeutic treatment. Harder and Haberkorn (Citation1989) showed that activities of some enzymes of the respiratory chain, such as succinate-cytochrome C reductase, nicotinamide adenine dinucleotide (NADH) oxidase and succinate oxidase from mouse liver, were reduced in the presence of toltrazuril. They also showed an inhibitory effect on the dihydroorotate-cytochrome C reductase from mouse liver. More recently, it has been suggested that toltrazuril might affect plastid-like organelles (Hackstein et al. Citation1995). Toltrazuril is efficacious against all intracellular stages (schizogony and gametogony) of all important Eimeria spp. in the chicken (Mehlhorn et al. 1984, 1988). It induces cidal changes in the organelles of the parasite at multiple levels and does not seem to impair the development of natural immunity (Greif and Haberkorn Citation1997; Greif Citation2000).
2.2.3. Products affecting cell membrane function
Polyether antibiotics influence the transport of mono- or divalent cations (Na+, K+ and Ca++) across cell membranes inducing osmotic damage (Berger Citation1951; Shumard and Callender Citation1967). These drugs are accumulated by extracellular stages (like sporozoites and merozoites) of the parasite in the lumen of the intestine (Long and Jeffers Citation1982).
Depending on the dose given, ionophore anticoccidials allow the development of immunity against coccidia (Jeffers Citation1989; Chapman and Hacker Citation1993; Chapman Citation1999b).
2.2.4. Products with unknown mode of action
Diclazuril is a nucleoside analogue thought to be involved in nucleic acid synthesis, possibly affecting later phases of coccidia differentiation (Verheyen et al. Citation1988). It has been shown to affect parasite wall synthesis resulting in the formation of an abnormally thickened, incomplete oocyst wall and zygote necrosis in both E. brunetti and E. maxima (Verheyen et al. Citation1989).
Halofuginone is a quinazolinone derivative with an unknown mode of action, which particularly affects the first generation of the schizogony.
2.3. Antimicrobial and growth-promoting properties
Ionophores have been found to inhibit Gram-positive organisms and mycoplasmas (Shumard and Callender Citation1967; Dutta and Devriese Citation1984; Stipkovits et al. Citation1987). Monensin and narasin were shown to inhibit Clostridium perfringens (types A and C) in chickens and turkeys (Elwinger et al. Citation1992; Vissiennon et al. Citation2000). Ionophores may therefore have contributed in some cases to the control of necrotizing enteritis (Martel et al. Citation2004).
Salinomycin has been shown to reduce the number of resistant coliforms (sulphadiazine) and Streptococci (erythromycin and lincomycin; George et al. 1982). It also seemed to reduce the number of resistant Salmonella typhimurium bacteria (Ford et al. Citation1981).
2.4. Incompatibilities
Ionophores are incompatible with some therapeutic antibiotics like tiamulin, chlorampheniol, erythromycin, oleandromycin and certain sulphonamides. Ionophores are also incompatible with some antioxidants (XAX-M, Duokvin, TD; Umemura et al. Citation1984; Prohaszka et al. 1987; Dowling Citation1992; Von Wendt et al. Citation1997).
2.5. European Union regulations
The future status of anticoccidial drugs is uncertain at this moment: their current status as feed additives may be maintained or new legislation and phasing-out of these drugs as feed additives could be proposed. Article 11 of Regulation 1831/2003 called for a report of the European Commission on this subject. In April 2008, the commission submitted a report (COM 2008) in which it clearly recommends the maintenance of the use of anticoccidial products, including ionophores as feed additives, due to a lack of alternatives and to safeguard the economical feasibility of the poultry industry. It is not clear whether the European Council will follow this recommendation or not.
Actual and forthcoming information of the obtained regulations can be found on the website of the European Commission animal nutrition feed additives (http://ec.europa.eu/food/food/animalnutrition/feedadditives/legisl_en.htm).
2.6. Resistance
The World Health Organization (WHO) defines drug resistance in antimalarial chemotherapy, which can also be applied to coccidiology, as ‘the ability of a parasite strain to survive and/or multiply despite the administration and absorption of a drug in doses equal to or higher than those usually recommended but within the limits of tolerance of the subject’ (WHO 1965).
Generally, drug resistance in coccidia can be complete, in which case increasing doses up the maximum tolerated by the host is ineffective (i.e. diclazuril and nicarbazin). In contrast, relative resistance to anticoccidial drugs is characterized by the fact that increasing doses tolerated by the host still will show efficacy (i.e. ionophores).
The worldwide intensive use of anticoccidial drugs to prevent coccidiosis has inevitably led to the development of resistance to all anticoccidial drugs as long-term exposure to any drug will result in loss of sensitivity. The widespread occurrence of resistance has been described in the United States of America, South America, Europe and China (Jeffers 1974a, 1974b, 1989; Chapman 1978, 1982, 1984, 1997; Ryley Citation1980; Hamet Citation1986; Litjens Citation1986; McDougald et al. 1986, 1987; Zeng and Hu Citation1996; Zhou et al. Citation2000; Peek and Landman Citation2003; Peek and Landman Citation2004). In some cases resistance is induced very quickly, as in the case of quinolones and pyridinols, which led to a decline in their use, while in other instances it may take several years as in the case of the ionophores.
Despite the widespread occurrence of resistance, at least in Europe, coccidiosis outbreaks seem to have had limited impact so far. This is explained by the fact that resistance in many cases will have allowed the occurrence of trickle infections, which are essential in the building up of immunity (Jeffers Citation1989; Chapman Citation1998; Peek and Landman Citation2003; McDougald and Shirley Citation2009).
2.6.1. Management of resistance
To minimize the occurrence of resistance, rotation (a given anticoccidial product is used during a maximum of 2 months or two fattening periods) of various anticoccidial drugs or shuttle programmes (two or more anticoccidial drugs are used within a fattening period) is used. The rationale behind this is the fact that, as said previously, the loss of sensitivity is correlated to the length of drug exposure, which should be kept short if possible. Due to the occurrence of cross-resistance between anticoccidial drugs, anticoccidial drugs with distinct mode of action should be used within rotation and shuttle programmes.
In order to optimize the use of prophylactic medication in the field, scientific information on the drug-sensitivity profiles of Eimeria spp. concerning field isolates is vital. This information can only be obtained by performing an in vivo Anticoccidial Sensitivity Test (AST) in battery cages. Despite the fact that the AST is the only accurate tool to detect anticoccidial drug resistance, the procedure is slow and expensive; isolates frequently originate from disease outbreaks (and may not always be representative for the field) and need to be propagated first in specified pathogen-free chickens for multiplication (which may result in the selection of non-relevant coccidia). Nevertheless, it is generally accepted by the scientific community that ASTs provide valuable information and should be given more attention in coccidiosis prevention programmes.
A more recent development in managing anticoccidial drug resistance is the rotation of anticoccidial drugs with live Eimeria spp. vaccines. Several studies have documented a higher incidence of sensitive Eimeria spp. field isolates when live anticoccidial vaccines and anticoccidial products are rotated. The exact mechanism resulting in an increase of sensitivity of Eimeria spp. field isolates is currently unknown, but it is explained by the fact that chicken houses are seeded with drug-sensitive vaccine oocysts (Jeffers Citation1976; Mathis and McDougald Citation1989; Chapman 1994, 1996; Newman and Danforth Citation2000; Mathis and Broussard Citation2006; Peek and Landman Citation2006). This may lead to the outgrowth of resistant strains by reproductively more advantageous drug-sensitive coccidia, interbreeding between field and vaccine parasites resulting in (more) sensitive interbreeds or a combination of both. Moreover, in case live attenuated coccidiosis vaccines are used, less virulent interbreed field isolates may be produced (Shimura and Isobe Citation1994; Williams Citation2002a).
3. Vaccines
Immunity to coccidiosis, which can be induced by passive or active immune responses, is generally defined as the occurrence of ‘resistance’ to a challenge infection with an Eimeria spp. and can be determined by a reduction of the pathogenic effects of coccidiosis: less macroscopically visible lesions and/or a decrease in oocyst production, and increased performance of birds.
The first study showing that chickens infected with E. tenella were resistant to homologous challenge was reported by Beach and Corl (Citation1925) and formed the basis of modern coccidiosis vaccinology. However, it took another 27 years before the first commercial live coccidiosis vaccine CocciVac® was registered in the USA (Edgar and King Citation1952).
During the past 20 years, various reports describing coccidiosis vaccines and their use in poultry have been published (Shirley Citation1988; Williams 1992, 1996, 1998, 1999, 2000; 2002a, 2002b; Dalloul and Lillehoj Citation2006; Shirley et al. Citation2007). In , an overview showing most available coccidiosis vaccines and their usage is given (Williams Citation2002a; Shirley et al. Citation2005).
Table 2. Overview of anticoccidial vaccines that are being used or being registered for use in chickens (modified from Williams 2002a; Shirley et al. Citation2005; manufacturer's technical information bulletins and websites).
Vaccination can be alternated with anticoccidial drugs in feed within rotation programmes and in combination with biosecurity.
3.1. Subunit vaccines
Subunit vaccines are composed of a purified antigenic determinant that is separated from the virulent organism. Such vaccines can be obtained by different technologies and may consist of native antigens or of recombinant proteins expressed from DNA of various developmental stages (sporozoites, merozoites and gametes) of the Eimeria parasite.
Despite an increasing number of manuscripts on exploring the feasibility of subunit vaccines against coccidiosis, no commercial products, except CoxAbic®, have been marketed to date (Brother et al. Citation1988; Jenkins et al. Citation1989; Miller et al. Citation1989; Jenkins et al. Citation1990; Crane et al. Citation1991; Bhogal et al. Citation1992; Jenkins Citation1998; Vermeulen Citation1998; Jenkins Citation2001; Vermeulen et al. Citation2001; Dalloul and Lillehoj Citation2006). A major limiting factor has been that until now no antigens able to induce potent protective immune response against Eimeria have been isolated. Systematic and detailed analysis of host–parasite interactions at the molecular and cellular levels including studies of basic immunology need to be completed before successful subunit vaccine products will be made available. In this regard, the E. tenella genome project may help to further understand how protective immune responses against Eimeria spp. are developed and help to identify antigens of vital importance in coccidial immunology (Shirley et al. Citation2007).
3.1.1. Maternal immunization, transmission blocking immunity
CoxAbic® is a vaccine against coccidiosis, which induces maternally derived antibodies to protect broiler chickens (Michael 2003, 2007; Finger and Michael Citation2005; Ziomko et al. Citation2005). It is an inactivated, subunit vaccine (oil emulsion) containing affinity-purified proteins (56, 82 and 230 kDa) from the oocyst wall-forming bodies of E. maxima gametocytes. Cross-protection resulting in lower oocyst shedding of E. maxima, E. acervulina and E. tenella has been described after the administration of low-dose challenge inocula using the aforementioned Eimeria spp. (Wallach et al. Citation1995; Wallach Citation1997). This is remarkable because after natural Eimeria infections, cross-immunity has not been described (Rose and Long Citation1962). However, Crane and co-workers (Citation1991) found cross-protection against four Eimeria spp. (E. acervulina, E. maxima, E. necatrix and E. tenella) after administration of a single recombinant antigen.
Offspring originating from vaccinated parent birds are fed an anticoccidial drug-free diet in order to ensure natural exposure to the parasites and subsequent development of active immunity after maternal antibodies have disappeared.
3.2. Live vaccines
Live Eimeria vaccines consist of sporulated oocysts and are either non-attenuated (wild-type strains of Eimeria spp.) or attenuated. Further differentiation is based on the Eimeria spp. included, their anticoccidial drug sensitivity profile and application. Protective immunity can be achieved if chickens are infected with either a single high dose or multiple low doses (trickle infections) of Eimeria (vaccine) parasites (Joyner and Norton 1973, 1976; Long et al. Citation1986). It is crucial that anticoccidial drugs are withdrawn from feed in case chicken flocks are vaccinated with drug-sensitive live vaccine parasites in order to avoid vaccination failures.
3.2.1. Non-attenuated (or wild-type strains of Eimeria spp.) vaccines
Non-attenuated vaccines consist of Eimeria parasites, which have not been modified in any way to change their pathogenicity and originate from laboratory or field strains. Examples of such vaccines are: CocciVac®, Immucox®, Inovocox® and ADVENT®.
Some of these vaccines (CocciVac® and Immucox®) are available as two different products and the choice of products depends on the poultry to be vaccinated, e.g. broilers versus breeders and layers.
During the usage of non-attenuated coccidiosis vaccines, it is crucial that all birds are given the required dose and the occurrence of clinical coccidiosis is avoided. Therefore, application protocols should be followed strictly (Chapman et al. Citation2002). In order to diminish the risk of coccidiosis outbreaks following vaccination, attenuated vaccines have been developed.
3.2.2. Attenuated vaccines
Attenuated vaccines consist of Eimeria spp. strains, which have been manipulated in the laboratory in order to decrease their virulence. Reduced virulence has been performed by serial passages of the parasite in chicken embryos; e.g. E. tenella in Livacox® vaccines. Selection for precocity is the second described method for attenuation; e.g. remaining Eimeria spp. in Livacox® and all lines in Paracox® vaccines (McDonald and Shirley Citation1984; Shirley and Millard Citation1986; Shirley et al. Citation1995; Shirley and Bedrnik Citation1997). Precocity is characterized by a shortened endogenous life-cycle due to the fact that the number of generations of schizogony is decreased because last generations of schizogony disappear by selecting early oocysts. As a consequence, the number of oocysts produced during infection is reduced. However, the immunizing potential is maintained (Jeffers 1975, 1986; McDonald et al. Citation1986; Shirley and Millard Citation1986).
A major drawback of live coccidiosis vaccines is their loss of infectivity with time affecting their expiry (Jeston et al. Citation2002). Other concerns are their high production costs and management shortcomings during their application such as dosage errors that may result in insufficient immune response or clinical coccidiosis in case non-attenuated vaccines are used, the erroneous addition of anticoccidial drugs to the feed frustrating effective vaccination of drug-sensitive strains and vaccination of sick birds. Furthermore, reversal of virulence of live vaccines may be another point of concern. Subunit vaccines offer possibilities to circumvent mentioned drawbacks and could provide a sustainable solution for the coccidiosis problem in commercial poultry if with the help of new technologies their immunogenicity can be increased.
A full list of anticoccidial vaccines, including, amongst others, their composition and route of administration, has been given in .
4. Other preventive strategies against coccidiosis
The prevention and control of coccidiosis in commercial poultry and mainly broilers is largely based on the administration of anticoccidial drugs in feed, although in some cases an anticoccidial drug administered shortly via drinking water is applied (Mathis et al. Citation2004). Moreover, biosecurity measures aiming at preventing the introduction of the parasite to the farm can be of additional strategic value against clinical coccidiosis. During the past decade, live coccidiosis vaccination has become increasingly popular as an alternative strategy to control Eimeria spp. In feed, products with supposed anticoccidial activity like herbs, essential oils, probiotics and prebiotics have been added more recently.
The development of resistance against all anticoccidial drugs used in feed to date and a loud call for residue-free poultry products, which in Europe has been translated first into a re-evaluation of all existing anticoccidial products and regulations safeguarding lower residues, have prompted the search for alternative control and prevention strategies.
4.1. Management and biosecurity
Good health and hence optimal immune response are essential in order to minimize the impact of infectious diseases. General management measures safeguarding the basic requirements of poultry should therefore always be implemented: birds should be provided with proper feed and drinking water intake, adequate bedding, temperature, humidity, lighting and ventilation.
Management and biosecurity measures for the control of coccidiosis should focus on the prevention of the introduction of the parasite into the premises, and control of its multiplication and spread in case flocks have been infected. However, strict biosecurity leading to coccidiosis-free flocks is almost impossible to attain in practice. Therefore, already in the early days of coccidiosis research gradual building up of immunity through repeated light (trickle) infections was advocated as a mean to control this disease (Chapman 2003).
4.1.1. Avoiding the introduction of the parasite
Eimeria parasites are ubiquitous and have enormous reproduction capabilities leading to high contamination levels of infected poultry houses and their surroundings. Moreover, the oocyst wall protects the parasite from desiccation and chemical disinfectants, ensuring long-term survival in the environment, from which it may be introduced to a farm through a variety of ways. On the other hand, oocysts may already be present in the farm house if cleaning and disinfection was not adequate.
Biosecurity measures aiming to prevent the introduction of Eimeria parasites to the farm are similar to those applied for the prevention of other infectious poultry diseases and should focus on:
| 1. | Isolation. Birds should be separated from the environment by fencing and other animals including rodents and insects should be kept out. | ||||
| 2. | Traffic control should not only be performed at the farm, but also the traffic between farms should be restricted. | ||||
| 3. | Sanitation includes disinfection of materials, people and equipment entering the farm and poultry house. | ||||
4.1.2. Environmental factors
In case coccidiosis infections occur, their multiplication cannot be restricted by influencing the climate environment of the chicken house as Eimeria spp. thrives in atmospheric conditions that are beneficial to the birds also.
In order for Eimeria spp. to become infective, it must sporulate after excretion in the faeces. The degree and rate of sporulation of excreted oocysts determine the infection pressure in a chicken flock. Sporulation of the oocyst depends mainly on the following basic factors: temperature, humidity and aeration (access to oxygen) (Kheysin 1972).
The best sporulation temperature is approximately 24–28°C (Edgar Citation1955), while a temperature above 35°C is lethal for oocysts (Schneider et al. Citation1979). Due to the fact that the ideal sporulation temperature is within the range of temperatures frequently encountered in the poultry environment and that high temperatures are detrimental to the birds, temperature is not a factor that can be used to control parasite multiplication.
A general misconception, regarding humidity is the belief that the drier the climate, the less coccidiosis occurs. Research data have shown that in the field sporulation in wet litter is suboptimal possibly due to the occurrence of ammonia and bacteria (Williams Citation1995); in fact, dry litter showed better sporulation rates (Graat et al. Citation1994; Waldenstedt et al. Citation2001). However, increasing the litter humidity as a control measure to reduce sporulation does not seem feasible as it might cause footpad lesions and skin burns in the birds.
Adequate ventilation of the poultry house is essential for good performance and health of the birds although proper aeration will also favour sporulation.
Theoretically, a higher bird density will result in greater oocyst accumulation in the litter and increase the chances of clinical coccidiosis (Williams et al. Citation2000). Thus, reducing bird density could help to control Eimeria infections.
4.1.3. Cleaning and disinfection
Cleaning and disinfection between flocks and maximizing the downtime period is important in order to significantly reduce the number of parasites in contaminated chicken houses. However, there are mixed points of view regarding the use of cleaning and disinfection for the control of coccidiosis. Some consider that the presence of oocysts in the poultry environment enabling early establishment of immunity in order to avoid outbreaks at later age is beneficial. In case coccidiosis vaccines are used to repopulate the houses with drug-sensitive strains, survival of vaccine parasites between vaccinated and non-vaccinated flocks is desired and therefore cleaning and disinfection should be skipped. Nevertheless, in case of severe clinical coccidiosis outbreaks especially due to anticoccidial drug-resistant strains, it is customary in Europe, where birds are not housed on deep litter, to clean and disinfect the chicken houses in order to reduce infection pressure.
Ammonium hydroxide has been reported as a highly effective disinfectant against sporulated and non-sporulated oocysts. It has been shown to be effective at a concentration of ≥5% in both fluid and vapour forms (Chroustova and Pinka Citation1987). Also, products that generate ammonia after mixing two components (ammonium salt and sodium hydroxide) have shown to be oocidal (Oocide®, Antec International Ltd, UK). More recently, a cresol-based product (Neopredisan® 135-1, Menno Chemie, Norderstedt, Germany) has been marketed in Germany for the control of coccidiosis by chemical disinfection. It has been shown to be efficient against various Eimeria spp. (Daugschies et al. Citation2002; Houdek et al. Citation2002). In a recent study, the efficacy of eight disinfectants against E. tenella unsporulated oocysts isolated from broilers was studied in vitro. The best disinfection efficacy was observed for the combination formol 37% and sodium dodecylbenzene sulphonate 12% (Gumarães et al. Citation2007).
In practice, poultry houses in the Netherlands are often disinfected using calcium hydroxide in combination with ammonium sulphate (per 500 m2 40 kg calcium hydroxide is spread and subsequently moisturized with approximately 500 L water, after which 80 kg ammonium sulphate is added).
4.2. Alternative coccidiosis control
Various alternative methods like homeopathy, phytotherapy and aromatherapy have been used during the past decennia for the treatment of various poultry diseases. Homeopathy is a system for treating disease based on the administration of minute doses of a drug that in massive amounts produces clinical signs in healthy individuals similar to those of the disease itself. The treatment is thus based on law of similars, i.e. similia similibus curentur = the most similar remedy will cure (Saine Citation2000). It is thought to enhance the body's natural defenses. Homeopathy is often confused with herbalism also known as botanical medicine, medicinal botany, medical herbalism, herbal medicine, herbology and phytotherapy. It is based on the use of plant and plant extracts for the treatment and prevention of disease. Its scope is sometimes extended to include fungi, bee products, minerals, shells and certain animal parts. Aromatherapy, which is closely related to phytotherapy, is the treatment or prevention of disease by means of essential oils, and other scented compounds from plants. It involves the use of distilled plant volatiles, a twentieth century innovation. Essential oils differ in chemical composition from other herbal products because the distillation process only recovers lighter phytomolecules.
Although many papers have been published on the efficacy of homeopathic treatment in humans, its effectiveness has remained a matter of debate to the scientific community. Studies assessing the methodological quality of a large number of human homeopathic experiments report a lack of evidence for efficacy of homeopathic treatments because of frequent low methodological quality and publication bias (Kleynen et al. Citation1991; Linde et al. Citation2001). According to Velkers et al. (Citation2005), ‘Efficacy of medicines should be beyond placebo effect and observation and interpretation should be without subjective elements. Therefore, all medicines, including homeopathic remedies, should be tested in a double blind randomized clinical trial’. Published studies on homeopathy in poultry are rare and similar to human studies, are frequently affected by low methodological quality (Velkers et al. Citation2005). There are no scientific reports on the homeopathic treatment of coccidiosis.
Some plant products or derived pharmaceutical drugs have been incorporated into mainstream medicine; nevertheless, most herbal treatments have been developed without modern scientific assessment. Although later on many herbs have been found efficacious in in vitro animal models and/or clinical studies (Srinivasan Citation2005), there are also many studies showing negative results (Pittler et al. Citation2000). However, evaluation of the literature on complementary/alternative therapies is difficult due to the occurrence of location bias in the corresponding controlled clinical trials. More positive than negative trials are published, except in high-impact mainstream medical journals. Furthermore, in complementary/alternative medicine journals, positive studies are of poorer quality than corresponding negative studies. The latter was not the case in mainstream medical journals publishing on a wider range of therapies.
Pre- and probiotics have also been included within alternative coccidiosis control strategies although they are quite distinct from phytotherapy and aromatherapy. Probiotics consist of live bacteria or yeasts administered in feed, which are supposed to be beneficial for the individual's health. In contrast, prebiotics are non-digestible food ingredients, which also have a beneficial effect on the host's health.
4.2.1. Plant (herb) extracts
Many different herbal compounds have been investigated for their potential use as a dietary supplement to control coccidiosis and are reported next in alphabetical order. The main conclusions obtained in the research of alternative coccidiosis control have been summarized in and .
Figure 1. Artemisinin extracts, citric fruits and different herb extracts seem to have an inhibitory effect on the development of Eimeria spp. Prebiotics and oregano have an indirect inhibitory effect on the development of Eimeria parasite. Betaine has an indirect effect on the development of the parasite through its osmoprotective properties on the intestinal mucosa and stimulation of the intraepithelial lymphocytes. Echinacea purpurea, mushrooms extracts, turmeric and probiotics have an indirect inhibitory effect on the development of Eimeria spp. through stimulation of the immune system.
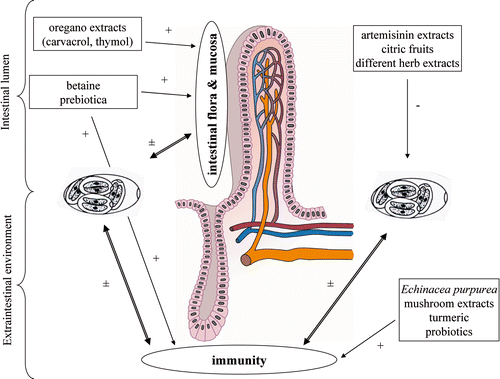
Table 3. Overview of the influence of alternative feed additives, including the active compound, dose per kg feed and mode of action, on a coccidiosis infection in poultry.
4.2.1.1. Artemisinin
Artemisinin is an herbal extract, which can be obtained from Artemisia annua (annual wormwood) and Artemisia sieberi. Positive effects were found for E. tenella (Oh et al. Citation1995; Allen et al. Citation1997b), mixed results for E. acervulina (Allen et al. Citation1997b; Arab et al. Citation2006) and negative results for E. maxima (Allen et al. Citation1997b; Arab et al. Citation2006).
4.2.1.2. Betaine
Betaine is a sweet crystalline alkaloid (trimethylglycine C5H11NO2), choline analogue and methyl donor occurring in sugar beets and other plants, which is used in the treatment of certain metabolic disorders (muscular weakness and degeneration). It has been shown to protect cells against osmotic stress by stabilizing cell membranes enabling the maintenance of osmotic pressure in the cells and ensuring normal metabolic activity (Rudolph et al. Citation1986). The osmoprotective effect of betaine is not restricted to the intestinal cells, but also affects the developing stages of the coccidia. It protects different cell types against chemical and environmental stresses (Kunin and Rudy Citation1991), including the asexual stages (sporozoites) of E. acervulina against the destructive action of salinomycin (Augustine and Danforth Citation1999). Nevertheless, feed efficacy and weight gain were significantly improved in birds infected with a mixture of E. acervulina, E. maxima and E. tenella if betaine (0.15%) and salinomycin (44 or 66 ppm) were supplemented separately, but much more if both components were given together suggesting a palliative effect of betaine on the coccidiosis infection. Betaine alone did not influence the severity of lesion scores and mortality (Augustine et al. Citation1997). Both products alone or in combination inhibited the invasion of E. acervulina and E. tenella (sporozoites) and the development of E. acervulina (second generation schizonts).
The ionophore-amplifying effect of betaine was only observed once and could not be found with other anticoccidial ionophore drugs like monensin and narasin (Matthews et al. Citation1997; Waldenstedt et al. Citation1999), while other scientists found contradictory results (Fetterer et al. Citation2003).
The weight gain and feed conversion-promoting capabilities found in some studies may be explained by betaine's osmoprotectant properties ensuring normal metabolism of intestinal cells. This may warrant normal development of the chicks despite the fact that the coccidiosis infection is not fully halted. Another contributing factor to the positive effect of betaine on coccidiosis infection may be the fact that it increases intraepithelial lymphocytes in the duodenum and the functional properties of phagocytes of Eimeria-infected chickens (Klasing et al. Citation2002).
4.2.1.3. Citric extracts
A product based on citric extracts and organic acids amongst others supplemented to broilers showed a moderate efficacy against a challenge with various Eimeria spp. considering coccidiosis lesion scores and oocyst production (Tamasaukas et al. 1996, 1997).
4.2.1.4. Echinacea purpurea
The anticoccidial effect of E. purpurea has been attributed to its immunomodulating properties, which have been widely documented (Stimple et al. Citation1984; Burger et al. Citation1997; See et al. Citation1997; Sun et al. Citation1999; Currier and Miller Citation2001; Goel et al. Citation2002). Ground root preparations of E. purpurea (0.1–0.5%) supplemented to broilers during 2 weeks reduced weight gain retardation and coccidial lesions after a mixed infection at the age of 28 days with E. acervulina, E. maxima, E. tenella and E. necatrix (Allen Citation2003).
4.2.1.5. Gentian violet
Gentian violet, also named crystal violet, methyl violet and hexamethyl pararosaniline chloride, is derived from coal tar and known for its antifungal and antibacterial properties. It has been shown to reduce coccidiosis lesion scores in the duodenum and improve weight gain in Eimeria spp. challenged birds. In combination with anticoccidial drugs, it improved feed conversion (Sharkey Citation1978).
4.2.1.6. Mushrooms and their extracts
Mushrooms and their extracts have gained interest in medicine and as dietary supplement due to their immune enhancing and antitumour properties. However, they should be used cautiously as mushrooms may harbour toxic levels of metals (arsenic, lead, cadmium and mercury) and radioactive contamination with 137Cs (Borchers et al. Citation2004).
Polysaccharide extracts originating from Lentinus edodes and Tremella fuciformes as well as the herb Astragalus membranaceus showed a positive effect on the cellular and humoural immunity of E. tenella infected female broilers (Guo et al. Citation2004). In another study, the birds treated with the same extracts had better growth during immunization in comparison with the non-treated vaccinated birds and had lower E. tenella oocyst countings after challenge (Guo et al. Citation2005).
Extracted lectin from the mushroom Fomitella fraxinea injected in 18-day-old embryos, protected broilers inoculated with E. acervulina at 1 week post-hatch against weight loss and was associated with a significant reduction in oocyst shedding compared to untreated embryos (Dalloul et al. Citation2006).
4.2.1.7. Oregano
The essential oils of Origanum vulgare are known for their antibacterial activity (Hammer et al. Citation1999) and effect against some parasites (Milhau et al. Citation1997). Furthermore, some essential oils of oregano, mainly carvacrol and thymol, have an anticoccidial effect against E. tenella although lower than lasalocid (Giannenas et al. Citation2003). However, in subsequently performed studies with diets supplemented with a mixture of the essential oils, oregano, thymol, eugenol, curcumin and piperin, a beneficial effect of these oils on a coccidiosis vaccination was not found (Oviedo-Rondón et al. Citation2006).
4.2.1.8. Turmeric (Curcuma longa)
Turmeric is a spice and colourant made from the rhizomes of the plant C. longa, a leafy plant belonging to the ginger family. Its phenolic compound curcumin has been shown to have antioxidative, anti-inflammatory and antitumour properties (Mukhopadhyay et al. Citation1982; Conney et al. Citation1991; Ammon et al. Citation1993; Brouet and Ohshima Citation1995).
Eimeria maxima-infected chicks fed with diets supplemented with 1% curcumin showed an improved weight gain and a reduction in the lesion scores and oocyst excretion. Nevertheless, the activity was only shown against E. maxima and not against E. tenella. A significant reduction of plasma and
concentrations was only found in E. maxima-infected and curcumin-treated birds and provide a possible explanation for the difference in anticoccidial activity found for both Eimeria spp. (Allen et al. Citation1998). A similar effect on lesion scores, oocyst shedding, growth, and plasma
and
concentrations was found for γ-tocopherol. The antioxidative properties of curcumin by inhibiting NOS induction by macrophages stimulated with lipopolysaccharide and interferon-γ has been shown previously (Brouet and Ohshima Citation1995). Although NO is an important defence mechanism against the invasion of different Apicomplexa parasites (Adams et al. Citation1990; Mellouk et al. Citation1991), it was suggested more recently that NO might itself promote the development of coccidial lesions (Allen 1997a, 1997b).
4.2.1.9. Incidental reports on the anticoccidial activity of other herb(s) extracts
The anticoccidial effect of a number of herbs and extracts has been documented in single manuscripts and summarized in for convenience. Although anticoccidial activity was reported, to our knowledge, none of these studies was repeated and has not led to the large-scale application of any of these compounds in practice.
4.2.2. Pre- and probiotics
The term prebiotic was introduced by Gibson and Roberfroid (Citation1995), who defined it as ‘a non-digestible food ingredient that beneficially affects the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon, and thus improves the host's health. The positive influence of prebiotics on the intestinal flora has been confirmed by a number of studies (Van Loo et al. Citation1999). Recently, the definition of the prebiotics was narrowed with the introduction of a prebiotic index by Roberfroid (2005), who stated that a preparation might be called prebiotic if it is capable to produce at least 4 × 108 colony-forming units of Bifidobacteria/gram faeces per daily dose (gram) ingested. Only three large groups meet this criterion: inulin and oliogofructose, galactose oligosaccharides and xylooligosaccharides.
Probiotics consist of beneficial live bacteria or yeasts supplemented to the diet. According to the Food and Agriculture Organization (FAO) and WHO probiotics are ‘live microorganisms, which when administrated in adequate amounts confer a health benefit on the host’ (FAO/WHO Citation2002). The most frequently used probiotics in humans are species of the genera Lactobacillus and Bifidobacteria, whereas species of Bacillus, Enterococcus and Saccharomyces yeasts have been the most commonly used organisms in livestock. In poultry production, probiotics are known for their capacity to restore the intestinal microflora after being disrupted by antibiotic treatment or enteric infections (Rada and Rychly Citation1995; Line et al. Citation1998; Pascual et al. Citation1999). They are also known for their ability to boost the immune system and used against allergies and other immune diseases (Zulkifli et al. Citation2000; Dalloul et al. Citation2003b; Kabir et al. Citation2004; Koenen et al. Citation2004).
A treatment with prebiotics can be easily combined with a probiotic in a so-called ‘synbiotic’ approach (Gibson and Roberfroid Citation1995). An advantage of this combination is the improved survival of probiotics when given in a medium of prebiotics. The use of pre- and probiotics in poultry has been reviewed by Patterson and Burkholder (Citation2003).
4.2.2.1. Prebiotics
Mannan oligosaccharides (MOS) are derived from the cell wall of the yeast Saccharomyces cerevisiae and are widely used in animal feed to promote gastrointestinal health and performance. MOS have been described as a prebiotic, but are thought to block the binding of pathogens to mannan receptors on the mucosal surface and stimulate the immune response (Spring et al. 2000).
In an experiment performed with coccidia, dietary MOS (1 g/kg feed) were able to reduce the severity of a single E. tenella infection with 3500 or 5000 sporulated oocysts (Elmusharaf et al. Citation2006). In another experiment, a dietary supplementation of MOS at a concentration of 10 g/kg feed reduced the oocyst excretion and diminished the severity of E. acervulina lesions in birds infected orally with a mixture of E. acervulina, E. maxima and E. tenella at subclinical doses of 900, 570 and 170 sporulated oocysts, respectively (Elmusharaf et al. Citation2007).
In contrast, in a study performed by McCann et al. (Citation2006) supplementation of MOS (0.5 g/kg feed) or tannin (0.5 g/kg feed) either individually or in combination did not reduce the severity of a mixed coccidiosis infection of E. acervulina, E. maxima and E. tenella given orally at clinical doses of 50,000, 15,000 and 15,000 sporulated oocysts, respectively. These contradictory results are possibly explained by the differences in MOS concentrations in feed and the magnitude of Eimeria spp. inoculation doses.
4.2.2.2. Probiotics
Lower intestinal invasion, development of coccidia and oocyst production, explained by enhanced local cell-mediated immunity, were observed with a Lactobacillus-based probiotic supplemented diet in E. acervulina-infected broilers (Dalloul et al. 2003a, 2003b, 2005).
More recently, in a study performed with a Pediococcus-based commercial probiotic (MitoGrow®) given to birds infected with either E. acervulina or E. tenella, increased resistance of birds against coccidiosis and a partial protection against growth retardation were demonstrated (Lee et al. Citation2007a). In another study, performed with a Pediococcus- and Saccharomyces-based probiotic (MitoMax®), less E. acervulina and E. tenella oocyst shedding and a better antibody response were found (Lee et al. Citation2007b).
5. Conclusions and perspectives
To date, coccidiosis control has relied mainly on chemoprophylaxis. However, the occurrence of resistance, consumer concerns and the increasing regulations as well as possible upcoming bans on the use of anticoccidial drugs as feed additives, have prompted the quest for alternative control strategies, amongst which vaccines, phytotherapy, aromatherapy, pre- and probiotics have been quite extensively studied. So far, live vaccines have proved to be the most solid and successful anticoccidiosis alternative approach. Although a number of drawbacks are associated with the production and use of live coccidiosis vaccines, their efficacy and ability to increase the sensitivity of Eimeria spp. isolates to anticoccidial drugs, have further stimulated their use. If the immunogenicity of subunit vaccines can be improved, they could represent the next generation of highly efficient and low-cost anticoccidial strategies.
References
- Adams , LB , Hibbs , JB , Taintor , RR and Krahenbuhl , JL . 1990 . Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii: role for synthesis of inorganic nitrogen oxides from L-arginine . J Immunol , 14 : 2725 – 2729 .
- Allen , PC . 1997a . Nitric oxide production during Eimeria tenella infections in chickens . Poult Sci , 76 : 810 – 813 .
- Allen , PC . 1997b . Production of free radical species during Eimeria maxima infections in chickens . Poult Sci , 78 : 1506 – 1509 .
- Allen , PC . 2003 . Dietary supplementation with Echinacea and development of immunity to challenge infection with coccidia . Parasitol Res , 91 : 74 – 78 .
- Allen , PC , Danforth , HD and Augustine , PC . 1998 . Dietary modulation of avian coccidiosis . Int J Parasitol , 28 : 1121 – 1140 .
- Allen , PC and Fetterer , RH . 2002 . Recent advances in biology and immunobiology of Eimeria species and in diagnosis and control of infection with these coccidian parasites in poultry . Clin Microbiol Rev , 15 : 58 – 65 .
- Allen , PC , Lydin , J and Danforth , HD . 1997 . Effects of components of Artemisia annua on coccidia infections in chickens . Poult Sci , 76 : 1156 – 1163 .
- Ammon , HPT , Safayhi , H , Mack , T and Sabieraj , J . 1993 . Mechanism of anti-inflammatory of curcumine and boswellic acids . J Ethnopharmacol , 38 : 113 – 119 .
- Arab , HA , Rahbari , S , Rassouli , A , Moslemi , MH and Khosravirad , F . 2006 . Determination of artemisinin in Artemisia sieberi and anticoccidial effects of the plant extracts in broiler chickens . Trop Anim Health Prod , 38 : 497 – 503 .
- Augustine , PC and Danforth , HD . 1999 . Influence of betaine and salinomycin on intestinal absorption of methionine and glucose and on the ultrastructure of intestinal cells and parasite development stages in chicks infected with E. acervulina . Avian Dis , 43 : 89 – 97 .
- Augustine , PC , McNaughton , JL , Virtanen , E and Rosi , L . 1997 . Effect of betaine on the growth performance of chicks inoculated with mixed cultures of Avian Eimeria species and on invasion and development of Eimeria tenella and Eimeria acervulina in vivo and in vitro . Poult Sci , 79 : 802 – 809 .
- Beach , JR and Corl , JC . 1925 . Studies in the control of avian coccidiosis . Poult Sci , IV : 83 – 93 .
- Berger , J , Rachlin , AI , Scott , EE , Sternbach , LH and Goldberg , MW . 1951 . The isolation of three new crystalline antibiotics from Streptomyces . J Am Chem Soc , 73 : 5295 – 5298 .
- Bhogal , BS , Miller , GA , Anderson , AC , Jessee , SJ , Strausberg , S and McCandliss , R . 1992 . Potential of a recombinant antigen as a prophylactic vaccine for day-old broiler chickens against Eimeria acervulina and Eimeria tenella infections . Vet Immunol Immunopathol , 31 : 323 – 335 .
- Borchers , AT , Keen , CL and Gershwin , ME . 2004 . Mushroom, tumors, and immunity: an update . Exp Biol Med , 229 : 393 – 406 .
- Brother , VM , Kuhn , I , Paul , LS , Gabe , JD , Andrew , WH , Sias , SR , McCaman , MT , Dragon , EA and Files , JG . 1988 . Characterisation of a surface antigen of Eimeria tenella sporozoites and synthesis from a cloned cDNA in Escherichia coli . Mol Biochem Parasitol , 28 : 235 – 247 .
- Brouet , I and Ohshima , H . 1995 . Curcumin, an anti-tumour promoter and anti-inflammatory agent, inhibits induction of nitric oxide synthase in activated macrophages . Biochem Biophys Res Commun , 206 : 533 – 540 .
- Burger , RA , Torres , AR , Warren , RP , Caldwell , VD and Highs , BG . 1997 . Echinacea-induced cytokine production by human macrophages . Int J Immunopharmacol , 19 : 371 – 379 .
- Challey , JR and Jeffers , TK . 1973 . Synergism between 4-hydroxyquinoline and pyridone coccidiostats . J Parasitol , 59 : 502 – 504 .
- Chapman , HD . 1978 . Drug resistance in coccidia , Paper presented at 13th Poultry Science Symposium. . Proceedings of the 13th Poultry Science Symposium; Edinburgh, UK, p. 387–412
- Chapman , HD . 1982 . “ Anticoccidial drug resistance ” . In The biology of the coccidia , Edited by: Long , PL . 430 – 451 . Baltimore, MD : Baltimore University Park Press . Chapter 2
- Chapman , HD . 1984 . Drug resistance in avian coccidia (review) . Vet Parasitol , 15 : 11 – 27 .
- Chapman , HD . 1994 . Sensitivity of field isolates of Eimeria to monensin following the use of a coccidiosis vaccine in broiler chickens . Poult Sci , 73 : 476 – 478 .
- Chapman , HD . 1996 . Restoration of drug sensitivity following the use of live coccidiosis vaccines , World Poultry, Special Supplement Coccidiosis 2. . Amsterdam (The Netherlands): Elsevier. p. 20–21
- Chapman , HD . 1997 . Biochemical, genetic and applied aspects of drug resistance in Eimeria parasites of the fowl . Avian Pathol , 26 : 221 – 244 .
- Chapman , HD . 1998 . Evaluation of the efficacy of anticoccidial drugs against Eimeria species in the fowl . Int J Parasitol , 28 : 1141 – 1144 .
- Chapman , HD . 1999a . The development of immunity to Eimeria species in broilers given anticoccidial drugs . Avian Pathol , 28 : 155 – 162 .
- Chapman , HD . 1999b . Anticoccidial drugs and their effects upon the development of immunity to Eimeria infections in poultry . Avian Pathol , 28 : 521 – 535 .
- Chapman , HD . 2000 . Practical use of vaccines for the control of coccidiosis in the chicken . World's Poult Sci J , 56 : 7 – 20 .
- Chapman , HD . 2003 . Origins of coccidiosis research in the fowl – the first fifty years . Avian Dis , 47 : 1 – 20 .
- Chapman , HD , Cherry , TE , Danforth , HD , Richards , G , Shirley , MW and Williams , RB . 2002 . Sustainable coccidiosis control in poultry production: the role of live vaccines . Int J Parasitol , 32 : 617 – 629 .
- Chapman , HD and Hacker , AB . 1993 . The effects of shuttle programs upon the growth of broilers and the development of immunity to Eimeria species . Poult Sci , 72 : 658 – 663 .
- Christaki , E , Florou-Paneri , P , Giannenas , I , Papazahariadou , M , Botsoglou , NA and Spais , AB . 2004 . Effect of a mixture of herbal extracts on broiler chickens with Eimeria tenella . Anim Res , 53 : 137 – 144 .
- Chroustova , E and Pinka , K . 1987 . The efficacy of disinfectants on the oocysts of Eimeria tenella . Acta Vet BRNO , 56 : 141 – 149 .
- COM 2008 . COM/2008/0233. Report from the Commission to the Council and the European Parliament on the use of coccidiostats and histomonostats as feed additives, submitted pursuant to article 11 of regulation (EC) no 1831/2003 of the European parliament and the Council of 22 September 2003 on additives for the use in animal nutrition. Available from: http://www.ipex.eu/ipex/cms/home/Ducuments/dossier_COM20080233 (accessed 23 December 2009)
- Conney , AH , Lysz , T , Ferraro , T , Abidi , TF , Manchand , PS , Laskin , JD and Huang , MT . 1991 . Inhibitory effect of curcumin and some related dietary compounds on tumour promotion and arachidonic metabolism in mouse skin . Adv Enzyme Regul , 31 : 385 – 396 .
- Conway , DP and Mckenzie , ME . 2007 . Poultry coccidiosis: diagnostic and testing procedures, , 3rd , Ames, IA : Blackwell Publishing Professional .
- Crane , MSJ , Goggin , B , Pellegrino , RM , Ravino , OJ , Lange , C , Karkhanis , YD , Kirk , KE and Chakraborty , PR . 1991 . Crossprotection against four species of chicken coccidia with a single recombinant antigen . Infect Immun , 59 : 1271 – 1277 .
- Currier , NL and Miller , SC . 2001 . Echinacea purpurea and melatonin augment natural-killer cells in leukemic mice and prolong life span . J Altern Complement Med , 7 : 241 – 251 .
- Dalloul , RA and Lillehoj , HS . 2006 . Poultry coccidiosis: recent advancements in control measures and vaccine development . Expert Rev Vaccines , 5 : 143 – 163 .
- Dalloul , RA , Lillehoj , HS , Lee , JS , Lee , SH and Chung , KS . 2006 . Immunopotentiating effect of a Formitella fraxinea-derived lectin on chicken immunity and resistance to coccidiosis . Poult Sci , 85 : 446 – 451 .
- Dalloul , RA , Lillehoj , HS , Shellum , TA and Doerr , JA . 2003a . Intestinal immunomodulation by vitamin A deficiency and lactobacillus-based probiotic in Eimeria acervulina infected broiler chickens . Avian Dis , 47 : 1313 – 1320 .
- Dalloul , RA , Lillehoj , HS , Shellum , TA and Doerr , JA . 2003b . Enhanced mucosal immunity against Eimeria acervulina in broilers fed a Lactobacillus-based probiotic . Poult Sci , 82 : 62 – 66 .
- Dalloul , RA , Lillehoj , HS , Shellum , TA and Doerr , JA . 2005 . Induction of local protective immunity to Eimeria acervulina by Lactobacillus-based probiotic . Comp Immunol Microbiol Infect Dis , 28 : 351 – 361 .
- Daugschies , A , Böse , R , Marx , J , Teich , K and Freidhoff , KT . 2002 . Development and application of a standardized assay for chemical disinfection of coccidia oocysts . Vet Parasitol , 103 : 299 – 308 .
- Dougherty , HW . 1974 . Inhibition of mitochondrial energy transduction of carbanilides . Fed Proc , 33 : 1657
- Dowling , L . 1992 . Ionophore toxicity in chickens: a review of pathology and diagnosis . Avian Pathol , 21 : 355 – 368 .
- Du , A and Hu , S . 2004 . Effects of a herbal complex against Eimeria tenella infection in chickens . J Vet Med B Infect Dis Vet Public Health , 51 : 194 – 197 .
- Dutta , GN and Devriese , LA . 1984 . Observations on the in vitro sensitivity of Gram-positive intestinal bacteria of farm animals to growth promoting antibacterials . J Appl Bacteriol , 56 : 117 – 123 .
- Edgar , SA . 1955 . Sporulation of oocysts at specific temperatures and notes on the prepatent period of several species of avian coccidia . J Parasitol , 41 : 214 – 216 .
- Edgar , SA and King , DE . 1952. Breeding and immunizing chickens for resistance to coccidiosis. 62nd and 63rd Annu rep Alabama Agr Exp Stat. p. 36–37
- Elmusharaf , MA , Bautista , V , Nollet , L and Beynen , AC . 2006 . Effect of a mannaoligosaccharide preparation on Eimeria tenella infection in broiler chickens . Int J Poult Sci , 5 : 583 – 588 .
- Elmusharaf , MA , Peek , HW , Nollet , L and Beynen , AC . 2007 . The effect of and in-feed mannaoligosaccharide preparation (MOS) on a coccidiosis infection in broilers . Anim Feed Sci Technol , 134 : 347 – 354 .
- Elwinger , K , Schneitz , C , Berndtson , E , Fossum , O , Teglöf , B and Engström , B . 1992 . Factors affecting the incidence of necrotic enteritis, ceacal carriage of Clostridium perfringes and bird performance in broiler chickens . Acta Vet Scand , 33 : 369 – 378 .
- FAO/WHO. 2002. Guidelines for the evaluation of probiotics in food. Report of a joint FAO/WHO working group on drafting guidelines for the evaluation of probiotics in food. Available from: http://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf
- Fetterer , RH , Augustine , PC , Allen , PC and Barfield , RC . 2003 . The effect of dietary betaine on intestinal and plasma levels of betaine in uninfected and coccidia-infected broiler chicks . Parasitol Res , 90 : 343 – 348 .
- Finger , A and Michael , A . 2005. Maternal protection against Eimeria challenge of CoxAbic® vaccinated chickens. Proceedings of the IXth International Coccidiosis Conference; 2005 Sep 19–23; Foz do Iguassu, Brasil. p. 146
- Ford , AM , Fagerberg , DJ , Quarles , CL , George , BA and McKinley , GA . 1981 . Influence of salinomycin on incidence, shedding, and antimicrobial resistance of Salmonella typhimurium in experimentally infected broiler chicks . Poult Sci , 60 : 2442 – 2453 .
- George , BA , Ford , AM , Fagerberg , DJ and Quarles , CL . 1982 . Influence of salinomycin on antimicrobial resistance of coliforms and streptococci from broiler chickens . Poult Sci , 61 : 1842 – 1852 .
- Giannenas , I , Florou-Paneri , P , Papazahariadou , M , Christaki , E , Botsoglou , A and Spais , AB . 2003 . Effect of dietary supplementation with oregano essential oil on performance of broilers after experimental infection with E. tenella . Arch Anim Nutr , 57 : 99 – 106 .
- Gibson , GR and Roberfroid , M . 1995 . Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics . J Nutr , 125 : 1401 – 1412 .
- Goel , V , Chang , C , Slama , JV , Barton , R , Bauer , R , Gahler , R and Basu , TK . 2002 . Alkylimides of Echinacea purpurea stimulate alveolar macrophage function in normal rats . Int Immunopharmacol , 2–3 : 381 – 387 .
- Graat , EAM , Henken , AM , Ploeger , HW , Noordhuizen , JPTM and Vertommen , MH . 1994 . Rate and course of sporulation of oocysts of Eimeria acervulina under different environmental conditions . Parasitology , 108 : 497 – 502 .
- Greif , G . 2000 . Immunity to coccidiosis after treatment with toltrazuril . Parasitol Res , 86 : 787 – 790 .
- Greif , G and Haberkorn , A . 1997 . Enhancement of immunity and protection against coccidiosis during therapeutic medication with toltrazuril , Paper presented at Control of Coccidiosis into the Next Millennium. . Proceedings of the VIIth International Coccidiosis Conference; 1997 Sep 1–5; Oxford (UK): Keble, p. 42
- Greif , G , Harder , A and Haberkorn , A . 2001 . Chemotherapeutic approaches to protozoa: coccidiae – current level of knowledge and outlook . Parasitol Res , 87 : 973 – 975 .
- Gumarães Jr , JS , Bogado , ALG , da Cunha , TCB and Garcia , JL . 2007 . In vitro evaluation of the disinfection efficacy on Eimeria tenella unsporulated oocysts isolated from broilers . Rev Bras Parasitol Vet , 16 : 67 – 71 .
- Guo , FC , Kwakkel , RP , Williams , BA , Paramentier , HK , Li , WK , Yang , ZQ and Verstegen , MW . 2004 . Effects of mushroom and herb polysaccharides on cellular and humoral immune responses of E. tenella-infected chickens . Poult Sci , 83 : 1124 – 1132 .
- Guo , FC , Kwakkel , RP , Williams , BA , Suo , X , Li , WK and Verstegen , MW . 2005 . Coccidiosis immunization: effects of mushroom and herb polysaccharides on immune responses of chickens with Eimeria tenella . Avian Dis , 4 : 70 – 73 .
- Hackstein , JHP , Mackenstedt , U , Mehlhorn , H , Meijerink , JPP , Schubert , H and Leunissen , JAM . 1995 . Parasitic apicomplexans harbor a chlorophyll a-D1 complex, the potential target for therapeutic triazines . Parasitol Res , 81 : 207 – 216 .
- Hamet , N . 1986 . Resistance to anticoccidial drugs in poultry farms in France from 1975 to 1984 , Paper presented at Research in Avian Coccidiosis. . Proceedings of the Georgia Coccidiosis Conference; 1985 Nov 18–20; Athens, GA, USA
- Hammer , KA , Carson , CF and Riley , TV . 1999 . Antimicrobial activity of essential oils and other plant extracts . J Appl Microbiol , 86 : 985 – 990 .
- Harder , A and Haberkorn , A . 1989 . Possible mode of action of toltrazuril: studies on two Eimeria species and mammalian and Ascaris suum enzymes . Parasitol Res , 76 : 8 – 12 .
- Hayat , B , Jabeen , F , Hayat , CS and Akhtar , M . 1996 . Comparative prophylactic effects of salinomycin and some indigenous preparations against coccidiosis in broiler chicks . Pakistan Vet J , 16 : 164 – 167 .
- Houdek , Z , Hortvikova , M and Cernik , F . 2002 . Efficacy of Neopredisan® 135-1 on the viability of chicken coccidian oocysts , Paper presented at Czech Section Soc Protozool. . Proceedings of the 32nd Annual Meeting
- James , S . 1980 . Thiamine uptake in isolated schizonts of Eimeria tenella and the inhibitory effects of amprolium . Parasitology , 80 : 313 – 322 .
- Jang , IJ , Jun , M , Lillehoj , HS , Dalloul , RA , Kong , IK , Kim , S and Min , W . 2007 . Anticoccidial effect of green tea-based diets against Eimeria maxima . Vet Parasitol , 144 : 172 – 175 .
- Jeffers , TK . 1974a . Eimeria tenella. Incidence and anticoccidial drug resistance of isolants in major broiler producing areas . Avian Dis , 18 : 74 – 84 .
- Jeffers , TK . 1974b . Eimeria acervulina and E. maxima. Incidence and anticoccidial drug resistance of isolants in major broiler producing areas . Avian Dis , 18 : 331 – 342 .
- Jeffers , TK . 1975 . Attenuation of Eimeria tenella through selection for precociousness . J Parasitol , 61 : 1083 – 1090 .
- Jeffers , TK . 1976 . Reduction of anticoccidial drug resistance by massive introduction of drug-sensitive coccidia . Avian Dis , 20 : 649 – 653 .
- Jeffers , TK . 1986 . Attenuation of coccidia-a review , Paper presented at Research in Avian Coccidiosis. . Proceedings of the Georgia Coccidiosis Conference; 1985 Nov 18–20; Athens, GA, USA
- Jeffers , TK . 1989 . Anticoccidial drug resistance: a review with emphasis on the polyether ionophores , Paper presented at Coccidia and Intestinal Coccidiomorphs. . Proceedings of the Vth International Coccidiosis Conference; 1989 October 17–20; Tours, France. p. 295–308
- Jenkins , MC . 1998 . Progress of developing a recombinant coccidiosis vaccine . Int J Parasitol , 28 : 1111 – 1119 .
- Jenkins , MC . 2001 . Advances and prospects for subunit vaccines against protozoa of veterinary importance . Vet Parasitol , 101 : 291 – 310 .
- Jenkins , MC , Danforth , HD , Lillehoj , HS and Fetterer , RH . 1989 . cDNA encoding an immunogenic region of a 22 kilodalton surface protein of Eimeria acervulina sporozoites . Mol Biochem Parasitol , 32 : 153 – 161 .
- Jenkins , MC , Lillehoj , HS , Barta , JR , Danforth , HD and Strohlein , DA . 1990 . Eimeria acervulina: cloning of a cDNA encoding an immunogenic region of several related merozoite surface and rhoptry proteins . Exp Parasitol , 70 : 353 – 362 .
- Jeston , PJ , Blight , GW , Anderson , GR , Molloy , JB and Jorgensen , WK . 2002 . Comparison of infectivity of Eimeria tenella oocysts maintained at 4, 12 or 28°C up to 10 months . Aust Vet J , 80 : 91 – 92 .
- Joyner , LP and Norton , CC . 1973 . The immunity arising from continuous low-level infections with Eimeria tenella . Parasitology , 67 : 907 – 913 .
- Joyner , LP and Norton , CC . 1976 . The immunity arising from continuous low-level infections with Eimeria maxima and Eimeria acervulina . Parasitology , 72 : 271 – 278 .
- Kabir , SML , Rahman , MM , Rahman , MB , Rahman , MM and Ahmed , SU . 2004 . The dynamics of probiotics on growth performance and immune response in broilers . Int J Poult Sci , 3 : 361 – 364 .
- Kendall , SB and Joyner , LP . 1956 . The potentiation of coccidiostatic drugs by Pyrimethamine . Vet. Rec , 68 : 119 – 121 .
- Kheysin , YM . 1972 . Life cycles of coccidia of domestic animals , London : William Heinerman Medical Books Ltd .
- Klasing , KC , Adler , KL , Remus , JC and Calvert , CC . 2002 . Dietary betaine increases intraepithelial lymphocytes in the duodenum of coccidial-infected chicks and increases functional properties of phagocytes . J Nutr , 132 : 2274 – 2282 .
- Kleynen , J , Knipschild , P and Ter Riet , G . 1991 . Clinical trails of homeopathy . Br Med J , 302 : 316 – 323 .
- Koenen , ME , Kramer , J , van der Hulst , R , Heres , L , Jeurissen , SHM and Boersma , WJA . 2004 . Immunomodulation by probiotic lactobacilli in layer and meat-type chickens . Br Poult Sci , 45 : 355 – 366 .
- Kunin , CM and Rudy , J . 1991 . Effect of NaCl induced osmotic stress on intracellular concentrations of glycine betaine and potassium in Escherichia coli, Enterococcus faecalis and staphylococci . J Lab Clin Med , 118 : 217 – 224 .
- Lee , SH , Lillehoj , HS , Dalloul , RA , Park , DW , Hong , YH and Lin , JJ . 2007a . Influence of Pediococcus-based probiotic on coccidiosis in broiler chickens . Poult Sci , 86 : 63 – 66 .
- Lee , SH , Lillehoj , HS , Park , DW , Hong , YH and Lin , JJ . 2007b . Effects of Pediococcus-based probiotic (MitoMax®) on coccidiosis in broiler chickens . Comp Immunol Microbiol Infect Dis , 30 : 261 – 268 .
- Linde , K , Jonas , WB , Melchart , D and Willich , S . 2001 . The methodological quality of randomized controlled trials of homeopathy, herbal medicines and acupuncture . Int J Epidemiol , 30 : 526 – 531 .
- Line , EJ , Bailey , SJ , Cox , NA , Stern , NJ and Tompkins , T . 1998 . Effect of yeast-supplemented feed on Salmonella and Campylobacter populations in broilers . Poult Sci , 77 : 405 – 410 .
- Litjens , JB . 1986 . The relationship between coccidiosis and the use of anticoccidials in broilers in the southern part of the Netherlands , Paper presented at Research in Avian Coccidiosis. . Proceedings of Georgia Coccidiosis Conference; 1985 Nov 18–20; Athens, GA. p. 442–448
- Long , PL and Jeffers , TK . 1982 . Studies on the stage of action of ionophorous antibiotics against Eimeria . J Parasitol , 68 : 363 – 371 .
- Long , PL , Johnson , J , McKenzie , ME , Perry , E , Crane , MS and Murray , PK . 1986 . Immunization of young broiler chickens with low level infections of Eimeria tenella, E. acervulina, or E. maxima . Avian Pathol , 15 : 271 – 278 .
- Mandal , SC , Sasma , NK and Ray , S . 1994 . Effect of IHP-250C (Zycox) on lesion scores of Eimeria necatrix infected chicks . Indian Vet J , 71 : 119 – 120 .
- Martel , A , Devriese , LA , Cauwerts , K , De Gussem , K , Decostere , A and Haesebrouck , F . 2004 . Susceptibility of Clostridium perfringens strains from broilers chickens to antibiotics and anticoccidials . Avian Pathol , 33 : 3 – 7 .
- Mathis , GF and Broussard , C . 2006 . Increased level of Eimeria sensitivity to diclazuril after using a live coccidial vaccine . Avian Dis , 50 : 321 – 326 .
- Mathis , GF , Froyman , R and Kennedy , T . 2004 . Coccidiosis control by administrating toltrazuril in the drinking water for a 2-day period . Vet Parasitol , 121 : 1 – 9 .
- Mathis , GF and McDougald , LR . 1989 . Restoration of drug sensitivity on turkey farms after introduction of sensitive coccidia during controlled exposure immunization , Paper presented at Coccidia and Intestinal Coccidiomorphs. . Proceedings of the Vth International Coccidiosis Conference; 1989 Oct 17–20; Tours, France, p. 17–20
- Matthews , JO , Ward , TL and Southern , LL . 1997 . Interactive effects of betaine and monensin in uninfected and Eimeria acervulina-infected chicks . Poult Sci , 76 : 1014 – 1019 .
- McCann , MEE , Newell , E , Preston , C and Forbes , K . 2006 . The use of Mannan-oligosaccharides and/or Tannin in broiler diets . Int J Poult Sci , 5 : 873 – 879 .
- McDonald , V and Shirley , MW . 1984 . Eimeria mitis: a comparison of the endogenous development stages of a line selected by early maturation of the parent strain . Parasitology , 88 : 37 – 44 .
- McDonald , V , Shirley , M and Millard , B . 1986 . A comparative study of two lines of Eimeria tenella attenuated either by selection for precocious development in the chicken or by growth in chicken embryos . Avian Pathol , 15 : 323 – 335 .
- McDougald , LR . 2003 . Coccidiosis , Diseases of Poultry. . 12th ed. Chapter 28, Ames (IA): Iowa State University Press. p. 1076–1085
- McDougald , LR , Da Silva , JM , Solis , J and Braga , M . 1987 . A survey of sensitivity to anticoccidial drugs in 60 isolates of coccidia from broiler chickens in Brazil and Argentina . Avian Dis , 31 : 287 – 292 .
- McDougald , LR , Fuller , AL and Solis , J . 1986 . Drug sensitivity of 99 isolates of coccidia from broiler farms . Avian Dis , 30 : 690 – 694 .
- McDougald , LR and Shirley , MW . 2009 . Past and future: vaccination against Eimeria . Parasitology , 136 : 1477 – 1489 .
- Mehlhorn , H , Ortmann-Falkenstein , A and Haberkorn , A . 1984 . The effects of symmetrical triazinetrione on developmental stages of Eimeria tenella and E. acervulina: a light and electron microscopical study . Z Parasitenkd , 70 : 173 – 182 .
- Mehlhorn , H , Schmahl , G and Haberkorn , A . 1988 . Toltrazuril effective against a broad spectrum of protozoan parasites . Parasitol Res , 75 : 64 – 66 .
- Mellouk , S , Green , SJ , Nacy , CA and Hoffmann , SL . 1991 . IFN-gamma inhibits development of Plasmodium berghei exoerythrocytic stages in hepatocytes by an L-arginine dependent effector mechanism . J Immunol , 146 : 3971 – 1976 .
- Michael , A . 2003 . The practical use of a maternal vaccine against coccidiosis , World Poultry 19. . Amsterdam (The Netherlands): Elsevier. p. 24–26
- Michael , A . 2007 . Maternal vaccination against coccidiosis is an option , World Poultry 23. . Amsterdam (The Netherlands): Elsevier. p. 36–37
- Milhau , G , Valentin , A , Benoit , F , Mallie , M , Bastide , JM , Pelissier , Y and Bessiere , JN . 1997 . In vitro antimalarial activity of eight essential oils . J Essent Oil Res , 9 : 329 – 333 .
- Miller , GA , Bhogal , BS , McCandliss , R , Strausberg , RL , Jessee , EJ , Anderson , AC , Fuchs , CK , Nagle , J , Likel , MH and Strasser , JM . 1989 . Characterization and vaccine potential of a novel recombinant coccidial antigen . Infect Immunol , 57 : 2014 – 2020 .
- Mukhopadhyay , A , Basu , N , Ghatak , N and Gujral , PK . 1982 . Anti-inflammatory and irritant activities of curcumin analogues in rats . Agents Actions , 12 : 508 – 515 .
- Newman , LJ and Danforth , HD . 2000 . Improved sensitivity of field oocyst populations to coccidiostat following vaccination with live oocyst vaccine . Poult Sci , 79 ( Suppl. 1 ) : 4
- Oh , HG , Youn , HG , Noh , HJ , Jang , JW and Kang , YB . 1995 . Anticoccidial effects of artemisin on the Eimeria tenella . Korean J Vet Res , 35 : 123 – 130 .
- Oviedo-Rondón , EO , Clemente-Hernández , S , Salvador , F , Williams , P , Losa , R and Stephan , F . 2006 . Essential oils on mixed coccidia vaccination and infection in broilers . Int J Poult Sci , 5 : 723 – 730 .
- Pascual , M , Hugas , M , Badiola , JI , Monfort , JM and Garriga , M . 1999 . Lactobacillus salivarius CTC2197 prevents Salmonella enteritidis colonization in chickens . Appl Environ Microbiol , 65 : 4981 – 4986 .
- Patterson , JA and Burkholder , KM . 2003 . Application of prebiotics and probiotics in poultry production . Poult Sci , 82 : 627 – 631 .
- Peek , HW and Landman , WJM . 2003 . Resistance to anticoccidial drugs of Dutch avian Eimeria spp. field isolates originating from 1996, 1999 and 2001 . Avian Pathol , 32 : 391 – 401 .
- Peek , HW and Landman , WJM . 2004 . Gevoeligheidsprofielen van Spaanse, Duitse en Nederlandse Eimeria spp. veldisolaten voor anticoccidiose middelen . Tijdschr Diergeneeskd , 129 : 210 – 214 .
- Peek , HW and Landman , WJM . 2006 . Higher incidence of Eimeria spp. field isolates sensitive for diclazuril and monensin associated with the use of live coccidiosis vaccinating with Paracox™-5 in broiler farms . Avian Dis , 50 : 434 – 439 .
- Pittler , MH , Abbot , NC , Harkness , EF and Ernst , E . 2000 . Location bias in controlled trials of complementary/alternative therapies . J Clin Epidemiol , 53 : 485 – 489 .
- Rada , V and Rychly , I . 1995 . The effect of Lactobacillus salivarius administration on coliforms and enterococci in the crop and ceca of chicken broilers . Vet Med (Praha) , 40 : 311 – 315 .
- Reid , WM . 1973 . Anticoccidials: differences in day of peak against Eimeria tenella , Paper presented at Symposium Coccidia and Related Organisms. . Proceedings of the Symposium Coccidia and related Organism; 1973; Guelph, Ontario, Canada, p. 119–134
- Reid , WM . 1975 . Progress in the control of coccidiosis with anticoccidials and planned immunization . Am J Vet Res , 36 : 593 – 596 .
- Roberfroid , MR . 2005 . Introducing inulin-type fructans . Br J Nutr , 93 ( Suppl. 1 ) : 513 – 525 .
- Rogers , EF , Clark , RL , Becker , HJ , Pessolano , AA , Leanza , WJ , McManus , EC , Andriuli , FL and Cuckler , AC . 1964 . Antiparasitic drugs V. Anticoccidial activity of 4-amino-2-theoxybenzoic acid and related compounds . Proc. Soc Exp Biol Med , 117 : 488 – 492 .
- Rose , M and Long , P . 1962 . Immunity to four species of Eimeria in fowls . Immunology , 5 : 79 – 92 .
- Rudolph , AS , Crowe , JH and Crowe , LM . 1986 . Effects of three stabilizing agents – praline, betaine and trehalose – on membrane phospholipids . Arch Biochem Biophys , 245 : 134 – 143 .
- Ryley , JF . 1980 . Drug resistance in coccidia . Adv Vet Sci Comp Med , 24 : 99 – 120 .
- Ryley , JF and Betts , MJ . 1973 . Chemotherapy of chicken coccidiosis . Adv Pharmacol Chemother , II : 221 – 293 .
- Saine , A . 2000 . Seminar homeopathy, the method: lectures on pure classical homeopathy II, part II: case handling , 52 Eindhoven, , The Netherlands : Lutra Services B.V .
- Schneider , D , Ayeni , AO and Dürr , U . 1979 . Sammelreferat: Zur physikalischen Resistenz der Kokzidienoocysten . Dtsch Tierärztl Wochenschr , 79 : 561 – 569 .
- See , DM , Broumand , N , Sahl , L and Tilles , JG . 1997 . In vitro effects of Echinacea and ginseng on natural killer and antibody-dependent cell cytoxicity in healthy subjects and chronic fatigue synchrome or acquired immunodeficiency patients . Immunopharmacology , 35 : 229 – 235 .
- Sharkey , DL . 1978 . Evaluation of coccidiosis in the presence of a mold inhibitor . Dissert Abstr Int , 39B : 2032
- Shimura , K and Isobe , T . 1994 . Pathogenicity and drug resistance of a recombinant line between a precocious line and a drug resistant field isolate of E. tenella , Paper presented at Asian Conference on Coccidiosis . Proceedings of the 2nd Asian Conference on Coccidiosis; 1994; Guangzhou, China, p. 119–123
- Shirley , MW . 1988 . Control of coccidiosis with vaccines . Post graduate committee in veterinary science. Proceedings of the 2nd Asian/Pacific Poultry Health Conference; 1988 Sep 23–25; University of Sydney . 1988 , Sydney, NSW. Australia p. 129–157
- Shirley , MW and Bedrnik , P . 1997 . Live attenuated vaccines against avian coccidiosis: success with precocious and egg-adapted lines of Eimeria . Parasitol Today , 13 : 481 – 484 .
- Shirley , M , Bushell , A , Bushell , J , McDonald , V and Roberts , B . 1995 . A live attenuated vaccine for the control of avian coccidiosis: trials in broiler breeders and replacements layer flocks in the United Kingdom . Vet Rec , 137 : 453 – 457 .
- Shirley , M and Millard , B . 1986 . Studies on the immunogenicity of seven attenuated lines of Eimeria given as a mixture to chickens . Avian Pathol , 15 : 629 – 638 .
- Shirley , MW , Smith , AL and Blake , DP . 2007 . Challenges in the successful control of the avian coccidia . Vaccine , 25 : 5540 – 5547 .
- Shirley , MW , Smith , AL and Tomley , FM . 2005 . The biology of avian Eimeria with an emphasis on their control by vaccination . Adv Parasitol , 60 : 285 – 330 .
- Shumard , RF and Callender , ME . 1967 . Monensin, a new biologically active compound. VI. Anticoccidial activity . Antimicrob Agents Chemother , 7 : 369 – 377 .
- Spring , P , Wenk , C , Dawson , KA and Newman , KE . 2000 . The effects of dietary mannanoligosaccharides on the cecal parameters and concentration of enteric bacteria in the ceca of salmonella-challenged broiler chicks . Poult Sci , 79 : 205 – 211 .
- Srinivasan , K . 2005 . Species as influences of body metabolism: an overview of three decades of research . Food Res Int , 38 : 77 – 86 .
- Stimple , M , Proksch , A , Wagner , H and Lohmann-Matthes , ML . 1984 . Macrophage activation and induction of macrophage cytoxicity by purified polysaccharides fractions from the plant Echinacea purpurea . Infect Immun , 46 : 845 – 849 .
- Stipkovits , L , Kobulej , T , Varga , Z and Juhasz , S . 1987 . In vitro testing of the anti-mycoplasma effect of some anti-coccidial drugs . Vet Microbiol , 15 : 65 – 70 .
- Sun , LZ , Currier , NL and Miller , SC . 1999 . The American corneflower: a prophylactic role involving nonspecific immunity . J Altern Complement Med , 5 : 437 – 446 .
- Tamasaukas , R , Ruiz , H , Theis , W and De Basillio , V . 1996 . Evaluation of the efficacy of Salstop and Digestor broilers (Citrate C): products derived from the seeds of citrus fruits for the control of avian coccidiosis: floor pen studies. (Esp.) . Parasitología al día , 20 : 118 – 124 .
- Tamasaukas , R , Ruiz , H , Theis , W and De Basillio , V . 1997 . Efficacy of a disinfectant composed by citric extracts in a floor-pen trail with broilers in Venezuela . Arch Latinoam Prod Anim , 5 ( Suppl. 1 ) : 612 – 615 .
- Tipu , MA , Pasha , TN and Ali , Z . 2002 . Comparative efficacy of salinomycin sodium and neem fruit (Azadirachta indica) as feed additive anticoccidials in broilers . Int J Poult Sci , 1 : 91 – 93 .
- Umemura , T , Nakamura , H , Goryo , M and Itakura , EA . 1984 . Histopathology of monensin-tiamulin myopathy in broiler chickens . Avian Pathol , 13 : 459 – 468 .
- Van Loo , J , Cummings , J , Delzenne , N , Englyst , H , Franck , A , Hopkins , M , Kok , N , Macfarlane , G , Newton , D Quigley , M . 1999 . Functional food properties of non-digestible oligosaccharides: a consensus report from the ENDO project (DGXII AIRII-CT94-1095) . Br J Nutr , 2 : 121 – 132 .
- Velkers , FC , Loo , AJH , Madin , F and van Eck , JHH . 2005 . Isopathic and pluralist homeopathic treatment of commercial broilers with experimentally induced colibacillosis . Res Vet Sci , 78 : 77 – 83 .
- Verheyen , A , Maes , L , Coussement , W , Vanparijs , O , Lauwers , F and Marsboom , R . 1989 . Ultrastructural evaluation of the effects of diclazuril on the endogenous stages of Eimeria maxima and E. brunetti in experimentally inoculated chickens . Parasitol Res , 75 : 604 – 610 .
- Verheyen , A , Maes , L , Coussement , W , Vanparijs , O , Lauwers , F , Vlaminckx , E , Borgers , M and Marsboom , R . 1988 . In vivo action of the anticoccidial diclazuril (Clinacox®) on the development stages of Eimeria tenella an ultrastructural evaluation . J Parasitol , 74 : 939 – 949 .
- Vermeulen , AN . 1998 . Progress in recombinant vaccine development against coccidiosis: a review and prospects into the next millennium . Int J Parasitol , 28 : 1121 – 1130 .
- Vermeulen , AN , Schaap , DC and Schetters , TP . 2001 . Control of coccidiosis in chickens by vaccination . Vet Parasitol , 100 : 13 – 20 .
- Vissiennon , T , Kröger , H , Khöler , T and Kliche , R . 2000 . Effect of avilamycin, tylosin, and ionophore anticoccidials on Clostridium perfringens entertoxaemia in chickens . Berl Münch Tierärztl Wochenschr , 113 : 9 – 13 .
- Von Wendt , M , Büsing , S and Bollwahn , W . 1997 . Zur Toxizität der Kombination van Salinomycin and Tiamutilin beim Schwein . Dtsch Tierärtzl Wochenschr , 104 : 405 – 410 .
- Waldenstedt , L , Elwinger , K , Lunden , A , Thebo , P and Uggla , A . 1999 . Effect of betaine supplement on broiler performance during an experimental coccidial infection . Poult Sci , 78 : 182 – 189 .
- Waldenstedt , L , Elwinger , K , Lunden , A , Thebo , P and Uggla , A . 2001 . Sporulation of Eimeria maxima oocysts in litter with different moisture contents . Poult Sci , 80 : 1412 – 1415 .
- Wallach , M . 1997 . The importance of transmission-blocking immunity in the control of infections by apicomplexa parasites . Int J Parasitol , 27 : 1159 – 1167 .
- Wallach , M , Smith , NC , Petracca , M , Miller , CM , Eckert , J and Braun , R . 1995 . Eimeria maxima gametocyte antigens: potential use in a sub-unit maternal vaccine against coccidiosis in chickens . Vaccine , 13 : 347 – 354 .
- Wang , CC . 1975 . Studies of the mitchondria from Eimeria tenella and inhibition of the electron transport by quinolone coccidiostats . Biochim Biophys Acta , 396 : 210 – 219 .
- Wang , CC . 1982 . “ Biochemistry and physiology of coccidia ” . In The biology of the coccidia , Edited by: Long , PL . 167 – 228 . Baltimore, MD : Baltimore University Park Press .
- Williams , RB . 1992 . The development, efficacy and epidemiological aspects of Paracox®, a new coccidiosis vaccine for chickens , Harefield, , Europe : Pittman-Moore .
- Williams , RB . 1995 . Epidemiological studies of coccidiosis in the domesticated fowl (Gallus gallus): II. Physical condition and survival of Eimeria acervulina oocysts in poultry-house litter . Appl Parasitol , 36 : 90 – 96 .
- Williams , RB . 1996 . The epidemiology of coccidiosis of chickens . World Poultry, Special Supplement Coccidiosis , 2 : 9 – 11 .
- Williams , RB . 1998 . Epidemiological aspects of the use of live anticoccidial vaccines for chickens . Int J Parasitol , 28 : 1089 – 1098 .
- Williams , RB . 1999 . Anticoccidial vaccines: the story so far . World Poultry, Special Supplement Coccidiosis , 3 : 20 – 22 .
- Williams , RB . 2002a . Anticoccidial vaccines for broiler chickens: pathway for success . Avian Pathol , 31 : 317 – 353 .
- Williams , RB . 2002b . Fifty years of anticoccidial vaccines for Poultry (1952–2002) . Avian Dis , 46 : 775 – 802 .
- Williams , RB , Johnson , JD and Andrew , SJ . 2000 . Anticoccidial vaccination of broiler chickens in various management programmes: relationship between oocyst accumulation in litter and the development of protective immunity . Vet Res Commun , 24 : 309 – 325 .
- Wong , DT , Horng , JS and Wilkinson , JR . 1972 . Robenidine, an inhibitor of phosphorylation . Biochem Biophys Res Commun , 46 : 621 – 627 .
- World Health Organization. 1965. Resistance of malaria parasites to drugs. WHO Technical Report. Geneva, Switzerland: World Health Organization. Vol. 296, p. 1–65
- Youn , HJ and Noh , JW . 2001 . Screening of the anticoccidial effects of herb extracts against Eimeria tenella . Vet Parasitol , 96 : 257 – 263 .
- Yvoré , P . 1968 . Chimioprévention des coccidiosis aviaries . Rec Med Vet , 144 : 485 – 503 .
- Zeng , M and Hu , Z . 1996 . The sensitivities of Eimeria tenella to three polyether ionophores antibiotics . Chin J Vet Sci , 16 : 390 – 393 .
- Zhou , R , Nie , K and Wang , J . 2000 . The sensitivity test of Eimeria tenella to four anticoccidial drugs . J Sichuan Inst Anim Husb Vet Med , 14 : 20 – 23 .
- Ziomko , I , Karamon , J , Cencek , T , Gornowicz , E , Skoracki , A and Ashash , U . 2005 . Prevention of broiler chick coccidiosis using the inactivated subunit vaccine CoxAbic® . Bull Vet Inst Pulawy , 49 : 299 – 302 .
- Zulkifli , I , Abdullah , N , Azrin , NM and Ho , YW . 2000 . Growth performance and immune response of two commercial broiler strains fed diets containing Lactobacillus cultures and oxytetracycline under heat stress conditions . Br Poult Sci , 41 : 593 – 597 .