Abstract
Background: Equine inflammatory small bowel disease (ISBD) is an idiopathic pathologic condition seeming to increase in prevalence.
Objective: To investigate the potential role of gluten in equine ISBD.
Animals & Methods: Antibodies known to be important in the diagnosis of human coeliac disease (CD): IgA antibodies to human recombinant and guinea pig tissue-transglutaminase (TGA), native gliadin (AGA), deamidated-gliadin-peptides (DGPA), and primate and equine endomysium (EMA) were assessed in blood samples from three different groups of horses: ISBD affected (n = 12) on a gluten-rich diet and controls either on gluten-rich (n = 22) or gluten-poor (n = 25) diets. Significant differences (p < 0.05) between groups were assessed using the Wilcoxon test.
Results: Both ISBD-affected horses and gluten-rich controls had significantly (p < 0.0004) higher hrTGA titers than gluten-poor controls. However, ISBD horses did not show significantly increased levels of any of the CD related antibodies when compared to gluten-rich controls. Nevertheless, markedly increased antibody levels (TGA, EMA and DGPA) were found in one of the ISBD horses. The introduction of a gluten-free ration in this 14-year-old warmblood stallion resulted after 6 months in the reduction of antibody levels and clinical recovery associated with improved duodenal histopathology.
Conclusion: To the best of our knowledge, this is the first study assessing gluten-related antibodies in horses and results suggest a potential pathogenic role of gluten in at least some cases of equine ISBD.
Clinical importance and impact for human medicine: Given serology and concurrent clinical findings, this study warrants further investigations into the immunologic basis of possible gluten-sensitive enteropathy in horses and analogy with human disease.
1. Introduction
The intestinal immune system is constantly exposed to a vast array of antigens, including those derived from food, components of the endogenous microbial flora, and pathogenic organisms. Important decisions must be made about the nature of the antigenic stimulus so that protective responses are mounted to pathogens but tolerance to harmless substances is preserved. If this delicate balance is interrupted, a state of chronic uncontrolled inflammation may ensue (German et al. Citation2003). A syndrome, marked by chronic weight loss and hypoalbuminaemia associated with chronic gastrointestinal disorders is well known in Standardbreds with two different pathomorphological entities, namely eosinophilic granulomatosis and granulomatous enteritis (Lindberg et al. Citation1985). Lymphocytic–plasmacytic enteritis also belongs to this complex of equine idiopathic inflammatory small bowel disease (ISBD). Clinical signs include weight loss, recurrent colic, soft feces, depression, oedema, and a dull hair coat (Schumacher et al. Citation2000). Lymphocytic–plasmacytic enteritis is regarded as an uncommon equine intestinal disease that is difficult to recognize and diagnose ante mortem and has a poor prognosis (Kemper et al. Citation2000). We noticed an increased prevalence of ISBD in equine elite athletes over the last decade in our veterinary teaching hospital with more than 70% of these horses used for dressage (Dansen et al. Citation2010). By definition, the exact cause of equine ISBD is unknown urging the need for studying diet and/or immune function in these equine elite athletes.
Coeliac disease (CD) is an auto-immune inflammatory disorder in humans characterized by a partial or total villous atrophy of the proximal small intestine occurring after ingestion of gluten in genetically predisposed patients (Working Group Amsterdam Citation2001; Tack et al. Citation2010). The classic form is much more frequent in children (Tack et al. Citation2010). The common factor for all patients with CD is the presence of a variable combination of gluten-dependent clinical manifestations, specific auto-antibodies directed to transglutaminase 2 (TGA) and endomysium (EMA), HLA-DQ2 and/or DQ8 genes, and different degrees of enteropathy. Recently, the so-called gluten sensitivity (GS) has received much interest in human medicine, although the limits and possible overlap between GS and CD remain poorly defined (Troncone and Jabri Citation2011). However, in GS antibodies to native gliadin (AGA) (the alcohol-soluble part of gluten) are more pronounced (Volta et al. Citation2011). For CD diagnostics in children, TGA with a sensitivity of almost 100% is now recommended as test of preference, while EMA and antibodies against deamidated gliadin can be used for confirmation or in doubtful cases (Husby et al. Citation2012). When tissue transglutaminase transforms gliadin by converting glutamine into glutamic acid, the remaining gliadin is termed “deamidated” gliadin. It should be realized that the clinical demand for both maximal sensitivity and maximal specificity cannot be achieved with a single test (Rozenberg et al. Citation2011). Also, in adult human patients with abdominal symptoms in primary care or other unselected populations, IgA TGA and IgA EMA have high sensitivity and specificity for diagnosing CD (van der Windt et al. Citation2010).
The objective of this report was to determine the serological status of elite horses admitted to an equine academic hospital evaluated for idiopathic chronically increased thickness of the wall of the jejunum regarding antibodies known to be important in the diagnosis of human CD.
2. Materials and methods
2.1. Animals
Twelve horses with signs of ISBD referred to the Equine Clinic of Utrecht University were included in the study. The history was taken, clinical examination was performed and peripheral venous blood was collected for routine haematological and biochemical examination as well as further assessment of specific immunological parameters. The (tentative) diagnosis of ISBD was based on chronic thickening of the wall of the jejunum as indicated by the repeated ability to palpate various (non-contractile) loops of the jejunum during rectal exploration and/or assessment of increased thickness of the jejunal wall (above 3.0 mm) by transabdominal ultrasound examination. The upper limit of 3.0 mm was based on the statement that the duodenum and jejunum have thin walls that rarely exceed 3 mm in thickness (Reef Citation1998). Horses suffering from acute (less than 2 weeks duration) diseases and horses showing diarrhea were excluded from the study as well as horses with abnormal lymphocyte morphology in peripheral blood. Horses with a strongyle egg count above 200 per gram feces were also excluded from the study. All affected horses were warmbloods except for one Friesian horse. They performed at the M-level of dressage or above (up to Prix St George/Intermédiaire I level and Olympics), as governed by the Federation Equestre Internationale (FEI). The affected horses comprised five mares, six geldings and one stallion ranging in age from 5–18 years (mean 10.8 ± 3.5 (SD)). All blood samples were taken between February 2010 and January 2011.
For comparison, 22 (gluten-rich) control horses belonging to the teaching herd of Utrecht University were used and were fed grass silage ad libitum and concentrates according to their nutrient requirements for maintenance and performance. The total diet of the control horses contained 10% ash, 14.5% crude protein, 1.3% crude fat, 20% crude fiber, and 56.2% other carbohydrates. On the average, individual gluten-rich control horses were fed 2 kg of concentrates daily. The cereals used in the concentrates were specified by the manufacturer as 25% wheat, 15 % wheat gluten feed, 15% wheat middlings, and 15% maize gluten feed. Water was provided ad libitum. The mean age of the gluten-rich control horses was 10.5 ± 4.3 years ranging from 8 to 16 years and the horses comprised 19 Dutch warmbloods (weighing 633 ± 60 kg), 2 Shetland ponies, and one Standardbred. The gluten-rich control horses included 21 mares and one gelding. All samples of the gluten-rich control horses were taken on 5 March 2010. Furthermore, a group of 25 Shetland ponies living year-round in a nature reserve in the Dutch province of Zeeland, the Netherlands, was used as a gluten-poor control group. They were kept in a gluten-poor (if not gluten-free) environment since their diet consisted exclusively of wild, non-agricultural grasses, and herbs. All 25 gluten-poor controls were mares ranging in age from 1 to 27 years (mean age unknown). All blood samples of the gluten-poor controls were taken on 6 October 2010.
The blood sampling from the gluten-rich control horses was approved by the Institutional Animal Care and Use Committee of the University of Utrecht. The samples from the other horses were taken on veterinary indication and approved by the owner.
2.2. Blood analysis
2.2.1. IgA antibodies against Escherichia coli
As CD serology is generally based on IgA antibodies, all horses were tested for the presence of IgA using an E. coli antibody ELISA in order to rule out IgA deficiency in individual cases. To this end, 96-wells microtiter plates (flat-bottom 96 well plate, Nunc Maxisorp©, Bioscience Inc., San Diego, CA, USA) were coated with E. coli strain EB1 and blocked with 1% bovine serum albumin (BSA) in PBS/Tween 0.05%. Sera were preincubated with the blocking reagent (45 min. at room temperature) and tested in a 1:20 dilution (120 min. at room temperature). After washing, the plates were incubated with goat anti-horse IgA:HRP (AbD Serotec, 1:5000 diluted, 60 min. at room temperature) and attached antibodies were detected by using orthophenylenediamine dihydrochloride (OPD) as substrate in a routine procedure. After 15 min, the reaction was terminated using H2SO4 and evaluated spectrophotometrically at 492 nm. Serum obtained from equine patient number 10 was used in all experiments as calibration curve and standard (by definition containing 100 arbitrary units (AU) of E. coli antibodies/mL).
2.2.2. IgA antibodies against human recombinant and guinea pig transglutaminase 2
TGA concentration was determined quantitatively in serum based on binding to either human recombinant transglutaminase 2 (hrTG2) or purified guinea pig transglutaminase 2 (gpTG2). 96-Well microtiter plates were coated with hrTG2 (1 µg/well in 0.05 M TRIS-HCL pH to 7.5, Diarect, Freiburg, Germany), or gpTG2 (1 µg/well in 0.05 M TRIS-HCL pH to 7.5, Sigma, St. Louis, MO, USA) according to standard operation procedures. After washing, open binding sites were blocked with 1% BSA and Tween-20 0.05%. The serum was diluted ranging from 1:20, 1:40, 1:80 till 1:160 in phosphate buffered saline (PBS) with 1% BSA and 0.05% Tween-20 and pre-incubated at room temperature for 30–60 min to allow potential anti-BSA to bind. The detection of TGA was performed as described for E. coli antibodies. Serum obtained from equine patient number 7, being strongly positive, was used as calibration curve and standard (by definition containing 100 AU of transglutaminase 2 (TG2)/mL).
2.2.3. IgA antibodies against native gliadin
Gliadin antibodies were tested by ELISA. 96-wells microtiter plates were coated with native gliadin (50 µg/well in 0.05 M Na-carbonate buffer, pH to 9.6; Sigma, St. Louis, MO, USA) without blocking. The serum was diluted ranging from 1:20, 1:40, 1:80 till 1:160. Bound antibodies were made visible as described above. Serum obtained from equine control animal number 28, being strongly positive, was used as calibration curve and standard (by definition containing 100 AU of AGA/mL).
2.2.4. IgA antibodies against deamidated gliadin
When tissue transglutaminase transforms gliadin by converting glutamine into glutamic acid, the remaining gliadin is termed “deamidated” gliadin. A commercial ELISA under development, kindly provided by Euro-Diagnostica AB (Nijmegen, the Netherlands) based on a set of deamidated gliadin peptides (DGPA) as target, was used for testing the presence of antibodies in equine serum directed against these DGPA. The “human” ELISA was adapted for measuring specific horse IgA, as described above. A 1:50 serum dilution was used. Unfortunately, the sample size of the kit was limited, so not all horses could be tested. Gluten-rich controls 34, 35, and 42 and gluten-poor controls 70–75 were not tested. Serum obtained from equine patient number 7, being strongly positive, was used as calibration curve and standard (by definition containing 100 AU of DGPA/mL).
2.2.5. IgA antibodies against endomysium
Endomysium (EMA) were evaluated in an indirect immunofluorescence analysis (IIFT) using unfixed cryostat sections of monkey (Macaca mulatta) and equine (obtained from a 1-day-old warmblood colt immediately following euthanasia) esophagus as substrate. Esophagus is used because the muscularis mucosae of the esophagus has a relatively high content of TG2. Serum was tested in a 1:2 dilution and PBS was used as a negative control. After washing, incubation was followed with goat anti-horse IgA:FITC-conjugate (AbD Serotec. 1:40). After washing, the glasses were covered, viewed under a fluorescence microscope, and scored for staining pattern and fluorescence intensity of the muscularis mucosae by at least two independent observers such as fluorescence intensity (−, +/−, +, ++ or +++) of the muscularis mucosae by at least two independent observers.
2.3. Statistical analysis
All results were analyzed using specialized software (MedCalc Software, Mariakerke, Belgium and SPPS Software, version 16.0, IBM Corporation, New York, USA). Differences between groups were statistically compared by means of the Wilcoxon test. p values <0.05 were considered significant.
3. Results
Clinical signs of horses with ISBD included intermittent colic (n = 6), weight loss (n = 4), and poor performance (n = 2). In 7 out of 12 cases, an oral glucose tolerance test (according to Benders et al. Citation2005) was performed with poor glucose absorption shown in only 2 cases. In addition, in 6 out of 12 cases gastro-duodenoscopy-assisted biopsies taken from the proximal duodenum were available for histopathology revealing predominantly very mild lymphocytic–plasmacytic enteritis. Routine blood analysis in these horses revealed normal mean values for total lymphocyte count (1.8 ± 1.2 ranging from 1.1 to 5.3 G/L), total protein concentration (64 ± 7.0 ranging from 51 to 73 g/L), and albumin concentration (35 ± 2.2 ranging from 31 to 38 g/L), whereas the mean hematocrit was slightly decreased (0.35 ± 0.034 ranging from 0.31 to 0.41 L/L) also taken into consideration the fact that the horses involved were elite horses. All horses included in this study tested positive for the presence of IgA antibodies directed against E. coli in serum. Remarkably, IgA antibody titers were significantly lower in gluten-poor control horses than in gluten-rich controls (p = 0.0004) or ISBD-affected horses (p = 0.0015).
As shown in ) serum antibody levels directed against hrTG2 were also significantly lower in gluten-poor controls as compared to both gluten-rich controls and ISBD-affected patients (p = 0.0004 and p = 0.0001, respectively). However, no significant differences were found in TGA levels between ISBD-affected horses and gluten-rich controls. Remarkably, one ISBD patient (number 7) showed an extremely high hrTGA level. Such high titers were not seen in either control groups. The three groups of horses did not differ significantly with respect to serum IgA antibodies directed against gpTG2 (). Again ISBD patient number 7 showed a relatively high gpTGA concentration, but also two gluten-rich controls had intermediate high levels.
Figure 1. CD-related IgA antibodies in equine ISBD patients and controls. Multiple comparison (box and dot) graph of serum IgA antibodies directed against hrTG2 (), gpTG2 (), native gliadin (), and deamidated gliadin () in 12 horses with inflammatory small bowel disease (ISBD glut +), 22 gluten-rich controls (CON glut +), and 25 gluten-poor controls (CON glut−). In the case of DGPA, 19 gluten-rich controls and 19 gluten-poor controls were tested. IgA antibodies are calibrated with a strong positive serum and expressed as arbitrary units (AU)/ml. The boxes represent the interquartile range (i.e., 25–75% range) and the horizontal bar in the box represents the median value. For each box plot, the T-bar extends to 1.5 times the interquartile range, if without a value in that range, to the minimum or maximum values. Statistical analysis was performed by the Wilcoxon test and P values are given in the figures.
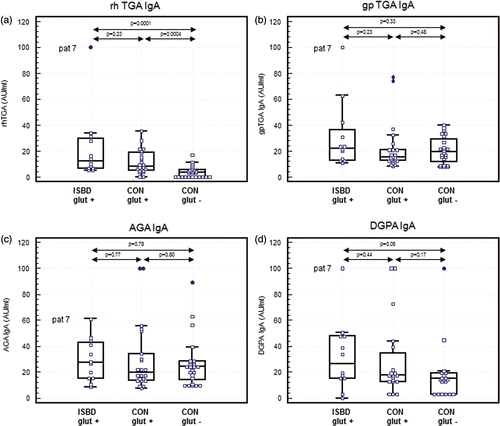
Similarly, differences between groups were not statistically significant regarding IgA antibodies directed against native gliadin () and DGPA (), despite incidental clear cut positive horses occurring in all groups.
Remarkably, control horse 32 (a 16-year-old warmblood mare) showed elevated serum IgA antibodies directed against DGPA as well as directed against equine oesophageal endomysium (EMA). Blood analysis in this mare revealed serum total protein concentrations of 72 g/L (with 37 g/L albumin) on 9 June 2004, 79 g/L (with 44 g/L albumin) on 14 February 2005, 60 g/L on 17 February 2010, and 63 g/L (with 34 g/L albumin) on 20 December 2010. A prompted oral glucose tolerance test revealed an increase of 6% only (normal range 44–174%).
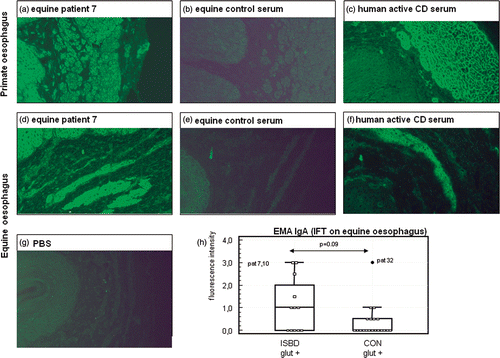
EMA (), with the classical reticular staining pattern of the muscularis mucosae () were not detectable when using monkey esophageal tissue as substrate (). However, the smooth muscle cells stained positive for some horses, including patient number 7. When evaluating the IIFT on equine esophageal tissue, the muscularis mucosae as well as the arterial walls in the mucosa stained quite positive for a number of horse sera (). The reticular staining pattern was however not very clear, since the smooth muscle cells also stained positive. This was, however, also the case for the human coeliac serum (). Based on the equine esophagus IIFT, EMA (i.e. positive staining of the muscularis mucosae) in ISBD patients tended to be higher than in the gluten-rich control group, but this did not reach significance (p = 0.09; ).
Figure 2. IIFTs for endomysium antibodies (EMA) IgA. EMA was tested on primate (a–c) and equine esophagus (d–h). Cryosections were incubated with serum from equine ISBD patient 7 (, ), from a gluten-rich control horse (patient 5; b and e), from a human active CD patient (c and f) and with PBS (g). (h) shows the multiple comparison (box and dot) graph of EMA IgA antibodies directed against equine oesophageal muscularis mucosae in 12 horses with inflammatory small bowel disease (ISBD glut +) and 22 gluten-rich controls (CON glut +). Antibody concentration is reflected by the fluorescence intensity of the muscularis mucosae on a scale from 0–3. The box represents the interquartile range (i.e., 25–75% range) and the horizontal bar in the box represents the median value. For each box plot, the T-bar extends to 1.5 times the interquartile range, if without a value in that range, to the minimum or maximum values. Note the high values in patients 7 and 10 as well as in control horse 32 (a 16-year-old warmblood mare). Statistical analysis was performed by the Wilcoxon test and p values are given in the figure.
3.1. Clinical course of patient number 7
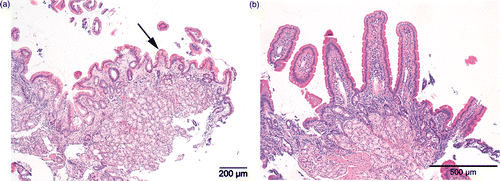
A 14-year-old warmblood stallion weighing 589 kg was admitted because of poor performance and based on findings during repeated rectal palpation was included in the study as patient number 7. Upon admission, serum total protein measured 66 g/L with 35 g/L albumin (see for additional blood values and its change over time). An oral glucose tolerance test performed upon admission indicated moderate glucose absorption (42% increase). Based on its high antibody concentrations (EMA, TGA, DGPA), a gluten-free ration was initiated to which the owner adhered strictly. The gluten-free ration was predominantly based on haylage, alfalfa, and black crushed oats containing 126 MJ digestable energy and 10.2 kg dry matter. Following feeding of the gluten-free ration, performance was normalized (return to FEI Intermédiaire I level). In contrast to the irrelevant E. coli antibodies, which remained strongly positive, all CD-associated antibodies (TGA, EMA, and DGPA) decreased following the gluten-free period of 6 months, though these did not yet normalize completely (). In addition, histopathology suggested increase in duodenal villous length ().
Figure 3. Follow up during gluten-free diet in an equine patient (#7) with ISBD. Change over time in titers of IgA antibodies to E. coli, hrTG2 (=hrTGA), gpTG2 (=gpTGA), native gliadin (AGA), deamidated-gliadin-peptides (DGPA), and endomysium (EMA) in a 14-year-old warmblood stallion with ISBD prior to and 6 months following a gluten-free diet (GFD) mainly based on haylage, alfalfa, and black crushed oats.
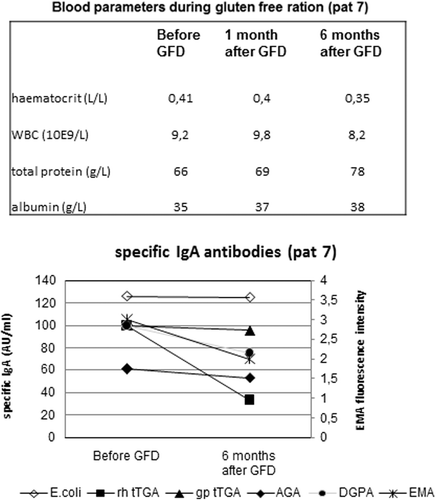
Figure 4. Histopathology of gastro-duodenoscopy-assisted biopsies taken from the proximal duodenum in patient 7 (a 14-year-old warmblood stallion) prior to (left) and following 6 months of a gluten-free diet (right). Arrow depicts a shortened villous in the initial duodenal biopsy. Note the marked increase in villous length in the control biopsy taken after 6 months of gluten-free feeding. H&E stain.
4. Discussion
Serological assessment of antibodies known to be important in the diagnosis of human CD disease in elite horses, evaluated for idiopathic chronically increased thickness of the wall of the jejunum, revealed that serum IgA antibodies directed against hrTG2 were significantly increased in ISBD-affected horses as compared to gluten-poor controls, but not as compared to control horses on a gluten-rich diet. No IgA deficiency, as based on IgA levels against E. coli, could be demonstrated in any of the horses. Regarding AGA and DGPA levels, no statistically significant differences were observed between the groups, though incidental clear cut positive horses were seen in all groups. The classical reticular staining pattern of human EMA on monkey esophageal tissue was not found in any of the horses. However, on equine esophageal tissue, bright staining of the muscularis mucosae and the arterial walls were seen in some of the horses. Since unequivocal discrimination between staining of the smooth muscle fibers and the endomysium was not possible on equine esophagus, these antibodies were considered to represent EMA in the present report. On the whole, EMA in ISBD patients tended to be higher than in the gluten-rich controls with some individual horses showing strong positive responses.
No significant differences between the ISBD group and the control horses on a comparable gluten containing ration were found for any of the CD-associated antibodies. Remarkably, one ISBD horse (number 7) showed extremely high IgA levels for all antibodies known to be important in the diagnosis of human CD, namely TGA, DGPA as well as EMA. To evaluate how far these antibodies were related to gluten consumption, this patient was followed up during a strict gluten-free diet. The high hrTGA, DGPA, and EMA levels decreased following a gluten-free ration for 6 months in this 14-year-old warmblood stallion suffering from ISBD with concurrent increase of duodenal villous length as visualized histopathologically. Although the majority of oral glucose absorption tests in ISBD horses revealed normal absorption, both the mean hematocrit and serum protein concentration were relatively low given the fact that elite horses were involved. In comparison, poor glucose absorption might also be demonstrated in human CD (van Elburg et al. Citation1995) although results are variable as shown in the current report in horses.
As the ELISA's used in this study are based on IgA antibodies, all horses were tested for the presence of IgA using an E. coli antibody ELISA. Although IgA deficiency has been reported in non-Arabian horses as a very rare disorder (Deem et al. Citation1979; Freestone et al. Citation1987; MacLeay et al. Citation1997), all horses included in this study tested positive for the presence of IgA antibodies directed against E. coli in serum. As a consequence, IgA deficiency as a confounding factor regarding serology in the current study can be ruled out. Remarkably, mean IgA antibody titer was significantly lower in gluten-poor control horses than in both gluten-rich and ISBD-affected horses suggesting lower environmental contamination in the nature reserve by E. coli and/or reduced serological response. To the authors’ best knowledge, no data are available whatsoever regarding the relationship between environmental contamination by E. coli and serology in horses.
It should be realized that some of the serological tests used were based on human assays modified for use in horses. Although cross-reactivity between species are obvious in this study (human coeliac antibodies reacting to monkey and guinea pig TG2 and equine antibodies reacting to human and guinea pig TG2), species-specific antigen differences might have influenced the outcome. Also, the partial reduction in antibodies, together with the improvement in clinical course and blood biochemistry as well as the results of proximal duodenal histopathology following the initiation of a gluten-free ration in equine patient number 7 support assay specificity. As a consequence, our findings suggest the presence of Gluten-Sensitive Enteropathy (GSE) in at least some horses suffering from ISBD. In addition, control horse 32 (a 16-year-old warmblood mare) retrospectively not only showed a decrease in serum total protein over time, but also had elevated DGPA and equine EMA titers. A prompted oral glucose tolerance test revealed a very insufficient glucose absorption (6%), thereby suggesting subclinical GSE. Of interest, almost by tradition horses in some geographic regions were fed oats grain in former times known as a gluten-free cereal. As our understanding of ISBD is far from complete, it is worthwhile to address its potential relationship with GSE.
To the authors’ knowledge, GSE has not been reported in the equine species yet. However, primary photosensitization observed in three Appaloosa mares associated with gluten ingestion has been reported (Yeruham et al. Citation1999). Dermatitis herpetiformis (DH) is a pruritic papulovesicular skin disorder of unknown cause, characterized by granular IgA deposits in the dermis along the dermoepidermal junction. It is associated with GSE and increased levels of TGA in man (Kárpáti Citation2012). We suggest a DH-like disease due to GSE in these Appaloosa horses manifested as primary photosensitization.
As mentioned before, an increased prevalence of ISBD in equine elite athletes over the last decade has been noticed in our equine academic hospital with more than 70% of these horses being used for dressage (Dansen et al. Citation2010). Given the fact that the intestinal immune system is constantly exposed to a vast array of antigens, including those derived from food (German et al. Citation2003) raises the question about possible alterations in the gluten content of equine diets over time. The high prevalence of dressage horses affected with ISBD might be explained by differences in feeding management as the equine species responds to differences in the quality of dietary protein (Reitnour and Salsbury Citation1976). On the other hand, competition induced a significant increase in cortisol and ACTH responses in both jumping and dressage horses being most pronounced in the latter (Cayado et al. Citation2006), thereby possibly negatively influencing the intestinal immune system.
Based on the current study, IgA antibodies against hrTG2, DGP, and equine endomysium in horses suffering from weight loss and/or recurrent colic with chronic increased thickness of the small bowel wall are the most likely candidates to support the tentative diagnosis of equine GSE besides assessment of the extent of the underlying enteropathy by means of histopathology. Last but not the least, clinical remission on a strict gluten-free diet is of importance. With reference to future research, it is necessary to explore the potential involvement of HLA-DQ2 and/or DQ8 (like) histocompatibility complex genes in horses with a tentative diagnosis of GSE.
Villous atrophy of the intestinal mucosa, in combination with increased numbers of intraepithelial lymphocytes and crypt hyperplasia has long been considered the hallmark of CD (Tack et al. Citation2010). In equine elite athletes such histopathology is best performed based on gastro-duodenoscopy-assisted biopsies taken from the proximal duodenum. However, sample size is small in the case of gastro-duodenoscopy-assisted biopsies thereby preventing optimal histopathological evaluation compared to full thickness biopsies. An additional disadvantage of gastro-duodenoscopy-assisted biopsies is that no information can be obtained on sufficient area of the small intestinal mucosa. The reference values of small intestine (duodenum and jejunum) wall thickness in Thoroughbreds have been reported to range from 0.27 to 0.33 mm (Bithell et al. Citation2010). In addition, it might be attractive to study small intestine wall thickness in horses on a long-term gluten-free diet also.
The question remains if there is a considerable lag time between improvement in serology and duodenal/jejunal wall thickness and initiation of a gluten-free diet. Preliminary findings derived from follow up of some GSE suspected horses (by the first author) suggest that reduction in duodenal/jejunal wall thickness is very limited (if not refractory) and takes perhaps years following the initiation of a gluten-free ration similar to findings in human CD serology (Esch et al. Citation2011) and histopathology (Mallant et al. Citation2007; Rubio-Tapia et al. Citation2010). In children with CD, about 80% were sero-negative for EMA and anti-TG2 after 2 years of the gluten-free diet (Esch et al. Citation2011). Of adults with biopsy-proven CD, mucosal recovery at two years following diagnosis was 34% and at 5 years was 66%. However, mucosal recovery was absent in a substantial portion of adults with CD after a gluten-free diet (Rubio-Tapia et al. Citation2010).
To date, there are no adequate in vivo models for the systemic complications of CD; in particular, there are no genetic knock-out models. However, models are available for GSE such as the Irish Setter dog (Daminet Citation1996) and Balb/c and BDF1 mouse strains (Stazi Citation2005). In addition, the rhesus macaque model of GS has been established (Bethune et al. Citation2008). Exclusion of dietary cereal from birth modified subsequent expression of the disease in Irish Setter dogs (Hall and Batt Citation1991c, Citation1992). In contrast to human CD, susceptibility to canine GSE does not appear to be determined by variation within the MHC class II gene cluster (Polvi et al. Citation1997, Citation1998).
Whether abnormal transport of gluten across the gut epithelium may participate in the pathogenesis of CD remains debatable. Recent data point to a key role for the transcellular pathway and highlights the “Trojan horse” role of secretory IgA which can hijack the transferrin receptor and allow the rapid translocation of intact gluten peptides into the mucosa (Heyman et al. 2012). In accord, intestinal permeability testing of puppies during controlled oral gluten challenge provides a practical screening test for gluten sensitivity in Irish Setter dogs at an early age (Hall and Batt Citation1991a, Citationb; Garden et al. Citation1998). Microvillar membrane proteins isolated from GSE-affected dogs revealed an essentially normal protein map with the exception being an 85 kDa protein (Pemberton et al. Citation1997).
In conclusion, preliminary findings indicate that gluten intake might induce clinical pathology in horses and this study warrants further investigations into the immunologic basis of possible GSE in horses. Although it cannot be excluded that equine ISBD must be regarded as a single clinical syndrome, it seems more likely that the syndrome is common to several different enteropathies with different etiologies among which GS might be identified.
Acknowledgements
The support of W. Goesten, DVM, E.P.R. Reijerkerk, DVM, E. Smiet, DVM, D.A. van Doorn, PhD, R. Amirthalingam, and A. Veenhof was greatly appreciated. We also thank Eurodiagnostica, Nijmegen, the Netherlands, for providing us with kits for antibodies to deamidated gliadin peptides.
References
- Benders , NA , Dyer , J , Wijnberg , ID , Shirazi-Beechey , SP and van der Kolk , JH . 2005 . Evaluation of glucose tolerance and intestinal luminal membrane glucose transporter function in horses with equine motor neuron disease . Am J Vet Res , 66 ( 1 ) : 93 – 99 .
- Bethune , MT , Borda , JT , Ribka , E , Liu , MX , Phillippi-Falkenstein , K , Jandacek , RJ , Doxiadis , GG , Gray , GM , Khosla , C and Sestak , K . 2008 . A non-human primate model for gluten sensitivity . PLoS One , 20.3 ( 2 ) : e1614
- Bithell , S , Habershon-Butcher , JL , Bowen , IM and Hallowell , GD . 2010 . Repeatability and reproducibility of transabdominal ultrasonographic intestinal wall thickness measurements in Thoroughbred horses . Vet Radiol Ultrasound , 51 ( 6 ) : 647 – 651 .
- Cayado , P , Muñoz-Escassi , B , Domínguez , C , Manley , W , Olabarri , B , Sánchez de la Muela , M , Castejon , F , Marañon , G and Vara , E . 2006 . Hormone response to training and competition in athletic horses . Equine Vet J Suppl , 36 : 274 – 278 .
- Daminet , SC . 1996 . Gluten-sensitive enteropathy in a family of Irish setters . Can Vet J , 37 ( 12 ) : 745 – 746 .
- Dansen , O and Rutten , J . van, Reijerkerk EPR, Kolk JH van der. 2010. Emerging idiopathic inflammatory bowel disease in horses: a report of 14 cases. Voorjaarsdagen proceedings, Amsterdam, the Netherlands
- Deem , DA , Traver , DS , Thacker , HL and Perryman , LE . 1979 . Agammaglobulinemia in a horse . J Am Vet Med Assoc , 175 ( 5 ) : 469 – 472 .
- Esch , CE , Wolters , VM , Gerritsen , SA , Putter , H , von Blomberg , BM , van Hoogstraten , IM , Houwen , RH , van der Lely , N and Mearin , ML . 2011 . Specific celiac disease antibodies in children on a gluten-free diet . Pediatrics , 128 ( 3 ) : 547 – 552 .
- Freestone , JF , Hietala , S , Moulton , J and Vivrette , S . 1987 . Acquired immunodeficiency in a seven-year-old horse . J Am Vet Med Assoc , 190 ( 6 ) : 689 – 691 .
- Garden , OA , Manners , HK , Sørensen , SH , Rutgers , HC , Daniels , S , Legrand-Defretin , V and Batt , RM . 1998 . Intestinal permeability of Irish setter puppies challenged with a controlled oral dose of gluten . Res Vet Sci , 65 ( 1 ) : 23 – 28 .
- German , AJ , Hall , EJ and Day , MJ . 2003 . Chronic intestinal inflammation and intestinal disease in dogs . J Vet Intern Med , 17 ( 1 ) : 8 – 20 .
- Hall , EJ and Batt , RM . 1991a . Abnormal intestinal permeability could play a role in the development of gluten-sensitive enteropathy in Irish setter dogs . J Nutr , 121 ( 11 Suppl ) : S150 – S151 .
- Hall , EJ and Batt , RM . 1991b . Abnormal permeability precedes the development of a gluten sensitive enteropathy in Irish setter dogs . Gut , 32 ( 7 ) : 749 – 753 .
- Hall , EJ and Batt , RM . 1991c . Delayed introduction of dietary cereal may modulate the development of gluten-sensitive enteropathy in Irish setter dogs . J Nutr , 121 ( 11 Suppl ) : S152 – 153 .
- Hall , EJ and Batt , RM . 1992 . Dietary modulation of gluten sensitivity in a naturally occurring enteropathy of Irish setter dogs . Gut , 33 ( 2 ) : 198 – 205 .
- Husby , S , Koletzko , S , Korponay-Szabó , IR , Mearin , ML , Phillips , A , Shamir , R , Troncone , R , Giersiepen , K , Branski , D and Catassi , C . et al.; for the ESPGHAN Working Group on Coeliac Disease Diagnosis, on behalf of the ESPGHAN Gastroenterology Committee 2012. European society for pediatric gastroenterology, hepatology, and nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 54(1):136–160
- Kárpáti , S . 2012 . Dermatitis herpetiformis . Clin Dermatol , 30 ( 1 ) : 56 – 59 .
- Kemper , DL , Perkins , GA , Schumacher , J , Edwards , JF , Valentine , BA , Divers , TJ and Cohen , ND . 2000 . Equine lymphocytic-plasmacytic enterocolitis: a retrospective study of 14 cases . Equine Vet J Suppl , 32 : 108 – 112 .
- Lindberg , R , Persson , SG , Jones , B , Thoren-Tolling , K and Ederoth , M . 1985 . Clinical and pathophysiological features of granulomatous enteritis and eosinophilic granulomatosis in the horse . Zentralbl Veterinarmed A , 32 ( 7 ) : 526 – 539 .
- MacLeay , JM , Ames , TR , Hayden , DW and Tumas , DB . 1997 . Acquired B lymphocyte deficiency and chronic enterocolitis in a 3-year-old quarter horse . Vet Immunol Immunopathol , 57 ( 1-2 ) : 49 – 57 .
- Mallant , M , Hadithi , M , Al-Toma , AB , Kater , M , Jacobs , M , Manoliu , R , Mulder , C and van Waesberghe , JH . 2007 . Abdominal computed tomography in refractory coeliac disease and enteropathy associated T-cell lymphoma . World J Gastroenterol , 13 : 1696 – 1700 .
- Ménard , S , Lebreton , C , Schumann , M , Matysiak-Budnik , T , Dugave , C , Bouhnik , Y , Malamut , G , Cellier , C Allez , M . 2012 . Paracellular versus transcellular intestinal permeability to gliadin peptides in active celiac disease . Am J Pathol , 180 ( 2 ) : 608 – 615 .
- Pemberton , PW , Lobley , RW , Holmes , R , Sørensen , SH and Batt , RM . 1997 . Gluten-sensitive enteropathy in Irish setter dogs: characterisation of jejunal microvillar membrane proteins by two-dimensional electrophoresis . Res Vet Sci , 62 ( 2 ) : 191 – 193 .
- Polvi , A , Garden , OA , Elwood , CM , Sørensen , SH , Batt , RM , Mäki , M and Partanen , J . 1997 . Canine major histocompatibility complex genes DQA and DQB in Irish setter dogs . Tissue Antigens , 49 ( 3 Pt 1 ) : 236 – 243 .
- Polvi , A , Garden , OA , Houlston , RS , Maki , M , Batt , RM and Partanen , J . 1998 . Genetic susceptibility to gluten sensitive enteropathy in Irish setter dogs is not linked to the major histocompatibility complex . Tissue Antigens , 52 ( 6 ) : 543 – 549 .
- Reef , V . 1998 . Equine diagnostic ultrasound , 1st , Saunders : Philadelphia .
- Reitnour , CM and Salsbury , RL . 1976 . Utilization of proteins by the equine species . Am J Vet Res , 37 ( 9 ) : 1065 – 1067 .
- Rozenberg , O , Lerner , A , Pacht , A , Grinberg , M , Reginashvili , D , Henig , C and Barak , M . 2011. A novel algorithm for the diagnosis of celiac disease and a comprehensive review of celiac disease diagnostics. Clin Rev Allergy Immunol. 2011 Jan 30. [Epub ahead of print]
- Rubio-Tapia , A , Rahim , MW , See , JA , Lahr , BD , Wu , TT and Murray , JA . 2010 . Mucosal recovery and mortality in adults with celiac disease after treatment with a gluten-free diet . Am J Gastroenterol , 105 : 1412 – 1420 .
- Schumacher , J , Edwards , JF and Cohen , ND . 2000 . Chronic idiopathic inflammatory bowel diseases of the horse . J Vet Intern Med , 14 ( 3 ) : 258 – 265 .
- Stazi , AV . 2005 . Coeliac disease and reproduction: possible in vivo models . Ann Ist Super Sanita , 41 ( 4 ) : 523 – 531 .
- Tack , GJ , Verbeek , WH , Schreurs , MW and Mulder , CJ . 2010 . The spectrum of celiac disease: epidemiology, clinical aspects and treatment . Nat Rev Gastroenterol Hepatol , 7 ( 4 ) : 204 – 213 .
- Troncone , R and Jabri , B . 2011 . Coeliac disease and gluten sensitivity . J Intern Med , 269 ( 6 ) : 582 – 590 .
- van der Windt , DA , Jellema , P , Mulder , CJ , Kneepkens , CM and van der Horst , HE . 2010 . Diagnostic testing for celiac disease among patients with abdominal symptoms: a systematic review . J Am Med Assoc , 303 ( 17 ) : 1738 – 1746 .
- van Elburg , RM , Uil , JJ , Kokke , FT , Mulder , AM , van de Broek , WG , Mulder , CJ and Heymans , HS . 1995 . Repeatability of the sugar-absorption test, using lactulose and mannitol, for measuring intestinal permeability for sugars . J Pediatr Gastroenterol Nutr , 20 ( 2 ) : 184 – 188 .
- Volta , U , Tovoli , F , Cicola , R , Parisi , C , Fabbri , A , Piscaglia , M , Fiorini , E and Caio , G . 2011. Serological tests in gluten sensitivity (nonceliac gluten intolerance). J Clin Gastroenterol. 2011 Dec 5. [Epub ahead of print]
- Working Group of the United European Gastroenterology Week in Amsterdam, the Netherlands . 2001 . When is a celiac a celiac? . Eur J Gastroenterol Hepatol , 13 ( 9 ) : 1123 – 1128 .
- Yeruham , I , Avidar , Y and Perl , S . 1999 . An apparently gluten-induced photosensitivity in horses . Vet Hum Toxicol , 41 ( 6 ) : 386 – 388 .