Abstract
Efficient artificial insemination (AI) is essential for future challenges in the pig industry. Knowledge on the exact relation between semen quality characteristics and fertility can have a major impact on both the genetic merit of future animals and the efficiency of AI. Variation in fertility is caused not only by farm- or sow-related parameters but also by boar- and semen-related parameters. In pig AI there is no gold standard concerning semen quality assessment. Assessing semen quality characteristics objectively and relating them to large field fertility datasets leads to an efficient production of insemination doses, which results in an efficient dissemination/descent of the breeding program required genes. Overall, this contributes to the development of semen quality assessments, which improves the prediction of porcine male fertility. Knowing which semen characteristics, and to what extent, contribute to male fertility and makes the field fertility more predictable.
1. History of artificial insemination
Artificial insemination (AI) was first mentioned around 1400 in undocumented tales about an Arabic chief inseminating his own mares with the semen of a stallion belonging to rival groups. Leeuwenhoek (Citation1678) was the first to report sperm cells. Another century passed before an Italian priest, Spallanzani (Citation1784), inseminated a bitch which whelped three pups (Siebenga Citation1937). The insemination in man was first attempted around 1800 by Hunter, followed by Sims in 1866. Heape (Citation1897) reported successful AI in studies with rabbits, dogs and horses. The Russian professor Elie Ivanov started working with sperm cells by the end of the nineteenth century (Ivanov Citation1907, Citation1922). All over the world, research on AI was stimulated. Milovanov (Citation1964) established major projects for sheep and cattle breeding and designed artificial vaginas, similar to those used today. In 1912, Ishikawa started a similar program in horses (Nishikawa Citation1962, Citation1964) and this developed AI is being applied in Japan in cattle, swine, goats, sheep and poultry. Growth of AI occurred in the 1940s in the United States and the developed procedures became established worldwide (Salisbury et al. Citation1978; ).
More studies on pig AI were conducted in the United States (McKenzie Citation1931), Japan (Niwa Citation1958) and in Western Europe (Polge Citation1956). Boars are easily trained on mounting dummies (Polge Citation1956). All artificial vaginas developed for semen collection provided a means of applying pressure to the glans (McKenzie Citation1931; Polge Citation1956) or a gloved hand (Hancock and Hovel Citation1959), minimising the amount of bacterial contamination in the collected semen (Althouse and Lu Citation2005). The development of a method to store semen long enough for shipment and use in the field was initiated in the United States where a yolk-phosphate semen extender was developed (Philips and Lardy 1940). Ito et al. (Citation1948) was the first to recommend a storage temperature of 15–20°C. With AI expanding rapidly, demands for semen increased. The simplest solution was to dilute each ejaculate further by using less sperm cells per insemination. The Beltsville Thawing Solution (BTS) was developed by the USDA laboratory in Beltsville (Pursel and Johnson Citation1975) and made the dilution of semen possible and increase the storage time up to 48 hours. The composition of BTS is still the basic for currently used semen diluters.
Pig AI became available in the Netherlands at the end of 1950s (Strikwerda Citation2007). The use of pig AI was stimulated by the Dutch Ministry of Agriculture, the Animal Health Service and was adopted by the regional herd books. The main reason was to prevent the spread of contagious diseases. The first pig AI stations were independent cooperative boar stations. The breeding companies were working closely together with the AI companies to make the boars with the highest genetic index available for AI and the exclusive use of those boars for the nucleus breeders. Pregnancy rates of 60% and litter sizes of six piglets were common in the beginning of the use of pig AI (Feitsma Citation2009). More research and development was funded since 1977 and in the years 1980–1990 the results improved. There was more knowledge on the oestrus cycle of the sow, timing of the insemination and treatment and dilution of boar semen. Once the results were similar to natural mating, commercial farms started using AI and the use of pig AI increased rapidly. These increased fertility results depended heavily on technicians trained at the AI centres who collected and inseminated semen. Later the demand for self-service AI increased in order to save labour (Strikwerda Citation2007). Currently, farrowing rate is 86% with 13.9 total number of piglets born ().
Table 1. Characteristics of sow production in the Netherlands.
2. Current status of pig AI in the Netherlands
Today, most sows (>98%) in the Netherlands are bred through AI, with one of the highest fertility results worldwide. In Europe, the pig AI rate in countries varies between 25% and 98% (Feitsma Citation2009). illustrates some characteristics of current sow fertility results in the Netherlands. In 1992, the Netherlands introduced the pooling of semen for commercial herds. Since the outbreak of classical swine fever in 1997, the use of pooled semen was prohibited by the Dutch government. Nowadays only single sire insemination doses are used, which is beneficial for analysing semen quality characteristics in relation with field fertility. In current research, individual ejaculate results are continuously merged with field fertility results, which make it possible for an AI station to monitor their boar population.
3. Monitoring field fertility
Economic losses due to reproductive inefficiency in male animals can be substantial (Roberts Citation1986; Lunenfeld and Insler Citation1993). Common problems in subfertile and infertile human patients and animals include low number of sperm cells and/or low semen quality. It is generally accepted that lower motility ejaculates have lower or limited potential to fertilise oocytes (Flowers Citation1997; Donadeu Citation2004). The question remains how differences in sperm properties from high fertile AI boars can explain variation in field fertility. Minimal differences in fertility may already lead in lower efficiency of producing piglets, and thus to a loss of economic prospects. Although the efficiency of piglet production is dependent on many factors, one of them that can be measured is the quality of semen. Ultimately one would like to design a semen quality test that could predict the fertilising potential of the semen of individuals (see also Section 2).
The most critical aspect of predicting the fertilisation potential of ejaculates is to have specific, precise and accurate fertility tests and precise and accurate fertility data. It is very difficult to obtain reliable field fertility data. The problems that can occur are (1) the boars or sows are not representative for the population, and are too few in number, (2) an insufficient number of sows are inseminated with sperm cells from each ejaculate, (3) too few ejaculates are assessed per boar, (4) a large number of cells is used for each insemination, which disables identifying detailed relations between semen quality and fertility and (5) the fertility outcome is not reported or documented properly. Predicting the fertilising potential of ejaculates on the basis of semen quality characteristics and analysing relationships with fertility needs high-quality fertility data to compare with standardised laboratory test results (Broekhuijse, et al. Citation2011a).
For successful implementation of AI, field fertility results of semen used are critical. In the Netherlands, data obtained at both pig AI centres and at the sow farms are recorded. Large sets of fertility data and ejaculate data are more suitable to analyse the effects of semen quality characteristics on field fertility. Due to the high throughput of data, a number of mentioned problems could be solved: (1) the number of boars or sows represent the actual population in the Netherlands, (2) the insemination doses produced from each ejaculate are transported to sow farms, (3) all routine ejaculate production per boar is used for AI, (4) each insemination is performed with a relatively low number sperm cells per dose and (5) fertility outcomes are reported in sow management systems with high accuracy. Variation in fertility in sows is large (Broekhuijse, et al. Citation2011a, Broekhuijse, et al. Citation2012a, Citation2012b) while the effect of semen factors is relatively small and therefore impossible to find in smaller data sets (Broekhuijse, et al. Citation2011a). By using large data sets, one can perform reliable statistical corrections on both sow- and boar-related parameters to get normalised and representative comparisons. Remaining sow fertility variation can then be assigned to semen quality parameters.
Following this procedure, we applied a standard calculation of technical results based on merged data from the TOPIGS breeding database and AI centre. This enabled us to analyse the relation between semen quality characteristics and fertility (Broekhuijse Citation2012a, Citation2012b). In contrast with retrospective data analysis, field trials can be designed with accurate data retrieval and for instance detectable differences in litter sizes as far as 0.1 piglets (Feitsma Citation2009). TOPIGS Research Center IPG B.V (Beuningen, the Netherlands) maintains the database of both breeding company TOPIGS B.V. (Vught, the Netherlands) and the cooperative pig AI centres Varkens KI Nederland B.V. (Deventer, the Netherlands) and Varkens KI Twenthe B.V. (Fleringen, the Netherlands). Results of over 12 million litters were recorded and semen quality information of over 1.2 million ejaculates is known (status in 2011). With this infrastructure semen quality characteristics can be validated for their relation with field fertility.
4. Semen quality assessment
Boars are introduced at Dutch AI centres via quarantine and strict health controls. Boars are collected in separate collection pens on dummy sows with the gloved-hand technique (Hancock and Hovel Citation1959) or in an automated semen collection system (Collectis®, IMV, L’Aigle, France). The semen is pre-diluted within 10 minutes after collection with Solusem® (Varkens KI Nederland, Deventer, the Netherlands) with similar temperature as the semen. Each semen quality assessment starts with the macroscopic evaluation: colour, smell, contamination with dirt, blood or urine and viscosity. After volume determination, the concentration and the motility are measured. Until 2006, the concentration was measured using a colorimeter or spectrophotometer and the motility was microscopically estimated by experienced laboratory technicians. Currently, a computer-assisted semen analysis (CASA) system is used for the evaluation of sperm motility characteristics and sperm concentration. After quality assessment, the semen is diluted (Solusem®, 20°C) to a current minimum level of 1.5 billion motile sperm cells in 80 mL (NEN-ISO Citation9001, Varkens KI Nederland, 2011). The insemination doses are transported to the distribution area. The transport temperature is 17 ± 2°C and the transport boxes are temperature controlled, since a further reduced storage temperature limits their viability (De Leeuw et al. Citation1990; Watson Citation1995; Paulenz et al. Citation2000). To maintain this temperature, the insemination doses are stored in temperature-controlled cabinets at the farms until use.
For the current AI companies, it is important to achieve the highest reproductive efficiency per sold insemination dose obtained and diluted from boars. Reproductive performance has a genetic basis, however it is highly affected by environment. For optimal genetic expression, the environmental effects should be reduced. Reducing the variation in fertility results caused by variation in semen quality will enhance genetic expression in fertility results at farm level. The selection criteria for semen approval need to be validated. With this knowledge, the production of semen doses can be optimised for fertility. Therefore, constant achieving fertilising efficiency in the field with insemination doses produced will result in a faster dissemination of desired genes by which the difference with the nucleus breeding top decreases (personal communication EHAT Hanenberg, TOPIGS).
From the initial stages of AI development until the present time, the assessment of the percentage moving (motile) semen is the most widely used test of semen quality (Salisbury et al. Citation1978). To improve quantifying the semen motility and sperm integrity bright field microscopy, differential interference contrast microscopy, epifluorescent microscopy with multiple fluorescent staining (Section 4.1) and CASA (Section 4.2) as well as sperm flow cytometry (Section 4.3) have contributed. The relationship of data obtained with these modern semen assessment techniques and field fertility has not been tested previously. Since the Dutch AI is eager to produce insemination doses as efficiently as possible without any fertility losses, we have evaluated the added predictive values of each of these novel techniques in the following subsections.
4.1. Microscopic semen motility analysis
Anthony van Leeuwenhoek (1632–1723) was a Dutch tradesman and is known to have made over 500 microscopes. Fewer than 10 have survived to the present day. His discoveries on the presence and movement of ‘animalculi’, which were sperm cells, were reported in 1677 (Leeuwenhoek Citation1678).
Nowadays the movement of semen is manually assessed with phase contrast microscopy by trained technicians. Usually there are two parameters for motility: quantity and quality of motile sperm cells. The cut-off values are set arbitrarily. Visual estimation of semen motility is a common laboratory test, but is the subject of discussion regarding the predictive value of fertility, and the subjectivity of the method. The World Health Organisation (WHO) recommends assessing by categorising semen as immotile (no movement), non-progressive motility and progressive motility (WHO Citation2010). The laboratory technicians of Varkens KI Nederland use a more detailed scoring system grading per 10% quality increase. It is difficult for a technician to grade the velocity of moving semen.
In one of our published studies (Broekhuijse et al. Citation2012a) an eight years’ time dataset of semen insemination doses from 110,186 ejaculates of 7429 boars were merged to fertility parameters of inseminations of 165,000 sows and these records were used for analysis. Only 6% of the total variation in fertility was due to boar- and semen-related parameters. Although semen motility is considered to be an important parameter to validate the quality of the ejaculate processed, microscopically assessed, it only minimally relates to fertility results under the current Dutch AI practice. Other boar- and semen-related parameters, like genetic line of the boar, are more relevant factors to select boars for AI purposes.
4.2. Computer-assisted semen analysis
In the 1940s, scientists started recognising the need for objective data on the movement of sperm cells (Amann and Katz Citation2004). Dott and Foster (Citation1979) first proposed a CASA approach to obtain an overall objective semen motility analysis. The first validated CASA system was presented at the Third International Conference on the Spermatozoon in 1978 (Amann Citation1979). Davis and Katz (Citation1993) reported an overview of over 120 papers that have been published, verifying CASA technology for semen analysis.
CASA is a computerised system, which visualises and digitises the image of sperm cell movement. By means of a stroboscopic principle with a frequency of 60 Hz pictures are taken from which the exact trajectory of each individual cell in the observed microscopic field is followed and recorded. Information on the kinematics of individual cells and ejaculate summary statistics can be calculated from CASA data. Next to motility and progressive motility (which are calculation results, based on settings of basic parameters), more movement parameters are assessed. Different CASA systems use different mathematical algorithms, so results are not automatically comparable among systems. But the results do give a perfect tool to link the CASA motility assessment scores with field fertility.
Despite the possibilities for objective and multiparametric motility analysis that are allowed by CASA, it is still of concern that different CASA systems intrinsically give different results (). Another concern is the intensive need for training of the users and evaluation and calibration of the settings of the equipment. CASA is currently one of the most popular methods to evaluate semen motility (Verstegen et al. Citation2002). In 2003, there were systems in use at approximately 1200 sites worldwide (Amann and Katz Citation2004), primarily in human andrology laboratories. Since then the highest market growth occurred in large human clinics and in larger production animal AI companies (e.g. pigs).
Figure2. Schematic representation of motility parameters recorded in a single sperm trajectory as recorded by CASA systems.
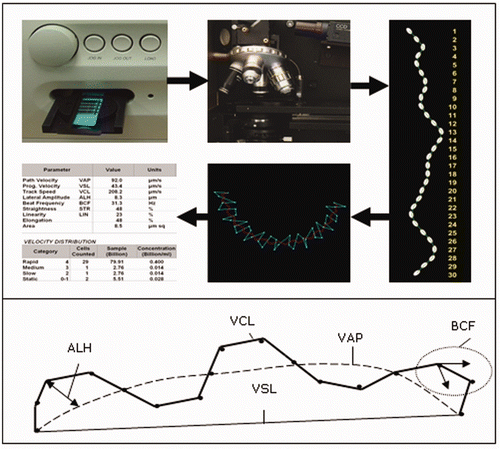
Using a CASA system in a high-productive pig AI laboratory had additional benefits over microscopic semen motility assessments (Broekhuijse et al. Citation2011b). The repeatability of CASA was enhanced by standardising for (1) an optimal sample temperature (39°C), (2) an optimal dilution factor, (3) optimal mixing of semen and dilution buffer by using mechanical mixing, (4) the slide chamber depth, and together with the previous points, (5) the optimal training of technicians working with the CASA system, (6) slide toxicity and (7) the use of a standard operating procedure (SOP). Once laboratory technicians were trained in using this SOP, they achieved a coefficient of variation of <5% which was superior to the variation found when the SOP was not strictly used (Broekhuijse, et al. Citation2011b).
Implementing a CASA system enables to establish the relation between semen motility and field fertility because more detailed semen motility parameters are studied (Broekhuijse et al. Citation2012b; ). In this study insemination records and semen parameters from a total of 45,532 ejaculates were collected over a three-year period. The boar- and semen-related variance (direct boar effect) was corrected for the effects of individual boar, genetic line of the boar, age of the boar, days between ejaculations, number of sperm cells in an ejaculate, number of sperm cells in an insemination dose and AI station. Boar- and semen-related parameters explained 5–6% of the variation in field fertility. Motility parameters, measured by CASA, explained 9–10% of the boar- and semen-related variation in field fertility. There were no significant differences between the effects of AI stations on fertility outcome, underscoring the objectivity of the CASA system used. Measuring motility parameters with CASA can be used to assess sperm motility in an objective manner and it enables to discriminate the fertilising capacity of ejaculates based on the motility pattern depending on genetic line of the boar in AI stations.
4.3. Flow cytometry
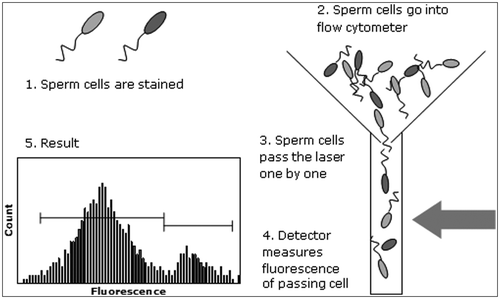
Another way for objective assessments of sperm samples is flow cytometry (). The principle of this technique is that a sperm cell suspension is introduced in fluid stream through a laser excitation source. Cells can be fluorescently labelled for certain cell characteristics. The cell size morphology is determined by scattered excitation light detection while the fluorescent properties per cell can be determined by fluorescence detectors. This technique allows rapid (up to 5000 cells/second) analysis of multiparametric properties (for a review about flow cytometry, see Nunez Citation2001). Semen motility only quantifies the movement of the cell, but does not give an indication about any other sperm cell characteristics. Other sperm quality characteristics could, for instance, thus be assessed by flow cytometry and may relate to field fertility results. Different parameters are assessed for thousands of sperm cells per second (for principle, see Nunez Citation2001). Flow cytometry is not only used for semen assessment but also routinely in the diagnosis of any given cell suspense, for instance, in human health for assessing blood cancer. The first flow cytometer was issued in 1953 to Wallace H. Coulter. The first fluorescent flow cytometer was developed in 1968 by Wolfgang Göhde and commercialised in 1969.
The strength of the flow cytometry technique compared to microscopic sperm assessments is that measurements on large numbers of sperm cells can be made within a short time frame. Different subsets of cells can be identified and quantified and sperm integrity parameters can be measured. Flow cytometry allows assessment of different semen quality characteristics. The system is relatively expensive and trained technicians are necessary to operate the system. Therefore, flow cytometry is not often used as the routine semen quality assessment method for pig AI.
Using flow cytometry in daily AI laboratory routine first needs examination whether or not currently used sperm integrity assessments with flow cytometry correlate with field fertility data. For this purpose 20 boars were followed for a 20-week period. From these ejaculates sperm cell integrity was assessed with respect to DNA and to membrane integrity, acrosome intactness, acrosome responsiveness and mitochondrial potential using established flow cytometric assays. This was done on freshly produced semen and on semen stored for up to 15 days (Broekhuijse et al. Citation2012c). Remarkably, none of the individual membrane integrity parameters were significantly related to fertility results. In contrast, the amount of DNA damage as assessed after >7 days of semen storage significantly related to field fertility. Thus, the detection of the degree of DNA damage in stored boar semen samples can be used to predict the fertilising capacity of boar ejaculates.
5. Discussion
Efficient dissemination of genes of high-indexed boars is one of the main goals of both AI and breeding companies. The effect of these high-indexed boars in the cost efficiency of meat production is evident. Hence the more sows bred per boar, the higher the impact on production costs. Optimisation of the fertilising capacity of boars in this context is critical. At the finisher production level it allows increased production efficiency and will thus increase feed efficiency and uniformity on finisher level. This results in decreasing the genetic lack between the top and the bottom of the breeding pyramid. Identifying and excluding terminal sires with relatively low fertility becomes more and more critical in the economic context. With knowledge on predicting porcine male fertility, the AI companies can make a step forward in efficient and guaranteed high semen quality production of insemination doses. Collecting field fertility data and merging these with ejaculate records is a very strong tool for both AI and breeding companies and can be used to identify sub-fertile boars. The data analyses performed are based on actual data, representing the relation between semen quality characteristics and field fertility. The AI company can use the results as a tool for efficient production and processing of insemination doses. Results show a clear relation with fertility, and with standardised objective methods such as CASA even more than with microscopic methods. Optional other tests (flow cytometry) are suggested. DNA fragmentation is indicated as a predictive test, and also membrane integrity tests are optional to be relevant in boar populations where less indirect selection on boar fertility was achieved. Finding a minimum set of tests with maximal functional coverage is an on-going process, along with the understanding of the quality characteristics essential in sperm cells for optimal fertilisation. The value of an accurate and reproducible semen analysis is clearly addressed: knowledge can be extremely helpful in the stepwise development of producing semen with the quality for further improvement in field fertility.
Acknowledgements
This research was supported by Varkens KI Nederland (Deventer, the Netherlands), Genossenschaft zur Förderung der Schweinehaltung (Ascheberg, Germany), Hamilton Thorne Inc. (Beverly, MA, USA) and Leja Products B.V. (Nieuw-Vennep, the Netherlands).
References
- Agricultural Economic Institute Wageningen University (LEI). 2011. Agrimonitor. Actuele informatie uit de land-en tuinbouw. [cited 2012 Feb]. Available from: www.lei.wur.nl
- Agrovision Kerngetalspiegel. 2010–2011. [cited 2012 May]. Available from: www.agrovision.nl
- Althouse , GC and Lu , KG . 2005 . Bacteriospermia in extended porcine semen . Theriogenology , 63 ( 2 ) : 573 – 584 .
- Amann , RP . 1979 . “ Computerized measurements of sperm velocity and percentage of motile sperm ” . In The spermatozoon , Edited by: Fawcett , DW and Bedford , JM . 431 – 425 . Baltimore : Urban and Schwarzenberg .
- Amann , RP and Katz , DF . 2004 . Reflections on CASA after 25 years . J Androl , 25 : 317 – 324 .
- Broekhuijse , MLWJ , Feitsma , H and Gadella , BM . 2011a . Field data analysis of boar semen quality . Reprod Dom Anim , 46 ( 2 ) : 59 – 63 .
- Broekhuijse , MLWJ , Soštarić , E , Feitsma , H and Gadella , BM . 2011b . Additional value of computer assisted semen analysis (CASA) compared to conventional motility assessments in pig artificial insemination . Theriogenology , 76 : 1473 – 1486 .
- Broekhuijse , MLWJ , Soštarić , E , Feitsma , H and Gadella , BM . 2012a . The value of microscopic semen motility assessment at collection for a commercial artificial insemination centre, a retrospective study on factors explaining variation in pig fertility . Theriogenology , 77 : 1466 – 1479 .
- Broekhuijse , MLWJ , Soštarić , E , Feitsma , H and Gadella , BM . 2012b . Application of computer assisted semen analysis to explain variations in pig fertility . J Anim Sci , 90 ( 3 ) : 779 – 789 .
- Broekhuijse MLWJ, Soštarić E, Feitsma H, Gadella BM. 2012c. Relation of flow cytometric sperm integrity assessments with boar fertility performance under optimized field conditions. J Anim Sci, Epub ahead of print 2012 July 24
- Davis , RO and Katz , DF . 1993 . Operational standard for CASA instruments . J Androl , 14 : 385 – 394 .
- De Leeuw , FE , Colenbrander , B and Verkleij , AJ . 1990 . The role membrane damage plays in cold shock and freezing injury . Reprod Domest Anim , l ( 1 ) : 95 – 104 .
- Donadeu , M . 2004 . Advances in male swine artificial insemination (AI) techniques . Pig J , 54 : 110 – 122 .
- Dott , HM and Foster , GC . 1979 . The estimation of sperm motility in semen, on a membrane slide, by measuring the area change frequency with an image analyzing computer . J Reprod Fertil , 55 : 161 – 166 .
- Dutch Association of Cooperative Pig AI Centres. 2010. Year report, Deventer, the Netherlands
- Feitsma , H . 2009 . Artificial insemination in pigs, research and developments in the Netherlands: a review . Acta Sci Vet , 37 ( 1 ) : 61 – 71 .
- Flowers , WL . 1997 . Management of boars for efficient semen production . J Reprod Fertil , 52 : 67 – 78 .
- Hancock , JL and Hovel , GLR . 1959 . The collection of boar semen . Vet Rec , 71 : 664 – 665 .
- Heape , W . 1897 . The artificial insemination of mammals and subsequent possible fertilization or impregnation of their ova . Proc R Soc Lond B , 61 : 52 – 63 .
- Ito , T , Niwa , T and Kudo , A . 1948 . Studies on artificial insemination in swine . Zootech Exp Sta Res Bul , 55 : 1 – 74 .
- Ivanov , EI . 1907 . De la fécondation artificielle chez les mammifères . Arch Sci Biol , 12 : 377 – 511 .
- Ivanov , EI . 1922 . On the use of artificial insemination for zootechnical purposes in Russia . J Agric Sci , 12 : 244 – 256 .
- Leeuwenhoek , A . 1678 . De natis è semine genitali animalculis . R Soc (Lond) Philos Trans , 12 : 1040 – 1043 .
- Lunenfeld , B and Insler , V . 1993 . “ Infertility: the dimension of the problem ” . In Infertility: male and female , Edited by: Insler , V and Lunenfeld , B . 3 – 7 . Edinburgh : Churchill Livingstone .
- McKenzie , FF . 1931 . A method for collection of boar semen . J Am Vet Med Assoc , 78 : 244 – 246 .
- Milovanov VK. 1964. Artificial insemination of livestock in the U.S.S.R. (Trans. by A Birron and ZS Cole. S Monson), Washington, DC: Jerusalem Technical Services, U.S. Department of Commerce
- NEN-ISO 9001. Nederlands Normalisatie-instituut – Internationale Organisatie voor Standaardisatie (NEN-ISO). 2011. Varkens KI Nederland kwaliteitshandboek
- Nishikawa Y. 1962. Fifty years of artificial insemination of farm animals in Japan. English Bull 2, Kyoto, Japan: Kyoto University
- Nishikawa Y. 1964. History and development of artificial insemination in the world. In: Proceedings of the 5th International Congress on Animal Reproduction and Artificial Insemination, Trento, Italy, Vol. 7, 163–259, Veterinary Institute, Skopje
- Niwa T. 1958. Artificial insemination with swine in Japan, Chiba-Shi, Japan: National Institute of Agricultural Science
- Nunez , R . 2001 . Flow cytometry: principles and instrumentation . Curr Issues Mol Biol , 3 ( 2 ) : 39 – 45 .
- Paulenz , H , Kommisrud , E and Hofmo , PO . 2000 . Effect of long-term storage at different temperatures on the quality of liquid boar semen . Reprod Dom Anim , 35 : 83 – 85 .
- Philips , PH and Lardy , HA . 1940 . A yolk-buffer pabulum for the preservation of bull semen . J Dairy Sci , 23 : 399 – 404 .
- Polge , C . 1956 . The development of artificial insemination service for pigs . Anim Breed Abstr , 24 : 209 – 217 .
- Pursel , VG and Johnson , LA . 1975 . Freezing of boar spermatozoa: fertilizing capacity with concentrated semen and a new thawing procedure . Anim Sci , 40 ( 1 ) : 99 – 102 .
- Roberts , SJ . 1986 . “ Infertility in male animals ” . In Veterinary obstetrics and genital diseases: theriogenology , 3rd , Edited by: Roberts , SJ . 752 – 893 . North Pomfret, Vermont : Edwards Brothers .
- Salisbury , GW , VanDemark , NL and Lodge , JR . 1978 . Physiology of reproduction and artificial insemination of cattle , 2nd , San Francisco : W. H. Freeman Co .
- Siebenga J. 1937. Kunstmatige inseminatie bij runderen. [Artificial insemination in cattle]. Universiteit Utrecht, Utrecht
- Spallanzani L. 1784. Dissertations relative to the natural history of animals and vegetables. (Trans. by T. Beddoes) in Dissertations Relative to the Natural History of Animals and Vegetables. London: J. Murray, 195–199
- Strikwerda R. 2007. Revolutie in het dierenrijk. [Revolution in the animal kingdom] Nationaal Veeteelt Museum, Beers
- Verstegen , J , Iguer-ouada , M and Onclin , K . 2002 . Computer assisted semen analyzers in andrology research and veterinary practice . Theriogenology , 57 : 149 – 179 .
- Watson , PF . 1995 . Cooling of spermatozoa and fertilizing capacity . Reprod Domest Anim. , 31 ( 1 ) : 135 – 140 .
- World Health Organisation. 2010. WHO laboratory manual for the examination and processing of human semen. 5th edition. Washington, DC: WHO