Abstract
The goal of this case-series was to increase our understanding of some complex within and between-host infection dynamics through the creation of mathematical and computational models that are able to capture the existing host and/or parasite heterogeneity. This goal was reached through a series of research projects (regarding experimental autoimmune encephalomyelitis (EAE) in mice, Mycobacterium avium subspecies paratuberculosis infection in cattle, Eimeria acervulina infection in chicken and human malaria) that gradually build up in complexity of both the system modelled and the modelling techniques used. In this case-series, the vast majority of model components have a direct link with reality. The results have shown some detailed examples of the valuable contribution that models have in understanding infection processes. The most satisfying achievements have come from those models that were able to, in hindsight, make complicated experimental results seem obvious and logical, and where the process of building the model was as insightful as the final results. The models created in these projects help to explain a wide range of sometimes contradictory experimental results and are used to predict the effect of control measures. In addition, they generate ideas for the development of new methods of control.
1. Introduction
In order to survive, infectious agents such as viruses, bacteria, protozoa and macroparasites (for convenience I will call them all parasites here) must succeed at two scales: the within-host scale and the between-host scale. Within-host survival involves processes such as reproduction and immune evasion, while between-host survival involves transmission and persistence in the host population. Within the host, the parasite needs to find a balance between exploiting the host's resources to grow, and keeping the host fit enough to transmit to a new host. At the same time, the host's immune system will apply a wide selection of attack mechanisms aimed at killing the parasite as soon as possible. To survive, parasites have evolved intricate techniques allowing them to hide from the immune system or evade getting killed, for as long as possible. At the between-host level the infecting parasites need to be able to transmit to and infect at least one other host to prevent infection from dying-out in the population. To persist in a population for a longer period of time, parasites have evolved mechanisms to find enough susceptible hosts to which they can transmit. For example, some parasites can change their outside appearance so that immune hosts cannot recognize them anymore, or hide inside host cells until transmission conditions are favorable.
When looking for measures to control infection, it is important to know which of the many components, interactions and influences that are involved in the above-mentioned infection processes are key in defining the infection. More insight in these allows us to focus our effort on understanding and learning how to influence these factors to our advantage. Using experimental methods alone it can be difficult, if not impossible, to find these key interactions as there are often practical, technical and financial limitations. This is where computational models can help out. For example, turning an interaction on and off to analyse its function may be an impossible task in reality, while in a computer model it can be as simple as a click. The difficulty, however, then lies in formulating a model with the limited knowledge available that is both sensible and useful. Models are caricatures of a much more complex reality and to be useful they need to be able to reproduce the essential characteristics of the real processes that are being studied. If that can be achieved, then using models, alongside experiments, opens up a myriad of new possibilities to both generate and test hypotheses.
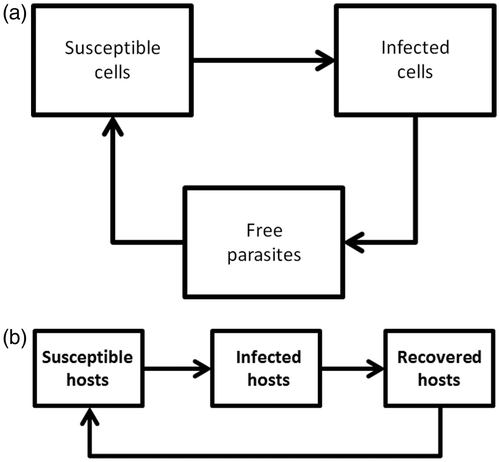
Models are particularly helpful to study systems where cause and consequence are so entangled that it makes the interpretation of experimental data very hard or experiments hard to perform. This is typically the case in systems where the interactions between the components form one or multiple interacting feedback loops. In the study of infectious disease, we find these feedback loops at both the within and between-host level. Two examples are given in . shows a typical within-host infection loop for a parasite with intracellular reproduction. Figure 1b the classical SIR-model, one of the simplest of epidemic models.
In terms of control measures, the aims for understanding within-host versus between-host processes are different. In general, efforts to understand within-host infection and immune processes are aimed at learning how to prevent a host from becoming infected, how to reduce clinical manifestation, or how to cure a host from infection. Efforts to understand between-host spread and infection processes are generally aimed at preventing parasites to cause an epidemic, or to slow down and stop outbreaks. To illustrate, a simple scenario could be that understanding the within-host processes tells us how to develop a vaccine whereas understanding the between-host processes tells us how many and which individuals we need to vaccinate. We therefore need understanding at both scales of infection, and their interaction, to eliminate a parasite from the population.
Within-host and between-host models can be made separately to help us gain such understanding. As such models describe processes at only one scale of the infection, they need to include some assumptions as to what happens at the other scale. For example, in a within-host model, infection is usually started by introducing a set dose of infection into the system. In reality, depending on how transmission took place, the initial dose of infection taken-up may vary greatly. As long as the dose of infection does not affect how the infection process continues, this assumption does not affect the results of the within-host model. For between-host models it is usually assumed that all hosts within a compartment, such as a ‘Recovered and Immune Hosts’ compartment, are equal. In reality, this is not the case, during infection individual hosts may use slightly different parts of the infecting parasite to generate an immune response, and therefore show differences in immune memory. Yet, as long as all these individuals become equally resistant against re-infections with that specific parasite, this assumption does not affect the validity of the between-host model.
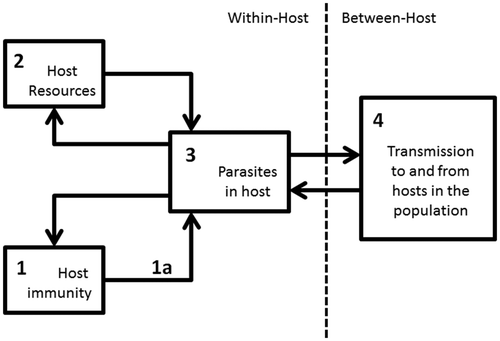
However, some parasites have more complex infection dynamics that entail feedback loops that breach the two scales of infection in such a way that the key processes at one scale are dependent on the processes of the other scale. Such a system of interacting feedback loops across the within-host and between-host scales is illustrated in . In infection processes where this is the case, studying infection at only one level may no longer be sensible or will no longer give realistic results (Mideo et al. Citation2008). Several studies have shown the importance of models that include feedback loops between infection processes at different scales (Coombs et al. Citation2007; Klinkenberg and Heesterbeek Citation2007; Boldin and Diekmann Citation2008). In many cases, these feedback loops that link the within-host and between-host scale cause heterogeneity in the host population. This can occur, for example, because differences in exposure between individuals cause differences in infection history, leading to heterogeneity in susceptibility and infectivity. Studies that take into account this kind of heterogeneity caused by processes across infection scales are scarce.
Figure 2. Schematic representation of an infection process that includes feedback loops between the parasites (3), the host resources (2), the host's immunity (1) and transmission (4).
The goal of this case-series is to increase our understanding of some of these complex within and between-host infection dynamics through the creation of mathematical and computational models that are able to capture the existing host and/or parasite heterogeneity. This goal is reached through a series of research projects that gradually build up in complexity of both the system modelled and the modelling techniques used. The models created in these projects help to explain a wide range of sometimes contradictory experimental results and are used to predict the effect of control measures. In addition, they generate ideas for the development of new methods of control.
2. Case series
2.1. Experimental autoimmune encephalomyelitis in mice
The first research project of this series focuses on experimental autoimmune encephalomyelitis (EAE), a mouse model of the human auto-immune disease multiple sclerosis (Raine Citation1984). The full project was published previously (Severins et al. Citation2008). In this project, the degree of complexity is limited to interacting feedback loops within the immune system itself, without an infectious parasite. In the context of , this project only involves feedback loops within compartment 1.
EAE is caused by auto-immune cells that build a response against a protein of the nervous system. Typically, adaptive immune responses, whether they are built against a foreign pathogen or a protein originating from the body itself, can be of type 1 (cellular response) or type 2 (antibody response). These responses can be controlled by regulatory cells that the immune system has at its disposal, which is often observed in chronic diseases. A large body of experimental data on EAE shows that the type of the auto-immune cells, and also the type of the cells that can control the autoimmune cells, are key to whether or not mice develop EAE (Kumar and Sercarz Citation1998). Therefore, although it is a non-infectious disease, understanding the interactions between the immune cells that are involved in EAE is very relevant to infectious diseases.
The model formulated in this research project was built to better understand these interactions between adaptive immune cells. The model is an ordinary differential equation (ODE) model that describes a set of interacting feedback loops formed by cell populations within the immune system that are involved in EAE. shows a visual representation of these components and highlights the different feedback loops.
Figure 3. Schematic representation of the EAE model. (a) The full model. (b) Highlights of the positive feedback loops within the immune cell populations. (c) Highlights of the interacting negative feedback loops between the immune cells.
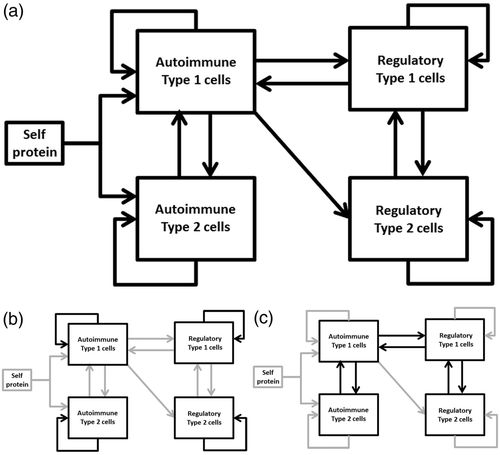
There are many more immune cells and interactions that influence EAE than the cells implemented in this model. However, this model can reproduce the results of a range of different vaccination experiments in mice with EAE. The strength of the model lies in just that. Because the results found in the vaccination experiments can be explained by the interactions between only the components used to build the model, it reduces the complexity of the experimental results to only those elements of the immune system implemented in the model.
The fact that regulatory cells could shift the autoimmune response from a type 1 to a type 2 following vaccination was initially conceived with surprise (Cohen Citation2001). Our modelling results show, however, how such a shift results from the interactions between regulatory cells and type 1 and type 2 autoimmune cells, without the need for any direct influence of regulatory cells on the type of autoimmune cells. The shift arises naturally because type 1 regulatory cells only suppress the type 1 autoimmune cells, and thus allow the type 2 autoimmune cells to grow in response to stimulation with self-protein.
2.2. Paratuberculosis in dairy cows
The second research project focuses on a bacterial infection in dairy cows that causes the disease paratuberculosis. Paratuberculosis, as EAE, is a chronic disease. However, in contrast with EAE it is caused by an infectious agent. Once initiated, infection is viewed as an autonomous process, where subsequent doses of infection later in life have little or no influence on the course of infection within the host. This allows the infection process to be studied at the within-host scale only. In this case, we can add a small step to the degree of complexity by having two interacting feedback loops describing within-host infection and immunity. One feedback loop describes parasite reproduction that is influenced differently by type 1 and type 2 immune responses, and the other describes the feedback loop between the parasite and the host's resources. The latter in this case also happens to be a part of innate immunity. These interacting feedback loop and their influences are illustrated in . In the context of , this project includes feedback loops between components 2 and 3 under the influence of 1a.
Figure 4. Schematic representation of the paratuberculosis model. (a) The full model. (b) Highlights of the within-host parasite reproduction cycle including a feedback loop with the host resources through the attraction of uninfected macrophages to the site of infection by free parasites. (c) Highlights of the differential influence of Type 1 and Type 2 adaptive immunity on the reproduction feedback loop.
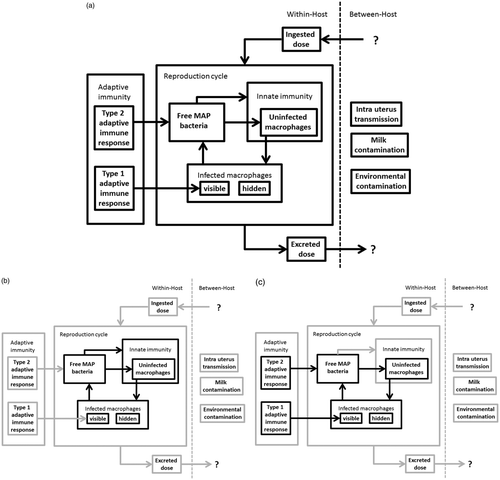
Paratuberculosis is caused by Mycobacterium avium subspecies paratuberculosis (MAP) (Behr and Collins Citation2010). When ingested by its host, MAP is able to cross the epithelial barrier lining the gut and infect macrophages inside the gut. Macrophages form part of our innate immune system and under normal conditions engulf and kill bacteria. MAP is able to avoid being killed after it is engulfed (de Chastellier Citation2009). On the contrary, it is able to reproduce inside the macrophages and establish a chronic infection for which no successful treatment is currently available. Cows differ greatly in the duration and the extent to which they can keep this chronic infection under control before it eventually progresses into severe disease.
It is unclear what causes the onset of clinical disease. A variety of studies indicate that cows build an early type 1 immune response that changes to a type 2 immune response during the course of the disease (Stabel Citation2006). It is thought that the type 1 response is protective while the type 2 response is permissive, and that the change in response could be the cause of progression into severe disease (Sweeney et al. Citation1998; Koets et al. Citation1999; Stabel Citation2000). However, direct evidence and a thorough understanding why one type of response would be able to control disease while the other fails, is lacking. The model in this project was built to clarify the role of the type of the adaptive immune response on the infection progression.
Type 1 adaptive immune responses kill parasite-infected host cells, while type 2 adaptive immune responses target free parasites in the body. This known functional difference in which the two types of adaptive immune responses combat infection is explicitly included in the model. In the model, it is easy to turn each of these responses on and off at any given time which allows for a detailed study of the effects these two responses have on MAP infections in dairy cows.
Through these analyses and by using the results from a longitudinal study in which infected cows were followed for four years, the model is able to indicate that there is no fundamental mechanical impediment to prevent a type 1 or a type 2 adaptive response from clearing the infection. The results show that the killing rate of the type 1 and type 2 adaptive immune responses together is much stronger than the sum of their individual killing rates. Surprisingly the results also show that the type 1 response observed experimentally is able to stop the initial growth of infection even if it does not have the critical killing rate needed to control the infection.
2.3. Coccidiosis in broiler chickens
The third research project focuses on coccidiosis in broiler chickens caused by the protozoan parasite Eimeria acervulina. The details of the full project were published previously (Severins et al. Citation2007). This project is the first to include parasite infection processes that breach the within-host and between-host scales. The within-host life cycle of this parasite is such that it allows for a less detailed modelling of the within-host processes than the previous projects, and we can therefore keep the degree of complexity more manageable. In the context of , this project includes feedback loops between components 1, 2 and 4 without explicitly describing the processes in component 3 through which these feedback loops interact.
Chickens become infected by picking-up Eimeria oocysts from their environment, which then replicate inside the gut of the animals and are excreted with the chickens’ faeces (Allen and Fetterer Citation2002). Eimeria reproduction inside the gut is a self-limiting process that cannot continue to re-infect gut cells without going through an environmental stage. How many parasites on average one Eimeria parasite can produce and excrete depends on how many parasites were picked-up at the same time. Excretion also depends on how many were picked-up previously, as these will be occupying some of the gut cells that would otherwise be available for infection (Williams Citation1973). In addition, chickens develop immune responses against Eimeria parasites such that every new case of infection protects the chickens better against infection and parasite reproduction, until they are fully immune (Chapman Citation1999; Lillehoj and Lillehoj Citation2000). Thus, the infection history of the chickens has an effect on the immune status of the chickens and also on the infectiousness of the chickens, i.e. on how many oocysts the chickens will excrete into the environment.
Ingesting small numbers of oocysts over a prolonged period of time will not cause much harm to the chickens. However, in stables of broiler chickens, characterized by a dense population of susceptible hosts, the environmental contamination can quickly grow to levels where chickens ingest high doses of oocysts, causing severe outbreaks of clinical disease. The cleaning intensity between cohorts of broilers is thought to affect the number of clinical cases, yet studies trying to show this revealed counterintuitive results (Henken et al. Citation1994; Graat et al. Citation1996; Klinkenberg and Heesterbeek Citation2007). The model built in this project is used to understand the progression of infection in flocks of commercial broiler chickens and to assess if there exist cleaning regimes that minimize the clinical disease cases.
To study the effect of the infection history on the infection processes, the model needs to be able to capture the infection history of the individual chickens, their immune status and the environmental contamination. To allow for this kind of heterogeneity the model is spatial and individual-based, meaning that each chicken can be tracked separately; we use the simulation software Netlogo (Northwestern University, Evanston, IL, USA) to implement the model. The two effects that the infection history has on the within-host infection are described by two interconnected feedback loops, as illustrated in .
Figure 5. Schematic representation of the coccidiosis model. (a) The full model. (b) Highlights of the within-between host infection feedback loop. (c) Highlights of the within-between host immunity feedback loop.
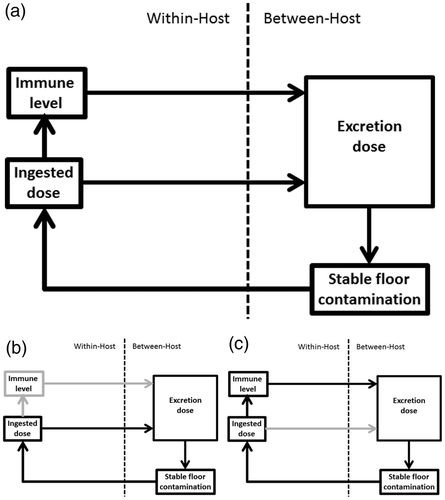
The different immune statuses, and ingestion and excretion doses, are limited in the model, which, together with the self-limiting within-host infection process of the Eimeria parasite, allows for the modelling of the excreted doses through the use of a limited set of fixed excretion patterns. The model uses specific excretion-templates, supported by experimental data, for every combination of infection and immune status. Furthermore, chickens switch back and forth between templates depending on the oocysts they ingest and their immune status. As a result, the heterogeneity in the chicken population is sufficiently large to capture its effect on the within-host infection and immune processes without having to model the mechanisms behind these processes explicitly.
In the model, different stable cleaning intensities and regimes can be simulated with different initial stable contaminations. The model shows a non-linear ‘wave-like’ relationship between the intensity of cleaning and the severity of Eimeria outbreaks in a flock where intermediate levels of cleaning intensity lead to the least clinical disease. This relationship explains the counterintuitive experimental findings and is caused by an increased heterogeneity in the host population at the peaks of the wave. The heterogeneity originates from stochastic parasite uptake that results in differences in infection between the chickens in the flock. There are cleaning intensities at which the feedback loops across the two infection scales either amplify or dampen the heterogeneity. This relationship could therefore not have been found from a model that does not allow for considerable host heterogeneity or that only models the infection dynamics at one of these scales.
2.4. Malaria in humans I
The fourth research project concentrates on Malaria in humans caused by the eukaryotic protist Plasmodium falciparum. Malaria parasites have an intricate infection process with many life stages in two different hosts: mosquitoes and humans. There are many different reactions of the immune system against malaria parasites. In turn, the parasites possess a wide range of immune evasion techniques to avoid the attacks by the immune system. In contrast to the previous project, this project describes in detail a within-host feedback loop between infection and adaptive immunity in addition to the interacting set of feedback loops across the within and between-host scales, as described in the previous project. In the context of , this projects includes feedback loops between components 1, 3 and 4 and a large amount of host and parasite heterogeneity to capture the effect of the interactions between these components.
When a parasite infects a cell, some parts of it usually show on the outside of the surface of the cell. The immune system uses these parts (called antigens) to identify infected cells and then build a response to kill them. This project concentrates on the variant surface antigens (VSA) that malaria parasites use to avoid these immune responses. The particular VSA considered here, P. falciparum erythrocyte membrane proteins 1 (PfEMP1's), are expressed on the surface of red blood cells (rbc) infected during the parasite's blood-stage. These VSA help parasites to survive in two ways: (1) by frequently changing the type of VSA expressed, the antibodies that the immune system has made against previously expressed VSA become less effective (Miller et al. Citation1994; Borst et al. Citation1995) and (2) VSA bind to receptors in organs which prevents the infected rbc from flowing through the spleen where infected rbc are recognized and killed (Ho and White Citation1999; Frank and Deitsch Citation2006). The strength of this binding affinity of the VSA therefore, at least partially, determines the relative growth rate of the parasites.
Each parasite carries a set of approximately 60 different types of VSA and different parasites carry different sets of VSA (Gardner et al. Citation2002). Due to several parasite recombination techniques, new types of VSA emerge continuously and therefore the number of different types of VSA that exist is immense (Taylor et al. Citation2000). Individuals living in malaria-endemic areas gradually build up a very large repertoire of antibodies against VSA associated with protection first from severe disease and eventually from clinical disease, although infections remain common even at old age (Beeson et al. Citation2008).
Experimental data show that the different types of VSA can be categorized into genetically similar groups (Kraemer and Smith Citation2003; Lavstsen et al. Citation2003). Data also suggest that every parasite does not carry a random set of VSA but that all VSA groups are proportionally represented in the set (Bull et al. Citation2005; Buckee et al. Citation2009). It is unclear why parasites carry representatives of all groups. One line of thought is that parasites need the VSA from the different groups to be able to cope under different circumstances. For example, when infecting young children with a relatively narrow anti-VSA antibody repertoire, the parasite can afford to express a VSA that maximizes its growth rate, while when infecting an older host with a broad antibody repertoire, the parasite has to express a very rare VSA for which the host has no antibodies yet. It has been suggested that the difference between a young host and an older host environment would benefit parasites that carry both ‘strong’ and ‘rare’ VSA (Kraemer et al. Citation2007; Bull et al. Citation2008). The model built in this project was developed to understand whether under these circumstances parasites with a strong-rare set of VSA are more successful than parasites with other sets of VSA.
To recreate the VSA-related difference in young hosts versus old hosts, the model needs to include the within-host parasite competition and immune responses that determine to which VSA the hosts build up antibodies, and to include the between-host competition for transmission to mosquitoes and susceptible hosts. The model also needs to include a diverse range of VSA-related host, vector and parasite heterogeneity. To accommodate all these processes and heterogeneity, the object-orientated programming language C++ is used to develop the model and run infection simulations. The model keeps track of, amongst other things, the VSA of each parasite in the parasite population, the VSA expressed by infected rbc and the anti-VSA antibodies of the hosts. illustrates the multiple interacting feedback loops, across both infection scales.
Figure 6. Schematic representation of malaria I model. (a) The full model with all interacting feedback loops. (b) Highlights of the within-host feedback loop between adaptive immunity and parasite reproduction. (c) Highlights of the feedback loop between host adaptive immunity and transmission. (d) Highlights of the feedback loop between parasite reproduction and transmission.
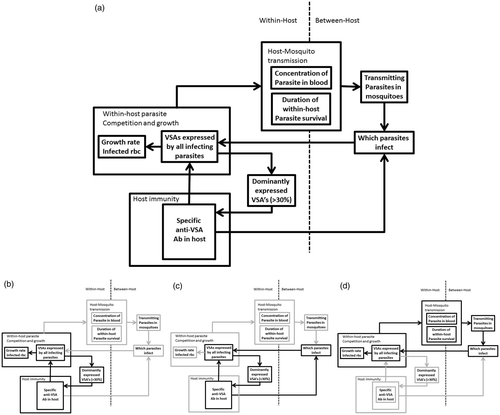
In the resulting model, the difference in the size of the anti-VSA immune repertoire between young hosts and older hosts indeed emerges. Additionally, the model shows that hosts do not acquire antibodies against VSA at random but that there is a general order in which they are acquired in which immunity against strong VSA is acquired earlier. An ordered acquisition of anti-VSA antibodies was also found experimentally (Cham et al. Citation2009) and the model now gives a potential explanation for the origin of this order. However, contrary to what has been suggested, the difference in a young versus older host environment, at least on its own, does not show a selective advantage for parasites with a strong-rare set of VSA over parasites with other, random, sets of VSA.
2.5. Malaria in humans II
The fifth and last research project also concentrates on the VSA used by malaria parasites. The full details of this project were published previously (Severins et al. Citation2012). However, the focus of this project lies on understanding the key selection forces on the parasites through their VSA and the influence that control measures can have on these. The selection forces work on all interactions shown in and therefore this project includes all components and interacting feedback loops illustrated in . As in the previous project, a large amount of host and parasite heterogeneity is allowed to capture the effect of the feedback loops between these components on infection.
As explained in the previous section, the binding strength of the VSA, at least partially, determines the relative growth rate of the parasites, which in turn has an effect on the severity of malaria. Disease severity is influenced by the type of VSA expressed in yet another way. The type of VSA expressed determines in which organ(s) the infected rbc can sequester, which has a large impact on the severity of disease, e.g. cerebral malaria is one of the most deadliest forms of malaria (Miller et al. Citation1994; Rottmann et al. Citation2006; Kaestli et al. Citation2006; Pasternak and Dzikowski Citation2009). These findings show that the VSA set carried by the parasites impacts on the virulence of the parasite.
VSA are implicated in many of the infection processes of malaria, making it difficult to predict how large-scale malaria control programs will influence the virulence of the circulating parasites. This will depend on which parasites are able to survive under all the selection forces that affect the parasites through the VSA they carry. The key selection forces on malaria parasites are: parasite competition (within and between-host), host immunity, mosquito abundance and host mortality (Mackinnon and Marsh Citation2010). The model developed in the previous project already included some of these, namely, within-host parasite competition, although only the relative competition between parasites, between-host competition ( and ), host immunity () and mosquito abundance. For the purposes of the current project, the previous model is extended with feedback loops for the within-host parasite competition to infect the host's rbc () and malaria-induced host mortality (), to include all key selection forces that act on the parasites.
Figure 7. Schematic representation of malaria II model. (a) The full model. (b) Highlights of the feedback loop between parasite reproduction and the host's resources. (c) Highlights of the feedback loop between malaria-induced host mortality, transmission and parasite reproduction.
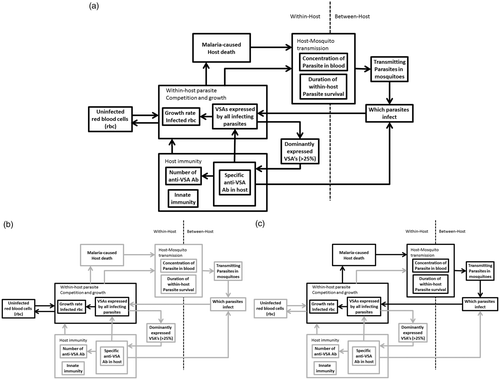
Like in the previous project, the model continues to show an ordered acquisition of anti-VSA antibodies. In addition, by including how the parasites compete to infect the available red blood cells, the model gives realistic life-time host parasitemia and provides a framework in which to study the within-host competition through VSA in detail.
The model is used to test the effect that a series of potential and currently implemented control measures can have upon the virulence of parasites circulating in the population. The results show that some seemingly sensible measures, such as reducing the reproduction rate of the parasites, could have the unwanted effect of increasing the frequency of highly virulent parasites, such as those causing cerebral malaria.
3. Final remarks
As mentioned above, the difficulty in modelling lies in formulating a model that is both sensible and useful. The chances for this are greatly improved when modellers and field experts work closely together. To facilitate these collaborations, it is important that modellers make models that are appealing to the expert community. The results and insights from a model that succeeds in being so, are much more likely to reach and be trusted by field experts.
A crucial element for the appeal of a biological model is for its components to have a clear biological meaning or interpretation. Some of the best models are those that do not require an expert in the modelling techniques used to understand it, but those that can be explained in biological terms. Such a direct link between the model and reality makes it easier to use existing data, or gather new data, to parameterize and validate the model. It also makes it straightforward for experimentalists to interpret the model results, give feedback and pinpoint errors.
In this case-series, the vast majority of model components have such a direct link with reality. The results have shown some detailed examples of the valuable contribution that models have in understanding infection processes. The most satisfying achievements have come from those models that were able to, in hindsight, make complicated experimental results seem obvious and logical, and where the process of building the model was as insightful as the final results.
References
- Allen , PC and Fetterer , RH . 2002 . Recent advances in biology and immunobiology of Eimeria species and in diagnosis and control of infection with these coccidian parasites of poultry . Clin Microbiol Rev , 15 : 58 – 65 .
- Beeson , JG , Osier , FHA and Engwerda , CR . 2008 . Recent insights into humoral and cellular immune responses against malaria . Trends Parasitol , 24 : 578 – 584 .
- Behr , MA and Collins , DM . 2010 . Paratuberculosis: organism, disease, control , Wallingford : CABI .
- Boldin , B and Diekmann , O . 2008 . Superinfections can induce evolutionarily stable coexistence of pathogens . J Math Biol , 56 : 635 – 672 .
- Borst , P , Bitter , W , McCulloch , R , Leeuwen , FV and Rudenko , G . 1995 . Antigenic variation in malaria . Cell , 82 : 1 – 4 .
- Buckee , CO , Bull , PC and Gupta , S . 2009 . Inferring malaria parasite population structure from serological networks . Proc R Soc B , 276 : 477 – 485 .
- Bull , PC , Berriman , M , Kyes , S , Quail , MA , Hall , N , Kortok , MM , Marsh , K and Newbold , CI . 2005 . Plasmodium falciparum variant surface antigen expression patterns during malaria . PLoS Pathog , 1 : e26
- Bull , PC , Buckee , CO , Kyes , S , Kortok , MM , Thathy , V , Guyah , B , Stoute , JA , Newbold , CI and Marsh , K . 2008 . Plasmodium falciparum antigenic variation: mapping mosaic var gene sequences onto a network of shared, highly polymorphic sequence blocks . Mol Microbiol , 68 : 1519 – 1534 .
- Cham , GKK , Turner , L , Lusingu , J , Vestergaard , L , Mmbando , BP , Kurtis , JD , Jensen , ATR , Salanti , A , Lavstsen , T and Theander , TG . 2009 . Sequential, ordered acquisition of antibodies to plasmodium falciparum erythrocyte membrane protein 1 domains . J Immunol , 183 : 3356 – 3363 .
- Chapman , HD . 1999 . Anticoccidial drugs and their effects upon the development of immunity to Eimeria infections in poultry . Avian Pathol , 28 : 521 – 535 .
- Cohen , IR . 2001 . T-cell vaccination for autoimmune disease: a panorama . Vaccine , 20 : 706 – 710 .
- Coombs , D , Gilchrist , MA and Ball , CL . 2007 . Evaluating the importance of within-and between-host selection pressures on the evolution of chronic pathogens . Theor Popul Biol , 72 : 576 – 591 .
- de Chastellier , C . 2009 . The many niches and strategies used by pathogenic mycobacteria for survival within host macrophages . Immunobiology , 214 : 526 – 542 .
- Frank , M and Deitsch , K . 2006 . Activation, silencing and mutually exclusive expression within the var gene family of Plasmodium falciparum . Int J Parasitol , 36 : 975 – 985 .
- Gardner , M J , Hall , N , Fung , E , White , O , Berriman , M , Hyman , RW , Carlton , JM , Pain , A , Nelson , KE Bowman . 2002 . Genome sequence of the human malaria parasite Plasmodium falciparum . Nature , 419 : 498 – 511 .
- Graat , EA , Ploeger , HW , Henken , AM , Reilingh , GDV , Noordhuizen , JP and Beek , PNV . 1996 . Effects of initial litter contamination level with Eimeria acervulina on population dynamics and production characteristics in broilers . Vet Parasitol , 65 : 223 – 232 .
- Henken , AM , Ploeger , HW , Graat , EA and Carpenter , TE . 1994 . Description of a simulation model for the population dynamics of Eimeria acervulina infection in broilers . Parasitology , 108 ( Pt 5 ) : 503 – 512 .
- Ho , M and White , NJ . 1999 . Molecular mechanisms of cytoadherence in malaria . Am J Physiol , 276 : C1231 – C1242 .
- Kaestli , M , Cockburn , IA , Cortés , A , Baea , K , Rowe , JA and Beck , HP . 2006 . Virulence of malaria is associated with differential expression of Plasmodium falciparum var gene subgroups in a case-control study . J Infect Dis , 193 : 1567 – 1574 .
- Klinkenberg , D and Heesterbeek , JAP . 2007 . A model for the dynamics of a protozoan parasite within and between successive host populations . Parasitology , 134 ( Pt7 ) : 1 – 10 .
- Koets , AP , Rutten , VP , Hoek , A , Bakker , D , van Zijderveld , F , Müller , KE and van Eden , W . 1999 . Heat-shock protein-specific t-cell responses in various stages of bovine paratuberculosis . Vet Immunol Immunopathol , 70 : 105 – 115 .
- Kraemer , SM , Kyes , SA , Aggarwal , G , Springer , AL , Nelson , SO , Christodoulou , Z , Smith , LM , Wang , W , Levin , E , Newbold , CI , Myler , PJ and Smith , JD . 2007 . Patterns of gene recombination shape var gene repertoires in Plasmodium falciparum: comparisons of geographically diverse isolates . BMC Genomics , 8 : 45
- Kraemer , SM and Smith , JD . 2003 . Evidence for the importance of genetic structuring to the structural and functional specialization of the Plasmodium falciparum var gene family . Mol Microbiol , 50 : 1527 – 1538 .
- Kumar , V and Sercarz , E . 1998 . Induction or protection from experimental autoimmune encephalomyelitis depends on the cytokine secretion profile of TCR peptide-specific regulatory CD4 T cells . J Immunol , 161 : 6585 – 6591 .
- Lavstsen , T , Salanti , A , Jensen , ATR , Arnot , DE and Theander , TG . 2003 . Sub-grouping of plasmodium falciparum 3d7 var genes based on sequence analysis of coding and non-coding regions . Malar J , 2 : 27
- Lillehoj , HS and Lillehoj , EP . 2000 . Avian coccidiosis: a review of acquired intestinal immunity and vaccination strategies . Avian Dis , 44 : 408 – 425 .
- Mackinnon , MJ and Marsh , K . 2010 . The selection landscape of malaria parasites . Science , 328 : 866 – 871 .
- Mideo , N , Alizon , S and Day , T . 2008 . Linking within-and between-host dynamics in the evolutionary epidemiology of infectious diseases . Trends Ecol Evol , 23 : 511 – 517 .
- Miller , LH , Good , MF and Milon , G . 1994 . Malaria pathogenesis . Science , 264 : 1878 – 1883 .
- Pasternak , ND and Dzikowski , R . 2009 . Pfemp1: an antigen that plays a key role in the pathogenicity and immune evasion of the malaria parasite Plasmodium falciparum . Int J Biochem Cell Biol , 41 : 1463 – 1466 .
- Raine , CS . 1984 . Biology of disease. Analysis of autoimmune demyelination: its impact upon multiple sclerosis . Lab Invest , 50 : 608 – 635 .
- Rottmann , M , Lavstsen , T , Mugasa , JP , Kaestli , M , Jensen , ATR , Müller , D , Theander , T and Beck , HP . 2006 . Differential expression of var gene groups is associated with morbidity caused by Plasmodium falciparum infection in Tanzanian children . Infect Immun , 74 : 3904 – 3911 .
- Severins , M , Borghans , JAM and de Boer , RJ . 2008 . T-cell vaccination , New York : Nova Science Publishers Inc .
- Severins , M , Klinkenberg , D and Heesterbeek , H . 2007 . Effects of heterogeneity in infection-exposure history and immunity on the dynamics of a protozoan parasite . J R Soc Interface , 4 : 841 – 849 .
- Severins , M , Klinkenberg , D and Heesterbeek , H . 2012 . How selection forces dictate the variant surface antigens used by malaria parasites . J R Soc Interface , 9 : 246 – 260 .
- Stabel , JR . 2000 . Cytokine secretion by peripheral blood mononuclear cells from cows infected with Mycobacterium paratuberculosis . Am J Vet Res , 61 : 754 – 760 .
- Stabel , JR . 2006 . Host responses to Mycobacterium avium subsp. paratuberculosis: a complex arsenal . Anim Health Res Rev , 7 : 61 – 70 .
- Sweeney , RW , Jones , DE , Habecker , P and Scott , P . 1998 . Interferon-gamma and interleukin 4 gene expression in cows infected with Mycobacterium paratuberculosis . Am J Vet Res , 59 : 842 – 847 .
- Taylor , HM , Kyes , SA and Newbold , CI . 2000 . Var gene diversity in Plasmodium falciparum is generated by frequent recombination events . Mol Biochem Parasitol , 110 : 391 – 397 .
- Williams , RB . 1973 . Effects of different infection rates on the oocyst production of Eimeria acervulina or Eimeria tenella in the chicken . Parasitology , 67 : 279 – 288 .