Abstract
Background: Periodontal disease in cats is a local disease that may have systemic consequences that are affected by treatment.
Objective: To test the hypotheses that systemic health indices would be correlated with the severity of periodontitis, and would improve with treatment.
Animals and methods: Apparently otherwise healthy cats from an in-bred colony were randomly assigned to a treatment group (n = 30), or a control group (n = 18), which was left untreated for 3 months. Periodontal disease was scored at baseline in the treatment group according to calculus, gingivitis, and alveolar bone loss measured from dental radiographs. Blood, urine and saliva were collected from both groups before, and 16, 45, and 90 days after dental treatment. Assays included haematology, urinalysis, serum biochemistry, serum IgG, salivary IgA, lymphocyte subsets and proliferation, and plasma malonyldialdehyde (MDA). Correlations between the severity of periodontitis and assays at baseline were assessed, and the effect of treatment determined using linear mixed model methodology.
Results: The severity of periodontitis was associated with age, bodyweight, total globulins (Globs), Alanine aminotransferase, and IgG, and negatively associated with albumin, haemoglobin, haematocrit, and Aspartate aminotransferase (AST). Treatment significantly reduced IgG, total Globs, AST, and eosinophils, and increased cholesterol. Other leucocyte assays and plasma MDA concentrations were not affected by the treatment. Cats ate dry food faster 1 week after, than they did 1 week before treatment.
Conclusions and clinical relevance: Although the clinical significance of these findings are unknown, we conclude that periodontitis is not simply a localized disease, but also impacts on systemic health and wellbeing.
1. Introduction
Dental disease can be considered the scourge of domesticated cats because of its prevalence, the morbidity it is associated with, the expense of treatment, and the difficulty of prevention. In a study in North America of 15,226 cats of all ages, calculus and gingivitis were reported to be present in 24% and 13% of all cats respectively, and “dental disease” was the most commonly reported disease (Lund et al. Citation1999). It is well recognized that periodontal disease in cats is associated with regional pain, but the systemic effects have not been well described, despite claims of significant effects by some authors (Gorrel Citation1998; Niemiec Citation2008).
In dogs, post-mortem examinations have revealed that there is a correlation between periodontal disease and a variety of degenerative and inflammatory diseases in the liver, kidney, and left AV heart valves (Pavlica et al. Citation2008). As the authors stated, this finding implies that periodontitis might contribute to the development of systemic pathology in dogs. In a very large cohort of primary practice admissions, found significant associations between severe periodontitis and endocarditis (Glickman et al. Citation2009). In another retrospective cohort study in dogs, the risk of azotaemia increased with increased severity of periodontitis (Glickman et al. Citation2011). Recently, a study of dogs with periodontal disease examined the correlation between several routine clinicopathological variables and the severity of disease (Rawlinson et al. Citation2011). In that study, the authors found that circulating platelet counts were positively correlated, and, surprising, that creatinine was negatively correlated with disease. Conventional routine indices of inflammation including leucocyte counts and serum C-reactive protein (CRP) were not correlated, and did not significantly change in response to treatment. However, the study by Rawlinson et al. (Citation2011) did not include an untreated control group, and evaluation of a response to treatment was not strictly possible, and may thus have been missed.
In man, there is a correlation between periodontal disease and myocardial infarction, stroke, and systemic markers of inflammation (Scannapieco et al. Citation2003; Craig Citation2004; Offenbacher et al. Citation2005). Circulating leucocytes, albumin (Alb):Glob ratios, and serum CRP and cytokines such as IFN-γ, TNF-α, IL-1β, IL-2, and IL-6, IgG1and IgG2 have been shown to be higher in patients with generalized periodontitis and localized periodontitis than controls (Scannapieco et al. Citation2003; Loos Citation2005). In one study, patients with chronic periodontitis had fewer circulating T cells (CD3 positive cells), and in vitro lymphocyte proliferation was reduced compared with healthy controls (Emingil et al. Citation2001). Other systemic markers of disease associated with periodontitis in humans include higher concentrations of acute-phase proteins and coagulation factors in plasma (Loos Citation2005). Thus, periodontitis in people is associated with systemic inflammation.
The etiology and pathogenesis of periodontal disease may involve dysfunctions in the cellular immune responses. Although relative counts of leucocyte subsets may not differ between normal humans and humans with severe periodontitis, lymphocytes isolated from patients with severe periodontitis have been shown to proliferate less when stimulated in vitro than those from healthy humans (Emingil et al. Citation2001). This has, in part, been attributed to the inhibitory action of some bacterial pathogens, which can differ between strains of the same species (do Vale et al. Citation2004).
Although suppression of some immunological functions has been demonstrated, the pathogenesis of periodontitis also involves destructive, potentially exaggerated host responses to the presence and products of periodontal pathogens (Borch et al. Citation2010). Several chronic or poorly regulated inflammatory diseases in humans have been associated with oxidative damage, including periodontitis. Damage from reactive oxygen species can occur from increased production by activated neutrophils, decreased antioxidant defence and abnormal mitochondrial production. Recent studies have revealed that humans with periodontal disease have an increase in plasma lipid peroxide products that is correlated with the severity of disease, and that the oxidative stress contributes to organ damage associated with periodontitis (Akalin et al. Citation2007).
Despite the prevalence of dental disease in domestic cats, the presence of the disease is not normally a primary reason for presentation to veterinarians. Accordingly, disease identification is usually made at the time of routine health checks, or as an incidental finding during examination for other disease. As such, owners may be reluctant to agree to aggressive treatment because of the absence of clinical signs that clearly indicate morbidity, or because of more apparently urgent problems. Conversely, dental treatment is not without cost or risk, and if there are no systemic effects of periodontal disease, it would not support aggressive early intervention. It is, therefore, important that we understand the systemic consequences of dental disease, and the improvement in those systemic consequences with appropriate treatment. In addition, identification of objective markers may allow disease grading, prognostication, and therapeutic guidance.
The objectives of this study were to assay a variety of biomarkers in cats with periodontal disease, and to compare those biomarkers in cats before and after appropriate treatment, with cats left untreated. Firstly, it was hypothesized that inflammatory markers would be altered in cats with dental disease, and secondly that they would improve following appropriate dental treatment. Lastly, it was hypothesized that with time, those makers would begin to recrudesce.
2. Materials and methods
2.1. Animals
The cats used in this study belonged to the Feline Nutrition Unit of Massey University, Palmerston North, New Zealand. The colony is a closed breeding unit used for the conduct of nutritional research. For the purpose of the study, approximately 100 cats were examined, and cats were selected for inclusion in the study on the basis of the gross appearance of dental calculus and/or gingivitis. The most severely affected 48 cats were selected for the study. All included cats were deemed otherwise healthy according to stable weight in the 8 weeks preceding the study, and a physical examination by one of the authors (NC). Cats were then randomized into treatment and control groups by assigning each cat a random number, ordering them, and dividing every consecutive triplet such that every 1st and 3rd cat was assigned to the treatment group (Group 1) up to a maximum of 30 cats, whilst the 2nd cat was assigned to the control group (group 2), and the final 2 cats were assigned to group 2. Thus, group 1 (dental treatment group) consisted of 30 cats, and group 2 (control group) consisted of 18 cats that were untreated for the first 3 months. This unbalanced design was a pragmatic compromise between our desire to treat and assay as many cats as possible within a defined experimental period, while maintaining an untreated control group for as short a time as possible. A semi-quantitative visual scoring system graded the apparent pre-existing dental disease by grading generalized calculus and gingivitis to produce a composite score from 0 to 4, and groups were compared at baseline prior to treatment to ensure there was no significant difference in apparent severity.
2.2. Diets
Cats were maintained on their staple canned diet throughout the experimental period, except in the peri-operative period. The staple diet was an American Association of Feed Control Officials (AAFCO) growth and maintenance protocol tested commercial moist diet. As estimated from reported guaranteed analysis of the diet, the percentage distribution of metabolizable energy from protein, fat, and carbohydrate in the diet was 36%, 58%, and 5%, respectively. We wished to determine if dental treatment had an effect on food intake. The study cats were known to be of stable bodyweight on ad libitum feeding before treatment, and we did not anticipate detecting a significant change on their staple diet. Therefore, to maximize the ability to detect changes in oral discomfort with eating, the cats were changed to an extruded, dry, AAFCO maintenance protocol tested commercial biscuit diet for the 7 days either side of treatment.
2.3. Experimental design and timeline
The experimental protocol and sample collection times are presented in . The study was reviewed and approved by the Massey University Animal Ethics Committee.
Table 1. Experimental timeline and protocol.
2.4. Sample collection
Blood was collected by jugular venipuncture using a 21 gauge butterfly needle and extension tube (BD Vacutainer Push Button Blood Collection Set, Becton Dickson Ltd, Auckland) directly into EDTA, sodium citrate, heparin, and plain collection tubes. Urine was collected from midline cystocentesis using a 25 gauge needle and 10 mL syringe. Routine haematology, serum biochemistry, serum T4 and cortisol quantification, urinalysis, and coagulation times, were assayed by a commercial diagnostic laboratory (New Zealand Veterinary Pathology, Palmerson North) on the day of collection. Serum and plasma not used was placed into plastic vials and stored in air-tight, screw-top plastic tubes at −80°C until they were assayed.
Saliva (between 80 and 250 µL) was collected using cotton-tipped swabs, held against the oral mucosa behind the lower molar. Swabs were held in place until soaked. Swab tips were placed into sieved, microcentrifuge tubes within larger tubes, and centrifuged at 14,000 × g for 10 min. The saliva supernatant was then aspirated and was stored at −80°C until assayed.
2.5. Blood analyses
2.5.1. Lymphocyte immunophenotyping
Two-color flow cytometric analysis was used to determine the level of expression of CD4+ (T-helper cells), CD8+ (cytotoxic T-cells), and CD21 (B cells) antigens on peripheral blood leucocytes and granulocytes. Antibodies (Serotec Raleigh, NC, USA) were either feline-specific (CD4, CD8) or canine-specific (CD21). Canine-specific antibodies had been shown to cross react with the relevant feline antigen by the supplier. Immunolabeling was performed according to the method of Gill et al. (Citation2000) and samples were analyzed using a FACSCalibur flow cytometer (Becton Dickinson Instruments, Cambridge, MA, USA). For each sample, data was collected for 10,000 gated events.
2.5.2. Lymphocyte proliferation
A whole blood cell proliferation assay was modified from previously reported methods (Cave et al. Citation2007). Briefly, lithium heparin-treated peripheral whole blood was diluted 1:4 in complete RPMI-1640 medium (RPMI-1640 medium supplemented with 10% fetal calf serum, 10 mM HEPES, 2 mM/L-glutamine, 100 U/mL penicillin, 100 µg/mL streptomycin sulphate and 50 µM 2-mercaptoethanol; all reagents from Gibco, Poole, UK). Diluted blood (100 µL) was added in quadruplicate to the wells of a 96-well, flat-bottomed tissue culture plate (Greiner, Neuberg, Germany) and cultured in the presence of either 5 µg/mL Concanavalin A (Sigma, USA), 1:49 diluted Phytohemagglutinin (Gibco, Poole, UK), or complete RPMI-1640 in place of the mitogen (control wells). The cells were then cultured for 48 h at 37°C in a 5% humidified CO2-air atmosphere, before being pulsed for 18 hrs with 0.5 µCi methyl-3H-thymidine (Amersham Biosciences, UK) per well. Each plate was then harvested onto a 96-well glass fibre mat using a Tomtek cell harvester 96 (Hamden, CT, USA) and counted using a Wallac MicroBeta Trilux 1450 liquid scintillation and luminescence counter (Turku, Finland). Stimulation index was calculated as counts per minute in wells with mitogen divided by counts per minute in wells without mitogen.
2.5.3. Immunoglobulin Enzyme-linked immunosorbant assay (serum IgG and salivary IgA)
To confirm that any effect of dental treatment on serum Glob was the result of an effect on immunoglobulin (Ig) production, serum IgG was quantified using a sandwich Enzyme-linked immunosorbant assay (ELISA). Salivary IgA was quantified to compare the local mucosal Ig response. Both Igs were detected with a validated commercial sandwich ELISA, according to the manufacturer's instructions (Bethyl Laboratories, Montgomery, TX, USA).
2.5.4. Plasma malonyldialdehyde assay
Malonyldialdehyde (MDA) was assayed using the high-pressure liquid chromatography (HPLC) method of Fukanaga et al. (Citation1998). 1,1,3,3-Tetramethoxypropane (TMP) and thiobarbituric acid (TBA) were purchased from Sigma-Aldrich (USA); and HPLC grade acetonitrile (CH3CN) was purchased from Merck (Germany). All the other chemicals were of analytical grade. Water was purified with a Millipore RiOs 8 system (USA), used for the preparation of reagent and HPLC mobile phase and obtained daily.
The TBA reagent was prepared as a 0.2 % (w/v) TBA solution in 0.1 M sodium acetate buffer, pH3.5. This solution was kept at 4°C with light protection using aluminum foil. TMP was used as the MDA standard without any preliminary hydrolysis prior to the TBA–MDA reaction. The stock solution of MDA was prepared by dissolving 100 mg of TMP in 50.0 mL of absolute ethanol, and stored at 4°C with light protection using aluminum foil. The working standards were prepared by diluting the stock solution with absolute ethanol to concentrations of 30, 60, and 300 µM for MDA. An absolute ethanol blank corresponding to nil µM was also used. The mobile phase; CH3CN, water and acetic acid (700:300:2, v/v) was filtered through a 0.45 µm nylon membrane and degassed using vacuum.
An aliquot of 100 µL plasma was transferred to a 1.5 mL Safe-Lock micro centrifuge tube (Eppendorf, Hamburg, Germany). Then 700 µL of 1% v/v phosphoric acid was added and mixed by vortex mixer (10 s at 20 Hertz). Finally, 200 µL of TBA reagent was added and mixed again by vortex mixer (under the same conditions). The tubes were capped and heated in an oven at 95 ± 0.1°C for 60 min. After cooling to room temperature, the reaction mixture was centrifuged at 4500 rcf at 20°C for 10 min. Finally, 900 µL of the supernatants were transferred to HPLC vials for the analysis. The HPLC analysis was performed using a Shimadzu Prominence Liquid Chromatograph (Kyoto, Japan) and using the following modules; Degasser DGU-20As, Pump LC-20AD, Auto Sampler SIL-20AC HT, Column Oven CTO-20AC and Fluorescence Detector RF-20AxS. The separation of the TBA–MDA adduct was performed on an Grace Davison Octadecylsilane (C18) column, GraceSmart RP18 of 5 µm particle size, 150 mm length and 4.6 mm internal diameter (Grace, Deerfield, IL, USA). A guard column with the same packing was used to prevent column contamination. An aliquot of 10 µL of sample and standards were injected in duplicate into the previously described HPLC Liquid Chromatograph. The flow-rate was 1.0 mL/min at 25°C throughout the chromatography analysis. The TBA–MDA reaction was monitored using the fluorescence detector with excitation at 515 nm and emission at 553 nm. The concentration of MDA in the samples was calculated taking into account the peak area and using the TMP standard curve as a reference. Results were expressed as micromoles of MDA per liter of plasma (µM). Peak areas were obtained using the Shimadzu LC Solutions software (Kyoto, Japan).
2.6. Anaesthesia dental examination, and treatment
Treated (group 1) cats were anaesthetized using sedation with buprenorphine (20 µg/kg BW, s/c) and acetyl-promazine (40 µg/kg BW, s/c), and induction with ketamine (5 mg/kg BW), and diazepam (0.5 mg/kg BW), intravenously. Anaesthesia was maintained by isoflurane inhalation, delivered following intubation, and intravenous lactated Ringer's was administered at a rate of 5 mL/kg BW/hr, commencing after induction. All cats were treated with amoxicillin/calvulanic acid (15 mg/kg BW, s/c) at the time of premedication.
The teeth and periodontium were radiographed in four quadrants, using intra-oral occlusal and apical unscreened films. The dental calculus, gingivitis, and periodontitis were scored using the scheme presented in , as previously published by Ingham et al. (Citation2002). Findings were recorded on standardized feline dental charts. The calculus and gingivitis grades from each quadrant were summed to produce one calculus and one gingivitis score per cat. Ultrasonic dental scaling with polishing was performed in all cats. Teeth were extracted if there was any periodontitis, tooth mobility, odontoclastic resorptive lesions, or fractures. In almost all cases, extraction involved mucosal flap creation, removal of alveolar bone using a high-speed dental burr, and flap closure with 5–0 polyglactin (Vicryl®, Ethicon Inc). Local nerve blocks were administered using 0.25% bupivacaine to cats requiring extraction. Cats that had teeth extracted were administered a second dose of buprenorphine (20 µg/kg BW, s/c) 6 h after extubation.
Table 2. Scoring system used to grade the severity of dental disease.
Alveolar bone loss was quantified from dental radiographs. Digital callipers were used to measure the distance from the cement-enamel junction to the alveolar bone margin on the rostral and caudal aspects of all the teeth except the incisors. Results were expressed as the sum of all measurements (total bone loss), and the mean bone loss per tooth, since there was variation in the number of teeth present at the start of treatment.
2.7. Food intake
Cats from group 1 were individually caged for 7 days before and after treatment. During this time, they were fed the dry diet, and daily food intake was measured by weighing residual offered food. During the last 3 days of each of those periods (days −3 to −1, and days 5–7), the amount of food ingested hourly was recorded for 6 h after the food was offered. Hourly intake was expressed as a proportion of the total amount ingested in 6 h.
2.8. Statistics
All data was assessed for normality using the Anderson–Darling test, and variables that were not normally distributed were log transformed, which achieved normality. Data were analyzed using linear mixed model methodology that accounted for repeated measures on the same cat and associated correlated errors (nlme Package in R version 2.8.1, R Foundation for Statistical Computing, Vienna, Austria). The model included the fixed effects of treatment (treated and control cats), time (day following treatment) and their interaction and the random effect of the cat within the treatment. The compound symmetry correlation model was determined as the most appropriate error variance structure using Akaike's information criterion. The predict function was used to obtain maximum likelihood estimates and their standard errors. We tested the hypothesis that the severity of dental disease is associated with changes in assay variables by testing for correlations between study variables in the 30 group-1 cats at baseline. A correlation matrix was constructed for all the study variables. Correlation analysis was used to crudely examine correlations of interest. Strong correlations subsequently identified were further investigated by simple linear regression analysis. Correlations of greatest interest were those between indices of severity of local disease (gingivitis and calculus scores, alveolar bone resorption, time to ingest food prior to treatment) and all other study variables. To test the hypothesis that dental treatment improves study variables, group 1 and group 2 cats were compared across the study period from days 0–90. To test for differences in time to consume food, repeated measures Analysis of variance (ANOVA) was performed. Finally, we retested group 1 cats at day 180 to subjectively evaluate for evidence of deterioration that is predicted to occur with recrudescence of plaque accumulation and gingivitis over time. In the absence of a control group at day 180, the significance of any apparent change could not be rigorously tested. The difference between day 90 and 180 was tested using a paired t-test. Unless otherwise noted, data are presented as mean ± standard deviation (SD).
3. Results
There were no significant differences in the initial dental disease scores, bodyweight, sex, or age between groups at baseline. At the time of dental treatment, there was also no difference in the scores of calculus (p = 0.51) or gingivitis (p = 0.86) between the group 1 cats (day 0), and the group 2 cats (90–120 days later). The mean (±SD) scores for disease severity in both groups combined were: gingivitis 2.2 (±0.4), calculus 1.5 (±0.26), whole mouth alveolar bone resorption 14.9 mm (±3.9), and average alveolar bone resorption 1.5 mm (±0.35). There were significant differences between the groups at baseline in serum Alb, calcium, and cortisol, which were accounted for in the linear mixed model. For all other variables, there was no significant difference between groups at baseline. The majority of cats required extensive dental treatment, and a median of three teeth (range 0–9) were extracted from each cat. One cat did not eat well for 48 h after treatment, and treatment with buprenorphine was continued for 3 days, and amoxicillin/clavulanic acid was continued for 7 days. By day 4, his food intake and demeanour returned to normal.
3.1. Hypothesis 1: There is a correlation between the severity of dental disease and markers of systemic inflammation
This hypothesis was tested on the 30 group-1 cats. Age and bodyweight were significantly positively associated (r = 0.405, p = 0.026). Thus both age and bodyweight were included as covariates in all subsequent models.
Dental disease was advanced in all the included cats. The cats had between 17 and 30 teeth present (median 26), with a mean gingivitis score of 2.2 (SD 0.37), and a mean calculus score of 1.5 (SD 0.26) per tooth. Bodyweight was positively correlated with gingivitis, and there was a trend towards a correlation with calculus (r = 0.3, p = 0.102). The significant baseline correlations are presented in .
Table 3. Significant correlations between variables in 30 cats with periodontal disease.
3.2. Hypothesis 2: Dental treatment will be associated with an improvement in the markers of systemic inflammation
This hypothesis was tested by comparing assay results between Group 1 (n = 30) and Group 2 (n = 18) cats over a 90-day period. The model that best described the data included age and bodyweight as covariates. For most variables assessed, there was a significant effect of time, whereby some variables increased, others decreased, and some changed from time point to time point. For example, serum Alb concentration decreased significantly in both groups, (p < 0.001), and the treated cats consistently had a higher Alb concentration relative to the control cats (, p = 0.027), but there was no effect of treatment over time (p = 0.632). presents the variables for which there was a significant, or near significant effect of treatment over time, and these data are graphed in Figures . Dental treatment had a significant effect on the time to ingest food, whereby cats more quickly ingested the 6 h total after treatment than before treatment (, p < 0.001), whereas the total amount of food eaten during that time was not significantly different between time points (p = 0.18).
Figure 1. Serum Alb concentration (with bar indicating SEM) in cats with periodontal disease that received dental treatment (n = 30), or were left untreated (n = 18). The effect of time was significant in both groups, (p < 0.001), and the treated cats consistently had a higher Alb relative to the control cats (p = 0.027), but there was no effect of treatment over time (p = 0.632).
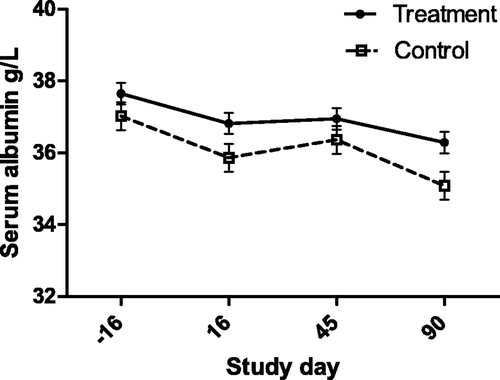
Figure 2. Serum Glob concentration (with bar indicating SEM) in cats with periodontal disease that received dental treatment (n = 30) or were left untreated (n = 18). The effect of treatment over time was significant (p < 0.0001).
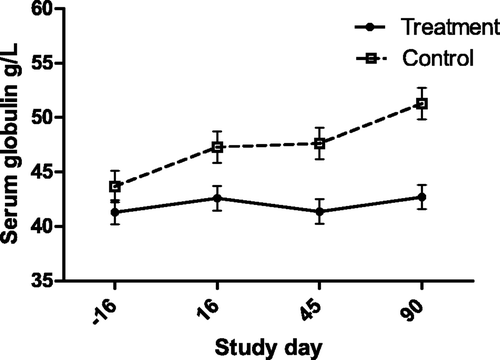
Figure 3. Serum total protein concentration (with bar indicating SEM) in cats with periodontal disease that received dental treatment (n = 30) or were left untreated (n = 18). The effect of treatment over time was significant (p = 0.0023).
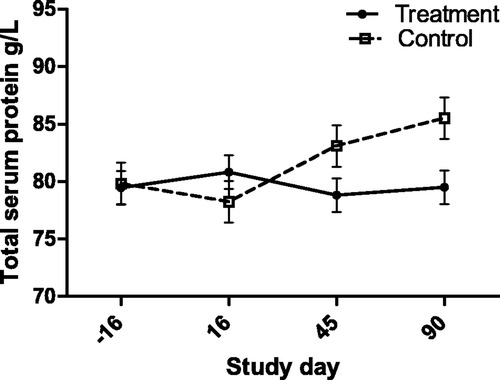
Figure 4. Serum Alb:Glob ratio (with bar indicating SEM) in cats with periodontal disease that received dental treatment (n = 30) or were left untreated (n = 18). The effect of treatment over time was significant (p = 0.0472).
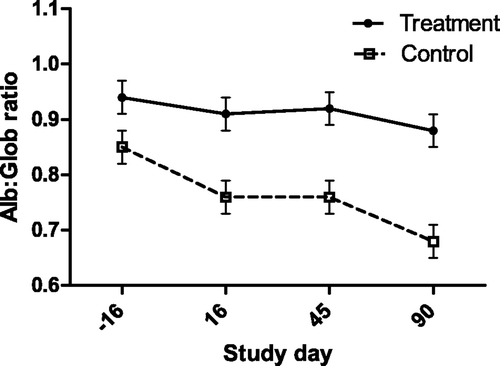
Figure 5. Serum IgG concentration (with bar indicating SEM) as measured by sandwich ELISA in cats with periodontal disease that received dental treatment (n = 30) or were left untreated (n = 18). The effect of treatment over time was significant (p = 0.0133).
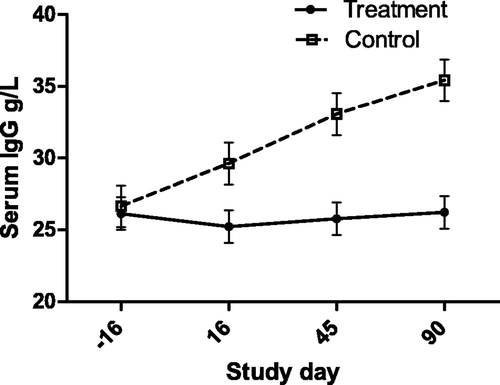
Figure 6. Serum cholesterol concentration (with bar indicating SEM) in cats with periodontal disease that received dental treatment (n = 30) or were left untreated (n = 18). The effect of treatment over time was significant (p = 0.0257).
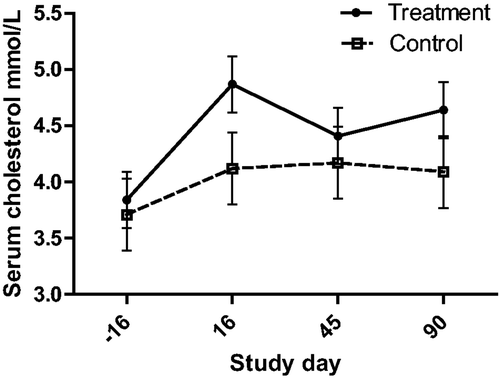
Figure 7. Serum CK activity (with bar indicating SEM) in cats with periodontal disease that received dental treatment (n = 30) or were left untreated (n = 18). The effect of treatment over time was significant (p = 0.0272).
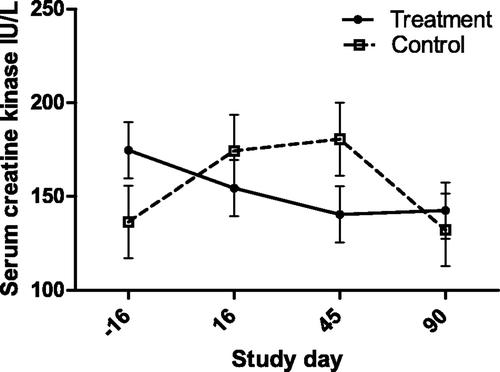
Figure 8. Circulating eosinophil count (with bar indicating SEM) in cats with periodontal disease that received dental treatment (n = 30) or were left untreated (n = 18). The effect of treatment over time was significant (p = 0.032).
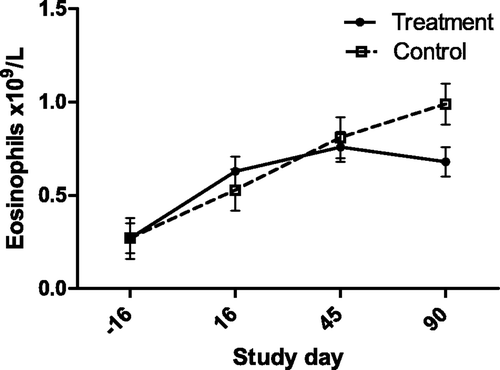
Figure 9. Serum AST activity (with bar indicating SEM) in cats with periodontal disease that received dental treatment (n = 30) or were left untreated (n = 18). The effect of treatment over time was significant (p = 0.0497).
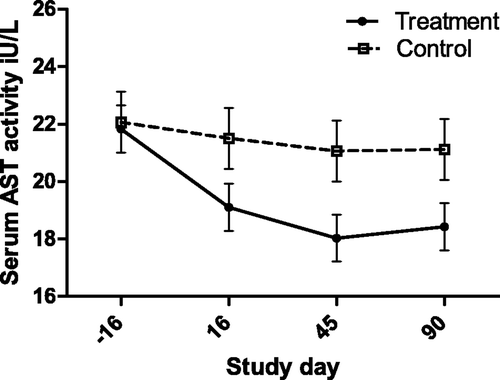
Figure 10. Intake of food (with bar indicating SEM) over first 6 h in 30 cats with periodontal disease, before, and after dental treatment (repeated measures ANOVA, p < 0.001). Each cat's intake was recorded hourly over the 6-h period after food was offered on days 3–1 (pre treatment), and days 5–7 (post treatment), and the hourly intake averaged over the 3 days. Data represent the average of all 30 cats “3 day average” at each time point. There was no significant difference in the total amount of food eaten during the 6-h time period between time points.
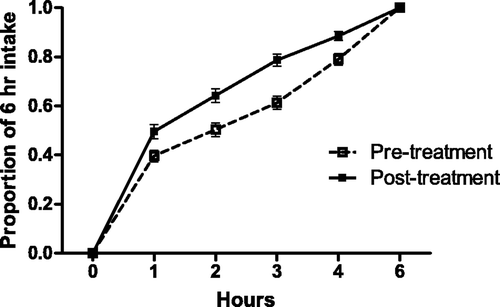
Table 4. Significance of effect of dental treatment (n = 30) on study variables compared with no treatment (n = 18) in cats with periodontal disease.
The total white blood cell (WBC) count approached significance (p = 0.087), but there was no clear pattern to the trend over time, and none of the variables predicted to be associated with a conventional inflammatory response were associated, other than the eosinophil count. Total neutrophil, monocyte, and lymphocyte numbers were not significantly associated with dental treatment. Lymphocyte subsets (CD4, CD8, B-cell) were not significantly affected either in absolute counts or relative proportions, and there was no effect of treatment on in vitro Con-A induced lymphocyte proliferation. The mean proliferation index for all cats at baseline was 28.0 (±26.9, range 8.7–161).
In contrast to serum IgG (), salivary IgA concentrations were not significantly affected by the treatment, and there was no correlation between salivary IgA and serum IgG concentrations. The mean salivary IgA concentration for all cats at baseline was 0.72 g/L (±0.41, range 0.17–1.79 g/L). The mean MDA concentration across all cats and sample times was 6.52 µM, (±4.98, range: 1.5–39.7). Although there were significant, but small correlations between lipid peroxidation and severity of dental disease, treatment did not have an effect on plasma MDA concentration.
3.3. Hypothesis 3. Variables that are affected by dental treatment will return to the diseased state over time
There was no significant return to baseline values at 90 days in the treatment group. At 180 days, the control group had been treated and was no longer available for comparison. Therefore, only variables significantly affected by treatment were evaluated for a return to baseline, namely Aspartate aminotransferase (AST), Alb, Glob, Alb:Glob, and IgG. Serum Alb concentration was significantly lower on day 180 than on day 90 (day 90: 36.8 ± 2.8, day 180: 34.7 ± 2.6, p < 0.001), but there was no significant difference for any of the other variables.
4. Discussion
Advanced periodontal disease in cats is associated with significant local inflammation, and is suspected to influence systemic responses and organ function in distant sites. This study provides support for the hypothesis that there are measurable systemic changes associated with advanced dental disease. Little research has previously been conducted in cats to support this hypothesis, and there is a considerable amount of accepted dogma.
Conventional wisdom predicts that white cell counts consistent with a systemic inflammatory response will be elevated, and there may be suppression of circulating lymphocytes due to recruitment, sequestration, or decreased production. This conventional thinking is supported by reports that the number of WBCs is higher in humans with periodontitis than healthy subjects (Inoue et al. Citation2005; Loos Citation2005). Although there was a trend towards a correlation with severity, and an effect of treatment, this study did not support this conventional wisdom. These findings are consistent with the similar study conducted in dogs, where there was no correlation between periodontal disease variables and total WBCs (Rawlinson et al. Citation2011). The logical conclusion is that either routine haematology is a crude and insensitive assay for any effects of dental disease on circulating cells, or the local response has no effect on circulating cells.
In addition to routine cell counts, we quantified the different lymphocyte subtypes. Both immunosuppression and exaggerated systemic inflammation have been postulated to occur in severe dental disease, and changes in the recruitment of lymphocytes has been consistently shown to occur in human periodontitis, which are thought to contribute to the cytokine-dependant activation of osteoclastic resorption (Taubman and Kawai Citation2001). Other chronic inflammatory diseases have been associated with absolute and relative shifts in the proportion of circulating lymphocyte subtypes, but, similar to findings in humans with periodontitis; this was not identified in this study (Emingil et al. Citation2001). In contrast with human studies, we did not detect a correlation between the severity of disease and the ability of lymphocytes to proliferate in vitro when stimulated with Con-A. This study cannot rule out any effect of dental disease on the study variables because it did not include a healthy control group. The proliferation indices in these cats at baseline were similar to those in other studies using healthy cats, but we cannot exclude the possibility that periodontal disease does have an effect on lymphocyte function (Cave et al. Citation2007).
The eosinophil count was not associated with the severity of disease in the group-1 cats, however, treatment was associated with a lower count. Eosinophils have not been reported to constitute a significant fraction of the inflammatory infiltrate in feline periodontal disease, with the exception of eosinophilic granulomas, and in inflammation induced by experimental ligation of the salivary gland ducts (Harrison and Garrett Citation1976). The significance of this correlation is unknown.
Despite an absence of detectable differences in circulating cells and lymphocyte proliferation, one variable that was most strongly associated with both severity of disease and response to treatment was serum Glob, and its derived values. The IgG ELISA confirmed that serum IgG was largely responsible. Serum IgG in these cats could be produced in regional lymphoid tissue, or in more distant tissue such as the spleen. The obvious question is “to what are the Igs specific?” There are several possible explanations that are non-exclusive, and may, to some extent, all be present. It is possible that the increased IgG is specific to oral pathogens. In humans with periodontitis, serum IgG to the lipoteichoic acid component of Gram-positive plaque bacteria is associated with the amount of periodontal bone loss (Monefeldt and Tollefsen Citation1989). It is also possible that polyclonal B cell activation might occur in a T cell-independent fashion, by extensive cross-linking of membrane IgM by circulating microbial antigens, or by bystander stimulation mediated by cytokines induced by the periodontitis (Fagarasan and Honjo Citation2000; Vos et al. Citation2000). It is also possible that the widespread tissue destruction and associated inflammation might result in the development of auto antibodies as has been shown in aggressive periodontitis in people (Hendler et al. Citation2010).
Dental treatment is expected to reduce both the number and range of species present in the mouth, and to reduce the potential for bacteria–leukocyte interaction. In response to that, serum IgG would be expected to decline. After treatment, tissue healing and a reduction in local inflammation would be predicted to lead to resolution of any autoimmune component. In both these cases, however, the divergence of serum IgG at day 16 after the treatment appears too rapid for either a decrease in bacteria-specific, or local autoantibody IgG production. Thus, it seems more likely that the increased IgG in the untreated cats was a polyclonal, non-specific response. In this last possibility, there is an immunological bystander effect whereby the production of IgG to unrelated antigens by plasma cells is increased by the adjacent, or systemic production of inflammatory cytokines. In this respect, IL-4 might be predicted to be involved, which in turn might be responsible for the apparent effect on circulating eosinophils. It is interesting that the effect of the treatment occurred as the result of serum IgG (and hence total Glob) increasing in the control group relative to baseline, rather than decreasing in the treatment group following treatment. This could indicate that the inflammatory response to dental disease was increasing rapidly in the control group, and that treatment only served to arrest, rather than improve the response. The absence of a group of cats without dental disease prevents firm conclusion. However, serum Glob remained within the reference range for all but a few cats throughout the study, and the afore-mentioned unexplained changes with time in other variables warns against too much extrapolation in this regard.
In contrast with the serum IgG response, we did not detect a correlation between salivary IgA and disease severity, or an effect of treatment. This finding is similar to that reported by Harley et al. (Citation2003), who reported that in cats with chronic gingivostomatitis, serum IgG was increased compared with normal controls, but salivary IgA was decreased (Harley et al. Citation2003). It is not possible to compare the concentrations reported in that or other feline studies with this present study, because previous studies have reported ELISA units, or absorbance values. Salivary IgA concentrations in normal humans has been reported to be 0.02–0.63 g/L in unstimulated saliva of normal individuals, and, similar to the situation in cats with chronic gingivostomatitis, it was less than 0.05 g/L in humans with early onset generalized periodontitis (Hagewald et al. Citation2000). It may be that the local inflammatory response in periodontal disease leads to a relative regional, and possibly systemic isotype shift resulting in an increased salivary IgG and reduced IgA concentration. However, it is interesting that there was a clear effect of treatment on serum IgG, but no effect on salivary IgA once the local inflammation had resolved. Immunosuppressive treatment of cats with chronic gingivostomatitis normalizes the isotype production (Harley et al. Citation2003). In this study, there may have been a persistence of the secretion of a given isotype by regional plasma cells despite a more quiescent cytokine milieu. A much longer time-period of sampling following a prolonged period of dental health may be required to normalize mucosal Ig production.
At baseline, the erythrocyte count, haemoglobin (HB) concentration, and haematocrit were all negatively associated with the severity of the disease (gingivitis, whole mouth alveolar bone resorption, and calculus). No cat was anemic, however these findings are consistent with suppression of bone marrow production of erythrocytes, a mechanism that could lead to the “anemia of chronic disease” (ACD). Similarly, humans with periodontitis have been found to have lower erythrocyte indices than healthy individuals (Gokhale et al. Citation2010). The cause for ACD is most likely multi-factorial, however it is currently thought that pro-inflammatory cytokines, especially IL-1, IL-6, and TNF-α, suppress erythropoiesis in the bone marrow (Jongen-Lavrencic et al. Citation1997).
Of the biochemical assays, AST activity was the most strongly associated with both severity (gingivitis) and treatment. Aside from primary hepatocellular disease in cats, elevated AST activity is seen in diverse responses including muscle injury from ovariohysterectomy, Feline leukemia virus infection, anorexia, distal aortic thromboembolism, and hyperthyroidism (Laste and Harpster Citation1995; Broussard et al. Citation1995; Fascetti et al. Citation1997; Alves et al. Citation2009; Gleich and Hartmann Citation2009). AST is found in a wide range of feline tissues, although hepatic and muscle sources are thought to be the only sources large enough to lead to elevated serum concentrations. In this study, creatine kinase (CK) activity was not associated with the severity of dental disease, and although there was a statistically significant effect of treatment, the biological significance of this is questioned due to the appearance over time (). Thus the source of the serum AST is thought to be hepatic, rather than muscular. This interpretation suggests that dental disease in cats produces hepatic changes that might be inflammatory leading to hepatocellular enzyme leakage, or a response to inflammation leading to enzyme induction. In support of this was the finding that alanine aminotransferase (ALT) activity was correlated with the severity of disease (gingivitis, and mean alveolar bone resorption). These findings contrast with the study of periodontal disease in dogs by Rawlinson et al., in which none of the liver enzyme serum activities were associated with disease (Rawlinson et al. Citation2011). However, in the current study serum ALT activity did not decrease in response to treatment. This may argue against ALT as an indicator of periodontitis induced liver disease, could indicate a longer time to normalization compared with AST, or simply be beyond the power of this study to detect a change following treatment. None-the-less, it appears likely that periodontal disease affects hepatocellular function, and may be associated with cellular damage.
Similar to CK, there may not be any biological significance to the changes in serum cholesterol described in this study. However, therapy for periodontal disease in humans has been associated with changes in circulating lipoprotein fractions (notably increased high density lipoprotein), and recently periodontitis has been suggested as a contributing cause of hyperlipidaemia in people (Taleghani and Shamaei Citation2010). Thus it is conceivable that the findings in this study are reflective of a true and biologically significant effect whereby the inflammatory mediators produced in response to the periodonitis have effects on lipid metabolism in cats. Whether this might contribute, or even protect against disease is beyond speculation at present.
It was hypothesized that the local and systemic increase in exposure to endogenously produced oxidants would be measurable by an increase in the production of MDA. The MDA assay was chosen because of its proven sensitivity and reliability in humans (Grune and Berger Citation2007). Although there was a small significant correlation between the amount of periodontal bone loss and MDA at baseline, there was no effect of treatment on MDA. In addition, plasma MDA and serum cholesterol were positively correlated at baseline. This could be simply a reflection of increased lipid peroxidation in samples with higher lipoprotein concentrations.
Serum MDA has proven to be a sensitive indicator of oxidative stress in numerous inflammatory disease states in humans including periodontitis (Akalin et al. Citation2007; Wei et al. Citation2010). In a study of 48 human patients with chronic periodontitis, the mean serum MDA concentration was 72 µM, and 68 µM in 35 healthy control subjects (Wei et al. Citation2010). After 16 weeks of periodontal therapy, the serum concentration reduced to 70 µM. We assayed plasma rather than serum MDA, however the two samples have not been shown to contain significantly different concentrations in healthy or diseased humans (Carbonneau et al. Citation1991). The generally low concentrations assayed in this study compared with human concentrations may indicate a higher level of protection against lipid peroxidation in cats, or may reflect an adequate level of dietary antioxidant ingestion. Other cats on diets less replete may be more susceptible. Alternatively, other measures of oxidative damage to molecules such as DNA or protein might be more sensitive. A previous study of plasma MDA in healthy and FIV infected cats found mean concentrations of 30.3–36.8 µM (Webb et al. Citation2008). Therefore, the concentration range in this study (1.5–39.7 µM) is low in comparison with previous human and feline studies. However, direct comparisons are difficult due to differences in sample handling and assaying techniques, although concerns regarding assay accuracy are usually directed towards ex vivo induction of lipid peroxidation rather than low sensitivity (Del Rio et al. Citation2005).
A correlation between bodyweight and dental disease in cats has not previously been reported. In this study, larger cats had higher gingivitis scores than smaller cats, which is the opposite to the correlation in dogs, in which smaller dogs tend to have more severe gingivitis, attachment loss and bone resorption than larger dogs (Harvey et al. Citation1994). Although there was a very strong correlation between the bone loss indices and bodyweight (data not shown), it is not possible to attribute that to increased bone loss rather than size of cat, since we did not normalize the bone loss to the size of the adjacent tooth. Future studies that attempt to do so may further elucidate the correlation between bodyweight and periodontal disease in cats.
It has been well established that there is a correlation between periodontal disease and chronic kidney disease in humans (Kshirsagar et al. Citation2005). However, it is not clear to what degree one precedes the other, since advanced renal disease is an independent risk factor for periodontitis (Davidovich et al. Citation2005). In the study of the effect of treatment of canine periodontal disease, the authors found a negative correlation between creatinine and attachment loss, and no correlation between severity of disease and any other renal or urinary variable (Rawlinson et al. Citation2011). In the present study, we found no correlation between severity of disease and urea or creatinine, nor any effect of treatment. These findings are surprising, because a study of the post mortem findings in 44 dogs with periodontal disease identified an increased risk of renal pathology with increasing severity of periodontitis, and in a large retrospective cohort, the risk of azotaemia was increased almost three-fold in dogs with severe periodontal disease (Pavlica et al. Citation2008; Glickman et al. Citation2011). It is likely that the measures of renal tubular function used in this study are not sensitive enough to detect an effect, and the authors do not argue that this study provides evidence to refute the proposed association.
For most variables assessed, there was a significant effect of time, whereby some variables increased, others decreased, and some changed from time point to time point. The inconsistency of these changes precludes a clear explanation (e.g., differences in collection or storage between assay days). Although the logistic regression accounts for the effect of time, it is likely that this effect masked some of the significance of variables that were near p = 0.05. None-the-less, these findings emphasise the need to control for unrelated variations over time when assessing for a response to treatment, and that an animal cannot serve as its own control.
This is the first study in either dogs or cats to evaluate systemic effects of periodontal disease by including an untreated control group. The 3-month period during which time we compared the treated and untreated groups was a compromise between a long term scientific goal, namely a desire to study longer term effects and to determine the time to recrudescence following treatment, and a short term ethical goal, namely to treat the dental disease in the control group cats. Untreated animals control for the effect of treatment, but do not control for the effect of disease. Thus, an ideal control group to truly evaluate for the systemic effects of periodontal disease would consist of healthy age, sex, and weight-matched animals. For that reason, there is a need to consider the significance of variables that were correlated with disease but did not change with treatment (e.g., ALT, red blood cells [RBC] variables (HCT, RBC, HB)).
It is possible that some disease markers do not change with treatment in the time period studied here. That may be true for the subtle suppression of erythropoeisis noted in this study. It is possible that once altered with disease, some variables remain altered. That might have been expected to be the case had serum creatinine been elevated with disease, but is unlikely to be the case with ALT or erythrocyte indices. It may also be simply the case that these correlations are coincidental, and that future, larger studies will not support the findings.
Finally, it may also indicate that the direction of the correlation is in reverse. The transition from gingivitis to periodontitis is the result of changes in the plaque microflora, inappropriate or inadequate host response to gingival infection, and in humans, various risk factors (e.g., systemic disease, stress, age, medications lack of oral hygiene maintenance, poor diet). As noted above, smaller size is a risk for periodontitis in dogs. Those variables that were associated at baseline but did not change with treatment may be causative and not effect, for example, MDA and mean bone loss. It may be that increased oxidative stress exacerbates the development of periodontal disease in certain individuals, rather than being a consequence of it.
Two of the baseline correlations were opposite in valence to that hypothesized. Serum AST activity was negatively correlated with gingivitis, and HB concentration was positively correlated with total alveolar bone resorption. This is especially hard to explain with AST given the clear effect of treatment on the serum activity. Similarly, as discussed above, most of the erythrocyte indices were negatively associated with disease scores. In the case of HB, it may be that this spurious result is evidence against the use of total alveolar bone resorption as a true indicator of disease, rather than the mean resorption. It is unknown if having 3 mm of alveolar bone loss around two teeth, is more or less disease than 6 mm of bone loss around one tooth. Given that there were three correlations with the mean bone loss that were consistent with the hypothesis, and one for total bone loss that was inconsistent, we suggest that the mean alveolar bone loss is the superior measure.
It is often surprising how severe periodontal disease can be, and yet have no apparent effect on food intake, and owners will often be unaware of oral discomfort in their pet. All of the cats in this study had stable body weights and apparently ate normally prior to treatment. The food intake data, collected on days 5–7 after dental treatment revealed a significantly faster ingestion of food during the initial 6 h after food was offered, than before treatment. Many of the study cats required multiple tooth extractions due to periodontitis or odontoclastic resorptive lesions, and yet within a short time, they were willing to prehend and ingest dry biscuits more rapidly. We interpret this finding as indicative of the relief of discomfort following dental treatment, despite the tissue trauma induced by gingival flap elevation, alveolar osteoplasty, and gingival suturing. This is compelling evidence for the persistent discomfort associated with dental disease, and is supportive of an aggressive prophylactic approach to minimize the development and progression of disease in patients under our care.
5. Conclusions
This study has provided support for both of the main hypotheses: namely that there are systemic markers that are associated with the severity of dental disease, and that there are markers that change with dental treatment. Thus, dental disease in cats has systemic consequences that can be altered with treatment. The key markers of the systemic response to periodontal disease in cats appear to be serum Globs and the derived values, serum IgG, and serum AST activity. Of those, serum IgG appears the most promising. Further studies should attempt to answer the several questions posed by this study. Definition of the antigenic specificities of the IgG produced in response to dental disease would elucidate if the response is non-specific polyclonal stimulation or specific to certain key pathogenic antigens. The nature of the hepatic response, and if that constitutes disease, needs to be clarified, as does the nature of the change in lipid metabolism associated with periodontal disease. It may be helpful to identify the significant changes in circulating cytokine concentrations that might produce these systemic effects, and to define the role, if any, that eosinophils play in the local and systemic manifestations of periodontal disease in cats. Finally, and most importantly, we have yet to clearly define what the clinically significant long term measures of health relating to these changes are. None-the-less, we conclude that periodontitis, like many chronic inflammatory diseases, should not be considered to be a localized disease process, but one that has an impact on systemic health and wellbeing.
Acknowledgments
The authors would like to acknowledge and thank Kay Rutherfurd-Markwick and Michele McGrath for their assistance with the lymphocyte and MDA assays, and to Margreet Hekman and Karin Weidgraaf for their tireless efforts caring for the colony cats.
References
- Akalin , FA , Baltacioglu , E , Alver , A and Karabulut , E . 2007 . Lipid peroxidation levels and total oxidant status in serum, saliva and gingival crevicular fluid in patients with chronic periodontitis . J Clin Periodontol , 34 ( 7 ) : 558 – 565 .
- Alves , AE , Ribeiro , AP , Filippo , PA , Apparicio , MF , Motheo , TF , Mostachio , GQ , Vicente , WR and Hotston Moore , A . 2009 . Evaluation of creatine kinase (CK) and aspartate aminotransferase (AST) activities after laparoscopic or conventional ovariectomy in queens . Schweiz Arch Tierheilkd , 151 ( 5 ) : 223 – 227 .
- Borch , TS , Holmstrup , P , Bendtzen , K and Nielsen , CH . 2010 . In vitro cytokine responses to periodontal pathogens: generalized aggressive periodontitis is associated with increased IL-6 response to Porphyromonas gingivalis . Scand J Immunol , 71 ( 6 ) : 440 – 446 .
- Broussard , JD , Peterson , ME and Fox , PR . 1995 . Changes in clinical and laboratory findings in cats with hyperthyroidism from 1983 to 1993 . J Am Vet Med Assoc , 206 ( 3 ) : 302 – 305 .
- Carbonneau , M , Peuchant , E , Sess , D , Canioni , P and Clerc , M . 1991 . Free and bound malondialdehyde measured as thiobarbituric acid adduct by HPLC in serum and plasma . Clin Chem , 37 ( 8 ) : 1423 – 1429 .
- Cave , NJ , Backus , RC , Marks , SL and Klasing , KC . 2007 . Modulation of innate and acquired immunity by an estrogenic dose of genistein in gonadectomized cats . Vet Immunol Immunopathol , 117 ( 1–2 ) : 42 – 54 .
- Craig , RG . 2004 . Inflammation, cardiovascular disease and destructive periodontal diseases . The evolving role of the dental profession. N Y State Dent J , 70 ( 5 ) : 22 – 26 .
- Davidovich , E , Davidovits , M , Eidelman , E , Schwarz , Z and Bimstein , E . 2005 . Pathophysiology, therapy, and oral implications of renal failure in children and adolescents: an update . Pediatr Dent , 27 ( 2 ) : 98 – 106 .
- Del Rio , D , Stewart , AJ and Pellegrini , N . 2005 . A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress . Nutr Metab Cardiovasc Dis , 15 ( 4 ) : 316 – 328 .
- do Vale , CH , de Oliveira Fraga , LA , Costa , AS , Tavares , CA , Martins-Filho , OA , de Macedo Farias , L and Roque de Carvalho , MA . 2004 . Antiproliferative activity of Actinobacillus (Haemophilus) actinomycetemcomitans and Fusobacterium nucleatum in peripheral blood mononuclear cells . Res Microbiol , 155 ( 9 ) : 731 – 740 .
- Emingil , G , Karaarslan , F , Keskinoglu , A , Coker , I and Atilla , G . 2001 . Phenotypic and functional analysis of peripheral blood mononuclear cells in generalised aggressive and chronic periodontitis patients . J Int Acad Periodontol , 3 ( 4 ) : 87 – 94 .
- Fagarasan , S and Honjo , T . 2000 . T-Independent immune response: new aspects of B cell biology . Science , 290 ( 5489 ) : 89 – 92 .
- Fascetti , AJ , Mauldin , GE and Mauldin , GN . 1997 . Correlation between serum creatine kinase activities and anorexia in cats . J Vet Intern Med , 11 ( 1 ) : 9 – 13 .
- Fukunaga , K , Yoshida , M and Nakazono , N . 1998 . A simple, rapid, highly sensitive and reproducible quantification method for plasma malondialdehyde by high-performance liquid chromatography . Biomed Chromatogr , 12 ( 5 ) : 300 – 303 .
- Gill , HS , Rutherfurd , KJ , Prasad , J and Gopal , PK . 2000 . Enhancement of natural and acquired immunity by Lactobacillus rhamnosus (HN001), Lactobacillus acidophilus (HN017) and Bifidobacterium lactis (HN019) . Br J Nutr , 83 ( 2 ) : 167 – 176 .
- Gleich , S and Hartmann , K . 2009 . Hematology and serum biochemistry of feline immunodeficiency virus-infected and feline leukemia virus-infected cats . J Vet Intern Med , 23 ( 3 ) : 552 – 558 .
- Glickman , LT , Glickman , NW , Moore , GE , Goldstein , GS and Lewis , HB . 2009 . Evaluation of the risk of endocarditis and other cardiovascular events on the basis of the severity of periodontal disease in dogs . J Am Vet Med Assoc , 234 ( 4 ) : 486 – 494 .
- Glickman , LT , Glickman , NW , Moore , GE , Lund , EM , Lantz , GC and Pressler , BM . 2011 . Association between chronic azotemic kidney disease and the severity of periodontal disease in dogs . Prev Vet Med , 99 ( 2–4 ) : 193 – 200 .
- Gokhale , SR , Sumanth , S and Padhye , AM . 2010 . Evaluation of blood parameters in patients with chronic periodontitis for signs of anemia . J Periodontol , 81 ( 8 ) : 1202 – 1206 .
- Gorrel , C . 1998 . Periodontal disease and diet in domestic pets . J Nutr , 128 ( 12 ) : 2712S – 2714S .
- Grune , T and Berger , MM . 2007 . Markers of oxidative stress in ICU clinical settings: present and future . Curr Opin Clin Nutr Metab Care , 10 ( 6 ) : 712 – 717 .
- Hagewald , S , Bernimoulin , JP , Kottgen , E and Kage , A . 2000 . Total IgA and porphyromonas gingivalis-reactive IgA in the saliva of patients with generalised early-onset periodontitis . Eur J Oral Sci , 108 ( 2 ) : 147 – 153 .
- Harley , R , Gruffydd-Jones , TJ and Day , MJ . 2003 . Salivary and serum immunoglobulin levels in cats with chronic gingivostomatitis . Vet Rec , 152 ( 5 ) : 125 – 129 .
- Harrison , JD and Garrett , JR . 1976 . Inflammatory cells in duct-ligated salivary glands of the cat: a histochemical study . J Pathol , 120 ( 2 ) : 115 – 119 .
- Harvey , CE , Shofer , FS and Laster , L . 1994 . Association of age and body weight with periodontal disease in North American dogs . J Vet Dent , 11 ( 3 ) : 94 – 105 .
- Hendler , A , Mulli , TK , Hughes , FJ , Perrett , D , Bombardieri , M , Houri-Haddad , Y , Weiss , EI and Nissim , A . 2010 . Involvement of autoimmunity in the pathogenesis of aggressive periodontitis . J Dent Res , 89 ( 12 ) : 1389 – 1394 .
- Ingham , KE , Gorrel , C and Bierer , TL . 2002 . Effect of a dental chew on dental substrates and gingivitis in cats . J Vet Dent , 19 ( 4 ) : 201 – 204 .
- Inoue , K , Kobayashi , Y , Hanamura , H and Toyokawa , S . 2005 . Association of periodontitis with increased white blood cell count and blood pressure . Blood Press , 14 ( 1 ) : 53 – 58 .
- Jongen-Lavrencic , M , Peeters , HR , Wognum , A , Vreugdenhil , G , Breedveld , FC and Swaak , AJ . 1997 . Elevated levels of inflammatory cytokines in bone marrow of patients with rheumatoid arthritis and anemia of chronic disease . J Rheumatol , 24 ( 8 ) : 1504 – 1509 .
- Kshirsagar , AV , Moss , KL , Elter , JR , Beck , JD , Offenbacher , S and Falk , RJ . 2005 . Periodontal disease is associated with renal insufficiency in the Atherosclerosis Risk In Communities (ARIC) study . Am J Kidney Dis , 45 ( 4 ) : 650 – 657 .
- Laste , NJ and Harpster , NK . 1995 . A retrospective study of 100 cases of feline distal aortic thromboembolism: 1977–1993 . J Am Anim Hosp Assoc , 31 ( 6 ) : 492 – 500 .
- Loos , BG . 2005 . Systemic markers of inflammation in periodontitis . J Periodontol , 76 ( 11-s ) : 2106 – 2115 .
- Lund , EM , Armstrong , PJ , Kirk , CA , Kolar , LM and Klausner , JS . 1999 . Health status and population characteristics of dogs and cats examined at private veterinary practices in the United States . J Am Vet Med Assoc , 214 ( 9 ) : 1336 – 1341 .
- Monefeldt , K and Tollefsen , T . 1989 . Serum IgG antibodies reactive with lipoteichoic acid in adult patients with periodontitis . J Clin Periodontol , 16 ( 8 ) : 519 – 524 .
- Niemiec , BA . 2008 . Periodontal disease . Top Companion Anim Med , 23 ( 2 ) : 72 – 80 .
- Offenbacher , S , Elter , JR , Lin , D and Beck , JD . 2005 . Evidence for periodontitis as a tertiary vascular infection . J Int Acad Periodontol , 7 ( 2 ) : 39 – 48 .
- Pavlica , Z , Petelin , M , Juntes , P , Erzen , D , Crossley , DA and Skaleric , U . 2008 . Periodontal disease burden and pathological changes in organs of dogs . J Vet Dent , 25 ( 2 ) : 97 – 105 .
- Rawlinson , JE , Goldstein , RE , Reiter , AM , Attwater , DZ and Harvey , CE . 2011 . Association of periodontal disease with systemic health indices in dogs and the systemic response to treatment of periodontal disease . J Am Vet Med Assoc , 238 ( 5 ) : 601 – 609 .
- Scannapieco , FA , Bush , RB and Paju , S . 2003 . Associations between periodontal disease and risk for atherosclerosis, cardiovascular disease, and stroke . A systematic review. Ann Periodontol , 8 ( 1 ) : 38 – 53 .
- Taleghani , F and Shamaei , M . 2010 . Association between chronic periodontitis and serum lipid levels . Acta Med Iran , 48 ( 1 ) : 47 – 50 .
- Taubman , MA and Kawai , T . 2001 . Involvement of T-lymphocytes in periodontal disease and in direct and indirect induction of bone resorption . Crit Rev Oral Biol Med , 12 ( 2 ) : 125 – 135 .
- Vos , Q , Lees , A , Wu , ZQ , Snapper , CM and Mond , JJ . 2000 . B-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms . Immunol Rev , 176 : 154 – 170 .
- Webb , CB , Lehman , TL and McCord , KW . 2008 . Effects of an oral superoxide dismutase enzyme supplementation on indices of oxidative stress, proviral load, and CD4:CD8 ratios in asymptomatic FIV-infected cats . J Feline Med Surg , 10 ( 5 ) : 423 – 430 .
- Wei , D , Zhang , XL , Wang , YZ , Yang , CX and Chen , G . 2010 . Lipid peroxidation levels, total oxidant status and superoxide dismutase in serum, saliva and gingival crevicular fluid in chronic periodontitis patients before and after periodontal therapy . Aust Dent J , 55 ( 1 ) : 70 – 78 .