Two tigers (Panthera tigris), six and seven years old, presented for laparoscopic ovariectomy. The tigers were privately owned and had never been bred. Both animals had been receiving 375 mg medroxyprogesterone acetatea intramuscularly every 6 months for contraception.
The tigers were immobilized with an intramuscular combination of 25 mcg/kg BW medetomidine HClb, 2.5 mg/kg BW ketamine HClc, and 0.1 mg/kg BW midazolam HCld. They were then intubated and maintained with isofluranee in oxygen. Physical examination revealed the animals were obese with a body condition score of 4/5 based on body condition parameters for domestic felids (Thatcher et al. Citation2000) weighing 178.5 and 168.0 kg, respectively. Complete blood count and serum biochemical panels were unremarkable. Methadonef and meloxicamg were administered intramuscularly at 0.1 mg/kg BW each for intraoperative and postoperative analgesia.
For surgery the tigers were positioned in dorsal recumbency and prepared aseptically. A 20 mm midline incision was made through the skin, subcutaneous tissue, and linea alba just caudal to the umbilicus using monopolar radiofrequency cauteryh in both cut and coagulation modes. A multicannulated porti was then inserted into the peritoneal cavity using carmalt forceps. Noticeable longitudinal stretching of the foam port was observed due to the thickness of the patient's body wall. Two 5 mm and one 10 mm inner cannulas were placed into the SILS™ port and the peritoneal cavity was insufflatedj using CO2 gas (Figure ) to a maximum peritoneal pressure setting of 8–10 mm Hg. Once peritoneal insufflation was accomplished a 5 mm, 0-degree laparoscopic telescopek was inserted via a 5 mm inner cannula and a brief scan of the peritoneal space was performed to rule out iatrogenic injury during port placement. Following initial exploration, the telescope was removed to prevent injury to viscera during position change and the tigers were manually rotated into approximately 45-degrees right, dorsal-oblique recumbency. Sandbags were used to maintain the tigers in this position, which allows for increased visualization of the left ovary as gravity pulls the other visceral contents away. The telescope was reinserted to explore the left gutter just caudal to the left kidney. A 5 mm laparoscopic Babcock forcepsl was then inserted into a separate 5 mm inner cannula and used to grasp and elevate the proper ovarian ligament ventrally (Figure ). Once the ovarian pedicle was elevated, a 10 mm bipolar ligation devicem was inserted in the 10 mm inner cannula to seal and divide the suspensory ligament, ovarian pedicle, and uterine horn immediately adjacent to the proper ligament. Multiple ligation cycles were required to attain an appropriate seal. A 10 mm specimen retrieval bagn was then inserted in place of the 10 mm bipolar device and the left ovary placed inside. Once the ovary was placed in the specimen bag, the telescope and Babcock forceps were removed and the peritoneal cavity was evacuated of CO2. The SILS™ port and specimen retrieval bag were then removed from the abdomen together. The SILS™ port was then re-inserted and peritoneal insufflation achieved. The tigers were then placed into approximately 45-degrees left, dorsal-oblique recumbency. The right ovariectomy was completed in the same manner as the left.
Figure 1. a. Intraoperative image of a tiger undergoing SILS™ port ovariectomy. The Ligasure Atlas™ and 10 mm inner cannula are to the left, the 5 mm telescope and camera are in the middle cannula, and the 5 mm Endo Grasp™ is to the right. b. Image of the SILS™ port with one 10 mm and two 5 mm inner cannulas.
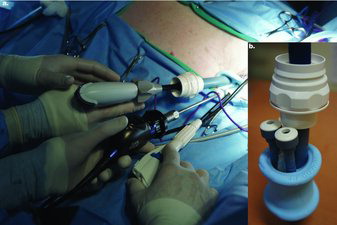
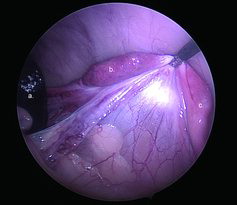
Figure 2. Intraoperative image of the ovarian pedicle in one of the tigers. a. Spleen. b. Ovary. c. Uterine horn. Notice the open ovarian bursa, which was observed with both ovaries of both tigers.
For the second tiger, a 5 mm rotating and articulating, disposable laparoscopic grasping forcepso was used to elevate and stabilize the ovarian pedicles during resection. This modification was performed to reduce clashing of instruments during the procedure. Once both ovaries were removed, the peritoneal cavity was evacuated of CO2 gas. The linea alba was closed using 2-0 polydioxanonep in a cruciate pattern and the subcutaneous tissue apposed using 3-0 glycomerq with an intradermal pattern. Surgical time (start of skin incision to end of skin closure) was 104 and 84 minutes for the first and second tiger, respectively.
No surgical complications were observed. Anesthetic monitoring revealed a minimal increase in heart rate and mean arterial pressure at the insertion of the SILS™ port into the abdominal wall. Ten minutes after the initiation of abdominal insufflation, an increase of 10mm Hg in end tidal CO2 from baseline was observed and confirmed by arterial blood gas analysis. Accordingly, controlled ventilation was adjusted to maintain normocapnia (PaCO2 of 35–45 mm Hg). Subjective postoperative pain assessment was performed by monitoring recumbency, posture, respiratory rate, and vocalization. Both tigers were sternal and ambulatory with normal respiratory rates and no abnormal vocalizing within two hours of recovery. Additionally, both tigers had good appetites six hours into recovery. At 8 months post-surgery both tigers were behaviorally normal with no apparent complications.
Ovaries from both tigers were submitted for histopathological examination and confirmation of complete removal. The right and left ovaries measured 3.2 × 1.4 × 1.7 mm and 3.2 × 1.1 × 1.4 mm, and 4.0 × 3.0 × 2.2 mm and 3.5 × 3.2 × 2.0 mm, respectively. The first tiger had a paraovarian cyst in the right ovary. The second tiger had bilateral follicular and rete ovarii cysts.
Prevention of unwanted reproduction is an important concern in the zoological medicine field and has become increasingly important for captive-bred privately owned tigers that are inter-subspecific crossed or “generic” tigers. While wild populations of tigers are in peril, the captive population of “generic” tigers is flourishing (Williamson & Henry Citation2008). This necessitates the use of contraception options that are reasonably safe and effective for this population. Nonsurgical methods of contraception in large cats have a number of problems, ranging from lack of efficacy to carcinogenesis (Harrenstien et al. Citation2004; McAloose et al. Citation2007). The major advantage of laparoscopic ovariectomy in tigers is the permanent prevention of pregnancy and removal of hormonal stimulus for pyometra, which is an important cause of morbidity in geriatric captive felids (McCain et al. Citation2009). Further, anecdotal complications associated with open ovariohysterectomy in large felids include incisional infection, self-induced trauma, dehiscence, or prolonged recoveries (Gass Citation1982). These complications are likely to be minimized with the single small incision (20 mm) used in this procedure.
In dogs, laparoscopic sterilization has been shown to be associated with less morbidity and pain when compared to traditional open techniques (Devitt et al. Citation2005). Laparoscopic ovariohysterectomy using multiple ports has been described in lions (Aguilar et al. Citation1997; Kolata Citation2002) and more recently laparoscopic ovariectomy has been reported in tigers (Steeil et al. Citation2012). However, single-port laparoscopic sterilization has not been previously described in these species. Minimization of surgical trauma by laparoscopic techniques may be even more important in tigers due to the difficulties associated with postoperative management.
A trend currently exists towards reduction in cannula number and size for laparoscopic sterilization in animals, with the overall goal being reduction in morbidity and postoperative discomfort (Van Nimwegen & Kirpensteijn Citation2007; Dupre et al. Citation2009; Case et al. Citation2011; Wilson & Monnet Citation2012). Since the SILS™ port ovariectomy allows the surgeon to use three instruments with independent maneuverability via a single 20 mm incision, it provides balance between operative efficiency and surgical wound size. Currently, publications on the use of the SILS™ port in veterinary medicine are limited, but favorable (Manassero et al. Citation2012; Wilson & Monnet Citation2012).
The surgical time ranged from 84 to 104 minutes but improvement was seen from the first case to the second. These operative times were thought to be reasonable given these preliminary experiences with single-port laparoscopy in tigers. The surgical times reported here are similar to those recently reported with multi-port laparoscopic ovariectomy in tigers, which were found to be significantly shorter than standard surgical ovariohysterectomy times (Steeil et al. Citation2012). It is likely that these operative times will continue to improve with successive cases.
From a technique standpoint, the SILS™ method appeared to offer certain advantages over previously reported laparoscopic ovariectomy techniques (Aguilar et al. Citation1997; Devitt et al. 2005; Kolata Citation2002; Dupre et al. Citation2009). First, the port accommodates three separate instruments which obviates the need for transabdominal stabilization of the ovarian pedicle which is required when performing a 2-cannula ovariectomy. Transabdominal stabilization using a weighted hook or suture would have been difficult in these tigers due to the thickness of the abdominal wall, which led to stretching of the foam port. This did not cause any noticeable difficulty, however, in placing the port's inner cannulas.
A second advantage of the SILS™ technique is the use of a single 20 mm incision. In both tigers the ovaries were removed without needing incision enlargement. The ability to remove the ovaries through the original incision eliminates the need for temporary dropping of the ovaries in the abdomen between sides and prevents further surgical trauma. Another benefit of this technique was the ability to place the ovarian tissue in a specimen retrieval bag prior to removal from the abdomen. Ectopic ovarian tissue has not been reported at port sites in domestic canids or felids following laparoscopic sterilization. However, it has been diagnosed at port sites in humans following laparoscopic oophorectomy (Chao Citation2008). As both tigers had open ovarian bursas, this was considered a potential risk. Additionally, the use of these bags eliminated the need to release the ovary into the abdomen prior to removal and the necessity to enlarge the abdominal incision to allow for removal.
While there may be many advantages with SILS™ ovariectomy in tigers, difficulties were also noted. First, instrument interference was common due to the straight laparoscopic instruments. The SILS™ port was designed to be used with articulating and rotating laparoscopic instruments. For example, the Endo Grasp™ allows crossing of the instrument shafts in the abdomen, shifting the instrument handles further away from each other. Subjectively, we did not find the use of the Endo Grasp™ to offer significant benefits over the straight laparoscopic Babcock forceps and clashing was easily minimized by specific placement of individual instruments and by rotating the instrument handles away from each other. Another difficulty was with ligation of the uterine horns using the bipolar device. Although the exact number was not recorded, many ligation cycles were required to seal and divide each of the thick uterine horns. A third limitation of this procedure is the increased cost associated with the necessary laparoscopy equipment, SILS™ port, and specimen retrieval bags. However, the authors feel that these limitations are greatly outweighed by the benefits of this procedure in tigers.
In conclusion, laparoscopic ovariectomy using a SILS™ port was performed safely and in a reasonable amount of time in two adult obese tigers. This technique appears to be an acceptable minimally-invasive option for sterilization in some large felids.
Sources and manufacturers:
aDepo-Provera, Pfizer Pharmaceuticals Group, New York, NY 10017, USA
bZoo Pharm, Laramie, WY 82702, USA
cKetaset, Fort Dodge Animal Health, Fort Dodge, IA 50501, USA
dAPP Pharmaceuticals, LLC, Schaumburg, IL 60173, USA
ePiramal Healthcare Limited, N.H.9, Digwal Village, Kohir Mandal, Medak Dist. 502 321, Andhra Pradesh, India
fBioniche Pharma USA LLC, Lake Forest, IL 60045, USA
gBoehringer Ingelheim Vetmedica, Inc., St. Joseph, MO 64506, USA
hConMed, Utica, NY 13502, USA
iSILS™, Covidien, Mansfield, MA 02048, USA
jKarl Storz, Endoflator, Veterinary Endoscopy, Goleta, CA 93117, USA
kKarl Storz, Hopkins II telescope, Veterinary Endoscopy, Goleta, CA 93117 USA
lKarl Storz, Veterinary Endoscopy, Goleta, CA 93117, USA
mLigasure Atlas™, Tyco-Covidien, Mansfield, MA 02048, USA
nEndo Catch™, Covidien-Tyco, Mansfield, MA 02048, USA
oEndo Grasp™, Covidien-Tyco, Mansfield, MA 02048, USA
pPDS II, Ethicon, Somerville, NJ 08876, USA
qBiosyn, Covidien-Tyco, Mansfield, MA 02048, USA
Acknowledgement(s)
The authors would like to thank Drs. Darryl Heard and Jim Wellehan for their helpful input with this paper. The authors would also like to thank John Huhn from Covidien for donating the SILSTM port used for this procedure. Additionally, we acknowledge the partial support by the National Institutes of Health-National Center for Research Resources Clinical and Translational Science Awards to the University of Florida (KL2 RR029888 and UL1 RR029890) and Feld Entertainment.
References
- Aguilar RF, Mikota SK, Smith J, Munson L, Freeman LJ, Kolata R. 1997. Endoscopic ovariohysterectomy in two lions (Panthera leo). J Zoo Wildl Med. 28(3): 290–297.
- Case JB, Marvel SJ, Boscan P, Monnet EL. 2011. Surgical time and severity of postoperative pain in dogs undergoing laparoscopic ovariectomy with one, two, or three instrument cannulas. J Am Vet Med Assoc. 239: 203–208.
- Chao HA. 2008. Ovarian remnant syndrome at the port site. J Minim Invasive Gynecol. 15(4): 505–507.
- Devitt CM, Cox RE, Hailey JJ. 2005. Duration, complications, stress, and pain of open ovariohysterectomy versus a simple method of laparoscopic-assisted ovariohysterectomy in dogs. J Am Vet Med Assoc. 227: 921–927.
- Dupre G, Fiorbianco V, Skalicky M, Gültiken N, Ay SS, Findik M. 2009. Laparoscopic ovariectomy in dogs: comparison between single portal and two-portal access. Vet Surg. 38: 818–824.
- Gass H. 1982. Felidae, viverridae, mustelidae. In: Klos HG, Lang EM, editors. Handbook of zoo medicine. New York: Van Nostrand Reinhold Co.; p. 119–120.
- Harrenstien LA, Munson L, Chassy LM, Liu IK, Kirkpatrick JF. 2004. Effects of porcine zona pellucida immunocontraceptives in zoo felids. J Zoo Wildl Med. 35: 271–279.
- Kolata RJ. 2002. Laparoscopic ovariohysterectomy and hysterectomy on African lions (Panthera leo) using the ultracision harmonic scalpel. J Zoo Wildl Med. 33(3): 280–282.
- Manassero M, Leperlier D, Vallefuoco R, Viateau V. 2012. Laparoscopic ovariectomy in dogs using a single-port multiple-access device. Vet Rec. 171(3): 69.
- McAloose D, Munson L, Naydan DK. 2007. Histologic features of mammary carcinomas in zoo felids treated with melengestrol acetate (MGA) contraceptives. Vet Pathol. 44: 320–326.
- McCain S, Ramsay E, Allender MC, Souza CS, Schumacher J. 2009. Pyometra in captive large felids: a review of eleven cases. J Zoo Wildl Med. 40(1): 147–151.
- Steeil JA, Sura PA, Ramsay EC, Reilly S, Seddighi R, Whittemore J. 2012. Laparoscopic-assisted ovariectomy of tigers (Panthera tigris) with the use of the Ligasure™ device. J Zoo Wildl Med. 43(3): 566–572.
- Thatcher CD, Hand MS, Remillard RL. 2000. Small animal clinical nutrition: an iterative process. In: Hand MS, Thatcher CD, Remillard RL, Roudebush P, editors. Small animal clinical nutrition. Marceline: Walsworth Publishing Company; p. 1–19.
- Van Nimwegen SA, Kirpensteijn J. 2007. Comparison of Nd:YAG surgical laser and Remorgida bipolar electrosurgery forceps for canine laparoscopic ovariectomy. Vet Surg. 36: 533–540.
- Williamson DF, Henry LA. 2008. Paper tigers?: the role of the U.S. captive tiger population in the trade in tiger parts. TRAFFIC North America, Washington, DC: World Wildlife Fund.
- Wilson DM, Monnet EL. 2012. The use of single incision laparoscopic surgery (SILS™ Port) in dogs: description of technique and initial impressions after 22 consecutive cases. Veterinary Endoscopy Society Proceedings; 2012 March 22–24; Park City, Utah.