Abstract
Bovine herpesvirus-1 (BHV-1) is known to cause several diseases worldwide. It is a double-stranded DNA virus consisting of 33 structural proteins out of which 13 are associated with the envelope. Based on genomic analysis and viral peptide patterns, BHV-1 virus can be divided into several subtypes like BHV-1.1, BHV-1.2, and BHV-1.3. However, all subtypes are antigenically similar. The symptoms of the related diseases are mainly non-life-threatening but have a rather wide host range that limits animal trade. The different modes of transmission as unique feature of this virus and the tendency to cause infection in the early age with latency development in trigeminal and sacral ganglion cause huge economic losses around the world. The virus also affects endangered bovine species like mithun (Bos frontalis) and yak (Poephagus grunniens). The disease can be diagnosed by using conventional procedures (like cell culture, immune-histopathology, and enzyme-linked immunosorbent assay (ELISA)) as well as highly sensitive modern techniques (like nested PCR and southern hybridization) with the virus neutralization test regarded as gold standard. With the currently available diagnostic tests it is not possible to identify animals which have a latent BHV-1 infection. Different types of modern and conventional vaccines are available for immunoprophylaxis. Inactivated vaccines are not as efficacious as modified live virus (MLV) vaccines. Marker vaccines allow the distinction between vaccinated and naturally infected animals. In this review the present status of BHV-1 around the world will be addressed besides the current knowledge with regard to its biology, epidemiology, diagnosis, and prophylaxis.
1. Introduction
Bovine herpesvirus-1 (BHV-1) belongs to the genus Varicellovirus in the subfamily Alphaherpesvirinae under the family Herpesviridae. The virus is responsible for severe economic damage to the cattle industry throughout the world. The clinical spectrum of the disease is complex and the severity of the infection and pathogenesis depends upon its virulence. Development of latency is a unique feature of the virus. In most cases, the carrier animals go unnoticed and remain silent shedders of the pathogen and a potent source of infection for other animals on the farm. Thus, the pathogen continues to exist in the environment unnoticed and inflicting major damage to the livestock. Usually, the infection is not life threatening but secondary bacterial infection may complicate the scenario. Even though mortality is low, the disease with its severe impact on growth, milk production, and international livestock trade may jeopardize the agro-economy of a country.
2. The virus
2.1. Subtypes
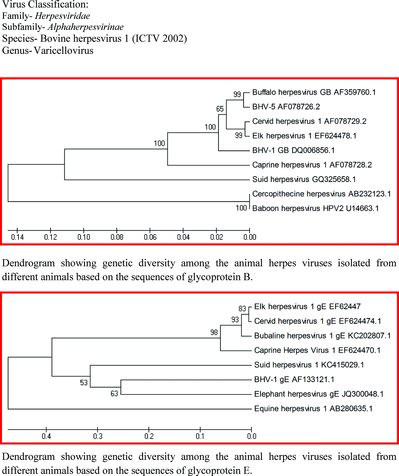
BHV-1 is a double-stranded DNA virus having 135–140 kbp size (Mayfield et al. Citation1983; Wyler et al. Citation1989), and codes for 70 proteins. The genomic sequence of the virus can be divided into a unique long (UL) segment of approximately 102–104 kbp and a unique short (US) segment of approximately 10.5–11 kbp with inverted repeat regions of approximately 24 kbp (Mayfield et al. Citation1983; Wyler et al. Citation1989; Schwyzer & Ackermann Citation1996). These UL, US, and inverted repeat regions are responsible for two isomeric forms of the viral genome (Mayfield et al. Citation1983). Based on the genomic analysis and viral peptide patterns, BHV-1 virus can be divided into several subtypes like BHV-1.1, BHV-1.2, and BHV-1.3. BHV-1.1 mostly is related to the respiratory syndrome, BHV-1.2 is related to genital infections, and BHV-1.3/BHV-5 is associated with neurological disorders of the cattle (Muylkens et al. Citation2007). However, all subtypes are antigenically similar. Antigenically BHV-1 is closely related to cervine herpesvirus-1 (CvHV-1), buffalo herpesvirus-1, and elk herpesvirus (Keuser et al. Citation2004)(see Table and Figure ). It is an enveloped virus and susceptible to disinfectants which destroy its lipid envelope. The virus can be isolated with the help of bovine cell cultures as well as cell lines of other species.
Table 1. Different prototypes of Herpesviridae, host range, and their associated infection in animals.
Figure 1. Phylogenetic relationships between animal alphaherpesviruses (gB: Glycoprotein B, gE: glycoprotein E). Available sequences from NCBI database were aligned using the Mega 4.1 program. The phylogenetic tree was obtained by unweighted pair group method with arithmetic mean (UPGMA) analysis. The phylogenetic distance was estimated by Kimura's two-parameter method using 1000 bootstrap replicates. Virus classification: Family: Herpesviridae; Subfamily: Alphaherpesvirinae; Species: Bovine herpesvirus 1 Genus: Varicellovirus. Dendrogram showing genetic diversity among the animal herpesviruses, isolated from different animals, based on the sequences of glycoprotein B. Dendrogram showing genetic diversity among the animal herpesviruses isolated from different animals based on the sequences of glycoprotein E.
2.2. Viral genome and proteins
Herpesviruses are large, enveloped, double-stranded DNA viruses (Harrison Citation2001). Typical herpesvirus virions consist of a core containing linear double-stranded DNA and an icosadeltahedral capsid of about 100 nm diameter containing 162 capsomeres. The viral DNA genome is wrapped around a fibrous spool-like core, which has the shape of a torus. Surrounding the capsid is a layer of globular material, known as the tegument, which is enclosed by a typical lipoprotein envelope with numerous small glycoprotein spikes (Roizman et al. Citation2007; MacLachlan & Dubovi Citation2010). The US sequence is flanked by inverted internal repeat (IR) and terminal repeat (TR) sequences. In BHV-1 genome, a total of 73 open reading frames (ORF) coding for proteins have been identified. BHV-1 genome codes for 33 structural proteins, 13 of which are probably associated with the envelope (Liang et al. 1996) and 10 of these have the potential to encode glycoproteins (Schwyzer & Ackermann Citation1996). Among the 10 glycoproteins, six are in the UL segment, namely gK (UL53), gC (UL44), gB (UL27), gH (UL22), gM (UL10), and gL (UL1), whereas the four remaining ones are in the US segment, namely gG (US4), gD (US6), gI (US7), and gE (US8) (Muylkens et al. Citation2007). The other major proteins of BHV-1 are vhs (virion host shut down), VP8, VP16, BICP0, and BICP4 protein. The functions of some of the major proteins are given in Table .
Table 2. Function of some of the major proteins of the bovine herpesvirus-1.
2.3. Physiochemical properties of the virus
BHV-1 is resistant to environmental influences. Inactivation of the virus in the environment depends on factors such as temperature, pH, light, and humidity. At 4°C, the virus is stable for 1 month. BHV-1 is inactivated at 56°C within 21 minutes, at 37°C within 10 days and at 22°C within 50 days (Gibbs & Rweyemamu Citation1977). The virus may survive for more than 30 days in feed. As the virus is enveloped, it is sensitive to organic solvents such as chloroform, ether, and acetone. The virus is sensitive to many disinfectants and is readily inactivated by 0.5% NaOH, 0.01% HgCl2, 1% chlorinated lime, 1% phenolic derivatives, 1% quaternary ammonium bases, and 10% Lugol's iodine. Formalin (5%) inactivates BHV-1 within 1 minute (Straub Citation1990; Nandi et al. Citation2009).
2.4. Host range
BHV-1 primarily affects cattle. Other Artiodactyla (e.g. goats, sheep, water buffaloes, and camelids) may be infected with BHV-1. BHV-1 has been shown to infect and cause disease in sheep and goats also (Whetstone & Everman Citation1988). Antibodies to BHV-1 have been detected in captive Asian elephants (Elephas maximus) without any apparent clinical symptoms (Metzler et al. Citation1990; Bhat et al. Citation1997). BHV-1 has also been isolated from apparently healthy pronghorn antelope, wildebeest, mink, and ferrets (Porter et al. Citation1975) as well as from the soft-shelled tick Ornithodoros coriaceus (Taylor et al. Citation1982). Face flies (Musca autumnalis) can carry BHV-1, but they do not transmit the virus to the cattle (Johnson et al. Citation1991). BHV-1 does not infect mice, rats, guinea pigs, or chick embryos (Gibbs & Rweyemamu Citation1977), although immunocompromised mice that lack interferon receptors can be infected if the virus is injected into the peritoneal cavity (Abril et al. Citation2004). Rabbits can be experimentally infected with BHV-1 if the virus is injected into the conjunctival sac of their eyes (Rock & Reed Citation1982; Rock et al. Citation1987, Citation1992), or through holes drilled into the sinuses (Brown & Field Citation1990a, Citation1990b). In vitro studies in cell culture showed that BHV-1 can bind weakly to human vascular endothelial cell (HveC) (nectin-1) or to the human poliovirus receptor (Geraghty et al. Citation1998; Connolly et al. Citation2001) without detectable viral replication. There are no reports of human infection with BHV-1.
2.5. Virus transmission
BHV-1 is shed in nasal discharge for 10–14 days during acute respiratory infection (Gibbs & Rweyemamu Citation1977) and transmission occurs by contact with mucosal droplets from infected cattle (Kahrs Citation2001). The virus is also shed following reactivation from latency. BHV-1 can also be transmitted to susceptible animals through contaminated materials including semen (Mars et al. Citation2000). Airborne transmission of BHV-1.1 can occur under experimental conditions at distances of 3.85 meters (Wentink et al. Citation1993), and is dependent on environmental temperature and relative humidity (Elazhary & Derbyshire Citation1979; Mars et al. Citation1999). Till date, no vectors have been described for BHV-1.
2.6. Virus entry into host cells
Herpesviruses enter cells by fusing with the cell plasma membrane in a complex process of attachment and penetration. Virus entry requires the presence of complementary binding partners on the virus and on the host cell. Studies show that the BHV-1 glycoproteins gB, gC, gD, gE, gH, gK, and gL are required for the virus entry (Li et al. Citation1995; Dasika & Letchworth Citation1999; Hanon et al. Citation1999; Schroder & Keil Citation1999). BHV-1.1 and BHV-1.2 subtypes differ in gC epitopes, which may alter viral attachment and virulence (Rijsewijk et al. Citation1999). Although the host cell proteins required for BHV-1 entry are not fully understood, the virus initially binds to cell surface heparan sulphate (Hanon et al. Citation1998; Tyler & Nathanson Citation2001) via BHV-1 gB and gC (Li et al. Citation1996). After this initial binding, BHV-1 gB and gD bind further quite strongly to cell surface receptors (Li et al. Citation1995). The nature and properties of these receptors are yet to be determined. Studies have shown that BHV-1 gD can weakly bind HveC or the human poliovirus receptor expressed in human or hamster cell lines (Geraghty et al. Citation1998; Connolly et al. Citation2001). This weak binding indicates that viral entry in a natural host may be mediated by receptors other than bovine HveC or poliovirus receptor homologues (Geraghty et al. Citation1998; Connolly et al. Citation2001).
2.7. Virus replication and release
After entry into the host cell, BHV-1 is transported along microtubules to the nucleus for replication using host cell proteins. The virus becomes enveloped as it buds through the nuclear envelope and is then transported within intracellular vesicles to the cytoplasmic membrane and released from the cell (Hunter Citation2001; Knipe et al. Citation2001). BHV-1 replication starts within 2 hours of infection in the cattle (Meurens et al. Citation2004b) with concomitant cell surface antigen expression within 3–4 hours after infection. Following assembling, the virus is released and it starts to infect other cells within 8 hours (Babiuk et al. Citation1996).
2.8. Latency development
Establishment of latency is a unique feature of this virus. BHV-1 virus can become latent following a primary infection with a field isolate or vaccination with an attenuated strain (Nandi et al. Citation2009). Latency is believed to develop in almost all animals that are infected with high or low doses of attenuated or virulent BHV-1 (Pastoret et al. Citation1982). Attenuated vaccine strains can remain in a latent state in the body and vaccination does not provide protection against establishment of a latent infection with a wild strain (Jones et al. Citation2000). Inoculation with live vaccine strains of BVH-1 can also lead to latent infection (Kit et al. Citation1985; SCAHW Citation2000). Vaccination in latently infected animals does not prevent re-excretion of a wild strain either. Young calves can have latent infections and have antibody responses due to infection in the presence of maternal antibodies (SCAHW Citation2000). Colostral anti-BHV-1 antibodies do not prevent initial virus replication in calves, and latency can persist following the decline in colostral immunity, with calves remaining seronegative (Homan & Easterday Citation1983). If the infection is initiated via the oral cavity, nasal cavity, or ocular orifices, the primary sites for latency are the sensory neurons within trigeminal ganglia (TG). Relatively high levels of viral gene expression (Schang & Jones 1997) or infectious virus (Inman et al. Citation2002) can be detected in TG within 1–6 days after infection. A hallmark of latency is the abundant level of transcription that occurs from the latency-related (LR) gene (Jones Citation1998, Citation2003; Jones et al. Citation2006) and open reading frame-E (ORF-E) (Inman et al. Citation2004). ORF-E is at the downstream of the gene, encoding the major viral transcriptional activator bICP0. After acute infection, some factors help in the establishment of latency, i.e., LR gene, ORF-E, neuronal factor, and cell-mediated immunity (CMI) response in TG (Jones and Chowdhury Citation2008). LR gene products induce latency by inhibiting apoptosis of virus-infected cells (Ciacci-Zanella et al. Citation1999; Henderson et al. Citation2004) and viral gene expression (Bratanich et al. Citation1992; Geiser et al. Citation2002). ORF-E may have a role in the establishment of latency because it can induce neurite-like outgrowth in mouse neuroblastoma cells (Perez et al. Citation2007). Consequently, ORF-E may be responsible for restoration of mature neuronal functions following the infection. Neuronal factors inhibit productive infection and CMI response in TG. It leads to abundant viral transcripts, viral protein, and viral DNA replication (Feldman et al. Citation2002), inducing latent infection. Maintenance of latent infection is mostly controlled by LR gene and ORF-E. LR gene inhibits bICP0 RNA expression, which is a viral promoter, and ORF-E maintains the neural health (Jones & Chowdhury Citation2008). The cattle that are seronegative for BHV-1 antibodies may be latently infected with BHV-1 (Hage et al. Citation1997). Latency may also occur in non-neural sites such as tonsillar lymphoid cells (Winkler et al. Citation2000), peripheral blood (Fuchs et al. Citation1999), and lymphnodes (Mweene et al. Citation1996).
3. BHV-1-related diseases
BHV-1 or infectious bovine rhinotracheitis (IBR) virus is an important causative agent of respiratory infection, abortion, conjunctival infection, and other respiratory-tract diseases. The clinical manifestation of BHV-1 infection is dependent on the nature of various subtypes. BHV-1 causes three different types of infection, namely respiratory infection (BHV-1.1), genital infection (BHV-1.2), and neurological disease (BHV-1.3). The respiratory infection is characterized by high fever, mucopurulent nasal discharge, and excessive salivation. The laesion includes rhinotracheitis, pharyngitis, and laryngotracheitis (Gibbs & Rweyemanu Citation1977; Yates Citation1982). The virus with co-infection of some bacteria might induce severe bronchopneumonia, pleuritis, thoracic pain, and audible rubbing. In genital infection, there is development of pustules, ulcers in the mucosa of vulva and vagina with frequent urination. Abortion occurs at the end of the fifth month of pregnancy, with expulsion of dead foetus and blood stained fluids. The nervous manifestation of this viral infection is mostly seen as a form of encephalitis in calves, with the virus presumed to have spread from nasal mucosa through trigeminal ganglion. The virus remains latent in trigeminal ganglion and sacral ganglion for several time periods. BHV-1 often causes enteritis in calves due to replication in the digestive tract.
3.1. Clinical course
In BHV-1 infection, after an incubation period of 2–4 days, serous nasal discharge, salivation, fever, anorexia, and depression become evident. Within a few days the nasal and ocular discharges become mucopurulent. Where natural mating is practised, genital infection can lead to pustular vulvovaginitis or balanoposthitis. However, most infections run a very mild or subclinical course (van Oirschot et al. Citation1993). An uncomplicated case of respiratory or genital disease caused by BHV-1 usually lasts for about 5–10 days. Secondary bacterial or viral agents may contribute to a multifactor disease complex resulting in severe respiratory diseases in young animals (‘shipping’ or ‘crowding fever’). After infection via the airborne route, BHV-1 replicates in high numbers in mucous membranes of the upper respiratory tract and tonsils. Subsequently, the virus disseminates to conjunctivae and reaches the trigeminal ganglia by neuro-axonal transport. After genital infection, BHV-1 replicates in the mucous membranes of the vagina or prepuce and becomes latent in the sacral ganglia. Viral DNA remains in the neurons of the ganglia, probably for the entire life of the host. The reactivation of the latent infection can be due to stressful conditions such as transport and parturition, and also due to the application of corticosteroids (Muylkens et al. Citation2007). Consequently, the virus may switch between latent and lytic infection, and may be shed intermittently into the environment and spread to in-contact animals.
3.2. Bovine respiratory disease complex
The Bovine Respiratory Disease Complex (BRDC) consists of at least three clinical entities, as well as several additional diseases which affect the respiratory tract secondarily or as part of a more generalized disease. The three clinical entities are as follows: (1) enzootic pneumonia of calves, (2) ‘shipping fever’ complex, and (3) atypical interstitial pneumonia. BHV-1 is one of the agents that cause BRDC. During shipment, cattle may be subjected to crowded conditions, exhaustion, irregular feeding and watering, climatic changes, and sick cattle. BRDC results from a combination of stress and infectious agents, in particular the viruses BHV-1, bovine viral diarrhoea virus (BVDV), parainfluenza-3 virus or bovine respiratory syncytial virus, and the bacteria Mannheimia haemolytica or Pasteurella multocida (Yates Citation1982). BHV-1 can initiate BRDC by causing immunosuppression (Winkler et al. Citation1999; Lovato et al. Citation2003), allowing secondary infections that lead to severe pneumonia and death (Hanon et al. Citation1998; Lovato et al. Citation2003). BRDC is a serious pneumonic condition that affects up to 50% of cattle in Australia, causing death of upto 5% of all the cattle (Meat & Livestock Australia 2001), costing the Australian feedlot industry around $60 million each year (CSIRO Citation2005). Control of bovine respiratory disease can be possible only with an increased understanding of both the respiratory and immune systems of the cow and of the interaction of the host, pathogen, and environment.
3.3. Pathogenesis, pathophysiology, and signalling pathway
After entry into the body, replication occurs at the portal of entry or within the nasal or genital mucosa. During the initial replication at the portal of entry, the herpesviruses may enter the axons of local nerve cells. Then, by intra-axonal transport, the viruses reach the neuron bodies in the regional ganglia where latency can be established (Nandi et al. Citation2009). Transmission into the body is achieved through blood, nerves, and infected tissues via cell-to-cell interaction. Following transient viraemia, secondary sites will be infected, such as the digestive tract, udder, foetus, and ovaries. From the site of infection, peripheral nerves are reached by retrograde axonal transportation, i.e., trigeminal ganglion in case of respiratory infection, and sacral ganglion after genital infection. Viral entry into cells is a multistep process involving several glycoproteins and at least two cellular receptors (Mettenleiter Citation1994). Glycoprotein gC of alphaherpesviruses initiates these steps by binding to heparan sulphate proteoglycans on the cell surface. The binding of gC to heparan sulfate moieties leads to a loose attachment that is followed by fixed binding of gD to the putative second cellular receptor. Binding of gD is necessary for initiation of viral entry (Karger et al. Citation1995), and for steps between virus binding and membrane fusion by interacting with other cellular or viral components. Viral entry into the cell is finally mediated by fusion of the viral envelope with the cell membrane, due to interactions of gB, gH, and gL (Liang et al. Citation1995). Nyaga and McKercher (Citation1980) have shown by in vitro examinations that BHV-1 can infect blood monocytes, where a limited virus replication and release is possible. In addition, BHV-1 is able to absorb to lymphocytes which may also serve as vehicles, at least as long as no neutralizing antibodies are present. The virus multiplies in the respiratory tract and causes inflammatory changes such as rhinitis, laryngitis, and tracheitis, leading to destruction of the tracheal microvilli. Infection of the cattle with BHV-1 impairs resistance to secondary bacterial infections such as M. haemolytica, P. multocida, and H. somni, leading to depression of cell-mediated immunity and subsequent fatality (Yates Citation1982; Leite et al. Citation2002). Laesions may extend from the nasal tract to the eyes through the nasolacrymal duct, and may give rise to conjunctivitis and nasal discharge. In yaks, BHV-1 infection is mostly associated with Moraxella bovis-, M. haemolytica-, and Neisseria-associated keratoconjunctivitis (Bandyopadhyay et al. Citation2010a, Citation2010b). The virus may enter the brain tissues from the nasal mucosa via the trigeminal nerves causing meningoencephalitis. The virus might also cause changes in the placenta and foetus, resulting in abortion.
4. Epidemiology
The first infection of BHV-1 was recorded in the form of genital disease as infectious pustular vulvo-vaginitis (IPV) in cattle in 1841 by Buchner & Trommsdrof in Germany. Viral association with this disease was first demonstrated in 1928 by Reisinger & Reimann. In the 1950s, emergence of the respiratory form of the disease as IBR was observed in North America. In 1958, the virus was isolated successfully for the first time and its antigenic identity was revealed. Later this viral agent was classified under the family of Herpesviridae. BHV-1 is currently widespread all over the world and reported for instance in USA, Canada, Zaire, Italy, Belgium, India, and Turkey (Castrucci et al. Citation1997; Bilgedagalap Citation1998; Boelaert et al. Citation2000; Yesilbag et al. Citation2003; Rajkhowa et al. Citation2004). Serological surveys in the cattle, carried out in Turkey, ascertained that this virus is widespread among the dairy and beef cattle in many regions of the country (Cabalar & Akca Citation1994; Alkan et al. Citation2000; Aly et al. Citation2003; Akca et al. Citation2004). Previous research by Cabalar and Can-Sahna (2000) revealed that the seropositivity rate of BHV-1 was between 20% and 74% in the cattle. BHV-1 especially infects domestic and wild cattle, and other ruminants may be similarly infected (International terrestial … Citation2004). In previous studies, cross-species infections by bovine and caprine herpesviruses were demonstrated (Lehmkuhl et al. Citation1985; Yesilbag et al. Citation2003). Detection of antibodies against BHV-1 in sheep suggests that sheep may play a crucial role in the epidemiology and maintenance of BHV-1 in the environment (Elazhary et al. Citation1984; Jetteur et al. Citation1990). However, sheep is unlikely to play any role in BHV-1 transmission (Hage et al. Citation1997). In the UK, the prevalence of BHV-1 has increased to 83% in unvaccinated herds (Woodbine et al. Citation2009). Control programs are ongoing in several other countries, like in Germany and Italy (OIE Citation2010). The seroprevalence of BHV-1 has been reported to be 14%–60% in Africa and 36%–48% in Central and South America (Straub Citation1990), 36% in China (Yan et al. Citation2008), 43% in England (Woodbine et al. Citation2009), and 63%–86% in Eygpt (Mahmoud et al. Citation2009). Several European countries successfully implemented the IBR eradication program. Countries like Austria, Denmark, Finland, Sweden, and Switzerland are officially free of IBR (Ackermann & Engels Citation2005). Like other countries, India was also affected by this disease as the virus was detected in many parts of the country, like Kerala (Sulochana et al. Citation1982), Gujarat (Singh et al. Citation1983), Tamil Nadu (Manickam & Mohan Citation1987), Orissa, Arunachal Pradesh (Bandyopadhyay et al. Citation2009), Nagaland (Rajkhowa et al. Citation2004), and Karnataka (Mohan Kumar et al. Citation1994). Given a grave concern regarding exotic bovine species like mithun and yak, the association with BHV was determined. The seroprevalence of IBR in yak is highest over the age of 3 years and lowest in the first year of life. The gender or the location of the yaks (farm or free ranging) was not a factor influencing the prevalence of the virus. The overall seroprevalence in yak was found to be 41% by virus neutralization test (VNT) and avidin–biotin enzyme-linked immunosorbent assay (AB-ELISA). The virus was also detected in the ocular swab of yaks suffering from infectious bovine keratoconjunctivitis (Bandyopadhyay et al. Citation2009; Bandyopadhyay et al. Citation2010a). The origin and geographic distribution of BHV-5 infection is largely unknown mainly due to its serological cross-reactivity with BHV-1 (Vogel et al. Citation2002). Sporadic cases of meningoencephalitis due to BHV-5 have been reported in Australia (Johnston et al. Citation1962), USA (Eugster et al. Citation1974), Italy (Moretti et al. Citation1964), and Hungary (Bartha et al. Citation1969). In contrast, BHV-5 infection and disease appear to be more frequent in Argentina and Brazil, where numerous outbreaks were described in the last decades (Carrillo et al. Citation1983; Weiblen et al. Citation1989; Roehe et al. Citation1997; Rissi et al. Citation2006). The rare occurrence of BHV-5 neurological disease in areas where BHV-1 infection is endemic may be explained by cross-protection induced by natural infection or vaccination (Thiry et al. Citation2006).
5. Immune response to BHV-1 infection
The immune response of cattle to BHV-1 infection is unique. While there are similarities in immune responses to other alphaherpesviruses, the bovine response to BHV-1 is not identical to that of herpes simplex virus in mice or in humans, and does not correlate precisely with experimental BHV-1 infection in mice (Babiuk et al. Citation1996). The immune response to BHV-1 infection is triggered when the virus begins to replicate (Babiuk et al. Citation1996). Adaptive cell-mediated and antibody-mediated immune response occurs 7 days after infection (Engels & Ackermann Citation1996). Antibody response is thought to be critical in preventing infection and viral spread, while cell-mediated immunity is involved in recovery from infection (Babiuk et al. Citation1996).
5.1. Humoral response
Experimental as well as natural infection and vaccination demonstrated that the antibodies are triggered against the gB, gC, gD, and gE glycoproteins (Tikoo et al. Citation1995a), protecting against viremia and associated severe disease (Mechor et al. Citation1987). The antibody response includes mostly neutralizing antibodies, and contributes to antiviral antibody-dependent cellular cytotoxicity (Tikoo et al. Citation1995a), which is often complement mediated (Rouse et al. Citation1977). Antibodies in the circulation may be recorded for an endurable period, like 3 years following BHV-1 vaccination (Hage et al. Citation1997). In contrast, the antibody response to the V155 strain of BHV-1 persists at maximal levels from 7 to 21 days after infection (Bagust 1972). Maternal antibody to BHV-1 persists for 123 days after weaning at 2 months of age (Fulton et al. Citation2003). Newborn calves are protected from BHV-1 infection after being fed colostrum collected from vaccinated cows (Mechor et al. Citation1987), although maternal antibody is not completely protective since calves can have latent BHV-1 infections early in life in the presence of maternal antibody too (SCAHW Citation2000).
5.2. Cell-mediated immune response
The cell-mediated immune response to BHV-1 infection is under the control of macrophages, interleukin-2, and interferon-γ production, natural killer cells and natural killer-like activity, proliferation of viral gC- and gD-specific CD4+ T-cells, and stimulation of cytotoxic T-lymphocyte activity (Hutchings et al. Citation1990; Tikoo et al. Citation1995b). Interferon-γ, interferon-α, and interferon-β have been shown to both protect against infection and to prevent viral spread in experimental infection in mice (Abril et al. Citation2004). Responses to BHV-1 infection are broad based and include both T helper cell 1 and T helper cell 2 responses (Babiuk et al. Citation1996), although, as with other intracellular pathogens, there is a skew towards a T helper cell 1-type response (Mena et al. Citation2002).
5.3. Allergenicity
There are no reports of BHV-1 encoding known allergens or causing an allergic response.
5.4. Immune-evasion, immune-modulation, and immunosuppression
BHV-1 evades the host immune response by interfering with MHC-mediated antigen processing and presentation following infection of monocytes and macrophages (Nyaga & McKercher Citation1980; Forman et al. Citation1982). Alphaherpesviruses, including BHV-1, also have immunomodulatory activity mediated by herpesvirus proteins that mimic key molecules of the host immune system (Raftery et al. Citation2000). For example, these viruses express proteins that bind complement C3 in a species-specific manner and thus alter the host immune response to allow viral infection (Huemer et al. Citation1993; Engels & Ackermann Citation1996). Additionally, latency is also established by immune evasion. During latency, viral proteins are not expressed and infection occurs at immunoprivileged sites that do not express major histocompatibility class I antigens (Tyler & Nathanson Citation2001). BHV-1 was also reported to cause immunosuppression in infected cattle, which often leads to secondary viral and bacterial infections (Winkler et al. Citation1999) contributing to BRDC. Immunosuppression is caused by impairment of macrophage, polymorphonuclear neutrophil, and lymphocyte function (Tikoo et al. Citation1995a), and by decreased IL-2 receptor expression, decreased mitogenic stimulation of peripheral blood mononuclear cells, and reduced numbers of circulating T lymphocytes (Winkler et al. Citation1999). Infection of monocytes and macrophages leads to impaired phagocytosis, reduced antibody-dependent cellular cytotoxicity function, and poor T cell stimulation (Forman et al. Citation1982). The effect of immunosuppression is partly mediated by the BHV-1 gG glycoprotein, a broad-spectrum chemokine-binding protein that blocks chemokine binding and activity (Bryant et al. Citation2003). BHV-1 also infects CD4+ T cells, inducing a loss of CD4 expression followed by apoptosis of these cells (Winkler et al. Citation1999; Lovato et al. Citation2003).
5.5. Immunity and relation to stress
Immunity to BHV-1 occurs mostly due to either natural infection or vaccination (Denis et al. Citation1994; Tikoo et al. 1995a). Immunity involves both cellular and humoral response to various glycoproteins (Babiuk et al. Citation1996). There are some factors facilitating elimination of infection from the body such as natural antibodies preventing the attachment of virus to cells and interaction with complement in order to lyse the cells infected by the virus. Monocytes or polymorphonuclear cells can also trigger the lysis of the infected cells with the aid of Fc-receptors. There are several glycoproteins promoting attachment and penetration of virus into cells, i.e., gB, gC, and gD. These glycoproteins also help in intercellular transmission of virus. After establishment of infection, early cytokines are released, i.e., interferon-α, TNF, interleukin-1, within a few hours and maximum levels are reached after 36 hours. Interferon-α recruits leukocytes at the sites of infection (Bielefeldt et al. Citation1991; Griebel et al. 1989; Griebel et al. Citation1998). Pro-inflammatory cytokines are released by epithelial cells and alveolar macrophages, leading to expression of adhesion molecules which retain leukocytes at the site of infection (Bochner et al. Citation1987; Lamontagne et al. Citation1985). This phenomenon causes increased vascular permeability and adhesion between cells leading to generation of reactive oxygen species (ROS), arachidonic acid, and other factors that prevent virus replication and promote killing of infected cells by different mechanisms. Killing of infected cells may also occur in the absence of antibody by natural killer cells and macrophages (Campos et al. Citation1994; Campos et al. Citation1989; Campos & Rossi Citation1986; Palmer et al. Citation1990). T-cells help in activation of macrophages regarding killing the BHV-infected cells by secretion of interferon-γ (Campos et al. Citation1989). In addition, macrophages and natural-killer (NK) cells produce cytokines that influence the development of specific immune responses. Activation of T-helper cells I and II (Th1/Th2) generate the production of other cytokines which improve T and B cell responses. Both antibody and cell-mediated immunity are important factors in ameliorating the disease. Elevated corticosteroid levels (due to stress) and/or immune suppression can initiate reactivation from latency. The stress associated with moving cattle from one location to another is one obvious stimulus that can trigger reactivation from latency and induction of BRDC. This reactivation from latency was also recorded in yaks following extreme stress like prolonged transportation in the mountainous tracts and severe nutritional deprivation as seen in harsh winter season (Bandyopadhyay et al. Citation2007). Interestingly, deficiency of important trace minerals like zinc and copper which are directly associated with intracellular oxidant/antioxidant defence mechanism and immune status was recorded to have higher correlation with BHV-1 infection in yaks as evidenced by higher seropositivity (Bandyopadhyay et al. 2007; Bandyopadhyay et al. Citation2009; Bandyopadhyay et al. Citation2010b). During reactivation from latency, three significant events occur: 1) productive viral gene expression is readily detected in sensory neurons, 2) both the ORF-E and LR gene expression decrease dramatically, and 3) infective virus is secreted from nasal or ocular swabs (Jones Citation1998, Citation2003; Jones et al. Citation2006). Administration of dexamethasone to calves or rabbits, latently infected with BHV-1, reproducibly leads to activation of viral gene expression and reactivation from latency (Rock et al. Citation1992; Jones Citation1998, Citation2003; Jones et al. Citation2000, Citation2006; Inman et al. Citation2002). The ability of dexamethasone to induce apoptosis of inflammatory cells in TG of calves latently infected with BHV-1 correlates well with reactivation from latency (Winkler et al. Citation2002). In yaks also, molecular detection of BHV-1 in the nasal discharge was possible following prednisolone or dexamethasone administration suggesting increased virus secretion from nasal discharge (Bandyopadhyay et al. Citation2007). Varying virus titers ranging from 103 to 106 TCID50 ml−1 (tissue-culture infective dose) during reactivation and re-excretion have been reported after stress (Pastoret et al. Citation1982; Straub Citation1990), transport (Thiry et al. Citation1987), infection with parainfluenza-3 (PI-3) virus (Mensik et al. Citation1976) or Dictyocaulus viviparous (Msolla et al. Citation1983), parturition, treatment with dexamethasone and adrenocorticotropic hormone (ACTH), and uptake of 3-methylindole. Spontaneous reactivation without clinical signs has also been observed at irregular intervals (Schultz et al. Citation1977; Grom et al. Citation2006).
6. Impact over livestock industry
Introduction of IBR into a cattle farm can cause severe economic losses due to weight loss, decrease in milk production, and restrictions in the international livestock trade (Nandi et al. Citation2009). Cattle recovering from IBR infection are very prone to become a silent carrier. These animals remain carriers of BHV-1 for the rest of their life and immunosuppressive treatments or conditions might reactivate virus replication, leading to spread of the infection to the rest of the herd (Van Oirschot Citation1997; Preston & Nicholl Citation2008). The same is true for rare bovine species like the yak and mithun. The yak (Bos grunniens) is a bovine species of high economic importance, living in the hills and snowbound areas at 3000–5000 m above the mean sea level in China, Mongolia, Bhutan, Nepal, Russia, and India (Weiner et al. Citation1994; Bandyopadhyay et al. Citation2009). They are mostly reared for milk, meat, and wool. The products obtained from the yak are considered to be organic due their geographical locations that are free from environmental pollutants (Bandyopadhyay et al. Citation2007). BHV-1 infection causes a sharp drop in milk production with loss of body weight, abortion, and decreased show value of animals due to bilateral conjunctivitis, chemosis, and extreme restlessness (Bandyopadhyay et al. Citation2010a). The mithun (Bos frontalis) is a ruminant species found in the hilly regions of northeast India, Myanmar, Bhutan, Bangladesh, China, and Malaysia (Rajkhowa et al. Citation2004). It is a source of meat and milk. Milk of mithun contains a higher amount of protein, fat, and solids-non-fat than that of buffalo but less lactose (Rajkhowa et al. Citation2004). Bovine rhinotracheitis causes a huge loss of productivity in mithun as well, and infection is prevalent in all varieties of mithun, namely the races of AP (39%), Mizoram (18%), and Nagaland (15%), according to Rajkhowa et al. (Citation2004).
7. Diagnosis
BHV infection can be diagnosed by cell culture, histopathology, serology, PCR, and electron microscopy.
7.1. Cell culture
BHV-1 can be readily isolated in cell culture of primary or secondary bovine kidney, lungs, testis, turbinate, or trachea and established cell lines such as Madin–Darby Bovine Kidney (MDBK) or CRIB cells (Flores & Donis Citation1995; Nandi et al. Citation2009). The virus can be isolated from nasal swabs, conjunctival swabs, vaginal swabs, preputial washing, placental cotyledons of aborted foetus, fetal liver, lung, spleen, kidney, lymph node, mucous membrane of the respiratory tract, tonsils, and lungs collected in virus transport medium. The presence of virus in specimens is detected by a cytopathic effect (CPE). The CPE of BHV-1 is characteristic and usually appears within 3 days of inoculation. There are grape-like clusters of rounded cells present around a microplaque in cell culture. Giant cells or syncytia are also observed. The virus is cytolytic if the cells are incubated for a prolonged time and there is total sloughing of the rounded cells from the plastic/glass surface of the container. The cell cultures inoculated with specimens are observed for 7 days. The cell culture should be passaged at least three times before the sample is considered negative (Straub Citation1990; Turin and Russo Citation2003).
7.2. Histopathology
Intranuclear viral inclusions of Cowdry type A can occasionally be identified in the epithelial cells of vaginal biopsy tissues collected in the early stage of IPV but not in cells collected in nasal discharge of cattle with IBR (Nandi et al. Citation2009). These inclusions are also present in the brain from cases of encephalitis, and in tissues of aborted foetuses. As these inclusions are transitory, the use of histological examination for diagnosis is of limited value (Turin & Russo Citation2003). Perivascular cuffing with neuronophagia, satellitosis, haemorrhage, and neuronal degeneration are seen in encephalitic form of BHV infection (Bagust & Cleark Citation1972; Belknap et al. Citation1994; Meyer et al. Citation2001; Perez et al. Citation2003).
7.3. Electron microscopy
The use of electron microscopy to identify virus particles in clinical material is a rapid method for the diagnosis of BHV-1 but it should be used in the early stage of the disease (Nandi et al. Citation2009).
7.4. Serology
Several serological tests are available for the detection of antibody and a rise in titre between the acute and convalescent phase of infection. The primary immune response to BHV-1 experimental inoculation of cattle is characterized by the formation of both IgM and IgG antibodies. Secondary immune responses are characterized primarily by the formation of IgG2 antibody. The VNT has been widely used and is the gold standard by which other techniques have been evaluated (Perrin et al. Citation1996). However, the ELISA is a specific, sensitive, and more practical test for detection of BHV-1 antibodies. A variety of ELISAs, namely indirect ELISA, c-ELISA, and AB-ELISA have been employed to screen serum samples of cattle and buffaloes in India (Nandi et al. 2004, 2007). The IgM-ELISA is useful for the diagnosis of recent infection with BHV-1 in calves (Ungar-Waron & Abraham Citation1991). Furthermore, a micro-ELISA is being used for the control program of BHV-1 infection in Switzerland. The test is simple, rapid, and convenient compared to the serum neutralization test, which requires cell culture facilities and is time-consuming. The only currently available assay that differentiates antibodies against BHV-1 from BHV-5 is a BHV-1 gE blocking ELISA (Wellenberg et al. Citation2001). Of note, an antibody-ELISA and VNT were successfully employed in yaks and mithuns also (Rajkhowa et al. 2004; Bandyopadhyay et al. Citation2009)
7.5. PCR
The PCR assay is as sensitive as virus isolation and is a practical alternative for the rapid detection of virus. The results are available in 1 day compared to virus isolation which requires 7 days. Virus could be detected in nasal swabs for upto 14 days after experimental infection of the cattle and the PCR assay can also detect the virus in bovine fetal serum and semen samples. The PCR assay is considered equivalent to that of standard virus isolation and dot blot hybridization (Xia et al. Citation1995). The PCR assay with southern blot hybridization is considered to be highly sensitive and can detect the virus in semen before detectable antibody development (Masri et al. Citation1996). Real-time PCR provides satisfactory reproducibility as well as high specificity and sensitivity in combination with significant reduction in time for detecting amplified products (Nandi et al. Citation2009). In order to differentiate between BHV-1 and BHV-5, several assays are available like nested PCR (Ashbaugh et al. Citation1997), multiplex PCR (Alegre et al. Citation2001; Claus et al. Citation2005) and multiple PCR sequencing assay (Del Medico Zajac Citation2007). Of interest, a highly sensitive nested PCR was reported to detect glycoprotein B and glycoprotein E of the virus in biological samples from yaks (Bandyopadhyay et al. Citation2010a).
8. Vaccination
With currently available diagnostic tests, it is not possible to identify animals which have a latent BHV-1 infection. The next best strategy is to use a well-planned vaccination program. Different types of vaccine are available, namely modified live virus (MLV) vaccines, inactivated vaccines, subunit vaccines, and marker vaccines.
8.1. Modified live virus vaccines
There are three types of MLV vaccine available. The parenteral vaccine is made from bovine fetal kidney tissue culture. Besides, there are two intranasal vaccines available either of rabbit tissue culture origin or of bovine tissue culture origin, containing the mutant form of BHV-1 (Nandi et al. Citation2009). The MLV vaccines offer a rapid immune response concomitant with relatively long-lasting local mucosal immunity (Whetstone et al. 1986). Both parenteral and intranasal vaccines stimulate the production of humoral antibody. The intranasal vaccine stimulates the production of local interferon and local antibody in the nasal mucosa and is safe for use in pregnant cows. Furthermore, it is highly effective in the prevention of abortion. Intranasal vaccines provide protection against respiratory form within 72 hours of administration. For intranasal vaccination, 2 ml of diluents containing vaccine should be administered in one nostril carefully because this vaccine only multiplies in the nasal mucosa. After intranasal vaccination, virus is shed for 7–14 days, with peak shedding occurring at 4 days. The parenteral MLV vaccine is potentially abortigenic and should not be used in non-immune pregnant animals. It is of importance to realize that the virus from MLV vaccine sometimes becomes latent following administration.
8.2. Inactivated vaccines
The inactivated vaccines have some advantages over MLV vaccines because they do not cause abortion, immunosuppression, or latency. However, they do not fully prevent latency by field strains. Usually two doses are administered at an interval of 10–14 days and protection is observed 7–10 days following the second dose (Patel Citation2005). Inactivated vaccines are not as efficacious as MLV vaccines because of potential destruction of some of the protective antigens during the inactivation process by alkylating agents. To improve efficacy, an adjuvant is always added (Johannes et al. Citation2004).
8.3. Subunit vaccine
A subunit vaccine contains one or more of the antigens of the virus necessary to evoke protective immunity, and lacks nucleic acid and other components that might cause unwanted side effects (Brunner et al. Citation1988). In BHV-1, gB, gC, and gD glycoproteins are immunogenic, and are separated from the virus-infected cells or the peptide is synthesized (Nandi et al. Citation2009). Calves are vaccinated in between 3–7 months of age with booster after 12 days of primary vaccination. The level of immunity based on serum antibody titres and protection against experimental challenge is much greater with individual glycoproteins than those immunized with commercially available inactivated vaccine. Another subunit BHV-1 vaccine containing only gD glycoprotein along with recombinant M. haemolytica vaccine was reported to be superior to MLV BHV-1 vaccine in reducing mortality due to respiratory disease (Nandi et al. Citation2009). It does not contain live virus, so there is no chance to cause abortion or establish the latent infection. Furthermore, there is potentiality to differentiate between vaccinated and naturally infected animals. However, its efficacy depends upon the adjuvant used and sufficient amount of glycoprotein required to induce immunity. The subunit vaccine was considered superior to the MLV BHV-1 vaccine in reducing mortality due to respiratory disease (Nandi et al. Citation2009).
8.4. Marker vaccine
Marker vaccine is based on deletion of one or more viral proteins, which allows the distinction between vaccinated and naturally infected animals based upon respective antibody responses (Oirschot et al. 1996). Different types of marker vaccines are available like gE-live, gE-killed, gG-killed, gC-live, gD-subunit, gB-subunit, and gD-replication-incompetent. Calves vaccinated at the age of 7 weeks showed reduction both in clinical signs and excretion of challenge virus as early as 7 days after IM injection or 3 days after intranasal vaccination. Antibodies against marker vaccine were detectable in milk within 2–3 weeks and persisted in the body more than 2–3 years if not probably lifelong. The gE-negative marker vaccines, live as well as killed, are already used in control or eradication programs in the EU (European Union Citation2000).
8.5. Multivalent vaccine
Multivalent vaccine is available for the control of co-infection associated with BHV-1. These vaccines contain other respiratory pathogens such as parainfluenza-3 (PI-3), bovine respiratory syncytial virus (BRSV) and bovine viral diarrhoea virus (BVDV). Sometimes it also contains antigens of Leptospira and Campylobacter sp. A combination vaccine containing gE-negative live vaccine, BRSV, and PI-3, administered at 2 and 6 weeks of age, has been shown to be effective in that it reduced severity of clinical signs and the amount of virus shedding after experimental challenge infection (Mars et al. Citation2000). The vaccination of calves with multivalent vaccines containing MLV or MLV and inactivated BHV-1 is associated with virus-specific interferon gamma production and protection from clinical disease due to challenge, 5 days after a single vaccination (Campos et al. Citation1989; Patel Citation2005; Nandi et al. Citation2009).
9. Conclusion
In a nutshell, BHV-1 is an emerging concern which has the potentiality to cripple the livestock industry. The diagnostic tools are time consuming and expensive. The available prophylactic strategies have their own limitations to develop a holistic control program against this transboundary pathogen. In-depth research with practical and implementable outcome is necessary to effectively tackle this pathogen which is slowly but deceptively spreading among our livestock population.
References
- Abril C, Engels M, Liman A, Hilbe M, Albini S, Franchini M, Suter M, Ackermann M. 2004. Both viral and host factors contribute to neurovirulence of bovine herpesviruses 1 and 5 in interferon receptor-deficient mice. J Virol. 78: 3644–3653.
- Ackermann M, Engels M. 2005. Pro and contra IBR eradication. Vet Mic. 113: 293–302.
- Akca Y, Burgu I, Gur S, Bilge-Dagalp S. 2004. A study on investigations of occurrence of some virus infection in buffaloes in Turkey. Revue Med Vet. 156: 268–271.
- Alegre M, Nanni M, Fondevila N. 2001. Development of a multiplex polymerase chain reaction for the differentiation of bovine herpesvirus-1 and -5. J Vet Med B. 48: 613–621.
- Alkan F, Ozkul A, Bilge-dagalap S, Yesilbag K, Oguzoglu TC, Akca Y and Burgu I. 2000. Virological and serological studies on the role of PI-3 virus, BRSV, BVDV and BHV-1 on respiratory infections of cattle:The detection of etiological agents by direct immunofluorescence technique. Dtsch Tierärztl Wochenschr. 107: 193–195.
- Aly NM, Shehab GG, Abd el-Rahim IH. 2003. Bovine viral diarrhoea, bovine herpesvirus and parainfluenza-3 virus infection in three cattle herds in Egypt in 2000. Rev Sci Tech. 22: 879–892.
- Anonymous. 2004. International terrestial animal health code. Paris: Office Int des Epiz. Available from: http//www.oie.int/
- Ashbaugh SE, Thompson KE, Belknap EB, Schultheiss PC, Chowdhury S, Collins JK. 1997. Specific detection of shedding and latency of bovine herpesvirus 1 and 5 using a nested polymerase chain reaction. J Vet Diagn Invest. 9: 387–394.
- Babiuk LA, van Drunen Littel-van den Hurk, Tikoo SK. 1996. Immunology of bovine herpesvirus 1 infection. Vet Microbiol. 53: 31–42.
- Bagust TJ. 1972. Comparison of the biological, biophysical and antigenic properties of four strains of infectious bovine rhinotracheitis herpesvirus. J Com Patho. 82: 365–374.
- Bandyopadhyay S, Biswas T K, Sasmal D, Samanta I, Ghosh M K. 2010b. Evaluation of methanolic extract of Allium sativum and Saussurea costus in yaks with infectious keratoconjunctivitis. Ind J Ani Sci. 80: 199–202.
- Bandyopadhyay S, Chakraborty D, Sarkar T, Pal B, Sasmal D, Biswas TK, Ghosh MK, Sarkar M. 2009. A serological survey of bovine herpes virus-1 antibodies in yaks (Poephagus grunniens). Rev Sci Tec Off Int Epiz. 28: 1045–1050.
- Bandyopadhyay S, Das S, Baruah KK, Chakravarty P, Chakrabarty D, Sarkar T, Pal B, De S, Pan D, Bera AK, et al. 2010a. Detection of bovine herpesvirus 1 sequences in yaks (Bos grunniens) with keratoconjunctivitis, using a highly sensitive nested polymerase chain reaction. Rev Sci Tec Off Int Epiz. 29: 695–703.
- Bandyopadhyay S, Saravanan BC, Ghosh MK, Sarkar M, Ramesha KP, Bhattacharya M. 2007. Common health ailments of Yaks (Poephagus grunniens L.). Technical bulletin. Dirang, AP: NRCY Publication.
- Bartha A, Hadju G, Aldasy G, Paczolay G. 1969. Occurrence of encephalitis caused by infectious rinotracheitis virus in cattle in Hungary. Acta Vet Hung. 19: 145–151.
- Belknap EB, Collins JK, Ayers VK, Schultheiss PC. 1994. Experimental infection of neonatal calves with neurovirulent bovine herpesvirus type 1.3. Vet Path. 31: 358–365.
- Bhat MN, Manickam R, Kumanan K. 1997. Serological evidence of bovine herpesviruses 1 and 2 in Asian elephants. J Wildl Dis. 33: 919–920.
- Bielefeldt Ohmann H, Babiuk LA, Harland R. 1991. Cytokine synergy with viral cytopathic effects and bacterial products during the pathogenesis of respiratory tract infection. Clin Immunol Immunopath. 60: 153.
- Bilgedagalap S. 1998. Kan ve süt serumlarinda IBR-IPV antikorlarinin nötralizasyon testiile saptanmasi ve süt örneklerinden virus izolasyonu. A Ü Vet Fak Derg. 45: 313–321.
- Bochner BS, Landy SD, Plant M, Dinarello CA, Schleimer RP. 1987. Interleukin-1 production by human lung tissue. I. Identification and characterization. J Immun. 139: 2297–2302.
- Boelaert F, Biront P, Soumare B, Dispas M, Vanopdenbosch E, Vermeersch JP, Raskin A, Dufey J, Berkvens D, Kerkhofs P. 2000. Prevalence of bovine herpesvirus-1 in the Belgian cattle population. Prev Vet Med. 45: 285–295.
- Bratanich AC, Hanson ND, Jones C. 1992. The latency-related gene of bovine herpesvirus 1 inhibits the activity of immediate-early transcription unit 1. Virol. 191: 988–991.
- Brown GA, Field HJ. (1990a). A reliable method for establishing viral infection in the rabbit by intranasal inoculation. J Viro Met. 27: 341–345.
- Brown GA, Field HJ. (1990b). Experimental reactivation of bovine herpesvirus 1 (BHV-1) by means of corticosteroids in an intranasal rabbit model. Arch Vir. 112: 81–101.
- Brunner D, Engels M, Schwyzer M, Wyler R. 1988. A comparison of three techniques for detecting bovine herpesvirus type 1 (BHV-1) in naturally and experimentally contaminated bovine semen. Rep Domes Ani. 23: 1–9.
- Bryant NA, Davis-Poynter N, Vanderplasschen A, Alcami A. 2003. Glycoprotein G isoforms from some alphaherpesviruses function as broad-spectrum chemokine binding proteins. EMBO J. 22: 833–846.
- Cabalar M, Akca Y. 1994. Fertilite problemli ineklerde infeksiyöz bovine rhinotracheitis, infeksiyöz pustuler vulvovaginitis (IBR/IPV) virus izolasyonu ve seroepidemiyolojisi. A Ü Vet Fak Derg. 41: 337–349.
- Çabalar M, Can-Sahna K. 2000. Dogu ve Güneydogu Anadolu bölgesinde süt sigirlarinda parainfluenza virus-3, bovine herpes virus-1 ve respiratory syncytial virüs enfeksiyonlarinin seroepidemiyolojisi. YYÜ Vet Fak Dergisi. 11: 101–105.
- Campos M, Bielefeldt Ohmann H, Hutchings D, Rapin N, Babiuk LA, Lawman MJP. 1989. Role of interferon gamma in inducing cytotoxicity of peripheral blood mononuclear leukocytes to bovine herpesvirus type 1 (BHV-1) infected cells. Cell Immunol. 120: 259–269.
- Campos M, Godson DL, Hughes HPA, Babiuk LA. 1994. Cytokine applications in infectious diseases. In: Goddeeris B, Morrisons 1, editors. Cell-mediated immunity in ruminants. Boca Raton: CRC Press; p. 229–240.
- Campos M, Rossi CR. 1986. Cytotoxicity of Bovine lymphocytes after treatment with lymphokines. Am J Vet Res. 47: 1524–1528.
- Carrillo BJ, Pospischil A, Dahme E. 1983. Pathology of bovine viral necrozing encephalitis in Argentina. Zentralbl Veterinarmed B. 30: 161–168.
- Castrucci G, Martin WB, Frigeri F, Ferrari M, Salvatori D, Tagliati, S, Cuter V. 1997. Serological survey of herpesvirus 1 infection in selected dairy herds in Northern and Central Italy. Comp Immunol Microbiol Infect Dis. 20: 315–317.
- Ciacci-Zanella J, Stone M, Henderson G, Jones C. 1999. The latency-related gene of bovine herpesvin∼s 1 inhibits programmed cell death. J Virol. 73: 9734–9740.
- Claus MP, Alfieri AF, Folgueras-Flatschart AV, Wosiacki SR, Médici, KC, Alfieri, AA. 2005. Rapid detection and differentiation of bovine herpesvirus 1 and 5 glycoprotein C gene in clinical specimens by multiplex-PCR. J Virol Methods. 128: 183–188.
- Connolly SA, Whitbeck JJ, Rux AH, Krummenacher C, van Drunen Littel-van den Hurk, Cohen G.H. Eisenberg RJ. 2001. Glycoprotein D homologs in herpes simplex virus type 1, pseudorabies virus, and bovine herpes virus type 1 bind directly to human HveC (nectin-1) with different affinities. Virol. 280: 7–18.
- [CSIRO] Commonwealth Scientific and Industrial Research Organisation 2005. New Vaccine for Cattle Pneumonia. [ Accessed on 2005 May 18]. Available from: http://www.farmsafe.com.au/pages/features/beef/csiro03.htm
- Dasika GK, Letchworth GJ. 1999. Cellular expression of bovine herpesvirus 1 gD inhibits cell-to-cell spread of two closely related viruses without blocking their primary infection. Virology. 254: 24–36.
- Del Médico Zajac MP. 2007. Herpesvirus bovino 5: estudio de la infección, la respuesta inmune y los recombinantes con herpesvirus bovino 1 en el hospedador natural [PhD Thesis]. Buenos Aires, Argentina: National Institute of Agricultural Technology.
- Denis M, Slaoui M, Keil G, Babiuk LA, Ernst E, Pastoret PP, Thiry, E. 1993. Identification of different target glycoproteins for bovine herpesvirus- specific cytotoxic T lymphocytes depending on the method of in vitro stimulation. Immunology. 78: 7–13.
- Elazhary MA, Derbyshire JB. 1979. Effect of temperature, relative humidity and medium on the aerosol stability of infectious bovine rhinotracheitis virus. Can J Comp Med. 43: 158–167.
- Elazhary MA, Silim A, Dea S. 1984: Prevalence of antibodies to bovine respiratory syncytial virus, bovine viral diarrhea virus, bovine herpesvirus-1, and bovine parainfluenza-3 virus in sheep and goats in Quebec. Am J Vet Res. 45: 1660–1662.
- Engels M, Ackermann M. 1996. Pathogenesis of ruminant herpesvirus infections. Vet Microbiol. 53: 3–15.
- Eugster AK, Angulo AB, Jones LP, 1974. Herpesvirus encephalitis in range calves. Proc Annu Meet Am Assoc Vet Lab Diag. 17: 267–290.
- European Union. 2000. Report on Bovine Herpes virus 1 (BHV 1) marker vaccines & the accompanying tests.
- Feldman LT, Ellison AR, Voytek CC, Yang L, Krause P, Margolis TP. 2002. Spontaneous molecular reactivation of herpes simplex virus type 1 latency in mice. Proc Natl Acad Sci USA. 99: 978–983.
- Flores EF, Donis RO. 1995. Isolation of a Mutant MDBK Cell Line Resistant to Bovine Viral Diarrhea Virus Infection Due to a Block in Viral Entry. Virology. 208: 565–575.
- Forman AJ, Babiuk LA, Misra V, Baldwin F. 1982. Susceptibility of bovine macrophages to infectious bovine rhinotracheitis virus infection. Infect Immun. 35: 1048–1057.
- Fuchs M, Hubert P, Detterer J, Rziha HJ. 1999. Detection of bovine herpesvirus type 1 in blood from naturally infected cattle by using a sensitive PCR that discriminates between wild-type virus and virus lacking glycoprotein E. J Cli Microbiol. 37: 2498–2507.
- Fulton RW, Saliki JT, Burge LJ, Payton ME. 2003. Humoral immune response and assessment of vaccine virus shedding in calves receiving modified live virus vaccines containing bovine herpesvirus-1 and bovine viral diarrhoea virus 1a. J Vet Med B Infect Dis Vet Pub Health. 50: 31–37.
- Geiser V, Inman M, Zhang Y, Jones C. 2002. The latency related (LR) gene of bovine herpes virus 1 (BHV-1) can inhibit the ability of bICPO to activate productive infection. J Gen Virol. 83: 2965–2971.
- Geraghty RJ, Krummenacher C, Cohen GH, Eisenberg RJ, Spear PG. 1998. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science. 280: 1618–1620.
- Gibbs EPJ, Rweyemamu MM. 1977. Bovine herpesviruses. Part I. Bovine herpesvirus 1. Vet Bull. 47: 317–343.
- Griebel PJ, Bielefeldt Ohmann H, Campos M, Qualtiere L, Davis WC, Lawman MJP, Babiuk LA. 1989. Bovine peripheral blood leukocyte population dynamics following treatment with recombinant bovine interferon-alpha. J Interferon Res. 9: 245–257.
- Griebel PJ, Qualtiere L, Davis WC, Gee A, Bielefeldt Ohmann H, Lawman MJP, Babiuk LA. 1998. Peripheral blood T lymphocyte population dynamics and function following a primary bovine herpesvirus infection. Viral Immunol. 1: 287.
- Grom J, Hostnik P, Toplak I, Barlic-Maganja D. 2006. Molecular detection of BHV-1 in artificially inoculated semen and in the semen of a latently infected bull treated with dexamethasone. Vet J. 171: 539–544.
- Hage JJ, Vellema P, Schukken YH, Barkema HW, Rijsewijk FAM, Oirschot JTV, Wentink GH. 1997. Sheep do not have a major role in bovine herpesvirus 1 transmission. Vet Microbiol. 57: 4l-54.
- Hanon E, Keil G, van Drunen Littel-van den Hurk, Griebel P, Vanderplasschen A, Rijsewijk FA, Babiuk L, Pastoret PP. 1999. Bovine herpesvirus 1-induced apoptotic cell death: role of glycoprotein D. Virology. 257: 191–197.
- Hanon E, Meyer G, Vanderplasschen A, Dessy-Doize C, Thiry E, Pastoret PP. 1998. Attachment but not penetration of bovine herpesvirus 1 is necessary to induce apoptosis in target cells. J Virol. 72: 7638–7641.
- Harrison SC. 2001. Principles of virus structure. Chapter 3. In: Knipe DM, Howley PM, editors. Fields’ virology. 4th ed. Volume 1. Philadelphia: Lippincott Williams & Wilkins; p. 53–85.
- Henderson G, Perng G-C, Nesburn A, Wechsler S, Jones C. 2004. The latency related gene of bovine herpesvirus 1 can suppress caspase 3 and caspase 9 during productive infection. J Neurovirol. 10: 64–70.
- Homan EJ, Easterday BC. 1983. Experimental latent and recrudescent bovine herpesvirus-l infections in calves. Am J Vet R. 44: 309–313.
- Huemer HP, Larcher C, van Drunen Littel-van den Hurk, Babiuk LA. 1993. Species selective interaction of Alphaherpesvirinae with the “unspecific” immune system of the host. Arch Virol. 130: 353–364.
- Hunter E. 2001. Virus assembly. Chapter 8. In: Knipe DM, Howley PM, editors. Fields’ virology. 4th ed. Volume 1. Philadelphia: Lippincott Williams & Wilkins; p. 171–197.
- Hutchings DL, van Drunen Littel-van den Hurk, Babiuk LA. 1990. Lymphocyte proliferative responses to separated bovine herpesvirus 1 proteins in immune cattle. J Virol. 64: 5114–5122.
- Inman M, Lovato L, Doster A, Jones C. 2002. A mutation in the latency related gene of bovine herpesvirus 1 interferes with the latency-reactivation cycle of latency in calves. J Virol. 76: 6771–6779.
- Inman M, Zhou J, Webb H, Jones C. 2004. Identification of a novel transcript containing a small open reading frame that is expressed during latency, and is antisense to the latency related gene of bovine herpes virus 1 (BHV-1). J Virol. 78: 5438–5447.
- Jetteur P, Thirty E, Pastoret PP. 1990. Serological survey concerning the IBR, CHV2, BVD, PI3, BRS and rinderpest viruses in small ruminants in Zaire. Rev Elev Med Vet Pays Trop. 43: 435–437.
- Johnston LA, Simmons GC, McGavin MD. 1962. A viral meningo-encephalitis in calves. Aus Vet J. 38: 207–215.
- Johnson GD, Campbell JB, Minocha HC, Broce AB. 1991. Ability of Musca autumnalis (Diptera: Muscidae) to acquire and transmit bovine herpesvirus-1. J Med Entomol. 28: 841–846.
- Johannes AK, Malcolm B, Martin B, Pierre K, Myriam P, Gerard JW, Van Oirschot JT. 2004. Evaluation of tests for antibodies against bovine herpesvirus 1 performed in national reference laboratories in Europe. Vet Microbol. 102: 169–181.
- Jones C. 1998. Alphaherpesvirus latency: its role in disease and survival of the virus in nature. Adv Virus Res. 51: 81–133.
- Jones C. 2003. Herpes simplex virus type 1 and bovine herpesvirus 1 latency. Cl Micro Rev. 16: 79–95.
- Jones C, Chowdhury S. 2008. A review of the biology of bovine herpesvirus type 1 (BHV-1), its role as a cofactor in the bovine respiratory disease complex and development of improved vaccines. Anim Health Res Rev. 8: 187–205.
- Jones C, Geiser V, Henderson G, Jiang Y, Meyer F, Perez S, Zhang Y. 2006. Functional analysis of bovine herpesvirus 1 (BHV-1) genes expressed during latency. Vet Micrbiol. 113: 199–210.
- Jones C, Newby TJ, Holt T, Doster A, Stone M, Ciacci-Zanella J, Webster CJ, Jackwood MW. 2000. Analysis of latency in cattle after inoculation with a temperature sensitive mutant of bovine herpesvirus 1 (RLB106). Vaccine. 18: 3185–3195.
- Kahrs RF. 2001. Infectious bovine rhinotracheitis and infections pustular vulvovaginitis. In: Viral Diseases of Cattle. 2nd ed. Ames, IA: Iowa State University Press; p. 159–170.
- Karger A, Saalmiiller A, Tufaro F, Banfield BW, Mettenleiter TC. 1995. Cell surface proteoglycans are not essential for infection by pseudo-rabies virus. J Virol. 69: 3482–3489.
- Kit S, Qavi H, Gaines JD, Billingsley P, McConnell S. 1985. Thymidine kinase-negative bovine herpesvirus type 1 mutant is stable and highly attenuated in calves. Arch Virol. 86: 63–83.
- Knipe DM, Samuel CE, Palese P. 2001. Virus-host cell interactions. Chapter 7. In: Knipe DM, Howley PM, editors. Fields’ virology. 4th ed. Volume 1. Philadelphia: Lippincott Williams & Wilkins; p. 133–170.
- Keuser V, Schynts F, Detry B, Collard A, Robert B, Vanderplasschen A, Pastoret P, Thirty E. 2004. Improved antigenic methods for differential of bovine diagnosis of bovine, caprine, and corvine Alphaherpesviruses related to bovine herpesvirus-1. J Clin Microbiol. 42: 1228–1235.
- Lamontagne L, Gauldie J, Stadnyk A, Richards C, Jenkins E. 1985. In vivo initiation of unstimulated in vitro interleukin-1 release by alveolar macrophages. Am Rev Respir Dis. 131: 326–330.
- Lehmkuhl HD, Cutlip RC, Bolin SR, Brogden KA. 1985. Seroepidemiologic survey for antibodies to selected viruses in the respiratory tract of lambs. Am J Vet Res. 46: 2601–2604.
- Leite F, Sylte MJ, O’Brien S, Schultz R, Peek S, van Reeth K, Czuprynski CJ. 2002. Effect of experimental infection of cattle with bovine herpesvirus (BHV-1) on the ex vivo interaction of bovine leukocytes with Mannheimia (Pasteurella) haemolytica leukotoxin. Vet Immunol Immunopathol. 84: 97–100.
- Li Y, Liang X, van Drunen Littel-van den Hurk, Attah-Poku S, Babiuk LA. 1996. Glycoprotein Bb, the N-terminal subunit of bovine herpesvirus 1 gB, can bind to heparan sulfate on the surfaces of Madin-Darby bovine kidney cells. J Virol. 70: 2032–2037.
- Li Y, van Drunen Littel-van den Hurk, Babiuk LA, Liang X. 1995. Characterization of cell-binding properties of bovine herpesvirus 1 glycoproteins B, C, and D: identification of a dual cell-binding function of gB. J Virol. 69: 4758–4768.
- Liang X, Chow B, Raggo C, Babiuk LA. 1996. Bovine herpesvirus 1 UL49.5 homolog gene encodes a novel viral envelope protein that forms a disulfide-linked complex with a second virion structural protein. J Virol. 70: 1448–1454.
- Lovato L, Inman M, Henderson G, Doster A, Jones C. 2003. Infection of cattle with a bovine herpesvirus 1 strain that contains a mutation in the latency-related gene leads to increased apoptosis in trigeminal ganglia during the transition from acute infection to latency. J Virol. 77: 4848–4857.
- Maclachlan N, Dubovi EJ. 2010. Fenner's veterinary virology. 4th ed. New York: Academic Press.
- Mahmoud MA, Mahmoud NA, Allam AM. 2009. Investigation on infectious bovine rhinotracheitis in Egyptian cattle and buffaloes. Glob Veterina. 3: 335–340.
- Manickam R, Mohan M. 1987. Seroepidemiological studies on infectious bovine rhinotracheitis (IBR) viral abortions in cows. Ind J Anim Sci. 57: 959–962.
- Mars MH, Bruschke CJ, Van Oirschot JT. 1999. Airborne transmission of BHV-1, BRSV, and BVDV among cattle is possible under experimental conditions. Vet Microbiol. 66: 197–207.
- Mars MH, de Jong MC, van Maanen C, Hage JJ, Van Oirschot JT. 2000. Airborne transmission of bovine herpesvirus 1 infections in calves under field conditions. Vet Microbiol. 76: 1–13.
- Masri SA, Olson W, Nguyen PT, Prins S, Deregt D. 1996. Rapid detection of bovine herpesvirus 1 in the semen of infected bulls by a nested polymerase chain reaction assay. Can J Vet Res. 60: 100–107.
- Mayfield JE, Good PJ, Vanoort HJ, Campbell AR, Reed DE. 1983. Cloning and cleavage site mapping of DNA from bovine herpesvirus 1 (cooper strain). J Virol. 47: 259–264.
- Meat & Livestock Australia. 2001. Annual reports of Australia's cattle, sheep and goat supply chains in the financial year 2000–2001.
- Mechor GD, Rousseaux CG, Radostits OM, Babiuk LA, Petrie L. 1987. Protection of newborn calves against fatal multisystemic infectious bovine rhinotracheitis by feeding colostrum from vaccinated cows. Can J Vet Res. 51: 452–459.
- Mena A, Ioannou XP, Van Kessel A, Van Drunen Little-Van Den Hurk, Popowych Y, Babiuk LA, Godson DL. 2002. Th1/Th2 biasing effects of vaccination in cattle as determined by real-time PCR. J Immunol Methods. 263: 11–21.
- Mensik J, Pospisil Z, Suchankova A, Cepica A, Rozkosny V, Machatkova M. 1976. Activation of latent infectious bovine rhinotracheitis after experimental infection parainfluenza 3 virus in young calves. Zentra Veterinarmed B. 23: 854–864.
- Mettenleiter TC. 1994. Initiation and spread of a-herpesvirus infections. Tren Microbiol. 2: 2–4.
- Metzler AE, Ossent P, Guscetti F, Rubel A, Lang EM. 1990. Serological evidence of herpesvirus infection in captive Asian elephants (Elephas maximus). J Wildl Dis. 26: 41–49.
- Meurens F, Schynts F, Keil GM, Muylkens B, Vanderplasschen A, Gallego P, Thiry E. 2004b. Superinfection prevents recombination of the alphaherpesvirus bovine herpesvirus 1. J Virol. 78: 3872–3879.
- Meyer G, Lemaire M, Ros C, Belak K, Gabriel A, Cassart D, Coignoul F, Belak S, Thiry E. 2001. Comparative pathogenesis of acute and latent infections of calves with bovine herpesvirus types 1 and 5. Arch Virol. 146: 633–652.
- Mohan Kumar KM, Rajasekhar M, Krishnappa G. (1994) Isolation of infectious bovine rhinotracheitis virus in Karnataka. Ind Vet J. 71: 109–112.
- Moretti B, Orfei Z, Mondino G, Perschino A. 1964. Infectious bovine rhinotracheitis clinical observations and isolation of virus. Vet Ital. 15: 676.
- Msolla PM, Allan EM, Selman JE, Wiseman A. 1983. Reactivation and shedding of bovine herpesvirus 1 following Dictyocaulus viviparous infection. J Comp Pathol. 93: 271–274.
- Muylkens B, Thiry J, Kirten P, Schynts F, Thiry E. 2007. Bovine herpesvirus 1 infection and infectious bovine rhinotracheitis. Vet Res. 38: 181–209.
- Mweene AS, Okazaki K, Kida H. 1996. Detection of viral genome in non-neural tissues of cattle experimentally infected with bovine herpesvirus 1. Jap J Vet Res. 44: 165–174.
- Nandi S, Pandey AB, Sharma K, Chauhan RS. 2004. Serological evidence of BHV-1 antibodies in cattle and buffalo from different states of India. Indian J Comp Microbiol Immunol Infect Dis. 25: 87–89.
- Nandi S, Pandey AB, Sharma K, Audarya SD, Chauhan RS. 2007. Seroprevalence of infectious bovine rhinotracheitis in cattle of an organized farm by indirect ELISA. The Indian Cow 7: 50–53.
- Nandi S, Kumar M, Manohar M, Chauhan RS. 2009. Bovine herpes virus infections in cattle. Anim Health Res Rev. 10: 85–98.
- Nyaga PN, McKercher DG. 1980. Pathogenesis of bovine herpesvirus-1 (BHV-1) infections: interactions of the virus with peripheral bovine blood cellular components. Comp Immunol Microbiol Infect Dis. 2: 587–602.
- Oirschot JTV. 1995. Bovine herpesvirus in semen of bulls and the risk of transmission: a brief overview. Vet Q. 17: 29–33.
- Oirschot JV, Kaashoek MJ, Rijsewijk FA. 1996. Advances in the development and evaluation of bovine herpesvirus 1 vaccines. Vet Microbiol. 53: 43–54.
- Palmer LD, Leary TP, Wilson DM, Splitter GA. 1990. Bovine natural killer-like cell responses against cell lines expressing recombinant bovine herpesvirus type 1 glycoproteins. J Immunol. 145: 1009–1014.
- Pastoret PP, Thiry E, Brochier B, Derboven G. 1982. Bovine herpesvirus 1 infection of cattle: pathogenesis, latency, consequences of latency. Ann Rech Vet. 13: 221–235.
- Patel JR. 2005. Relative efficacy of inactivated bovine herpesvirus 1 (BHV-1) vaccines. Vaccine. 23: 4054–4061.
- Perrin B, Calud T, Cordioli P, Coudert M, Edwards S, Eloit M, Guérin B, Kramps JA, Lenihan P, Paschaleri E, et al. 1996. Selection of European Union standard reference sera for use in the serological diagnosis of infectious bovine rhinotracheitis. Revue scientifique et technique de l’OIE. 13: 947–960.)
- Perez S, Meyer F, Henderson G, Jiang Y, Sherman S, Doster A, Inman M, Jones C. 2007. A protein encoded by the bovine herpesvirus 1 ORF E gene induces neurite-like morphological changes in mouse neuroblastoma cells and is expressed in trigelninal ganglionic neurons. J Neurovirol. 13: 139–149.
- Perez SE, Vagnozzi A, Sur JH, Odriozola E, Campero CM, Odeón AC. 2003. Análisis restrospectivodecasos condiagnóstico denecrosis cerebrocortical y surelacióncon herpesvirus bovino tipo 5. Rev Argen Microbiol. 35: 69–73.
- Porter DD, Larsen AE, Cox NA. 1975. Isolation of infectious bovine rhinotracheitis virus from Mustelidae. J Clin Microbiol. 1: 112–113.
- Preston CM, Nicholl MJ. 2008. Induction of cellular stress overcomes the requirement of herpes simplex virus type 1 for immediate-early protein ICP0 and reactivates expression from quiescent viral genomes. J Virol. 82: 11775–11783.
- Raftery M, Muller A, Schonrich G. 2000. Herpesvirus homologues of cellular genes. Virus Genes. 21: 65–75.
- Rajkhowa S, Rajkhowa C, Rahman H, Bujabaruah KM. 2004. Seroprevalence of infectious bovine rhinotracheitis in mithun in India. Rev Sci Tech. 23: 821–829.
- Rijsewijk FA, Kaashoek MJ, Langeveld JP, Meloen R, Judek J, Bienkowska-Szewczyk K, Maris-Veldhuis MA, Van Oirschot JT. 1999. Epitopes on glycoprotein C of bovine herpesvirus-1 (BHV-1) that allow differentiation between BHV-1.1 and BHV-1.2 strains. J Gen Virol. 80: 1477–1483.
- Rissi DR, Oliveira FN, Rech RR, Pierezan F, Lemos RAA, Barros CSL. 2006. Epidemiologia, sinais clínicos e distribuic a,a˜o das leso˜es encefélicas em bovinos afetados por meningoencefalite por herpesvírus bovino-5. Pesq Vet Bras. 26: 123–132.
- Roehe PM, Silva TC, Nardi NB, Oliveira LG, Rosa JCA. 1997. Diferenciac a,a˜o entre o vírus da rinotraqueíte infecciosa bovina (BHV-1) e o herpesvírus da encefalite bovina (BHV-5). Pesq Vet Bras. 17: 41–44.
- Rock DL, Beam SL, Mayfield JE. 1987. Mapping bovine herpesvirus type 1 latency-related RNA in trigeminal ganglia of latently infected rabbits. J Virol. 61: 3827–3831.
- Rock D, Lokensgard J, Lewis T, Kutish G. 1992. Characterization of dexamethasone-induced reactivation of latent bovine herpesvirus 1. J Virol. 66: 2484–2490.
- Rock DL, Reed DE. 1982. Persistent infection with bovine herpesvirus type 1: rabbit model. Infect Immun. 35: 371–373.
- Roizman B, Knipe DM, Whitley RJ. 2007. Herpes Simplex Virus. In: David M, Knipe DM, Howley PM, editors. Fields’ virology. 5th ed. Volume 2. Philadelphia: Lippincott Williams & Wilkins.
- Rouse BT, Grewal AS, Babiuk LA, Fujimiya Y. 1977. Enhancement of antibody-dependent cell-mediated cytotoxicity of herpesvirus-infected cells by complement. Infect Immun. 18: 660–665.
- [SCAHW] Scientific Committee on Animal Health and Welfare, European Commission Health & Consumer Protection Directorate-General. 2000. Report on Bovine Herpesvirus 1 (BHV1) marker vaccines and the accompanying diagnostic tests.
- Schang LM, Jones C. 1997. Analysis of bovine herpesvirus 1 transcripts during a primary infection of trigeminal ganglia of cattle. J Virol. 71: 6786–6795.
- Schroder C, Keil GM. 1999. Bovine herpesvirus 1 requires glycoprotein H for infectivity and direct spreading and glycoproteins gH(W450) and gB for glycoprotein D-independent cell-to-cell spread. J Gen Virol. 80: 57–61.
- Schultz RD, Hall CE, Sheffy BE, Kahrs RF, Bean BH. 1977. Current status of IBR-IPV infection in bulls. In: Proceedings of the 80th Annual Meeting of US Animal Health Association; p. 159–168.
- Schwyzer M, Ackermann M. 1996. Molecular virology of ruminant herpesviruses. Vet Microbiol. 53: 17–29.
- Singh B, Ramakhant K, Tongaonakar SS. 1983. Adaptation of infectious bovine rhinotracheitis virus in MDBK cell line and testing of buffalo sera for neutralizing antibodies. Ind J Comp Microbiol Immunol Infect Dis. 4: 6–10.
- Straub OC. 1990. Infectious bovine rhinotracheitis virus. In: Dinter, Z. and Morein, B., editors. Virus infections of ruminants. Oxford: Elsevier Science Publishers BV; p. 71–108.
- Sulochana S, Pillai RM, Nair GK, Abdulla PK. 1982. Serological survey on the occurrence of infectious bovine rhinotracheitis in Kerala. Ind J Comp Microbiol Immunol Infect Dis. 3: 7–11.
- Taylor RE, Seal BS, St Jeor S. 1982. Isolation of infectious bovine rhinotracheitis virus from the soft-shelled tick, Ornithodoros coriaceus. Science. 216: 300–301.
- Thiry E, Muylkens B, Meurens F, Gogev S, Thiry J, Vanderplasschen A, Schynts F. 2006. Recombination in the alphaherpesvirus bovine herpesvirus 1. Vet Microbiol. 113: 171–177.
- Thiry E, Saliki JT, Bublot M, Pastoret PP. 1987. Reactivation of infectious bovine rhinotracheitis virus by transport. Comp Immunol Microbiol Infect Dis. 10: 59–63.
- Tikoo SK, Campos M, Babiuk LA. 1995a. Bovine herpesvirus 1 (BHV-1): biology, pathogenesis, and control. Adv Virus Res. 45: 191–223.
- Tikoo SK, Campos M, Popowych YI, van Drunen Littel-van den Hurk, Babiuk LA 1995b. Lymphocyte proliferative responses to recombinant bovine herpes virus type 1 (BHV-1) glycoprotein gD (gIV) in immune cattle: identification of a T cell epitope. Viral Immunol. 8: 19–25.
- Turin L, Russo S. 2003. BHV-1 infection in cattle: an update. Vet Bull. 73: 16–21.
- Tyler KL, Nathanson N. 2001. Pathogenesis of viral infections. In: Knipe DM, Howley PM, editors. Fields’ virology. 4th ed. Volume 1. Philadelphia: Lippincott Williams & Wilkins; p. 199–243.
- Ungar-Waron H, Abraham A. 1991. Immunoglobulin M (IgM) indirect enzyme-linked immunosorbent assay and the involvement of IgM-rheumatoid factor in the serodiagnosis of BHV-1 infection. Vet Microbiol. 26: 53–63.
- Van Oirschot JT, Kaashoek MJ, Maris-Veldhuis MA, Weerdmeester K, Rijsewijk FA. 1997. An enzyme-linked immunosorbent assay to detect antibodies against glycoprotein E of bovine herpesvirus 1 allows differentiation between infected and vaccinated cattle. J Virol Methods. 67: 23–34.
- Van Oirschot JT, Straver PJ, Van Lieshout JAH, Quak J, Westenbrink F, Van Exsel ACA. 1993. A subclinical infection of bulls with bovine herpesvirus type 1 at an artificial insemination centre. Vet Rec. 132: 32–35.
- Vogel FSF, Flores EF, Weiblen R, Kunrath CF. 2002. Atividade neutralizante anti-herpesvírus bovino tipos 1 (BHV-1) e 5 (BHV-5) no soro de bovinos imunizados com vacinas contra o BHV-1. Ciênc Rural. 32: 881–883.
- Weiblen R, Barros CSL, Canabarro TF, Flores EF. 1989. Bovine meningo-encephalitis from IBR virus. Vet Rec. 124: 666–667.
- Weiner MP, Costa GL, Schoettlin W, Cline J, Mathur E, Bauer JC. 1994. Site-directed mutagenesis of double-stranded DNA by the polymerase chain reaction. Gene. 151: 119–123.
- Wellenberg GJ, Mars MH, Van Oirschot JT. 2001. Antibodies against bovine herpesvirus (BHV) 5 may be differentiated from antibodies against BHV1 in a BHV1 glycoprotein E blocking ELISA. Vet Microbiol. 78: 79–84.
- Wentink GH, Van Oirschot JT, Verhoeff J. 1993. Risk of infection with bovine herpes virus 1 (BHV1): a review. Vet Q. 15: 30–33.
- Whetstone CA, Wheeler JG, Reed DE. 1986. Investigation of possible vaccine-induced epizootics of infectious bovine rhinotracheitis, using restriction endonuclease analysis of viral DNA. Am J Vet Res. 47: 1789–1795.
- Whetstone CA, Evermann JF. 1988. Characterization of bovine herpesviruses isolated from six sheep and four goats by restriction endonuclease analysis and radioimmunoprecipitation. Am J Vet Res. 49: 781–785.
- Winkler MT, Doster A, Jones C. 1999. Bovine herpesvirus 1 can infect CD4(+) T lymphocytes and induce programmed cell death during acute infection of cattle. J Virol. 73: 8657–8668.
- Winkler MTC, Doster A and Jones C. 2000. Persistence and reactivation of bovine herpesvirus 1 in the tonsil of latently infected calves. J Virol. 74: 5337–5346.
- Winkler MT, Doster A, Sur JH, Jones C. 2002. Analysis of bovine trigeminal ganglia following infection with bovine herpesvirus 1. Vet Microbiol. 86: 139–155.
- Woodbine KA, Medley GF, Moore SJ, Ramirez-Villaescusa AM, Mason S, Green LE. 2009. A four year longitudinal sero-epidemiological study of bovine herpesvirus type-1 (BHV-1) in adult cattle in 107 unvaccinated herds in south west England. BMC Vet Res. 5: 5.
- [OIE] World Organisation for Animal Health. 2010. Infectious bovine rhinotracheitis/infectious pustular vulvovaginitis. In: OIE terrestrial manual. France: OIE.
- Wyler R, Engles M, Schwyzer M. 1989. Infectious bovine rhinotracheitis/vulvovaginitis (BHV-1). In: Wittmann G, editor. Herpesvirus diseases of cattle, horses and pigs., Developments In Veterinary Virology. Boston: Kluwer Academic Publishers; p. 1–172.
- Xia JQ, Yason CV, Kibenge FS. 1995. Comparison of dot blot hybridization, polymerase chain reaction, and virus isolation for detection of bovine herpesvirus-1 (BHV-1) in artificially infected bovine semen. Can J Vet Res. 59: 102–109.
- Yan BF, Chao YJ, Chen Z, Tian KG, Wang CB, Lin XM, Chen HC, Guo AZ. 2008. Serological survey of bovine herpesvirus type-1 infection in China. Vet Microbiol. 127: 136–141.
- Yates WD. 1982. A review of infectious bovine rhinotracheitis, shipping fever pneumonia and viral-bacterial synergism in respiratory disease of cattle. Can J Comp Med. 46: 225–263.
- Yesilbag K, Bilge-dagalap S, Okur-gumusova S, Gungor B. 2003. Studies on herpesvirus infections of goats in Turkey: Prevalence of antibodies to bovine herpesvirus 1. Revue Med Vet. 154: 772–774.