Abstract
Since the introduction of the standard mastitis prevention program in the late 1960s, enormous progress has been made in decreasing the average bulk milk somatic cell count (BMSCC). In many countries, reduction of BMSCC has been encouraged through premium payments or penalty systems. However, the success of the program depends heavily on consistent implementation of management practices. The approach to problem solving in a herd with high BMSCC must include the following elements: (1) problem definition using primary udder health parameters; (2) detection of cows causing the problem; (3) definition of short- and long-term goals; (4) formulation and implementation of a herd management plan; and (5) evaluation of the results. Findings and plans are recorded for use at follow-up visits. Every high BMSCC problem can be solved if farmers are sufficiently motivated, if farm advisors are sufficiently knowledgeable, and if farmer and advisors work together according to a jointly determined plan.
1. Introduction
Since the introduction of a standard mastitis prevention program by Neave et al. (Citation1969), enormous progress has been made in decreasing the prevalence of intramammary infection (IMI), resulting in an associated decrease in average bulk milk somatic cell count (SCC). In a Finnish study (Pitkala et al. Citation2004), an important decrease in IMI prevalence between 1988 and 2001 was found for Streptococcus agalactiae, Streptococcus dysgalactiae, Staphylococcus aureus, and Streptococcus uberis. During the same period, the prevalence of minor pathogens causing subclinical IMI [coagulase-negative staphylococci (CNS) and Corynebacterium bovis] in Finland increased considerably, while bulk milk SCC (BMSCC) decreased from 330,000 cells/ml in 1988 to 135,000 cells/ml in 2001. A concurrent decrease in BMSCC and similar shift in pathogens was observed in Canada, Denmark, The Netherlands, the UK, and Belgium, from over 350,000 cells/ml in 1980 to approximately 200,000 cells/ml in 2006 (e.g. Piepers et al. Citation2007; Sampimon et al. Citation2009).
Most European countries and Canada have experienced a decrease in BMSCC as a result of widespread adoption of the standard mastitis prevention program. Adoption of this program was partly the result of the introduction of penalty limits for BMSCC at 500,000 or 400,000 cells/ml and premiums for low BMSCC (e.g. Barkema et al. Citation1998a; Sargeant et al. Citation1998). In other countries, such as the USA, where the regulatory limit for BMSCC is 750,000 cells/ml, average BMSCC has remained at a higher level; average BMSCC differs considerably among states in the USA though, and average herd SCC (HSCC) of herds enrolled in Dairy Herd Improvement (DHI) programs decreased to 200,000 cells/ml in 2012 (Norman et al. 2013). In 2009, lowering the regulatory limit has been proposed by the National Mastitis Council. Since January 2012, the European Union (EU) SCC requirements have taken effect. Via a structural approach and more consistent application of the standard mastitis prevention program, the number of farms with a BMSCC > 400,000 cells/ml is expected to now drop fast in these countries as well (Allore et al. Citation1998; Rodrigues & Ruegg Citation2005; Norman et al. Citation2011). In every region of the world, however, high BMSCC problem herds can be found. In Canada, where the average BMSCC in 2008 was 230,000 cells/ml, 5% of the herds had an average HSCC (HSCC) > 400,000 cells/ml, while in 32% of the herds at least once a year HSCC was > 400,000 cells/ml (Olde Riekerink unpublished observations). In this review article, a herd level approach to manage high BMSCC problems is presented, based on peer-reviewed literature and the authors’ experiences.
2. Detecting high BMSCC problems
Due to the relatively strict milk quality regulations within the EU and Canada, there is widespread adoption of individual cow SCC measurements at regular intervals (Barkema et al. Citation1998b; Olde Riekerink et al. Citation2006). These measurements are carried out on milk samples collected for determination of production, fat, and protein levels as part of DHI programs. The proportion of cows that are tested varies considerably, ranging from less than 25% in Poland to 100% in Switzerland (International Committee for Animal Recording Citation2012). In some countries, such as the Netherlands, cow level SCC measurements are used to identify cows with infection and subsequent collection of selected milk samples for bacteriological culturing is recommended. The proportion of herds in which cases of either subclinical or clinical mastitis (CM) are sampled for bacteriological culturing on a regular basis is much lower than the proportion of herds in which cows are tested for SCC (Sol Citation2002).
A problem can only be addressed if it is recognized. The sooner a problem is detected, the easier it will be to solve it. Veterinary practitioners can become aware of BMSCC problems through: (1) questions during individual animal calls; (2) inspection of milk recording data before every herd health visit; and (3) tracking of drug use, particularly intramammary injectors, especially in countries where antimicrobials for intramammary use are only prescribed or sold by veterinarians and not over the counter.
The approach to a high BMSCC problem is basically the same as for outbreak investigations of other infectious diseases. It is essential to approach every problem in a systematic way. Every high BMSCC problem, and most CM problems, can be solved if the farmer is sufficiently motivated, advisors have sufficient knowledge for solving the problem, information can be obtained from farm records or laboratory tests, and farmer and advisor work together to formulate and implement a jointly determined plan. Transfer of the knowledge via communication with the farmer and his environment is at least as important as the technical knowledge itself. It is essential though to understand what the farmer drives to improve the udder health at his farm. Everyone, including farmers, has his/her own learning style and mindset, and this should be kept in mind when giving advice and achieving to transfer and share knowledge (Jansen et al. Citation2010). A pro-active, personal farmer-tailored approach is therefore very important (Jansen & Lam 2012). A high BMSCC problem cannot be solved while doing pregnancy checks or ambulatory work (e.g. treating cows for milk fever, caesarian section, etc.). A herd health visit or an individual animal call may provide the opportunity to become aware of a BMSCC problem, but a separate appointment with the farmer(s) is needed to address it.
2.1. Primary parameters
The three primary udder health parameters that can be used to monitor the udder health situation in a herd are as follows: (1) BMSCC; (2) incidence rate of clinical mastitis (IRCM); and (3) percentage of cows culled for udder health reasons (Brand et al. Citation1996). In most countries, BMSCC is determined by the regulatory milk quality laboratory. This count is measured at least once a month. BMSCC has, however, a moderate correlation with the average HSCC, particularly in herds with a high BMSCC (Lievaart et al. Citation2007). An important reason for this discrepancy is that producers frequently withhold milk from problem cows from the bulk tank and e.g. feed it to the calves (Lievaart et al. Citation2009). Thus, relying solely on BMSCC may seriously underestimate herd infection levels. Therefore, if DHI SCCs are available, HSCC is the preferred parameter, if the BMSCC is too high.
Data on IRCM and culling must be obtained from farm records. If treatment records are not available in the electronic format, the farmer can mark on the most recent DHI sheets those cows that had CM. The list of cows that had CM in the last three months should include cows that are still present on the farm and cows that have been culled.
2.1.1 Bulk milk somatic cell count
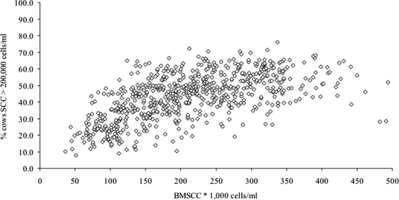
If records of BMSCC are not filed at the farm, BMSCC is available through the milk quality laboratory or the milk processor. Data of the last two years should be requested. The association between BMSCC and the prevalence of high SCC cows is not linear (Lievaart et al. Citation2007; Figure ). It is possible, though, to give a farmer a rough estimate of the proportion of high SCC cows in the herd based on the farms’ BMSCC (Eberhart et al. Citation1982). For example, on farms with a BMSCC > 350,000 cells/ml, on average 40% of the lactating cows will have a high SCC (>200,000 cells/ml). This often surprises the farmer. In herds with high milk production, the percentage will be higher, because of lower SCC in high producing cows, regardless of infection status (Green et al. Citation2006). Average SCC also differs among the bacteria that cause the IMI: SCC of a S. aureus IMI is, on average, lower than that of a S. agalactiae or S. uberis IMI (Djabri et al. Citation2002). Therefore, in herds with predominantly S. agalactiae or S. uberis IMI, prevalence of high SCC cows will be lower than that in a S. aureus problem herd at a specific BMSCC level.
2.1.2 Incidence rate of clinical mastitis
An exact determination of the incidence of CM is only possible after months of registration, especially on farms with a small herd size. Before an accurate list can be made, a definition of CM must be established. If fore-stripping is not used routinely, mild CM may go undetected and IRCM will be underestimated (Olde Riekerink et al. Citation2008). Some farmers only use the term ‘mastitis’ for cows that are systemically ill. Any visible abnormality of the milk, the udder, or both, whether or not it is accompanied by systemic signs, should be considered CM. Of course, signs are only visible to those who pay attention (fore-strip). An estimate of IRCM can be obtained if the farmer maintains a list of cows that had CM in the last months. Treatment records or a farmer's recollection may be the only information available at the first visit. In a dataset from 274 Dutch dairy herds, approximately 2% of cows had CM per month (Barkema et al. Citation1998a). If the IRCM is >2% per month, the herd is considered to be a problem herd. The IRCM and BMSCC levels are not necessarily correlated (Barkema et al. Citation1998a). Still, even when clinical cure after treatment is achieved, bacteriological cure is rarely better than 70% (Sol et al. Citation2000; Pinzón-Sánchez et al. Citation2011). Indeed, CM cases might develop into subclinical mastitis characterized by an elevated SCC in the months thereafter and thus potentially increasing the BMSCC. In turn, high SCCs in dairy cows may also develop into CM. Van den Borne et al. (Citation2011) recently found that cows with a high SCC had a two- to four-fold higher hazard for subsequent CM than cows with a low SCC. Based on their calculations, approximately 25% of the first subsequent CM cases after an SCC DHI record can potentially be avoided when cows are prevented from getting high SCC or when high SCC cows are removed from the population.
2.1.3 Culling
An udder health problem can be masked through frequent culling of cows with CM or a high SCC. On average, 5% of cows are culled because of udder health (high SCC or clinical mastitis) (Barkema et al. Citation1998a). If the percentage is higher than 5%, this should be considered a problem and needs to be evaluated.
2.2. Secondary parameters
If the BMSCC is too high, the percent chronic infections and the new infection rate (NIR) should be evaluated. Other parameters that can be determined are the percent high fresh cows and heifers (see further). These two parameters can be calculated using DHI data. These data can be downloaded electronically in herd health programs such as DairyComp 305, PCDArt, or VAMPP, or they can be entered manually, using the last two DHI recording sheets. Control of high BMSCC problems is impossible without individual cow SCC data. Therefore, problem herds that do not participate in a milk quality recording program should start sampling all cows individually, preferably with a monthly interval. To prevent reoccurrence of the problem, monitoring of individual cow SCC, and HSCC, should also be continued in the next months following the peak in BMSCC.
2.2.1 Percent chronic infections
The percent chronic infection is calculated as the number of cows with an SCC ≥200,000 cells/ml or linear score ≥4 both at the previous and current milk test, multiplied by 100 and divided by all lactating cows on the herd. One should strive for a percent chronic infection ≤5% with an upper limit of 10%.
2.2.2 New infection rate
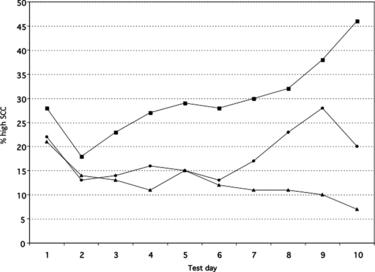
The NIR is calculated as the number of cows that experienced an increase in SCC typically from <200,000 cells/ml or a linear score <4 at the previous milk test to ≥200,000 cells/ml or a linear score ≥4 at the current milk test, multiplied by 100 and divided by the number of cows with low SCC (typically <200,000 cells/ml or linear score <4) at the previous milk test (cows ‘at risk’). New high SCC cases of adult cows at the first milk test after calving that had a low last test-day SCC before dry-off are not included in the NIR, which makes this parameter not useful for detection of problems occurring in the dry period or in early lactation. The same is true for heifers that calved and that have a high SCC at first milk test. It is, therefore, important to check that the proportion of these cows with a high SCC is acceptable (see further). The NIR is influenced by the interval between tests. On average, herds with a monthly DHI test and a BMSCC around 200,000 cells/ml have an NIR of approximately 10% (Olde Riekerink et al. Citation2007). Figure shows an example of a herd with a low NIR.
2.3. Analysis and interpretation of the data
Using the data that are now available the following questions can be answered.
When did the increase in BMSCC occur and is this a significant increase? The significance of the increase depends on the size of the herd and the BMSCC goals that the herd has had before. An example of a method to check whether an increase is significant is presented in Table . Because normal variation will be smaller in larger herds, alarm values will be closer to target values than in small herds.
Table 1. Tables to determine whether an increase of bulk milk somatic cell count (cells/ml) should prompt intervention (Lam et al. Citation1998)a.
Did an increase occur around the same time last year?
How many cows contribute to the high BMSCC?
Is the NIR high or is the high BMSCC only due to a cumulation of chronically infected cows?
If only a few cows contribute (<2%) to the high BMSCC and the NIR remained acceptable (<8%) over the last couple of milk tests, the problem is mostly due to a cumulation of (chronically) infected cows and can be solved by taking a decision for each of the high SCC cows in consultation with the farmer. The contribution of each individual cow to the herd average SCC can be calculated as the daily milk yield at milk recording multiplied by the SCC divided by the daily milk yield of all lactating cows multiplied by the average HSCC at the same milk recording. High SCC cows might be cultured and then treated, dried off, culled, or segregated. Only those cows that are still likely to cure should be treated. Cows with a low probability of cure should not be treated anymore, but culled or segregated. The different options are discussed in more detail below.
If more than 2% of the cows contribute to the BMSCC and too many new infections (NIR > 8%) occur in between two milk recordings, a thorough analysis of the individual SCC records along with the bacteriological culture data is required to specifically diagnose and tackle the root of the problem (‘evidence based medicine’). The timing the infections occur needs to be established (early lactation versus mid- to late-lactation), the animals that are affected need to be identified (heifers versus multiparous cows) and the origin of the infections needs to be assessed [contagious (spread from cow to cow) versus environmental]. The distribution of parity and days in milk (DIM) of cows with high SCC and/or CM can strongly differ from herd to herd. The following situations or combinations thereof are most common.
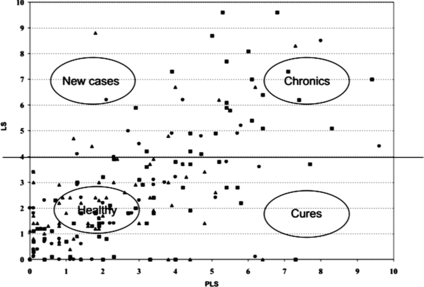
Increase in the proportion of cows with a high SCC with increasing parity (Figure ).
Increase in the proportion of cows with a high SCC with DIM (Figure ).
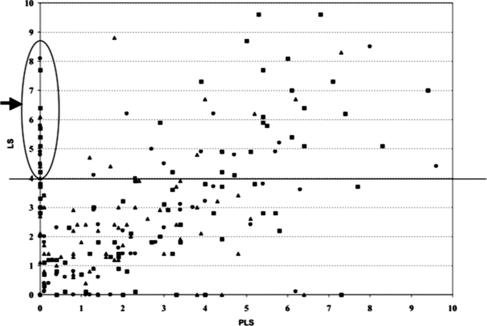
Peak in high SCC prevalence and CM incidence in heifers in the first month of lactation (Figure ).
Besides the percent chronic infections and NIR, the percent high fresh cows and heifers need to be evaluated. All parameters should be calculated and evaluated before the first herd visit at which the BMSCC problems will be discussed takes place.
2.3.1 High SCC in fresh adult cows (‘high fresh cows’)
The percentage of high SCC fresh adult cows can be calculated as the number of adult cows with an SCC ≥200,000 cells/ml at first milk recording after calving from five DIM on divided by all animals that had their first SCC record at that milk recording (Dufour & Dohoo Citation2012). A percent high fresh cows >15% indicate that too many cows either contracted a new IMI or did not cure from an existing IMI during dry period. On farms with a small herd size, it is recommended to calculate this parameter based on the data of the last six months to one year or even two years when available, as only a small number of cows might have freshened in between two milk recordings, thereby blurring the interpretation. The different situations can be distinguished from each other based on the last milk recordings of previous lactation. A new IMI is deemed to have occurred during the dry period when SCC was <200,000 cells/ml at the last milk recordings before dry-off and was ≥200,000 cells/ml at the first milk recording after calving (Dufour & Dohoo Citation2012). Animals with a high SCC at dry-off, but not more than three times in the last three milk recordings, are considered to be persistently infected and expected to cure during dry period, assuming that they were dried off with long-acting antibiotics. If not, the resistance against the antimicrobial that was used or the presence of a highly virulent mastitis pathogen that is known to be difficult to successfully treat (e.g. S. aureus, Klebsiella spp., etc.) can be suspected. Bacteriological culturing and sensitivity testing of the isolated pathogens can give a decisive answer herein. Chronically infected animals (>3 times high SCC at the last milk recordings before dry-off) have a low probability of cure, independently of the pathogen that is involved and the dry cow therapy that was applied.
2.3.2 High SCC in fresh heifers (‘high fresh heifers’)
IMIs in dairy heifers are highly prevalent. In several heifer mastitis surveys conducted throughout the world, up to 60% of the quarters harbored an IMI at the time of calving (e.g. Cook et al. Citation1992; Parker et al. Citation2007; Piepers et al. Citation2010). Most of these IMI reveal themselves as subclinical mastitis characterized by an elevated SCC without any other visible symptoms of inflammation (De Vliegher et al. Citation2012). In a Belgian study, 30% of heifers had an SCC >150,000 cells/ml in the first 14 days after calving (De Vliegher et al. Citation2004). The proportion of heifers calving with a high SCC varies considerably among herds (Borm et al. Citation2006). A herd is considered to have a heifer mastitis problem if > 15% of the heifers have an SCC >150,000 cells/ml at the first milk recording from 10 DIM on. Figure shows a herd that does not have a high BMSCC problem, but which nevertheless has too many heifers and cows with a high SCC at their first milk recording.
2.3.3 Bacteriological culture
Recent milk culture results are often not available. As a start, a bulk tank sample can be submitted for bacteriological culture to determine whether one of the contagious pathogens, S. aureus, S. agalactiae, or Mycoplasma spp., plays a role in the problem. Occasionally, mastitis due to S. uberis or Escherichia coli can also be detected through use of bulk tank milk samples (Zadoks et al. Citation2004; Zadoks et al. Citation2005). To determine the pathogen distribution in high SCC cases, quarter milk samples of a subset of high SCC cows should be cultured. Composite samples of the four quarters can be used for this purpose, but quarter samples are preferred. The sensitivity of composite milk samples for detection of mastitis pathogens is lower than the culture of quarter milk samples, especially for detection of S. aureus (Lam et al. Citation1996). Furthermore, if composite samples are cultured, the four quarters of these cows may subsequently need to be cultured individually to determine which and how many quarters are affected. An exact SCC threshold that always permits separation of infected cows from non-infected cows does not exist. For initial herd screening purposes, selecting cows with an SCC >400,000 cells/ml (linear score 5) for milk culture has been suggested for high BMSCC herds (McDermott et al. Citation1982). Even under the best conditions, producers should be aware that some cows with SCC >400,000 cells/ml will be culture-negative, while some with SCC of less than 400,000 cells/ml will be culture-positive. Screening of quarter level SCCs can help select quarters that should be sampled. Tools for SCC measurement, such as the California Mastitis Test, may have limited sensitivity and specificity, but their low cost, speed, ease of use, and quarter level results make them valuable in a herd screening and sampling program. Frequent use of the California Mastitis Test has in literature been consistently associated with low HSCC(Dufour et al. Citation2011). Quarter level information on infection status can be important for treatment or culling decisions (Barkema et al. Citation2006) (see further). Thorough instruction of the farmer or technical personnel on aseptic sampling technique is needed to prevent contamination of samples during collection. Information about seasonality can be important too. For example, in the Netherlands and in Norway, a recurrent increase in BMSCC during the summer pasture season is likely due to S. uberis mastitis or, in Norway, S. aureus mastitis, whereas a similar increase in BMSCC during the winter housing season is more likely to be due to S. dysgalactiae mastitis (Barkema Citation1999; Osteras et al. Citation2006; Olde Riekerink et al. Citation2007).
Using the data that are now available the following questions can be answered.
What is the parity and DIM distribution of high SCC and CM cows? For example, does the distribution point at a problem in heifers, in high producing cows, or around the dry period?
What is the most important pathogen involved?
3. First herd visit
The objectives of this visit are to: (1) identify the problem and the factors that are involved; (2) define short-term and long-term goals; and (3) plan and implement management changes and monitoring tools. It is essential that the owner and herdsperson (often the same) is involved in all three components, otherwise buy-in and implementation of changes will not be adopted.
It is important to sit down with the farmer, tour the farm, and observe milking practices. The order of these three elements depends on the time of day: at a morning visit, the milking is observed first, then the farm is toured, and finally one sits down with the farmer. At an afternoon visit, the farm is toured first, then the milking is observed, and at the end one sits down with the farmer. Often, other people have already been involved and some strategies have already been implemented to remedy the problem. An inventory of what has been done by whom, and what effect this has had, is important. If it is clear before the visit that in the opinion of the farmer, the milking machine or nutrition plays a role, the milking machine dealer or a nutritionist should be present. To be successful, it is important that all persons and organizations in the farmers’ environment articulate the same message (Lam et al. Citation2011). If the partner of the farmer or a herd manager is involved in the farming activities, this person should also be present.
3.1. Farm tour
The farm tour is preferably done in the company of the farmer or herd manager. Even if the farm is visited on a regular basis in a herd health program, a farm tour with focus on udder health risk factors is necessary. Existing data collection forms such as those of the New York State Cattle Health Assurance Program (http://nyschap.vet.cornell.edu/module/mastitis/mastitis.asp) and the Wisconsin Milk Money program (http://milkquality.wisc.edu/) can be used to ensure that no points are overlooked. During the farm tour, extra focus can be put on specific areas expected to be responsible for causing the problem based on the conclusions of the data analysis. For example, the environmental conditions of the lactating cows when a high NIR was detected along with a high proportion of IMI caused by environmental pathogens such as S. uberis, dry cow, and heifer management when a high rate of new IMI in dry cows or heifers, respectively, was diagnosed, etc.
3.2. Milking
Before milking starts, the milking parlor is observed and the milking machine is examined (e.g. type, cleanliness, presence of automatic take-offs). Try to determine when the milking machine was last serviced and how frequently this is done. Milking practices that have consistently been associated with low SCC herds are wearing gloves during milking, using automatic take-offs, using post-milking teat dipping, milking problem cows last, yearly inspection of the milking system, and use of a technique to keep cows standing following milking (Dufour et al. Citation2011). If infected cows, in particular those with S. aureus, cannot be milked last, each teat cup should be manually disinfected by flushing it with hot water (>75°C) for several seconds or by immersing the unit into a hypochlorite, iodine, or peracetic acid solution after milking an infected cow. Automatic teat cup sanitizers or backflush units on each milking unit could be installed as well (Ohnstad et al. Citation2012). The gloves/hands should be disinfected as well.
The goal of observations during milking is the registration of the following: (1) actual milking practices and milking hygiene (e.g. Johnson Citation2000); (2) general impression of the functioning of the milking machine (e.g. Mein Citation1998); and (3) behavior of the cows during milking. The presence of an observer will disturb milking. Therefore, first, make the milkers at ease with small-talk and be as invisible and as inaudible as possible to the cows that need to come in the parlor. Collecting milk samples during this visit will disturb milking considerably. During this visit, the milkers can be taught sterile sampling technique, so that additional sampling can be performed by them later on. Important points to pay attention to during milking are as follows.
Does the milking routine consists of udder stimulation, pre-stripping, pre-milking teat disinfection (if permitted in the country), a 60–90 second interval between udder stimulation and attachment, timely take-off (automatic or not), and post-milking teat disinfection? If there is an indication that intervals between pre-milking treatments and attachments or between milk-out and detachment are irregular or long, a time study should be performed. The adequacy of the milk flow process can be determined by using an instrument such as the Lactocorder® (Wallace et al. Citation2003).
What is the number of cows per pre-treatment towel?
What is the level of hygiene during milking?
Do the milkers wear gloves?
Are all teats well covered with post-milking teat disinfectant?
What is the milking order for pens or cows with known SCC or infection status?
When are cows with CM milked and what is done with the milking equipment after milking them?
What is the condition of the teats after milking (color and hyperkeratosis) (Mein Citation1998)?
What is the frequency of liner slips and falling teat cups (Mein Citation1998)?
Are the cows at ease during milking?
After milking, the status of the milk filter should be checked. If necessary, the filter can be used as evidence of poor hygiene or failure to detect clinical mastitis. Of course, automatic milking systems require an adapted protocol (Hovinen & Pyörälä Citation2011). However, most of the points raised on checking a conventional milking system also apply to automatic milking systems.
3.3. Sitting down
When the farm tour and milking have been completed, one sits down with the farmer(s), reviews and completes the history, defines the problem, states realistic short-term and long-term goals, and develops an intervention plan. For each of the high SCC cows, a decision needs to be taken in conversation with the farmer. High SCC cows can be cultured and then treated, dried off, culled or segregated.
3.4. Culturing and treating
Antibiotic treatment of cows, based only upon high SCC, is definitely not recommended (Seymour et al. Citation1989). Treating animals with high SCC is most successful in herds where S. agalactiae is the main cause of mastitis (Loeffler et al. Citation1995), although it can also be considered in herds with mastitis caused by other streptococci (St. Rose et al. Citation2003; Swinkels et al. Citation2005) or S. aureus (Zadoks et al. Citation2002; Barkema et al. Citation2006). Still, some cow-related factors strongly affecting the probability of cure such as previous history of high SCC or CM, parity, stage of lactation, quarter position, and number of infected quarters, should be taken into account before appropriate treatment can be decided. Older cows (≥3 lactations) are more difficult to cure than younger ones. The treatment response is also lower when treatment is administered to chronic infections (≥3 times high SCC at milk test). A hind quarter is more difficult to cure than a front quarter. Cows in early- and mid-lactation have a lower probability of cure than cows in later lactation. When multiple quarters of a cow are infected, cure rate is lower at both the cow and quarter levels. Cows from which only minor pathogenic bacteria such as CNS or Corynebacterium bovis are isolated do not require immediate treatment, except in those cases that the SCC remains persistently high (Supré et al. Citation2011).
3.5. Drying-off
Drying off cows with long-acting antibiotics in all four quarters offers an excellent opportunity for curing existing IMI (Halasa et al. Citation2009). The response to treatment is much more effective during the dry period than during lactation. This is partly because much higher doses of antibiotics can be infused into the dry quarter without concerns over witholding milk. The difference is particularly apparent for S. aureus where the response to lacational treatment may be very poor. Drying off cows sooner might increase the chance of eliminating the infection from that cow. Any Holstein–Friesian cow producing less than 10 kg can be dried off. The dry period can be extended by 30–60 days.
3.6. Culling
Culling of high SCC cows has an immediate effect on the prevalence of cows with IMI and results in a decrease of BMSCC. Additionally, the number of new cases of contagious mastitis is predominantly determined by the number of infected cows present in the herd (e.g. White et al. Citation2006). The more cows that shed bacteria into towels, teat cup liners, and hands of milkers, the more likely it is that additional cows will become infected. Farmers often think that implementation of measures that decrease the NIR, such as post-milking teat disinfection, also immediately lowers the BMSCC. However, this decrease will only occur if culling or cure decreases the number of existing subclinical mastitis cases. Availability of sufficient young stock to replace culled cows is a prerequisite for culling. If 50% of calves born are heifers, if the large majority of the calves is not sold, and if calf mortality is not too high, approximately 25% of cows can be culled on a yearly basis. This is, however, the average culling rate of a non-problem herd. To overcome a high BMSCC problem, the culling rate, and the number of young stock available, may need to be increased (Jones Citation1999). The feasibility of such increases will depend on other management and health aspects, e.g. herd fertility, participation in disease eradication programs, and involuntary culling due to disease in young stock or cows. If purchasing cows cannot be avoided, their udder health history should be evaluated and they should be examined and tested for contagious diseases (Barkema et al. Citation2009). In countries with milk quota, the quota situation also influences short-term culling decisions.
3.7. Segregating
Some IMI such as those caused by S. aureus can be easily spread from cow to cow at milking time either via the teat liners or milkers’ hands, assuming that common towels are not used during washing and drying. High SCC cows that are chronically infected with contagious mastitis pathogens such as S. aureus and that can neither be treated or culled should be isolated into a separate mastitis group and milk last or sorted into a milking group at the beginning of the milking process and hold until last before milking this group. If the cows cannot be separated, the milking routine should be rigorously optimized to limit the spread of IMI as much as possible (see Milking).
Other issues that can be discussed are the dry cow therapy, heifer management, and nutrition and health.
3.8. Dry cow therapy
Blanket dry cow therapy is essential in herds with a high BMSCC (Halasa et al. Citation2009). It is, however, practized on only 75% of dairy farms in Canada (Olde Riekerink et al. Citation2006). Implementation of blanket dry cow treatment is less common in herds with a high BMSCC than in herds without a BMSCC problem (Barkema et al. Citation1998b; Olde Riekerink et al. Citation2006). To decrease the risk for new IMI with environmental pathogens during dry period, the long-acting antibiotics might be combined with an internal wax sealant to prevent new IMI (Halasa et al. Citation2010).
3.9. Heifer management
Specific recommendations to prevent and control mastitis in late gestation in periparturient heifers are not part of the current standard prevention and control program. Control and prevention of heifer mastitis is currently based on avoidance of inter-suckling among young stock, fly control, optimal nutrition, and implementation of hygiene control and comfort measures, especially around calving (De Vliegher et al. Citation2012). More risks have been identified (e.g. season, location of herd, stage of pregnancy); however, they do not lend themselves to the development of specific intervention strategies designed to prevent the disease. Prepartum treatment can be implemented only as a short-term measure to assist in the control of a significant heifer mastitis problem under supervision of the herd veterinarian. When CNS are the major cause of IMI in heifers, productivity is not affected, making prepartum treatment redundant and even unwanted.
3.10. Nutrition and health
In addition to the level of exposure, the number of new IMIs is also determined by the ability of the animals to cope with the disease. Although the level of exposure to bacteria is of overriding importance in the establishment of IMI, the relevance of the host immune response should not be underestimated. Nutrition is an important factor in this, and it is too often forgotten when dealing with a problem of a high NIR caused by environmental mastitis pathogens such as E. coli (e.g. Goff Citation2006) or extremely poor treatment responses of cows newly infected with e.g. S. aureus (Kruze et al. Citation2007). Evaluate and ask the farmer about nutrition of the lactating cows, and also about dry cows and heifers. Specifically address supplementation with minerals such as selenium and vitamin E (e.g. Weiss et al. Citation1990). If laboratory capacity for testing is available, blood samples should be taken to check the selenium or glutathion-peroxidase activity levels at the individual animal level when deficiencies are suspected. Metabolic diseases related to nutritional deficiencies such as negative energy balance and (sub)clinical rumenacidosis, and viral diseases such as Bovine Viral Diarrhea might impair the host immune response as well and be involved in the high BMSCC problem (Laureyns et al. Citation2013).
4. Formulating an action plan
The intervention plan should be divided into practices that will achieve the following: (1) decrease the NIR; (2) decrease the duration of infection; and (3) decrease the IRCM. Keep the managerial capacities of the farmer and farm workers and the situation on the farm (e.g. expansion plans, shortage of young stock, milk quota situation, financial situation, management characteristics of the farmer) in mind when deciding on a realistic time line and goals for intervention (e.g. Barkema et al. Citation1999). Do not try to change everything at once. Recommendations should be prioritized using the following three criteria: (1) ease of implementation; (2) effect of the change; and (3) cost of the recommendation. Highest on the list will be cheap recommendations that are easy to implement and have a large effect. Examples include the use of post-milking teat disinfection to reduce NIR and the culling of chronically infected animals to decrease the duration of infection and lower the BMSCC. On most farms, no more than five recommendations should be given. Otherwise, the farmer may be overwhelmed and none of the recommendations may be implemented. Additional data collection is often necessary. Warn for possible culture-negative results and explain what they mean.
The success of interventions depends heavily on the motivation of the farmer and whether he/she applies control measures rigorously. Explain clearly why certain practices should be changed, what the effect of the change should be, and when these effects can be expected to show. Keep in mind that different farmers learn in different ways and that everyone has a preferred means of obtaining information. Some may prefer theoretical information such as numbers and figures from test-day records and management software, whereas others derive their information from practical examples (Lam et al. Citation2011). If farm personnel do not speak the same language as the farmer, make sure that management changes and their underlying reasons are explained to farm workers in a manner that they understand. In most countries, organic dairy farms tend to have a higher average BMSCC than conventional dairy farms, except for regions in the USA where the BMSCC limit for bulk milk is lower on organic farms (400,000 cells/ml) than on conventional farms (750,000 cells/ml). The approach to BMSCC problems in organic dairy herds is in essence the same as for conventional herds. However, one should make him/herself familiar with the rules and philosophies of organic farms, which differ between countries. It is not helpful to try and force these farms to adopt measures that are not in line with organic farming methods. It is certainly possible to produce milk with a low BMSCC on organic dairy farms. However, it requires an excellent farmer to run the farm because fewer short-term and therapeutic solutions are available. Therefore, mastitis control relies heavily on prevention of new infections and the time frame for solving of high BMSCC problems may be longer than on conventional farms.
5. Follow-up
A (short) report of every visit is essential for the follow-up. Included in the report should be: (1) the current situation; (2) the short-term and long-term goals, with a timeline for each goal; (3) the plan with the recommendations; (4) other actionable items, e.g. an agreement that the farmer will send a copy of DHI results or collect samples for bacteriological culture, a commitment from the veterinarian to propose a treatment protocol, etc.; and (5) date and time of the next meeting.
A high SCC problem cannot be solved with one single visit. The adoption of recommendations and their effect needs to be checked. Consistent attention from veterinarians or herd advisors to udder health management has repeatedly been shown to contribute to improvement of the situation (Hillerton et al. Citation1995; Wilson et al. Citation1997; Zadoks et al. Citation2002). Therefore, a second visit when sufficient new data are available is needed. The authors most often visit again after two months. However, if the herd is large, sufficient new data may be available sooner. Also, if the problem is urgent (e.g. pick-up of bulk milk is in jeopardy), an earlier visit may be necessary. If the situation changes, adjustment of goals and plans may be necessary.
Contact the farm in the interval between visits to check whether data collection (sampling for culture, treatments, etc.) that has been agreed upon is taking place. Stay in touch with the farmer and contact the farmer when new BMSCC data, DHI records, or culture results are available. If the sharing of information or collection of milk samples that was agreed upon during the herd visit does not take place, contact the farmer pro-actively. When the short-term goal has been met, farmers often do not give enough priority to these agreements anymore and achievement of long-term goals falls by the wayside.
6. Summary
Control of mastitis, whether measured through BMSCC, NIR, percentage of heifers with mastitis in early lactation, or culling, is feasible. Many studies have shown an impact of mastitis control programs in reduction of the prevalence and incidence of mastitis (Barkema et al. 1998b; Neave et al. Citation1969; Hillerton et al. Citation1993; Hillerton et al. Citation1995; Zadoks et al. Citation2002). Success is particularly easy to achieve in the case of S. agalactiae mastitis (Loeffler et al. Citation1995). Control of S. aureus mastitis is often deemed to be difficult, but many herds have been successful in this regard through implementation of the standard mastitis prevention program (e.g. Hillerton et al. Citation1995; Zadoks et al. Citation2002). The standard mastitis prevention program also affects the prevalence and incidence of S. dysgalactiae and S. uberis (Neave et al. Citation1969), regardless of the often used and misleading label of ‘environmental streptococci’. Additional measures may be necessary to control specific forms of mastitis that can lead to high BMSCC, e.g. mastitis in heifers before calving or mastitis caused by Mycoplasma spp. Control measures that are considered impractical or even impossible by some farmers are readily adopted by others. Thorough knowledge and understanding of factors that contribute to high BMSCC and of management measures and changes that affect BMSCC are essential for the motivation and the ability of farmers to lower BMSCC. Legality, economic and technical feasibility of control measures may differ between countries and management systems (e.g. Allore et al. Citation1998). However, such arguments should not be used as an excuse for high BMSCC levels if the true problem is that farmers, their personnel, or their veterinarians lack the willingness, or the understanding necessary to resolve the high BMSCC problem. We have the knowledge and the tools to solve most BMSCC problems. All we need is the decision to address them and the stamina to put our decisions into practice.
Acknowledgements
We thank Dr. Henk Hogeveen for providing the data and analyses to construct Table . We also thank Dr. Ynte Hein Schukken for providing the data for Figures to .
References
- Allore HG, Erb HN, Schruben LW, Oltenacu PA. 1998. A simulation of strategies to lower bulk tank somatic cell count below 500,000 cells per milliliter. J Dairy Sci. 81: 694–702.
- Barkema HW. 1999. Udder health on dairy farms. 1. Results of a longitudinal study on 300 Dutch farms. Tijdschr Diergeneeskd. 124: 338–344.
- Barkema HW, Green MJ, Bradley AJ, Zadoks RN. 2009. Invited review: The role of contagious disease in udder health. J Dairy Sci. 92: 4717–4729.
- Barkema HW, Ploeg JD van der, Schukken YH, Lam TJ, Benedictus G, Brand A. 1999. Management style and its association with bulk milk somatic cell count and incidence of clinical mastitis. J Dairy Sci. 82: 1655–1663.
- Barkema HW, Schukken YH, Lam TJGM, Beiboer ML, Wilmink H, Benedictus, G, Brand A. 1998a. Incidence of clinical mastitis in dairy herds grouped in three categories by bulk milk somatic cell counts. J Dairy Sci. 81: 411–419.
- Barkema HW, Schukken YH, Lam TJGM, Beiboer ML, Benedictus G, Brand A. 1998b. Management practices associated with low, medium, and high somatic cell counts in bulk milk. J Dairy Sci. 81: 1917–1927.
- Barkema HW, Schukken YH, Zadoks RN. 2006. Invited review: The role of cow, pathogen, treatment and study characteristics in the success of treatment of bovine Staphylococcus aureus mastitis. J Dairy Sci. 89: 1877–1895.
- Borm AA, Fox LK, Leslie KE, Hogan JS, Andrew SM, Moyes KM, Oliver SP, Schukken YH, Hancock DD, Gaskins CT, et al. 2006. Effects of prepartum intramammary antibiotic therapy on udder health, milk production, and reproductive performance in dairy heifers. J Dairy Sci. 89: 2090–2098.
- Brand A, Noordhuizen JPTM, Schukken YH. 1996. Herd health and production management in dairy practice. Wageningen: Wageningen Pers.
- Cook WF, Fiez EA, Fox LK. 1992. Mastitis in first lactation southwest Idaho dairy cows. J Dairy Sci. 75 (Suppl. 1): 158.
- De Vliegher S, Barkema HW, Stryhn H, Opsomer G, De Kruif A. 2004. Impact of early lactation somatic cell count in heifers on somatic cell counts over the first lactation. J Dairy Sci. 87: 3672–3682.
- De Vliegher S, Fox LK, Piepers S, McDougall S, Barkema HW. 2012. Invited review: Mastitis in dairy heifers: Nature of the disease, potential impact, prevention, and control. J Dairy Sci. 95: 1025–1040.
- Djabri B, Bareille N, Beaudeau F, Seegers H. 2002. Quarter milk somatic cell count in infected dairy cows: a meta-analysis. Vet Res. 33: 335–357.
- Dufour S, Dohoo IR. 2012. Monitoring dry period intramammary infection incidence and elimination rates using somatic cell count measurements. J Dairy Sci. 95: 7173–7185.
- Dufour S, Fréchette A, Barkema HW, Mussell A, Scholl DT. 2011. Invited review: effect of udder health management practices on herd somatic cell count. J Dairy Sci. 94: 563–579.
- Eberhart RJ, Hutchinson LJ, Spencer SB. 1982. Relationships of bulk tank somatic cell counts to prevalence of intramammary infection to indices of herd production. J Food Prot. 45: 1125–1128.
- Goff JP. 2006. Major advances in our understanding of nutritional influences on bovine health. J Dairy Sci. 89: 1292–1301.
- Green LE, Schukken YH, Green MJ. 2006. On distinguishing cause and consequence: Do high somatic cell counts lead to lower milk yield or does high milk yield lead to lower somatic cell count? Prev Vet Med. 76: 74–89.
- Halasa T, Nielen M, van Werven T, Hogeveen H. 2010. Simulation model to calculate costs and benefits of dry period interventions in dairy cattle. Livest Prod Sci. 129: 80–87.
- Halasa T, Nielen M, Whist AC, Osteras O. 2009. Meta-analysis of dry cow management for dairy cattle. Part 2. Cure of existing intramammary infections. J Dairy Sci. 92: 3150–3157.
- Hillerton JE, Bramley AJ, Staker RT, McKinnon CH. 1995. Patterns of intramammary infection and clinical mastitis over a 5 year period in a closely monitored herd applying mastitis control measures. J Dairy Res. 62: 39–50.
- Hillerton JE, Shearn MF, Teverson RM, Langridge S, Booth JM. 1993. Effect of pre-milking teat dipping on clinical mastitis on dairy farms in England. J Dairy Res. 60: 31–41.
- Hovinen M, Pyörälä S. 2011. Invited review: Udder health of dairy cows in automatic milking. J Dairy Sci. 94: 547–562.
- International Committee for Animal Recording. 2012. Yearly enquiry on the situation of milk recording in member countries–Results for the years 2010 and 2011. [cited 2013 March]. Available from: www.icar.org/Documents/Yearly%20inquiry/2010-2011/Cow%20Survey%202010- 2011.pdf
- Jansen J, Steuten CDM, Renes RJ, Aarts N, Lam TJGM. 2010. Debunking the myth of the hard-to-reach farmer: Effective communication on udder health. J Dairy Sci. 93: 1296–1306.
- Jansen, J, Lam, TJGM. 2012. The role of communication in improving udder health. Vet Clin North Am Food Anim Pract. 28: 363–379.
- Johnson AP. 2000. A proper milking routine: the key to quality milk. [monograph on the Internet]. NMC, Verona, WI, US. [Cited 2013 March]. Available from: www.nmconline.org/articles/keyqlty.htm
- Jones GM. 1999. Guidelines to culling cows with mastitis. Virginia State University, VI, USA. [Cited 2013 March]. Available from: www.ext.vt.edu/pubs/dairy/404-204/404-204.html
- Kruze J, Ceballos A, Stryhn H, Mella A, Matamoros R, Contreras pA, Leyan V, Wittwer F. 2007. Somatic cell count in milk of selenium-supplemented dairy cows after an intramammary challenge with Staphylococcus aureus. J Vet Med A. 54: 478–483.
- Lam TJGM, Grijsen EG, Schukken YH, Kaal-Lansbergen L, Hogeveen H. 1998. Mastitis management planner: a new approach to mastitis in dairy herds. Proceedings 20th World Buiatrics Conference; Sydney, Australia, 251–254.
- Lam TJGM, Jansen J, van den Borne BHP, Renes RJ, Hogeveen H. 2011. What veterinarians need to know about communication to optimise their role as advisors on udder health in dairy herds. New Zeal Vet J. 59: 8–15.
- Lam TJGM, Wuijckhuise LA, Franken P, Morselt ML, Hartman EG, Schukken YH. 1996. Use of composite milk samples for diagnosis of Staphylococcus aureus mastitis in dairy cattle. J Am Vet Med Assoc. 208: 1705–1708.
- Laureyns J, Piepers S, Ribbens S, Sarrazin S, De Vliegher S, Van Crombrugge JM, Dewulf J. 2013. Association between herd exposure to BVDV-infection and bulk milk somatic cell count of Flemish dairy farms. Prev Vet Med. 109: 148–151.
- Lievaart JJ, Barkema HW, Hogeveen H, Kremer WDJ. 2009. Reliability of the bulk milk somatic cell count as an indication of average herd somatic cell count. J Dairy Res. 76: 490–496..
- Lievaart JJ, Kremer WDJ, Barkema HW. 2007. Comparison of bulk milk, yield-corrected, and average somatic cell counts as parameters to summarize the subclinical mastitis situation in a dairy herd. J Dairy Sci. 90: 4145–4148.
- Loeffler SH, Lam TJ, Barkema HW, Scholten D, Hessels AL, van Kestel AM. 1995. The dairying veterinarian approach to a high bulk milk somatic cell count caused by Streptococcus agalactiae. Tijdschr Diergeneeskd. 120: 458–463.
- McDermott MP, Erb HN, Natzke RP. 1982. Predictability by somatic cell counts related to prevalence of intramammary infections within herds. J Dairy Sci. 65: 1535–1539.
- Mein GA. 1998. Simple checks of a milking system. Verona, WI, USA: NMC. [Cited 2013 March]. Available from: www.nmconline.org/articles/simplechks.htm
- Neave FK, Dodd FH, Kingwell RG, Westgarth DR. 1969. Control of mastitis in the dairy herd by hygiene and management. J Dairy Sci. 52: 696–706.
- Norman HD, Lombard JE, Wright JR, Kopral CA, Rodriguez JM, Miller RH. 2011. Consequence of alternative standards for bulk tank somatic cell count of dairy herds in the United States. J Dairy Sci. 94: 6243–6256.
- Norman HD, Miller RH, Ross, Jr., FA. 2013. Somatic cell counts of milk from Dairy Herd Improvement herds during 2012. USDA AIPL Research Report SCC14 (2-13). [Cited 2013 March]. Available from: aipl.arsusda.gov/publish/dhi/dhi13/sccrpt.htm
- Ohnstad I, Olde Riekerink RG, Hogewerf P, de Koning CA, Barkema HW. 2012. Short communication: Effect of automatic postmilking teat disinfection and cluster flushing on the milking work routine. J Dairy Sci. 95: 2567–2570.
- Olde Riekerink RGM, Barkema HW, Kelton DF, Keefe GP, Scholl DT. 2006. Risk factors for herd-level infections of contagious mastitis pathogens on Canadian dairy farms. Proceedings of the 11th Symposium of the International Society of Veterinary Epidemiology and Econonics; Cairns, Australia, 598.
- Olde Riekerink RGM, Barkema HW, Kelton DF, Scholl DT. 2008. Incidence rate of clinical mastitis on Canadian dairy farms. J Dairy Sci. 91: 1366–1377.
- Olde Riekerink RGM, Barkema HW, Stryhn H. 2007. The effect of season on somatic cell count and the incidence of clinical mastitis. J Dairy Sci. 90: 3733–3741.
- Osteras O, Solverod L, Reksen O. 2006. Milk culture results in a large Norwegian survey–effects of season, parity, days in milk, resistance, and clustering. J Dairy Sci. 89: 1010–1023.
- Parker KI, Compton CW, Anniss FM, Heuer C, McDougall S. 2007. Subclinical and clinical mastitis in heifers following the use of a teat sealant precalving. J Dairy Sci. 90: 207–218.
- Piepers S, De Meulemeester L, de Kruif A, Opsomer G, Barkema HW, de Vliegher S. 2007. Prevalence and distribution of mastitis pathogens in subclinically infected dairy cows in Flanders, Belgium. J Dairy Res. 74: 478–483.
- Piepers S, Opsomer G, Barkema HW, de Kruif A, De Vliegher S. 2010. Heifers infected with coagulase-negative staphylococci in early lactation have fewer cases of clinical mastitis and higher milk production in their firs lactation than non-infected heifers. J Dairy Sci. 93: 2014–2024.
- Pinzón-Sánchez C, Cabrera VE, Ruegg PL. 2011. Decision tree analysis of treatment strategiesfor mild and moderate cases of clinical mastitis occuring in early lactation. J Dairy Sci. 94: 1873–1892.
- Pitkala A, Haveri M, Pyorala S, Myllys V, Honkanen-Buzalski T. 2004. Bovine mastitis in Finland 2001–prevalence, distribution of bacteria, and antimicrobial resistance. J Dairy Sci. 87: 2433–2441.
- Rodrigues AC, Ruegg PL. 2005. Actions and outcomes of Wisconsin dairy farms completing milk quality teams. J Dairy Sci. 88: 2672–2680.
- Sampimon O, Barkema HW, Berends I, Sol J, Lam TJGM. 2009. Prevalence of intramammary infection in Dutch dairy herds. J Dairy Res. 76: 129–136.
- Sargeant JM, Schukken YH, Leslie KE. 1998. Ontario bulk milk somatic cell count reduction program: progress and outlook. J Dairy Sci. 81: 1545–1554.
- Seymour EH, Jones GM, McGilliard ML. 1989. Effectiveness of intramammary antibiotic therapy based on somatic cell count. J Dairy Sci. 72: 1057–1061.
- Sol J. 2002. Cure of Staphylococcus aureus mastitis in Dutch dairy cows [ PhD thesis]. The Netherlands: Utrecht University.
- Sol J, Sampimon OC, Barkema HW, Schukken YH. 2000. Factors associated with cure after therapy of clinical mastitis caused by Staphylococcus aureus. J Dairy Sci. 83: 278–284.
- St Rose SG, Swinkels JM, Kremer WD, Kruitwagen CL, Zadoks RN. 2003. Effect of penethamate hydriodide treatment on bacteriological cure, somatic cell count and milk production of cows and quarters with chronic subclinical Streptococcus uberis or Streptococcus dysgalactiae infection. J Dairy Res. 70: 387–394.
- Supré K, Haesebrouck F, Zadoks RN, Vaneechoutte M, Piepers S, De Vliegher S. 2011. Some coagulase-negative Staphylococcus species affect udder health more than others. J Dairy Sci. 94: 2329–2340.
- Swinkels JM, Rooijendijk RG, Zadoks RN, Hogeveen H. 2005. Use of partial budgeting to determine the economic benefits of antibiotic treatment of chronic subclinical mastitis caused by Streptococcus uberis or Streptococcus dysgalactiae. J Dairy Res. 72: 75–85.
- Van den Borne BHP, Vernooij JCM, Lupindu AM, van Schaik G, Frankena K, Lam TJGM, Nielen M. 2011. Relationship between somatic cell count status and subsequent clinical mastitis in Dutch dairy cows. Prev Vet Med. 102: 265–273.
- Wallace JA, Schukken YH, Welcome F. 2003. Measuring stimulation's effect with milk flow curves. Proceedings of the 42ndAnnual Meeting of the National Mastitis Council, Forth Worth, TX, USA, 86–97. National Mastitis Council; Verona, WI, USA.
- Weiss WP, Hogan JS, Smith KL, Hoblet KH. 1990. Relationships among selenium, vitamin E, and mammary gland health in commercial dairy herds. J Dairy Sci. 73: 381–390.
- White LJ, Lam TJGM, Schukken YH, Green LE, Medley GF, Chappell MJ. 2006. The transmission and control of mastitis in dairy cows: A theoretical approach. Prev Vet Med. 74: 67–83.
- Wilson DJ, Das HH, Gonzalez RN, Sears PM. 1997. Association between management practices, dairy herd characteristics, and somatic cell count of bulk tank milk. J Am Vet Med Assoc. 210: 1499–1502.
- Zadoks RN, Allore HG, Hagenaars TJ, Barkema HW, Schukken YH. 2002. A mathematical model of Staphylococcus aureus control in dairy herds. Epidemiol Infect. 129: 397–416.
- Zadoks RN, Gonzalez RN, Boor KJ, Schukken YH. 2004. Mastitis-causing streptococci are important contributors to bacterial counts in raw bulk tank milk. J Food Prot. 67: 2644–2650.
- Zadoks RN, Schulte HF, Tikofsky-Garrison LL. 2005. Molecular tools enhance the value of bulk tank milk monitoring. Proceedings 44th Annual Meeting of the NMC, Orlando, FL, USA, 86–93, NMC, Verona, WI, USA.